Grapevine Diversity and Genetic Relationships in Northeast Portugal Old Vineyards
Abstract
:1. Introduction
2. Results and Discussion
2.1. Genetic Identification Based on nSSR and SNP Markers
2.2. Nuclear SSR and SNP Diversity
2.3. Chlorotype Diversity
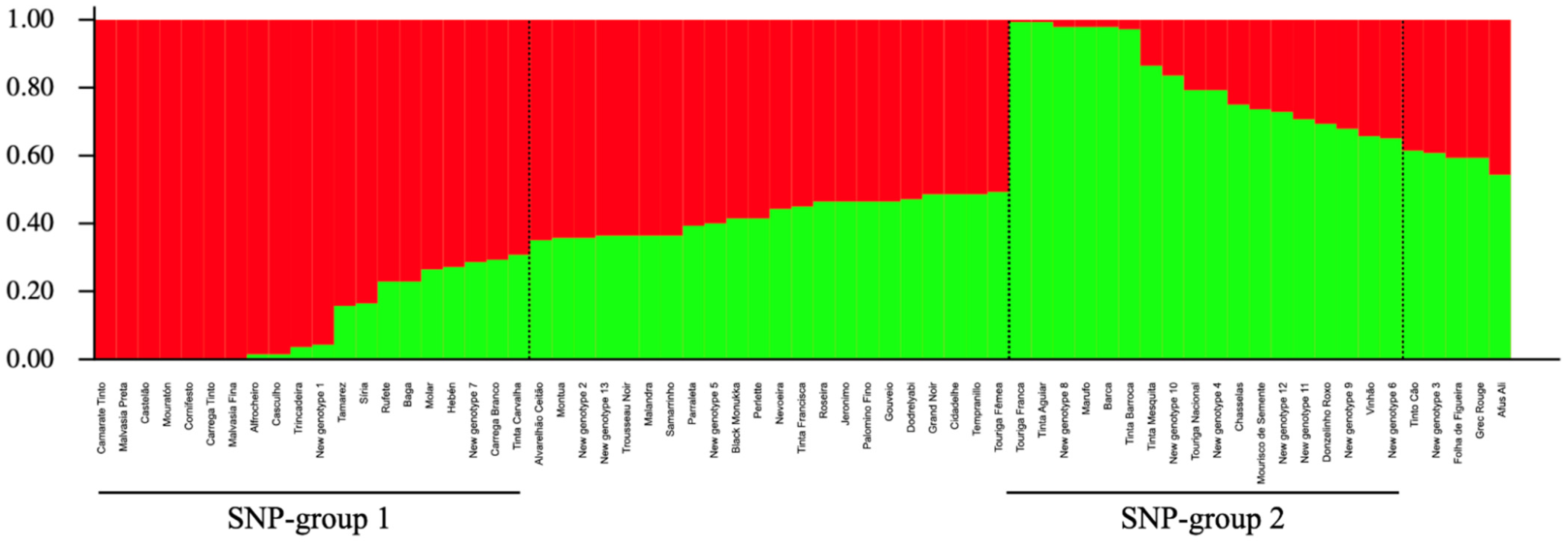
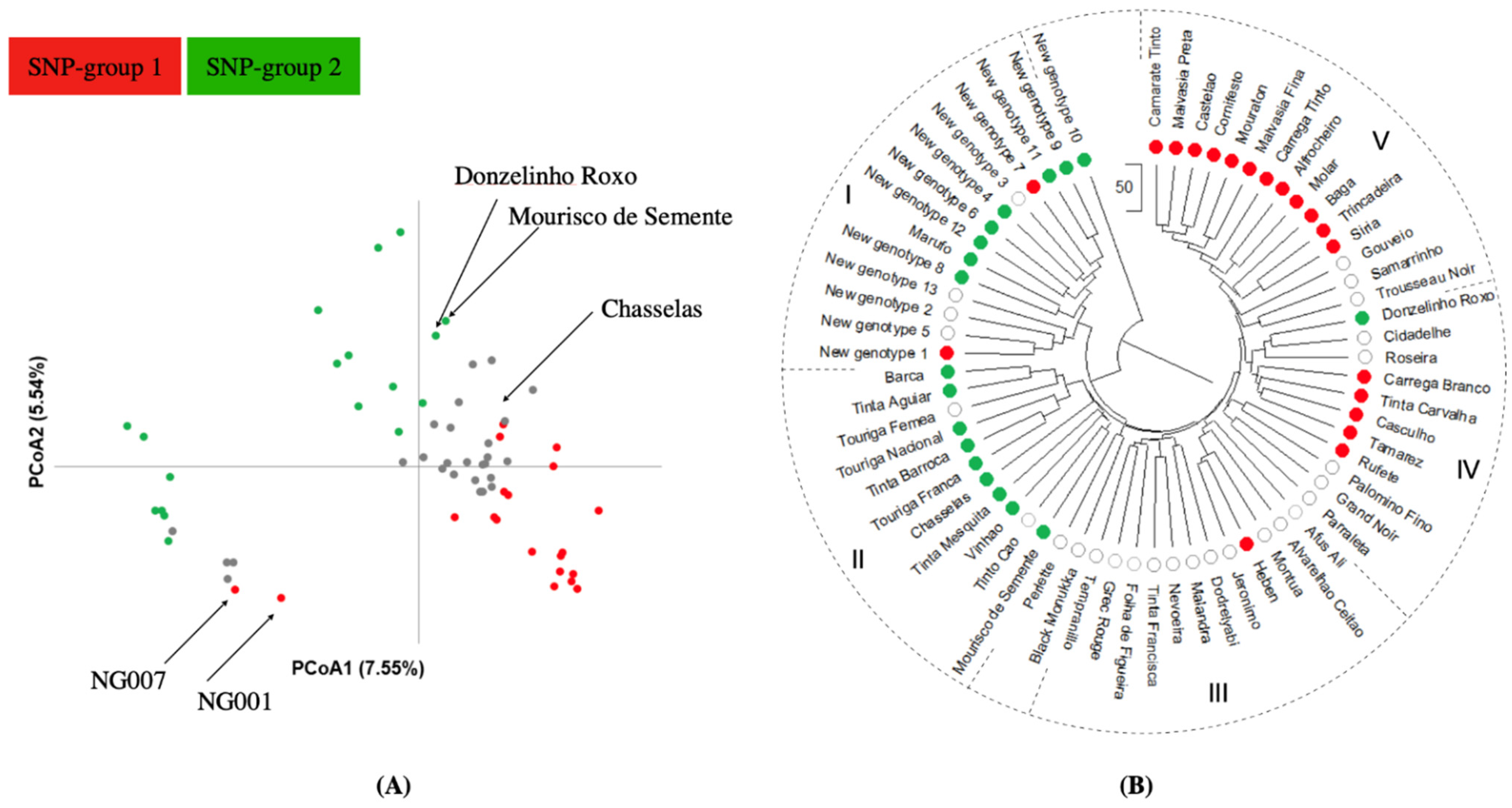
2.4. Population Structure Analysis
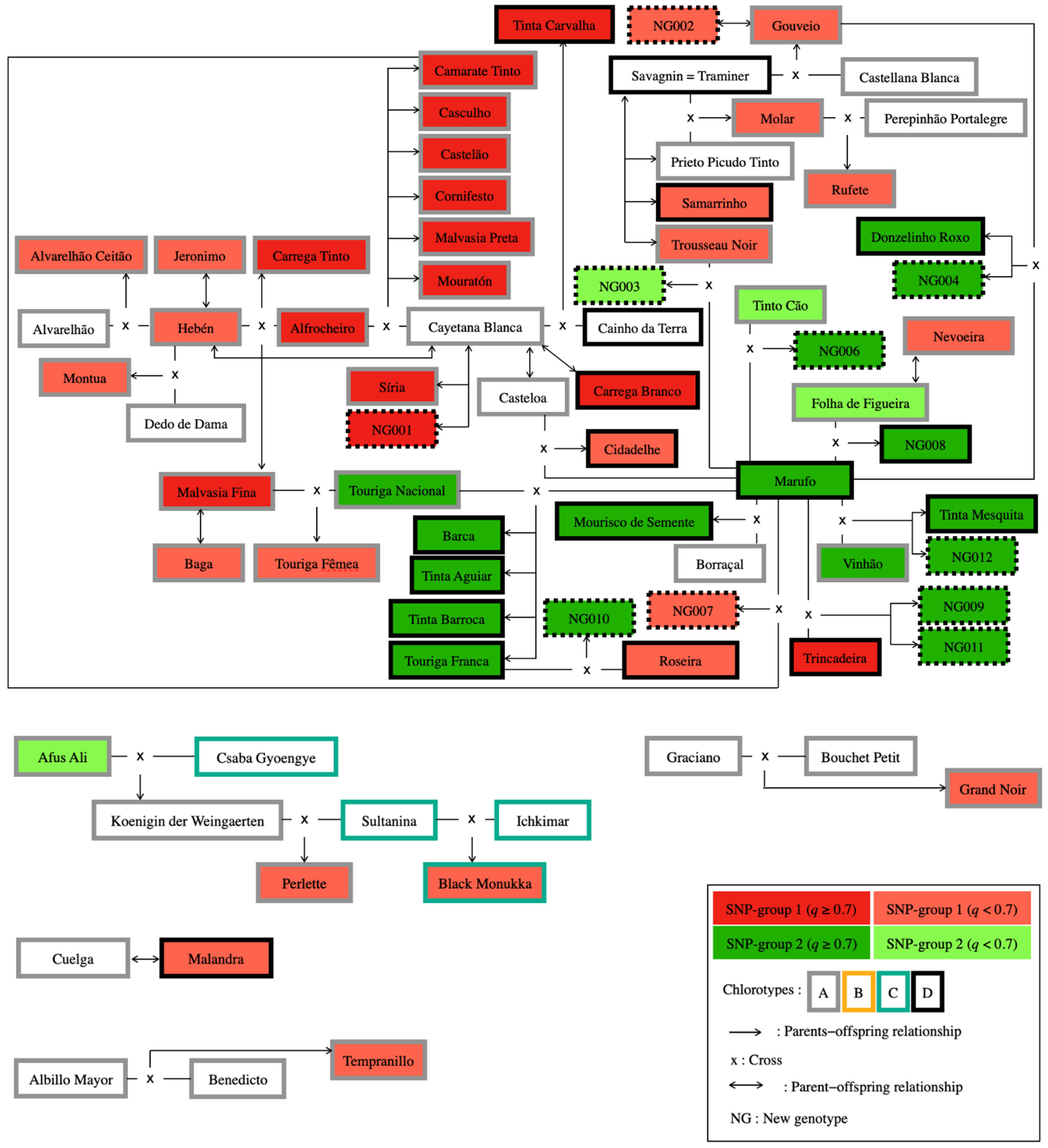
2.5. Pedigree Analysis
3. Materials and Methods
3.1. Sampling and DNA Extraction
3.2. Genotyping and Varietal Identification through SSR and SNP Markers
3.2.1. SSR Markers
3.2.2. SNP Markers
3.3. Data Analyses
3.3.1. Genetic Diversity Analysis
3.3.2. Population Structure Analysis
3.3.3. Pedigree Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IVV. Anuario de Vinhos e Aguardentes de Portugal; Instituto da Vinha e do Vinho: Lisbon, Portugal, 2018; pp. 111–121.
- Graca, A. Conservar a Biodiversidade da Vitis vinifera em Portugal: Um suporte estratégico da exportação dos vinhos portugueses. ENOLOGIA Assoc. Portug. Enol. 2012, 59–60, 3–8. [Google Scholar]
- Veloso, M.M.; Almadanim, M.C.; Baleiras-Couto, M.; Pereira, H.S.; Carneiro, L.C.; Fevereiro, P.; Eiras-Dias, J.E. Microsatellite database of grapevine (Vitis vinifera L.) cultivars used for wine production in Portugal. Cien. Tecn. Vitivinic. 2010, 25, 53–61. [Google Scholar]
- Castro, I.; Martín, J.P.; Ortiz, J.M.; Pinto-Carnide, O. Varietal discrimination and genetic relationships of Vitis vinifera L. cultivars from two major Controlled Appellation (DOC) regions in Portugal. Sci. Hortic. 2011, 127, 507–514. [Google Scholar] [CrossRef]
- Ferreira, V.; Pinto-Carnide, O.; Mota, T.; Martín, J.P.; Ortiz, J.M.; Castro, I. Identification of minority grapevine cultivars from Vinhos Verdes Portuguese DOC Region. Vitis 2015, 54, 53–58. [Google Scholar] [CrossRef]
- Cunha, J.; Ibáñez, J.; Teixeira-Santos, M.; Brazão, J.; Fevereiro, P.; Martínez-Zapater, J.M.; Eiras-Dias, J.E. Characterisation of the Portuguese grapevine germplasm with 48 single-nucleotide polymorphisms. Aust. J. Grape Wine Res. 2016, 22, 504–516. [Google Scholar] [CrossRef]
- Emanuelli, F.; Lorenzi, S.; Grzeskowiak, L.; Catalano, V.; Stefanini, M.; Troggio, M.; Myles, S.; Martínez-Zapater, J.M.; Zyprian, E.; Moreira, F.M.; et al. Genetic diversity and population structure assessed by SSR and SNP markers in large germplasm collection of grape. BMC Plant Biol. 2013, 13, 39. [Google Scholar] [CrossRef] [Green Version]
- Pelsy, F. Molecular and cellular mechanisms of diversity within grapevine varieties. Heredity 2010, 104, 331–340. [Google Scholar] [CrossRef]
- Cervera, M.; Cabezas, J.; Sancha, J.; De Toda, F.; Martínez-Zapater, J.M. Application of AFLPs to the characterization of grapevine Vitis vinifera L. genetic resources. A case study with accessions from Rioja (Spain). Theor. Appl. Genet. 1998, 97, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Sefc, K.M.; Lopes, M.S.; Lefort, F.; Botta, R.; Roubelakis-Angelakis, K.A.; Ibáñez, J.; Pejic, I.; Wagner, H.W.; Glössl, J.; Steinkellner, H. Microsatellite variability in grapevine cultivars from different European regions and evaluation of assignment testing to assess the geographic origin of cultivars. Theor. Appl. Genet. 2000, 100, 498–505. [Google Scholar] [CrossRef]
- Sefc, K.M.; Regner, F.; Turetschek, E.; Glössl, J.; Steinkellner, H. Identification of microsatellite sequences in Vitis riparia and their applicability of genotyping of different Vitis species. Genome 1999, 42, 367–373. [Google Scholar] [CrossRef] [PubMed]
- This, P.; Jung, A.; Boccacci, P.; Borrego, J.; Botta, R.; Costantini, L.; Crespan, M.; Dangl, G.S.; Eisenheld, C.; Ferreira-Monteiro, F.; et al. Development of a standard set of microsatellite reference alleles for identification of grape cultivars. Theor. Appl. Genet. 2004, 109, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-García, R.; Ruiz-García, L.; Bolling, L.; Ocete, R.; López, M.A.; Arnold, C.A.; Ergul, A.; Söylemezoglu, G.; Uzun, H.I.; Cabello, F.; et al. Multiple origins of cultivated grapevine (Vitis vinifera L. ssp. sativa) based on chloroplast DNA polymorphisms. Mol. Ecol. 2006, 15, 3707–3714. [Google Scholar] [CrossRef] [Green Version]
- Lopes, M.S.; Sefc, K.M.; Eiras-Dias, J.E.; Steinkellner, H.; Laimer da Câmara Machado, M.; Câmara Machado, A. The use of microsatellites for germplasm management in a Portuguese grapevine collection. Theor. Appl. Genet. 1999, 99, 733–739. [Google Scholar] [CrossRef]
- Lopes, M.S.; Rodrigues dos Santos, M.; Eiras-Dias, J.E.; Mendonça, D.; Câmara Machado, A. Discrimination of Portuguese grapevines based on microsatellite markers. J. Biotech. 2006, 127, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Baleiras-Couto, M.M.; Eiras-Dias, J.E. Detection and identification of grape varieties in must and wine using nuclear and chloroplast microsatellite markers. Anal. Chim. Acta 2006, 563, 283–291. [Google Scholar] [CrossRef]
- Almadanim, M.C.; Baleiras-Couto, M.M.; Pereira, H.S.; Carneiro, L.C.; Fevereiro, P.; Eiras-Dias, J.E.; Morais-Cecílio, L.; Viegas, W.; Veloso, M.M. Genetic diversity of the grapevine (Vitis vinifera L.) cultivars most utilized for wine production in Portugal. Vitis 2007, 46, 116–119. [Google Scholar]
- Cunha, J.; Teixeira-Santos, M.; Carneiro, L.C.; Fevereiro, P.; Eiras-Dias, J.E. Portuguese traditional grapevine cultivars and wild vines (Vitis vinifera L.) share morphological and genetic traits. Genet. Res. Crop Evol. 2009, 56, 975–989. [Google Scholar] [CrossRef]
- Arroyo-García, R.; LeFort, F.; de Andrés, M.T.; Ibáñez, J.; Borrego, J.; Jouve, N.; Cabello, F.; Martínez-Zapater, J.M. Chloroplast microsatellite polymorphisms in Vitis species. Genome 2002, 45, 1142–1149. [Google Scholar] [CrossRef] [Green Version]
- Cunha, J.; Ibáñez, J.; Teixeira-Santos, M.; Brazão, J.; Fevereiro, P.; Martínez-Zapater, J.M.; Eiras-Dias, J.E. Genetic relationships among Portuguese cultivated and wild Vitis vinifera L. germplasm. Front. Plant Sci. 2020, 11, 127. [Google Scholar] [CrossRef] [Green Version]
- Ghaffari, S.; Hasnaoui, N.; Zinelabidine, L.H.; Ferchichi, A.; Martínez-Zapater, J.M.; Ibáñez, J. Genetic diversity and parentage of Tunisian wild and cultivated grapevines (Vitis vinifera L.) as revealed by single nucleotide polymorphism (SNP) markers. Tree Genet. Genomes 2014, 10, 1103–1112. [Google Scholar] [CrossRef]
- De Lorenzis, G.; Mercati, F.; Bergamini, C.; Cardone, M.F.; Lupini, A.; Mauceri, A.; Caputo, A.R.; Abbate, L.; Barbagallo, M.G.; Antonacci, D.; et al. SNP genotyping elucidates the genetic diversity of Magna Graecia grapevine germplasm and its historical origin and dissemination. BMC Plant Biol. 2019, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, O.; Aury, J.M.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C.; et al. French-Italian public consortium for grapevine genome characterization. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 2007, 449, 463–467. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Troggio, M.; Cartwright, D.A.; Cestaro, A.; Pruss, D.; Pindo, M.; FitzGerald, L.M.; Vezzulli, S.; Reid, J.; et al. A High quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS ONE 2007, 2, e1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lijavetzky, D.; Cabezas, J.A.; Ibáñez, A.; Rodríguez, V.; Martínez-Zapater, J.M. High throughput SNP discovery and genotyping in grapevine (Vitis vinifera L.) by combining a re-sequencing approach and SNPlex technology. BMC Genom. 2007, 8, 424–435. [Google Scholar] [CrossRef] [Green Version]
- Myles, S.; Chia, J.-M.; Hurwitz, B.; Simon, C.; Zhong, G.Y.; Buckler, E.; Ware, D. Rapid genomic characterization of the genus Vitis. PLoS ONE 2010, 5, e8219. [Google Scholar] [CrossRef] [Green Version]
- Myles, S.; Boyko, A.R.; Owens, C.L.; Brown, P.J.; Grassi, F.; Aradhya, M.K.; Prins, B.; Reynolds, A.; Chia, J.-M.; Ware, D.; et al. Genetic structure and domestication history of the grape. Proc. Natl. Acad. Sci. USA 2011, 108, 3530–3535. [Google Scholar] [CrossRef] [Green Version]
- Le Paslier, M.-C.; Choisne, N.; Bacilieri, R.; Bounon, R.; Boursiquot, J.-M.; Bras, M.; Brunel, D.; Di Gaspero, G.; Hausmann, L.; Lacombe, T.; et al. The GrapeReSeq 18k Vitis Genotyping Chip. In Proceedings of the IX International Symposium on Grapevine Physiology and Biotechnology, La Serena, Chile, 21–26 April 2013; p. 23. Available online: https://hal.inrae.fr/hal-02811055 (accessed on 10 December 2021).
- Cabezas, J.A.; Ibáñez, J.; Lijavetzky, D.; Vélez, D.; Bravo, G.; Rodríguez, V.; Carreño, I.; Jermakow, A.M.; Carreño, J.; Ruiz-García, L.; et al. A 48 SNP set for grapevine cultivar identification. BMC Plant Biol. 2011, 11, 153. [Google Scholar] [CrossRef] [Green Version]
- Marrano, A.; Birolo, G.; Prazzoli, M.L.; Lorenzi, S.; Valle, G.; Grando, M.S. SNP-discovery by RAD-sequencing in a germplasm collection of wild and cultivated grapevines (V. vinifera L.). PLoS ONE 2017, 12, e1070655. [Google Scholar] [CrossRef] [PubMed]
- Degu, A.; Morcia, C.; Tumino, G.; Hochberg, U.; Toubiana, D.; Mattivi, F.; Schneider, A.; Bosca, P.; Cattivelli, L.; Terzi, V.; et al. Metabolite profiling elucidates communalities and differences in the polyphenol biosynthetic pathways of red and white Muscat genotypes. Plant Physiol. Biochem. 2015, 86, 24–33. [Google Scholar] [CrossRef]
- De Lorenzis, G.; Chipashvili, R.; Failla, O.; Maghradze, D. Study of genetic variability in Vitis vinifera L. germplasm by high-throughput Vitis18kSNP array: The case of Georgian genetic resources. BMC Plant Biol. 2015, 5, 154. [Google Scholar] [CrossRef] [Green Version]
- De Lorenzis, G.; Squadrito, M.; Rossoni, M.; Simone Di Lorenzo, G.; Brancadoro, L.; Scienza, A. Study of intra-varietal diversity in biotypes of Aglianico and Muscat of Alexandria (Vitis vinifera L.) cultivars. Aust. J. Grape Wine Res. 2017, 23, 32–42. [Google Scholar] [CrossRef]
- Mercati, F.; De Lorenzis, G.; Brancadoro, L.; Lupini, A.; Abenavoli, M.R.; Barbagallo, M.G.; Di Lorenzo, R.; Scienza, A.; Sunseri, F. High-throughput 18K SNP array to assess genetic variability of the main grapevine cultivars from Sicily. Tree Genet. Genomes 2016, 12, 59. [Google Scholar] [CrossRef] [Green Version]
- Ruffa, P.; Raimondi, S.; Boccacci, P.; Abbà, S.; Schneider, A. The key role of “Moscato bianco” and “Malvasia aromatica di Parma” in the parentage of traditional aromatic grape varieties. Tree Genet. Genomes 2016, 12, 50. [Google Scholar] [CrossRef]
- Sunseri, F.; Lupini, A.; Mauceri, A.; De Lorenzis, G.; Araniti, F.; Brancadoro, L.; Dattola, A.; Gullo, G.; Zappia, R.; Mercati, F. Single nucleotide polymorphism profiles reveal an admixture genetic structure of grapevine germplasm from Calabria, Italy, uncovering its key role for the diversification of cultivars in the Mediterranean Basin. Aust. J. Grape Wine Res. 2018, 24, 345–359. [Google Scholar] [CrossRef]
- Laucou, V.; Launay, A.; Bacilieri, R.; Lacombe, T.; Adam-Blondon, A.-F.; Bérard, A.; De Andrés, M.T.; Hausmann, L.; Ibáñez, J.; Le Paslier, M.-C.; et al. Extended diversity analysis of cultivated grapevine Vitis vinifera with 10K genome-wide SNPs. PLoS ONE 2018, 13, e0192540. [Google Scholar] [CrossRef] [PubMed]
- Maraš, V.; Tello, J.; Gazivoda, A.; Mugoša, M.; Perišić, M.; Raičević, J.; Štajner, N.; Ocete, R.; Bozovic, V.; Popović, T.; et al. Population genetic analysis in old Montenegrin vineyards reveals ancient ways currently active to generate diversity in Vitis vinifera. Sci. Rep. 2020, 10, 15000. [Google Scholar] [CrossRef] [PubMed]
- Maul, E.; Töpfer, R.; Röckel, F.; Brühl, U.; Hundemer, M.; Mahler-Ries, A.; Walk, M.; Kecke, S.; Wolck, A.; Ganesch, A. Vitis International Variety Catalogue. 2021. Available online: www.vivc.de (accessed on 2 July 2021).
- European Union. European Court of Auditors Special Report No. 09—Is the EU Investment and Promotion Support to the Wine Sector Well Managed and are its Results on the Competitiveness of EU Wines Demonstrated? Publications Office of the European Union: Luxembourg, 2014; pp. 1–11. [Google Scholar]
- OIV. Liste Internationale des Variétés de Vigne et de leurs Synonymes; OIV: Paris, France, 2013.
- Ebadi, A.; Ghaderi, N.; Vafaee, Y. Genetic diversity of Iranian and some European grapes as revealed by nuclear and chloroplast microsatellite and SNP molecular markers. J. Hortic. Sci. Biotech. 2019, 94, 599–610. [Google Scholar] [CrossRef]
- Mercati, F.; De Lorenzis, G.; Mauceri, A.; Zerbo, M.; Brancadoro, L.; D’Onofrio, C.; Morcia, C.; Barbagallo, M.G.; Bignami, C.; Gardiman, M.; et al. Integrated Bayesian Approaches Shed Light on the Dissemination Routes of the Eurasian Grapevine Germplasm. Front. Plant Sci. 2021, 12, 692661. [Google Scholar] [CrossRef]
- D’Onofrio, C.; Tumino, G.; Gardiman, M.; Crespan, M.; Bignami, C.; de Palma, L.; Barbagallo, M.G.; Muganu, M.; Morcia, C.; Novello, V.; et al. Parentage Atlas of Italian Grapevine Varieties as Inferred from SNP Genotyping. Front. Plant Sci. 2021, 11, 605934. [Google Scholar] [CrossRef]
- Díaz-Losada, E.; Salgado, A.; Ignacio, O.; Ramos-Cabrer, A.; Pereira-Lorenzo, S. New Synonyms and Homonyms for Cultivars from Northwestern Spain. Am. J. Enol. Vitic. 2013, 64, 156–162. [Google Scholar] [CrossRef]
- Martín, J.P.; Santiago, J.L.; Pinto-Carnide, O.; Leal, F.; Martínez, M.C.; Ortiz, J.M. Determination of relationships among autochthonous grapevine varieties (Vitis vinifera L.) in the Northwest of the Iberian Peninsula by using microsatellite markers. Genet. Resour. Crop Evol. 2005, 53, 1255–1261. [Google Scholar] [CrossRef]
- Díaz-Losada, E.; Salgado, A.; Ramos-Cabrer, A.; Segade, S.; Diéguez, S.; Pereira-Lorenzo, S. Twenty microsatellites (SSRs) reveal two main origins of variability in grapevine cultivars from Northwestern Spain. Vitis 2010, 49, 55–62. [Google Scholar]
- Ministério da Agricultura. Partaria N° 380/2012, de 22 de Novembro. Ministério da Agricultura, do Mar, do Ambiente e do Ordenamento do Território (MAMAOT); Diário da República, 1.a Série—N.° 226; Ministério da Agricultura: Lisbon, Portugal, 2012.
- Ministério da Agricultura. Partaria N° 1204/2006, de 9 de Novembro. Ministério da Agricultura, do Desenvolvimento Rural e das Pescas (MADRP); Diário da República, 1.a Série— N.° 216; Ministério da Agricultura: Lisbon, Portugal, 2006.
- Ministério da Agricultura. Partaria N° 383/2017, de 20 de Dezembro. Agricultura, Florestas e Desenvolvimento Rural (AFDR); Diário da República, 1.a Série— N.° 243; Ministério da Agricultura: Lisbon, Portugal, 2017.
- Cardoso, M.R. Casa do Douro, 2nd ed.; Peso da Régua, Portugal, 1995. [Google Scholar]
- Böhm, J.; Antunes, M.T.; Andrade, R.; Barroso, J.M.; Cabrita, M.J.; Cardoso, H.; Eiras-Dias, J.E.; Fernandes, L.; Fevereiro, P.; Figueiredo, A.; et al. Portugal Vitícola, o Grande Livro das Castas: Enciclopédia dos Vinhos de Portugal, 2nd ed.; Chaves Ferreira Publicações: Lisbon, Portugal, 2007. [Google Scholar]
- Cunha, J.; Teixeira-Santos, M.; Veloso, M.M.; Carneiro, L.; Eiras-Dias, J.E.; Fevereiro, P. The Portuguese Vitis vinifera L. Germplasm: Genetic Relations between Wild and Cultivated Vines. Cienc. E Tec. Vitivinic. 2010, 25, 25–37. [Google Scholar]
- Bowers, J.E.; Dangl, G.S.; Vignani, R.; Meredith, C.P. Isolation and characterization of new polymorphic simple sequence repeat loci in grape (Vitis vinifera L.). Genome 1996, 39, 628–633. [Google Scholar] [CrossRef]
- Singh, N.; Choudhury, D.R.; Singh, A.K.; Kumar, S.; Srinivasan, K.; Tyagi, R.K.; Singh, N.K.; Singh, R. Comparison of SSR and SNP markers in estimation of genetic diversity and population structure of Indian rice varieties. PLoS ONE 2013, 8, e84136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moita Maçanita, A.; Santos, R.; Gomes, A.C.; Santos, A. Unravelling the origin of Vitis vinifera L. Verdelho. Aust. J. Grape Wine Res. 2018, 24, 450–460. [Google Scholar] [CrossRef] [Green Version]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacombe, T.; Boursiquot, J.; Laucou, V.; Vecchi-Staraz, M.; Péros, J.; This, P. Large-scale parentage analysis in an extended set of grapevine cultivars (Vitis vinifera L.). Theor. Appl. Genet. 2013, 126, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Zinelabidine, L.H.; Haddioui, A.; Rodriguez, V.; Cabello, F.; Eiras-Dias, J.E.; Martínez-Zapater, J.M.; Ibáñez, J. Identification by SNP Analysis of a Major Role for “Cayetana Blanca” in the Genetic Network of Iberian Peninsula Grapevine Varieties. Am. J. Enol. Vitic. 2012, 63, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Cunha, J.; Zinelabidine, L.H.; Teixeira-Santos, M.; Brazão, J.; Fevereiro, P.; Martínez-Zapater, J.M.; Ibáñez, J.; Eiras-Dias, J.E. Grapevine cultivar ‘Alfrocheiro’ or ‘Brunal’ plays a primary role in the relationship among Iberian grapevines. Vitis 2015, 54, 59–65. [Google Scholar]
- Cipriani, G.; Spadotto, A.; Jurman, I.; Di Gaspero, G.; Crespan, M.; Meneghetti, S.; Frare, E.; Vignani, R.; Cresti, M.; Morgante, M.; et al. The SSR-based molecular profile of 1005 grapevine (Vitis vinifera L.) accessions uncovers new synonymy and parentages, and reveals a large admixture amongst varieties of different geographic origin. Theor. Appl. Genet. 2010, 121, 1569–1585. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, J.; Vélez, M.; De Andrés, M.T.; Borrego, J. Molecular markers for establishing distinctness in vegetatively propagated crops: A case study in grapevine. Theor. Appl. Genet. 2009, 119, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, J.; Muñoz-Organero, G.; Zinelabidine, L.H.; De Andrés, M.T.; Cabello, F.; Martínez-Zapater, J.M. Genetic origin of the grapevine cultivar Tempranillo. Am. J. Enol.Vitic. 2012, 63, 549–553. [Google Scholar] [CrossRef]
- Lacerda Lobo, C. Memória sobre a cultura das vinhas de Portugal. In Memórias Económicas da Academia Real das Ciências de Lisboa, para o Adiantamento da Agricultura, das Artes, e da Indústria de Portugal, e Suas Conquistas (1789–1815); Academia Real das Ciências de Lisboa: Lisbon, Portugal, 1790; Volume II. [Google Scholar]
- Antunes, M.T.; Lehmann, J.; Eiras-Dias, J.E.; Böhm, J. Atlas das Castas da Península Ibérica: História, Terroir, Ampelografia; Daniel Gouveia, D., Iglésias, A.M.G., Eds.; Dinalivro: Lisbon, Portugal, 2011. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Weising, K.; Gardner, R.C. A set of conserved PCR primers for the analysis of simple sequence repeat polymorphisms in chloroplast genomes of dicotyledonous angiosperms. Genome 1999, 42, 9–19. [Google Scholar] [CrossRef]
- Castro, I.; Pinto-Carnide, O.; Ortiz, J.M.; Martín, J.P. Chloroplast genome diversity in Portuguese grapevine (Vitis vinifera L.) cultivars. Mol. Biotechnol. 2013, 54, 528–540. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [Green Version]
- Botstein, D.; White, R.L.; Skalnick, M.H.; Davies, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphism. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- Bińkowski, J.; Miks, S. Gene-Calc. 2018. Available online: www.gene-calc.pl. (accessed on 8 February 2021).
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure: Extensions to linked loci and correlated allele frequencies. Genetics 2003, 164, 1567–1587. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Dominant markers and null alleles. Mol. Ecol. Notes 2007, 7, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Hubisz, M.; Falush, D.; Stephens, M.; Pritchard, J.K. Inferring weak population structure with the assistance of sample group information. Mol. Ecol. Resour. 2009, 9, 1322–1332. [Google Scholar] [CrossRef] [Green Version]
- Earl, D.A.; von Holdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy; Freeman: San Francisco, CA, USA, 1973. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
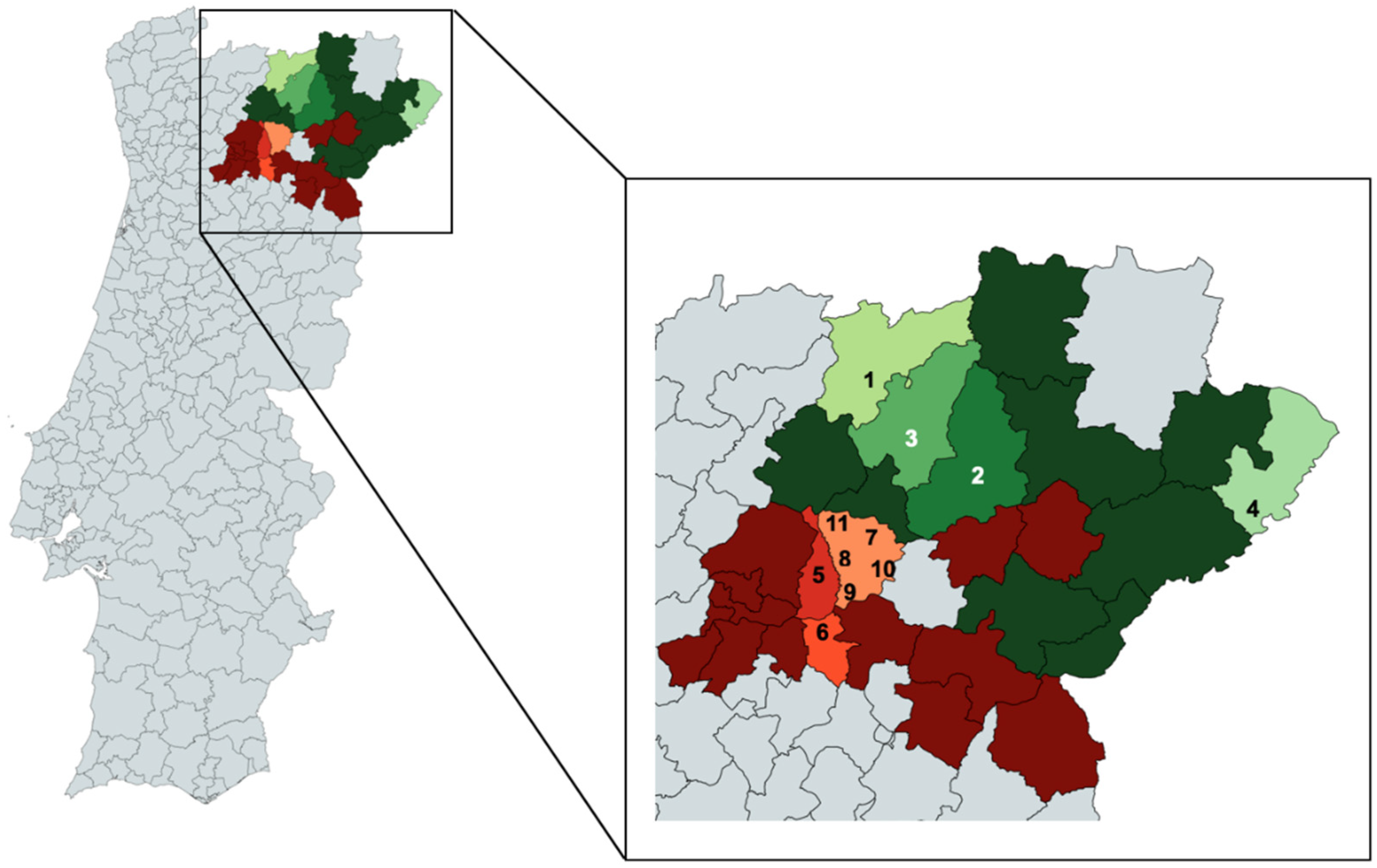
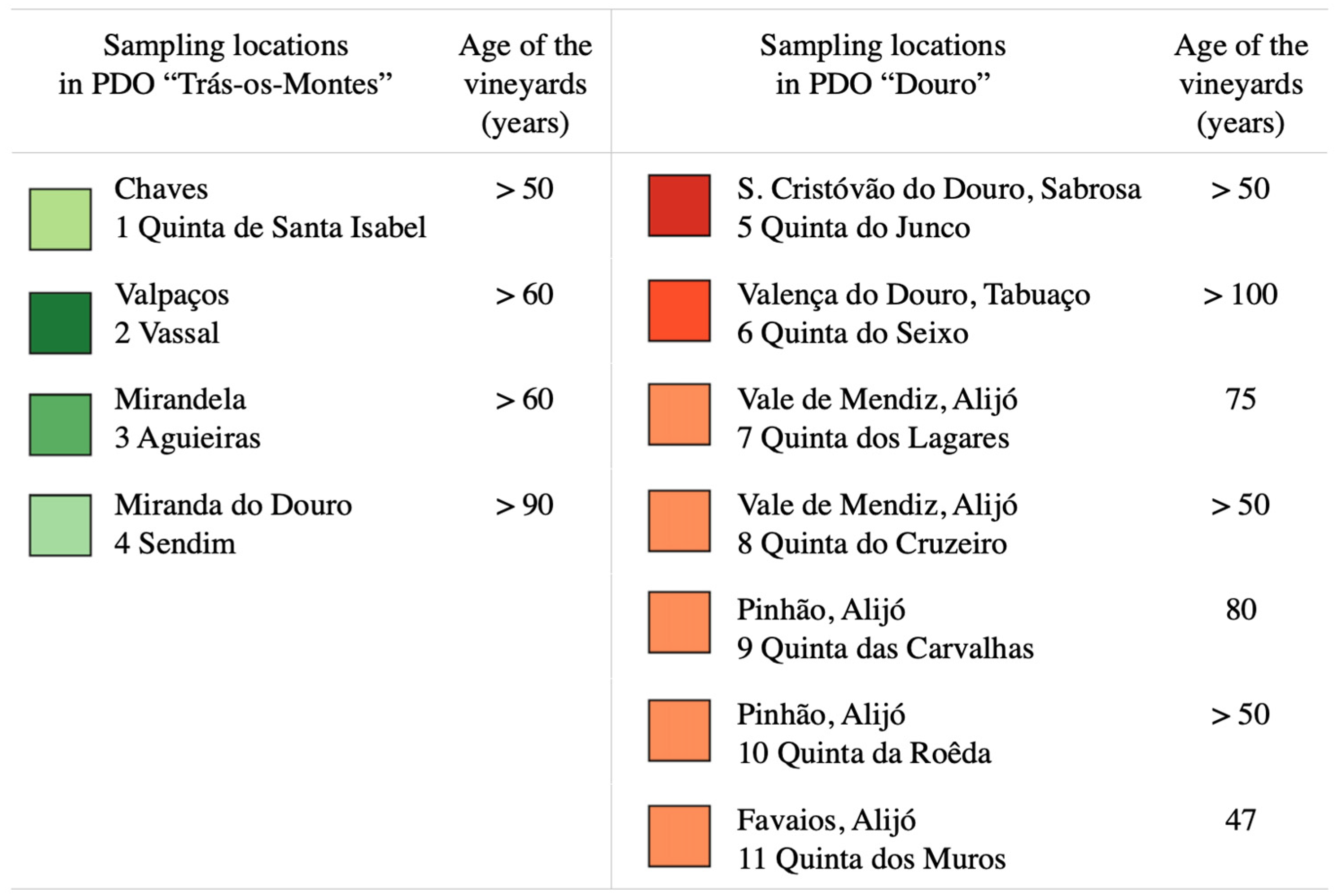
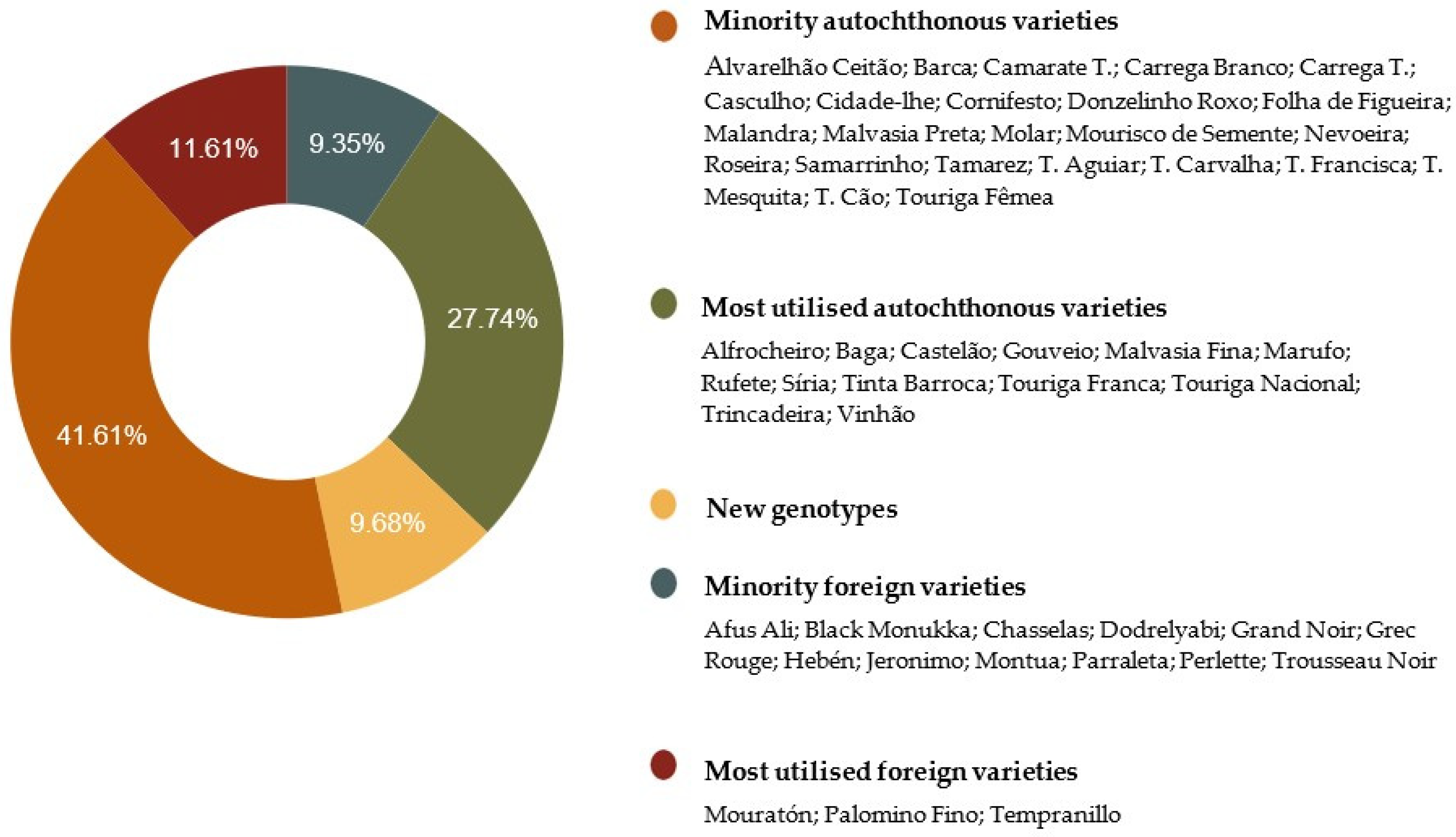
| Sample Origin 1 (Number of Samples Analysed) | Cultivar Prime Name 2 | VIVC Variety No. | ICVV-SNP Genotype No. | Synonymies in Portugal | Colour of Berry Skin 3 | Grape Use |
|---|---|---|---|---|---|---|
| QC (1) | Afus Ali | 122 | GEN_DNA_2036 | B | Wine/Table | |
| J (1) | Alfrocheiro (D) * | 277 | GEN_DNA_2173 | Tinta Bastardinha | N | Wine |
| QSI (1) | Alvarelhão Ceitão (D) * | 368 | GEN_DNA_2837 | R | Wine | |
| Qs (1) | Baga (D,T) * | 885 | GEN_DNA_1267 | N | Wine | |
| QC (1), M (1) | Barca (D) * | 17359 | GEN_DNA_3010 | N | Wine | |
| QSI (1), Vs (1) | Black Monukka | 17452 | GEN_DNA_2127 | N | Table | |
| V (2), Qs (1), QC (6), J (1) | Camarate Tinto * | 2018 | GEN_DNA_0634 | N | Wine | |
| Ag (2), Sd (9) | Carrega Branco (D,T) * | 2124 | GEN_DNA_2199 | B | Wine | |
| R (1) | Carrega Tinto (D) * | 2125 | GEN_DNA_1270 | Tinta Grossa | N | Wine |
| Sd (13), Qs (1), QC (9) | Casculho (D) * | 14149 | GEN_DNA_2976 | N | Wine | |
| V (1), QC (1) | Castelão (D,T) * | 2324 | GEN_DNA_1168 | N | Wine/Table | |
| Vs (1), QSI (1) | Chasselas (D) | 2473 | GEN_DNA_2055 | B | Wine/Table | |
| Qs (1) | Cidadelhe (D) * | 12476 | GEN_DNA_2997 | N | Wine | |
| Sd (1), QC (1), J (1) | Cornifesto (D,T) * | 2846 | GEN_DNA_1229 | N | Wine | |
| QSI (4) | Dodrelyabi | 3616 | GEN_DNA_0984 | N | Wine/Table | |
| Vs (2) | Donzelinho Roxo * | 17677 | GEN_DNA_2964 | R | Wine | |
| Ag (1), V (1) | Folha de Figueira (D) * | 14142 | GEN_DNA_3002 | Dona Branca | B | Wine/Table |
| Ag (2), Sd (2) | Gouveio (D,T) * | 12953 | GEN_DNA_1133 | B | Wine | |
| Qs (1) | Grand Noir (D) | 5012 | GEN_DNA_1110 | N | Wine | |
| J (1) | Grec Rouge | 4962 | GEN_DNA_1212 | Rabigato Franco | R | Wine/Table |
| QSI (2) | Hebén (D) | 5335 | GEN_DNA_1258 | Mourisco Branco | B | Wine/Table |
| Vs (1) | Jeronimo | 5692 | GEN_DNA_2236 | N | Wine/Table | |
| M (3), J (2) | Malandra (D) * | 12487 | GEN_DNA_2967 | N | Wine | |
| QC (1) | Malvasia Fina (D,T) * | 715 | GEN_DNA_2245 | B | Wine | |
| QC (11) | Malvasia Preta (D,T) * | 15647 | GEN_DNA_2347 | N | Wine | |
| Ag (1), Vs (4), QSI (1), Sd (1), V (2), Qs (1), QC (3) | Marufo (D,T) * | 8086 | GEN_DNA_1205 | N | Wine/Table | |
| Ag (1) | Molar * | 15678 | GEN_DNA_2128 | Tinta Negra | N | Wine/Table |
| Vs (2) | Montua (D) | 2520 | GEN_DNA_0621 | Diagalves | B | Wine/Table |
| Ag (1), Vs (3), Sd (12), Qs (1) | Mouratón (T) | 8082 | GEN_DNA_2201 | Tinta Gorda | N | Wine |
| QC (7) | Mourisco de Semente (D) * | 12471 | GEN_DNA_2999 | N | Wine | |
| QC (6) | Nevoeira (D) * | 8504 | GEN_DNA_3008 | N | Wine | |
| QSI (6) | Palomino Fino (D) | 8888 | GEN_DNA_1063 | Malvasia Rei | B | Wine/Table |
| QC (1) | Parraleta (D) | 8951 | GEN_DNA_1003 | Tinta Caiada | N | Wine |
| Sd (2) | Perlette | 9168 | GEN_DNA_0148 | B | Table/Raisin | |
| Qs (6), QC (10) | Roseira (D) * | 12497 | GEN_DNA_2971 | N | Wine | |
| Qs (1) | Rufete (D,T) * | 10331 | GEN_DNA_2106 | Tinta Pinheira | N | Wine |
| Vs (1) | Samarrinho (D,T) * | 15684 | GEN_DNA_0856 | Budelho | B | Wine |
| Vs (2), QSI (7), Sd (8) | Síria (D,T) * | 2742 | GEN_DNA_1154 | Roupeiro, Códega | B | Wine/Table |
| R (1) | Tamarez (D) * | 12231 | GEN_DNA_2224 | Molinha | B | Wine |
| Vs (1), Sd (2), V (8), QC (2) | Tempranillo (D,T) | 12350 | GEN_DNA_1316 | Aragonez, Tinta Roriz | N | Wine/Table |
| V (1), C (1) | Tinta Aguiar (D) * | 12459 | GEN_DNA_2968 | N | Wine | |
| Sd (1), QC (1) | Tinta Barroca (D,T) * | 12462 | GEN_DNA_1167 | N | Wine | |
| Vs (2), V (2), QC (2), M (1) | Tinta Carvalha (D,T) * | 12467 | GEN_DNA_1123 | N | Wine | |
| V (4), Qs (1), QC (2) | Tinta Francisca (D) * | 15686 | GEN_DNA_2348 | N | Wine | |
| C (1) | Tinta Mesquita (D) * | 12489 | GEN_DNA_3215 | N | Wine | |
| Vs (1), Sd (1), QC (2) | Tinto Cão (D,T) * | 12500 | GEN_DNA_0651 | N | Wine | |
| QC (4) | Touriga Fêmea (D) * | 12592 | GEN_DNA_2969 | Touriga Brasileira | N | Wine |
| Sd (1), QC (5), M (1) | Touriga Franca (D,T) * | 12593 | GEN_DNA_0493 | N | Wine | |
| V (2), Qs (1), QC (3) | Touriga Nacional (D,T) * | 12594 | GEN_DNA_0760 | N | Wine | |
| Vs (2), QSI (9), Sd (4), V (8), QC (2), J (3) | Trincadeira (D,T) * | 15685 | GEN_DNA_1239 | Tinta Amarela, Trincadeira Preta | N | Wine |
| Vs (1), QSI (3), Sd (4), Qs (1) | Trousseau Noir (D,T) | 12668 | GEN_DNA_2156 | Bastardo | N | Wine |
| Qs (1), QC (2) | Vinhão (D,T) * | 13100 | GEN_DNA_2240 | Sousão | N | Wine |
| Ag (3) | NG001 | GEN_DNA_4342 | B | Wine | ||
| Ag (1) | NG002 | GEN_DNA_4343 | B | Wine | ||
| Ag (1) | NG003 | GEN_DNA_4344 | N | Wine | ||
| Ag (1) | NG004 | GEN_DNA_4345 | R | Wine | ||
| Sd (1) | NG005 | GEN_DNA_4346 | B | Wine | ||
| QC (4) | NG006 | GEN_DNA_4347 | N | Wine | ||
| QC (4) | NG007 | GEN_DNA_4335 | N | Wine | ||
| QC (4) | NG008 | GEN_DNA_4336 | N | Wine | ||
| QC (4) | NG009 | GEN_DNA_4337 | N | Wine | ||
| QC (1) | NG010 | GEN_DNA_4348 | N | Wine | ||
| Qs (1) | NG011 | GEN_DNA_4349 | B | Wine | ||
| C (1) | NG012 | GEN_DNA_4350 | N | Wine | ||
| J (7) | NG013 | GEN_DNA_4338 | N | Wine |
| 6 nSSR Markers | 226 SNP Markers | |||||
|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean ± SE | Minimum | Maximum | Mean ± SE | |
| Na | 7 (VVMD7) | 12 (VVS2) | 9.500 ± 0.671 | - | - | 2.000 |
| Ne | 3.928 (VVMD7) | 6.995 (VVMD5) | 4.962 ± 0.480 | 1.016 (SNP625_278) | 1.999 (SNP1327_56) | 1.604 ± 0.020 |
| Ho | 0.785 (VVMD7; VrZAG62) | 0.954 (VVMD5) | 0.859 ± 0.027 | 0.016 (SNP625_278) | 0.714 (SNP251_159) | 0.378 ± 0.011 |
| He | 0.745 (VVMD7) | 0.820 (VVS2) | 0.790 ± 0.018 | 0.016 (SNP625_278) | 0.499 (SNP895_382; VMFT_595; Vvi_1187; Vvi_10992) | 0.351 ± 0.009 |
| PIC | 0.736 (VVMD7) | 0.844 (VVMD5) | 0.773 ± 0.015 | 0.006 (SNP817_209) | 0.492 (SNP853_312) | 0.280 ± 0.009 |
| PI | 0.037 (VVMD5)) | 0.095 (VrZAG79) | 0.072 ± 0.022 | 0.375 (SNP1495_148) | 0.969 (SNP625_278) | 0.510 ± 0.152 |
| Chlorotype | Loci | Ccmp3 | Ccmp5 | Ccmp10 | Frequency (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Allele Sizes (bp) | 105 | 106 | 103 | 102 | 110 | 111 | 112 | ||
| A | Combination of ccmp alleles | x | x | x | 50.77 | ||||
| B | x | x | x | 1.54 | |||||
| C | x | x | x | 1.54 | |||||
| D | x | x | x | 46.15 | |||||
| Frequency (%) | 53.85 | 46.15 | 53.85 | 46.15 | 50.77 | 47.69 | 1.54 | ||
| Offspring | Parent 1 | Parent 2 | Trio Loci Compared | M 2 | Trio LOD Score | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICVV-SNP Genotype Number | VIVC Number | Chl1 | Variety/New Genotype Name | ICVV-SNP Genotype Number | VIVC Number | Chl1 | Variety Name | ICVV-SNP Genotype Number | VIVC Number | Chl1 | Variety Name | ||||
| 2837 | 368 | A | Alvarelhão Ceitão | 1258 | 5335 | A | Hebén | 815 | 1650 | A | Alvarelhão | 225 | 0 | 77.42 | [20] |
| 3010 | 17359 | D | Barca | 1205 | 8086 | D | Marufo | 760 | 12594 | A | Touriga Nacional | 216 | 0 | 78.02 | [20,58] |
| 2127 | 17452 | C | Black Monukka | 2126 | 12051 | C | Sultanina | 463 | 5477 | C | Ichkimar | 228 | 2 | 81.71 | [58] |
| 634 | 2018 | A | Camarate Tinto | 1089 | 5648 | A | Cayetana Blanca | 2173 | 277 | A | Alfrocheiro | 230 | 0 | 83.17 | [20,58,59,60] |
| 1270 | 2125 | A | Carrega Tinto | 1258 | 5335 | A | Hebén | 2173 | 277 | A | Alfrocheiro | 226 | 0 | 81.29 | [20,59,60] |
| 2976 | 14149 | A | Casculho | 1089 | 5648 | A | Cayetana Blanca | 2173 | 277 | A | Alfrocheiro | 218 | 1 | 74.67 | [20,60] |
| 1168 | 2324 | A | Castelão | 1089 | 5648 | A | Cayetana Blanca | 2173 | 277 | A | Alfrocheiro | 236 | 0 | 94.09 | [20,58,59,60] |
| 2997 | 12476 | D | Cidadelhe | 1205 | 8086 | D | Marufo | 2960 | 23126 | A | Casteloa | 217 | 1 | 66.16 | [20] |
| 1229 | 2846 | A | Cornifesto | 1089 | 5648 | A | Cayetana Blanca | 2173 | 277 | A | Alfrocheiro | 230 | 0 | 75.51 | [20,58,59,60] |
| 2964 | 17677 | D | Donzelinho Roxo | 1205 | 8086 | D | Marufo | 1133 | 12953 | A | Gouveio | 220 | 1 | 68.57 | [20] |
| 1133 | 12953 | A | Gouveio | 2397 | 40016 | A | Castellana Blanca | 2099 | 17636 | D | Savagnin = Traminer | 234 | 0 | 65.72 | [20,58] |
| 1110 | 5012 | A | Grand Noir | 1321 | 4935 | A | Graciano | 2204 | 1619 | A | Bouschet Petit | 232 | 0 | 79.31 | [58] |
| 2245 | 715 | A | Malvasia Fina | 1258 | 5335 | A | Hebén | 2173 | 277 | A | Alfrocheiro | 238 | 1 | 77.80 | [20,58,59,60] |
| 2347 | 15647 | A | Malvasia Preta | 1089 | 5648 | A | Cayetana Blanca | 2173 | 277 | A | Alfrocheiro | 236 | 0 | 90.48 | [20,58,59,60] |
| 2128 | 15678 | A | Molar | 939 | 9694 | A | Prieto Picudo Tinto | 2099 | 17636 | D | Savagnin = Traminer | 233 | 1 | 56.90 | [20] |
| 621 | 2520 | A | Montua | 1258 | 5335 | A | Hebén | 2306 | 14842 | C | Dedo de Dama | 238 | 0 | 101.41 | [20,58,59] |
| 2201 | 8082 | A | Mouratón | 1089 | 5648 | A | Cayetana Blanca | 2158 | 277 | A | Alfrocheiro | 236 | 1 | 83.62 | [20,59,60] |
| 2999 | 12471 | D | Mourisco de Semente | 1205 | 8086 | D | Marufo | 896 | 1564 | A | Borraçal | 220 | 4 | 54.53 | [20,58] |
| 148 | 9168 | A | Perlette | 2035 | 6350 | A | Koenigin der Weingaerten | 2126 | 12051 | C | Sultanina | 223 | 1 | 78.13 | [59,61,62] |
| 2106 | 10331 | A | Rufete | 2128 | 15678 | A | Molar | 3960 | 21437 | A | Perepinhão Portalegre | 237 | 0 | 77.41 | [20,58] |
| 1316 | 12350 | A | Tempranillo | 2410 | 1131 | A | Benedicto | 2228 | 12581 | A | Albillo Mayor | 234 | 0 | 100.00 | [20,63] |
| 2968 | 12459 | D | Tinta Aguiar | 1205 | 8086 | D | Marufo | 760 | 12594 | A | Touriga Nacional | 219 | 0 | 68.59 | [20] |
| 1167 | 12462 | D | Tinta Barroca | 1205 | 8086 | D | Marufo | 760 | 12594 | A | Touriga Nacional | 236 | 0 | 75.72 | [20,58] |
| 1123 | 12467 | D | Tinta Carvalha | 3088 | 26692 | D | Cainho da Terra | 1089 | 5648 | A | Cayetana Blanca | 222 | 1 | 59.15 | [20] |
| 3215 | 12489 | D | Tinta Mesquita | 1205 | 8086 | D | Marufo | 2240 | 13100 | A | Vinhão | 225 | 1 | 61.44 | [20] |
| 2969 | 12592 | A | Touriga Fêmea | 2245 | 715 | A | Malvasia Fina | 760 | 12594 | A | Touriga Nacional | 215 | 1 | 64.23 | [20,58] |
| 493 | 12593 | D | Touriga Franca | 1205 | 8086 | D | Marufo | 760 | 12594 | A | Touriga Nacional | 236 | 1 | 69.51 | [4,20] |
| 4344 | D | NG003 | 1205 | 8086 | D | Marufo | 2156 | 12668 | A | Trousseau Noir | 213 | 2 | 52.20 | this study | |
| 4345 | D | NG004 | 1205 | 8086 | D | Marufo | 1133 | 12953 | A | Gouveio | 210 | 1 | 58.71 | this study | |
| 4347 | D | NG006 | 1205 | 8086 | D | Marufo | 651 | 12500 | A | Tinto Cão | 212 | 2 | 58.39 | this study | |
| 4335 | D | NG007 | 1205 | 8086 | D | Marufo | 634 | 2018 | A | Camarate Tinto | 210 | 0 | 64.63 | this study | |
| 4336 | D | NG008 | 1205 | 8086 | D | Marufo | 3002 | 14142 | A | Folha de Figueira | 213 | 2 | 76.00 | this study | |
| 4337 | D | NG009 | 1205 | 8086 | D | Marufo | 1239 | 15685 | D | Trincadeira | 213 | 1 | 58.54 | this study | |
| 4348 | D | NG010 | 2971 | 12497 | D | Roseira | 493 | 12593 | D | Touriga Franca | 162 | 0 | 58.91 | this study | |
| 4349 | D | NG011 | 1205 | 8086 | D | Marufo | 1239 | 15685 | D | Trincadeira | 213 | 1 | 67.72 | this study | |
| 4350 | D | NG012 | 1205 | 8086 | D | Marufo | 2240 | 13100 | A | Vinhão | 212 | 1 | 65.43 | this study | |
| Offspring | Parent 1 | Pair Loci Compared | Pair Loci Mismatching | Pair LOD Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ICVV-SNP Genotype Number | Chlorotype | Genotype Name | Loci Typed | ICVV-SNP Genotype Number | Chlorotype | Genotype Name | Loci Typed | |||
| 4342 | D | NG001 | 213 | 1089 | A | Cayetana Blanca | 235 | 210 | 0 | 33.50 |
| 4343 | D | NG002 | 212 | 1133 | A | Gouveio | 236 | 210 | 1 | 30.71 |
| 2199 | D | Carrega Branco | 218 | 1089 | A | Cayetana Blanca | 215 | 0 | 30.77 | |
| 2967 | D | Malandra | 214 | 2450 | A | Cuelga | 210 | 0 | 28.40 | |
| 3008 | A | Nevoeira | 230 | 3002 | A | Folha de Figueira | 221 | 0 | 25.86 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augusto, D.; Ibáñez, J.; Pinto-Sintra, A.L.; Falco, V.; Leal, F.; Martínez-Zapater, J.M.; Oliveira, A.A.; Castro, I. Grapevine Diversity and Genetic Relationships in Northeast Portugal Old Vineyards. Plants 2021, 10, 2755. https://doi.org/10.3390/plants10122755
Augusto D, Ibáñez J, Pinto-Sintra AL, Falco V, Leal F, Martínez-Zapater JM, Oliveira AA, Castro I. Grapevine Diversity and Genetic Relationships in Northeast Portugal Old Vineyards. Plants. 2021; 10(12):2755. https://doi.org/10.3390/plants10122755
Chicago/Turabian StyleAugusto, Diana, Javier Ibáñez, Ana Lúcia Pinto-Sintra, Virgílio Falco, Fernanda Leal, José Miguel Martínez-Zapater, Ana Alexandra Oliveira, and Isaura Castro. 2021. "Grapevine Diversity and Genetic Relationships in Northeast Portugal Old Vineyards" Plants 10, no. 12: 2755. https://doi.org/10.3390/plants10122755
APA StyleAugusto, D., Ibáñez, J., Pinto-Sintra, A. L., Falco, V., Leal, F., Martínez-Zapater, J. M., Oliveira, A. A., & Castro, I. (2021). Grapevine Diversity and Genetic Relationships in Northeast Portugal Old Vineyards. Plants, 10(12), 2755. https://doi.org/10.3390/plants10122755







