Thonningia sanguinea Extract: Antioxidant and Cytotoxic Activities Supported by Chemical Composition and Molecular Docking Simulations
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolic Content
2.2. Biological Activities
2.2.1. Antioxidant Activity
2.2.2. Cytotoxic Activity
2.2.3. Apoptosis Investigation
Annexin-V-FITC/PI Staining and Cell Cycle Analysis
RT-PCR
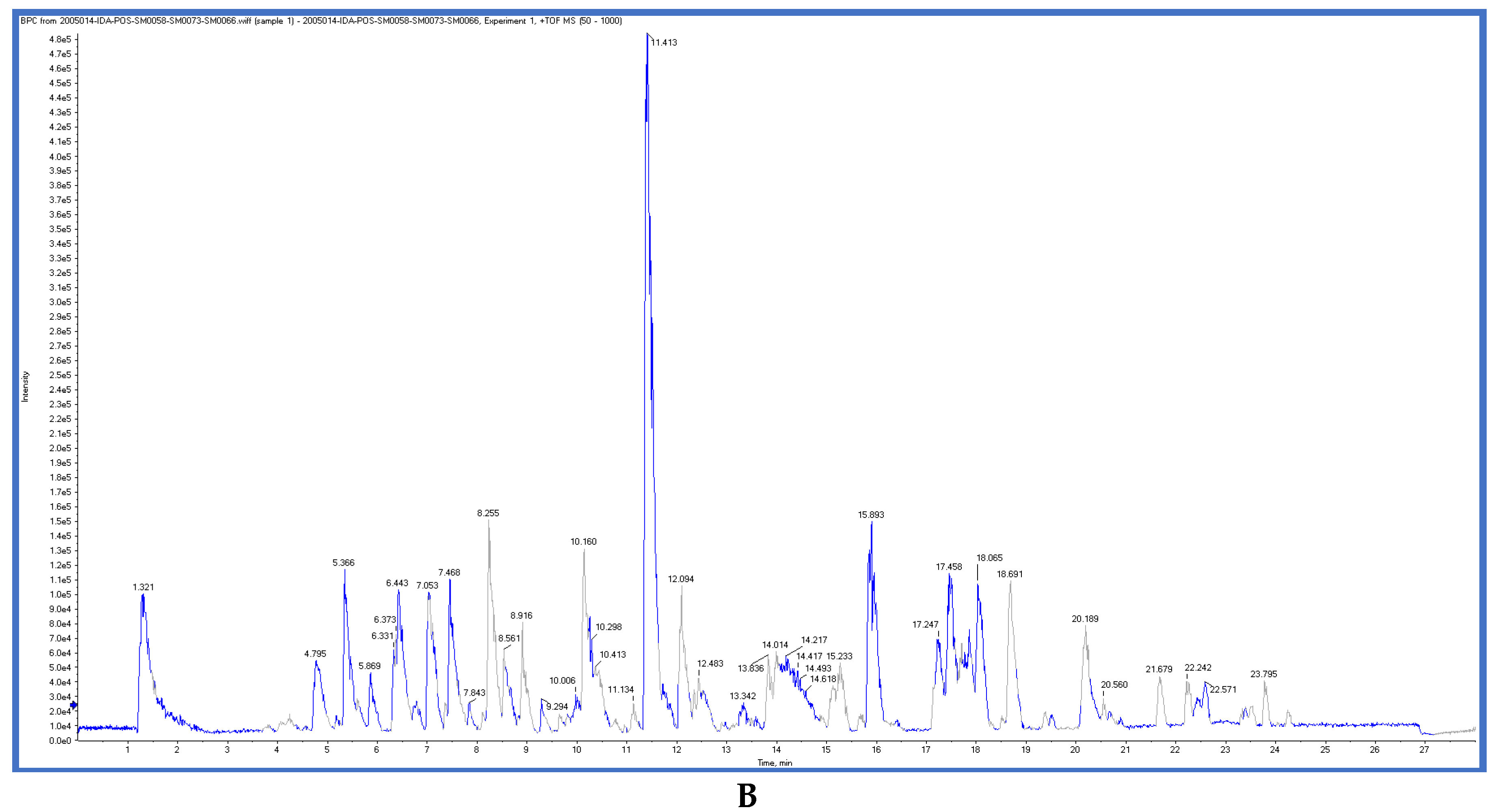
2.3. LC-ESI-TOF-MS/MS Analysis
2.4. Molecular Docking Studies
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Plant Extract
3.3. Determination of Total Phenolic Content
3.4. Preparation of Phenolic Extract
3.5. Determination of Antioxidant Activity
3.5.1. Initial Screening Step
3.5.2. Determination of IC50
3.5.3. Trolox Standard Preparation
3.5.4. DPPH• Assay
3.5.5. Data Analysis
3.6. LC-ESI-TOF-MS/MS Analysis
3.7. Biological Evaluation
3.7.1. Cytotoxic Activity
3.7.2. Apoptotic Investigation
Flow Cytometric Analysis
RT-PCR
3.7.3. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Dasgupta, P.; Henshaw, C.; Youlden, D.R.; Clark, P.J.; Aitken, J.F.; Baade, P.D. Global trends in incidence rates of primary adult liver cancers: A systematic review and meta-analysis. Front. Oncol. 2020, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Kufe, D.W.; Pollock, R.E.; Weichselbaum, R.R.; Bast, R.C.; Gansler, T.S.; Holland, J.F. (Eds.) Holland-Frei Cancer Medicine, 6th ed.; BC Deker: Hamilton, ON, Canada, 2003. Available online: https://www.ncbi.nlm.nih.gov/books/NBK12354/ (accessed on 1 March 2021).
- Malik, A.; Rasool, R.; Khan, S.R.; Waquar, S.; Iqbal, J.; Hassan, S.S.U.; Qazi, M.H.; Qazi, A. Development of anti-cancer stem cells as theranostic agents in the treatment of different cancer types: An update. J. Carcinog. Mutagen. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Musial, C.; Siedlecka-Kroplewska, K.; Kmiec, Z.; Gorska-Ponikowska, M. Modulation of Autophagy in Cancer Cells by Dietary Polyphenols. Antioxidants 2021, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Thomford, K.; Mensah, M.; Dickson, R.; Sarfo, B.; Edoh, D.; Mills-Robertson, F.; Annan, K. A randomized double-blind study evaluating the safety and effectiveness of a Ghanaian polyherbal product for the management of superficial mycoses. J. Herb. Med. 2015, 5, 140–146. [Google Scholar] [CrossRef]
- Nichols, R.L.; Graham, D.R.; Barriere, S.L.; Rodgers, A.; Wilson, S.E.; Zervos, M.; Dunn, D.L.; Kreter, B. Treatment of hospitalized patients with complicated gram-positive skin and skin structure infections: Two randomized, multicentre studies of quinupristin/dalfopristin versus cefazolin, oxacillin or vancomycin. J. Antimicrob. Chemother. 1999, 44, 263–273. [Google Scholar] [CrossRef]
- Senekal, M. Current resistance issues in antimicrobial therapy. Contin. Med Educ. 2010, 28, 54–57. [Google Scholar]
- Gaikwad, S.; Srivastava, S.K. Role of phytochemicals in perturbation of redox homeostasis in cancer. Antioxidants 2021, 10, 83. [Google Scholar] [CrossRef]
- Yaseen, G.; Ahmad, M.; Sultana, S.; Alharrasi, A.S.; Hussain, J.; Zafar, M. Ethnobotany of medicinal plants in the Thar Desert (Sindh) of Pakistan. J. Ethnopharmacol. 2015, 163, 43–59. [Google Scholar] [CrossRef]
- Bender, A.; Fauzi, F.; Koutsoukas, A.; Lowe, R.; Joshi, K.; Fan, T.-P.; Glen, R. Linking Ayurveda and Western medicine by integrative analysis. J. Ayurveda Integr. Med. 2013, 4, 117–119. [Google Scholar] [CrossRef][Green Version]
- Donia, M.S.; Fricke, W.F.; Ravel, J.; Schmidt, E.W. Variation in tropical reef symbiont metagenomes defined by secondary metabolism. PLoS ONE 2011, 6, e17897. [Google Scholar] [CrossRef]
- Conforti, F.; Ioele, G.; Statti, G.A.; Marrelli, M.; Ragno, G.; Menichini, F. Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food Chem. Toxicol. 2008, 46, 3325–3332. [Google Scholar] [CrossRef]
- Moloney, M.G. Natural products as a source for novel antibiotics. Trends Pharmacol. Sci. 2016, 37, 689–701. [Google Scholar] [CrossRef]
- Soares-Bezerra, R.J.; Calheiros, A.S.; da Silva Ferreira, N.C.; da Silva Frutuoso, V.; Alves, L.A. Natural products as a source for new anti-inflammatory and analgesic compounds through the inhibition of purinergic P2X receptors. Pharmaceuticals 2013, 6, 650–658. [Google Scholar] [CrossRef]
- Sanjita, D.; Dinakar, S.; Basu, S. Antispasmodic and anthelmintic activity of Nyctanthes arbortristis Linn. Int. J. Pharm. Sci. Res. 2010, 1, 51–55. [Google Scholar]
- Mali, R.G.; Mehta, A.A. A review on anthelmintic plants. Nat. Prod. Rad. 2008, 7, 466–475. [Google Scholar]
- Zhu, J.; Huang, X.; Gao, H.; Bao, Q.; Zhao, Y.; Hu, J.-F.; Xia, G. A novel glucagon-like peptide 1 peptide identified from Ophisaurus harti. J. Pept. Sci. 2013, 19, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Kinghorn, A.D.; Chin, Y.-W.; Swanson, S.M. Discovery of natural product anticancer agents from biodiverse organisms. Curr. Opin. Drug Discov. Dev. 2009, 12, 189–196. [Google Scholar]
- Khan, R.A. Natural products chemistry: The emerging trends and prospective goals. Saudi Pharm. J. 2018, 26, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Eltamany, E.; Elhady, S.; Nafie, M.; Ahmed, H.; Abo-Elmatty, D.; Ahmed, S.; Badr, J.; Abdel-Hamed, A. The antioxidant Carrichtera annua DC. Ethanolic extract counteracts cisplatin triggered hepatic and renal toxicities. Antioxidants 2021, 10, 825. [Google Scholar] [CrossRef]
- Elhady, S.; Abdelhameed, R.; El-Ayouty, M.; Ibrahim, A.; Habib, E.; Elgawish, M.; Hassanean, H.; Safo, M.; Nafie, M.; Ahmed, S. New antiproliferative triflavanone from Thymelaea hirsuta—Isolation, structure elucidation and molecular docking studies. Molecules 2021, 26, 739. [Google Scholar] [CrossRef] [PubMed]
- Eltamany, E.; Elhady, S.; Ahmed, H.; Badr, J.; Noor, A.; Ahmed, S.; Nafie, M. Chemical profiling, antioxidant, cytotoxic activities and molecular docking simulation of Carrichtera annua DC. (Cruciferae). Antioxidants 2020, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Elhady, S.; Eltamany, E.; Shaaban, A.; Bagalagel, A.; Muhammad, Y.; El-Sayed, N.; Ayyad, S.; Ahmed, A.; Elgawish, M.; Ahmed, S. Jaceidin flavonoid isolated from Chiliadenus montanus attenuates tumor progression in mice via VEGF inhibition. In vivo and in silico studies. Plants 2020, 9, 1031. [Google Scholar] [CrossRef] [PubMed]
- Elhady, S.; Abdelhameed, R.; Zekry, S.; Ibrahim, A.; Habib, E.; Darwish, K.; Hazem, R.; Mohammad, K.; Hassanean, H.; Ahmed, S. VEGFR-mediated cytotoxic activity of Pulicaria undulata isolated metabolites: A biological evaluation and in silico study. Life 2021, 11, 759. [Google Scholar] [CrossRef] [PubMed]
- N’guessan, J.; Bidie, A.; Lenta, B.; Weniger, B.; Andre, P.; Guédé-Guina, F. In vitro assays for bioactivity-guided isolation of antisalmonella and antioxidant compounds in Thonningia sanguinea flowers. Afr. J. Biotechnol. 2007, 6, 1685–1689. [Google Scholar]
- Pompermaier, L.; Heiss, E.H.; Alilou, M.; Mayr, F.; Monizi, M.; Lautenschlaeger, T.; Schuster, D.; Schwaiger, S.; Stuppner, H. Dihydrochalcone glucosides from the subaerial parts of Thonningia sanguinea and their in vitro PTP1B inhibitory activities. J. Nat. Prod. 2018, 81, 2091–2100. [Google Scholar] [CrossRef]
- Thomford, A.K.; Abdelhameed, R.F.A.; Yamada, K. Chemical studies on the parasitic plant Thonningia sanguinea Vahl. RSC Adv. 2018, 8, 21002–21011. [Google Scholar] [CrossRef]
- Pompermaier, L.; Marzocco, S.; Adesso, S.; Monizi, M.; Schwaiger, S.; Neinhuis, C.; Stuppner, H.; Lautenschläger, T. Medicinal plants of northern Angola and their anti-inflammatory properties. J. Ethnopharmacol. 2018, 216, 26–36. [Google Scholar] [CrossRef]
- N’guessan, J.D.; Dinzedi, M.R.; Guessennd, N.; Coulibaly, A.; Dosso, M.; Djaman, A.J.; Guede-Guina, F. Antibacterial activity of the aqueous extract of Thonningia sanguinea against extended-spectrum-b-lactamases (ESBL) producing Escherichia coli and Klebsiella pneumoniae strains. Trop. J. Pharm. Res. 2007, 6, 779–783. [Google Scholar] [CrossRef]
- Ohiri, F.; Uzodinma, V. Antimicrobial properties of Thonningia sanguinea root extracts. Fitoterapia 2000, 71, 176–178. [Google Scholar] [CrossRef]
- Ayim, J.A.; Bayor, M.T.; Phillips, R.M.; Shnyder, S.D.; Wright, C.W. The evaluation of selected Ghanaian medicinal plants for cytotoxic activites. J. Sci. Technol. Ghana 2008, 27, 16–22. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Yang, L.; Jiang, J.-G. Effects of thonningianin A in natural foods on apoptosis and cell cycle arrest of HepG-2 human hepatocellular carcinoma cells. Food Funct. 2015, 6, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Jiang, M.-H.; Jiang, J.-G.; Zhang, R.-F.; Zhang, M.-W. Isolation and identification of compounds from Penthorum chinense Pursh with antioxidant and antihepatocarcinoma properties. J. Agric. Food Chem. 2012, 60, 11097–11103. [Google Scholar] [CrossRef]
- Rezaei-Seresht, H.; Cheshomi, H.; Falanji, F.; Movahedi-Motlagh, F.; Hashemian, M.; Mireskandari, E. Cytotoxic activity of caffeic acid and gallic acid against MCF-7 human breast cancer cells: An in silico and in vitro study. Avicenna J. Phytomedicine 2019, 9, 574–586. [Google Scholar]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. Rsc Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Lee, I.R.; Yang, M.Y. Phenolic compounds from Duchesnea chrysantha and their cytotoxic activities in human cancer cell. Arch. Pharmacal Res. 1994, 17, 476–479. [Google Scholar] [CrossRef]
- Chen, B.-N.; Yang, G.-E.; Li, J.-K.; Du, H.-J.; Li, Q.-S.; Zhang, Z.-M. Cytotoxic constituents from Viscum coloratum. Chem. Nat. Compd. 2009, 45, 547–549. [Google Scholar] [CrossRef]
- Gad, E.M.; Nafie, M.S.; Eltamany, E.H.; Hammad, M.S.; Barakat, A.; Boraei, A.T. Discovery of new apoptosis-inducing agents for breast cancer based on ethyl 2-amino-4,5,6,7-tetra hydrobenzo [b] thiophene-3-carboxylate: Synthesis, in vitro, and in vivo activity evaluation. Molecules 2020, 25, 2523. [Google Scholar] [CrossRef]
- Nafie, M.S.; Arafa, K.; Sedky, N.K.; Alakhdar, A.A.; Arafa, R.K. Triaryl dicationic DNA minor-groove binders with antioxidant activity display cytotoxicity and induce apoptosis in breast cancer. Chem. Interact. 2020, 324, 109087. [Google Scholar] [CrossRef]
- Wang, L.; Halquist, M.S.; Sweet, U.H. Simultaneous determination of gallic acid and gentisic acid in organic anion transporter expressing cells by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2013, 937, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Lc-esi-qtof-ms/ms characterization of seaweed phenolics and their antioxidant potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef]
- Cao-Ngoc, P.; Leclercq, L.; Rossi, J.-C.; Hertzog, J.; Tixier, A.-S.; Chemat, F.; Nasreddine, R.; Al Hamui Dit Banni, G.; Nehmé, R.; Schmitt-Kopplin, P.; et al. Water-based extraction of bioactive principles from blackcurrant leaves and Chrysanthellum americanum: A comparative study. Foods 2020, 9, 1478. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. A Reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Nakabayashi, R.; Mori, T.; Yamada, Y.; Takahashi, M.; Rai, A.; Sugiyama, R.; Yamamoto, H.; Nakaya, T.; Yamazaki, M. A cheminformatics approach to characterize metabolomes in stable-isotope-labeled organisms. Nat. Methods 2019, 16, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, A.M.; Basam, S.M.; Marzouk, H.S.; El-Hawary, S. LC–MS/MS and GC–MS profiling as well as the antimicrobial effect of leaves of selected Yucca species introduced to Egypt. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Shang, Z.; Li, Q.; Huang, M.; He, W.; Wang, Z.; Zhang, J. Rapid screening and identification of daidzein metabolites in rats based on UHPLC-LTQ-orbitrap mass spectrometry coupled with data-mining technologies. Molecules 2018, 23, 151. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Clasquin, M.F.; Melamud, E.; Amador-Noguez, D.; Caudy, A.; Rabinowitz, J.D. Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a stand alone orbitrap mass spectrometer. Anal. Chem. 2010, 82, 3212–3221. [Google Scholar] [CrossRef]
- Available online: https://mona.fiehnlab.ucdavis.edu/spectra/display/PR020067 (accessed on 1 March 2021).
- Sánchez-Rabaneda, F.; Jáuregui, O.; Lamuela-Raventós, R.M.; Viladomat, F.; Bastida, J.; Codina, C. Qualitative analysis of phenolic compounds in apple pomace using liquid chromatography coupled to mass spectrometry in tandem mode. Rapid Commun. Mass Spectrom. 2004, 18, 553–563. [Google Scholar] [CrossRef]
- Zeković, Z.; Kaplan, M.; Pavlić, B.; Olgun, E.O.; Vladić, J.; Canlı, O.; Vidović, S. Chemical characterization of polyphenols and volatile fraction of coriander (Coriandrum sativum L.) extracts obtained by subcritical water extraction. Ind. Crop. Prod. 2016, 87, 54–63. [Google Scholar] [CrossRef]
- Available online: https://massbank.eu/MassBank/RecordDisplay?id=PB006261&dsn=IPB_Halle (accessed on 1 March 2021).
- Available online: https://mona.fiehnlab.ucdavis.edu/spectra/display/JP011904 (accessed on 1 March 2021).
- Available online: https://mona.fiehnlab.ucdavis.edu/spectra/display/VF-NPL-QEHF013798 (accessed on 1 March 2021).
- Su, X.-L.; Lin, R.-C.; Wong, S.-K.; Tsui, S.-K.; Kwan, S.-Y. Identification and characterisation of the Chinese herb Langdu by LC-MS/MS analysis. Phytochem. Anal. 2003, 14, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Singh, A.; Kumari, A.; Tyagi, C.; Goyal, S.; Jamal, S.; Grover, A. Natural polyphenolic inhibitors against the antiapoptotic BCL-2. J. Recept. Signal Transduct. 2017, 37, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar]
- El-Shaer, N.S.; Badr, J.M.; Aboul-Ela, M.A.; Gohar, Y.M. Determination of lawsone in henna powders by high performance thin layer chromatography. J. Sep. Sci. 2007, 30, 3311–3315. [Google Scholar] [CrossRef]
- Boly, R.; Lamkami, T.; Lompo, M.; Dubois, J.; Guissou, I. DPPH free radical scavenging activity of two extracts from Agelanthus dodoneifolius (Loranthaceae) leaves. Int. J. Toxicol. Pharmacol. Res. 2016, 8, 29–34. [Google Scholar]
- Chen, Z.; Bertin, R.; Froldi, G. EC50 estimation of antioxidant activity in DPPH assay using several statistical programs. Food Chem. 2013, 138, 414–420. [Google Scholar] [CrossRef]
- Nafie, M.S.; Amer, A.M.; Mohamed, A.K.; Tantawy, E.S. Discovery of novel pyrazolo[3,4-b] pyridine scaffold-based derivatives as potential PIM-1 kinase inhibitors in breast cancer MCF-7 cells. Bioorganic Med. Chem. 2020, 28, 115828. [Google Scholar] [CrossRef]
- Tantawy, E.S.; Amer, A.M.; Mohamed, E.K.; Alla, M.M.A.; Nafie, M.S. Synthesis, characterization of some pyrazine derivatives as anti-cancer agents: In vitro and in Silico approaches. J. Mol. Struct. 2020, 1210, 128013. [Google Scholar] [CrossRef]
- Youssef, E.; El-Moneim, M.A.; Fathalla, W.; Nafie, M.S. Design, synthesis and antiproliferative activity of new amine, amino acid and dipeptide-coupled benzamides as potential sigma-1 receptor. J. Iran. Chem. Soc. 2020, 17, 2515–2532. [Google Scholar] [CrossRef]
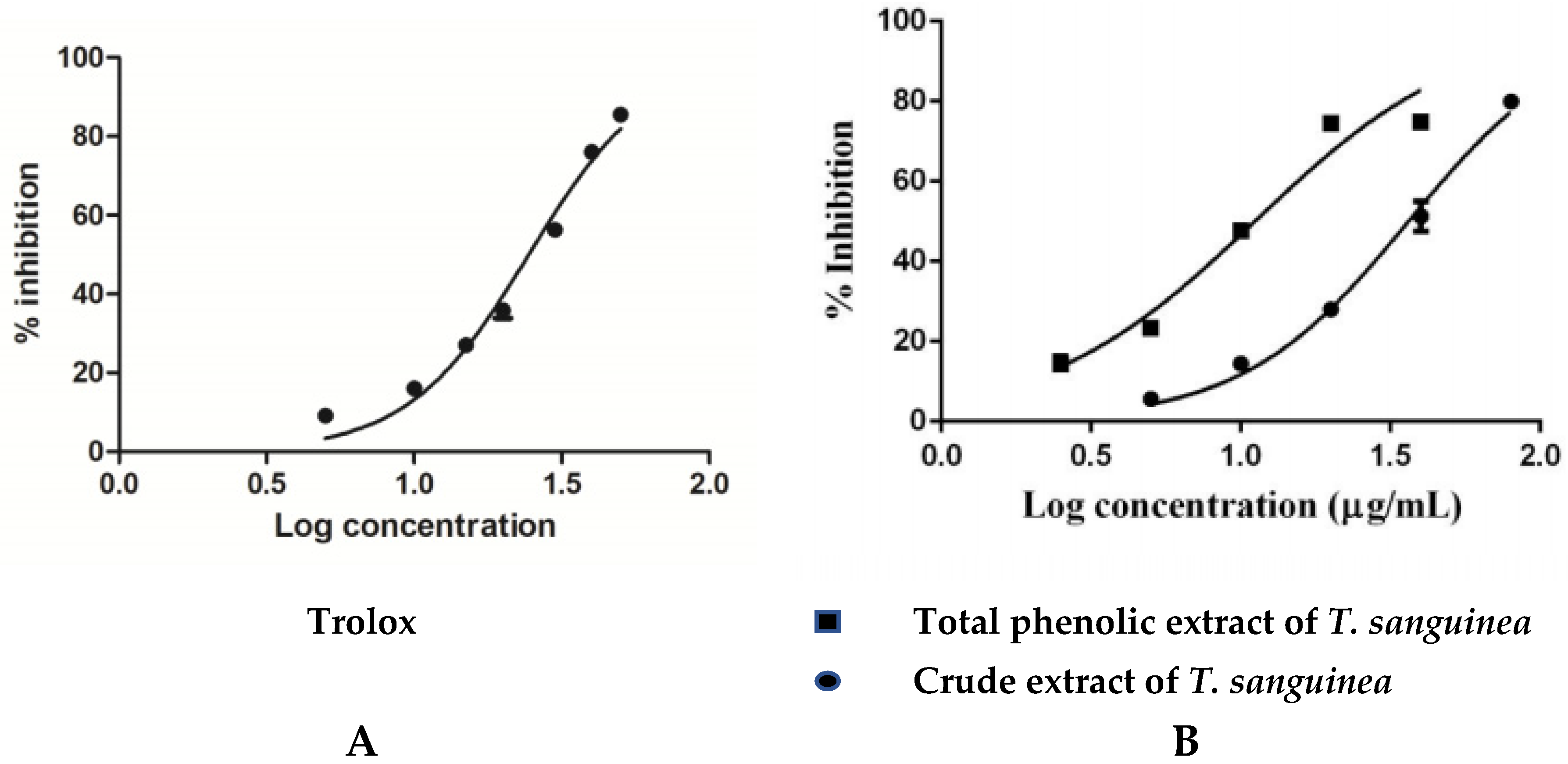
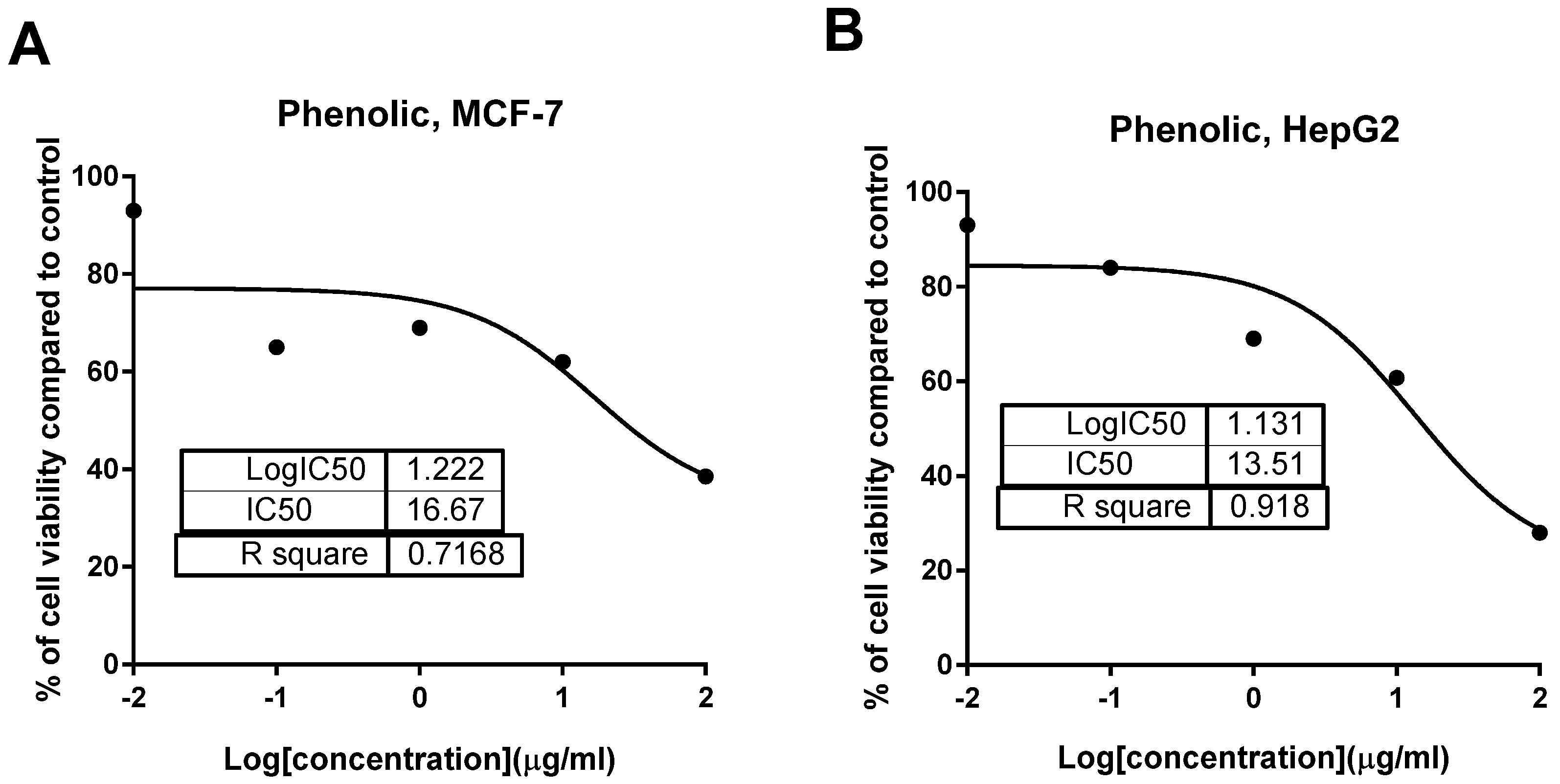


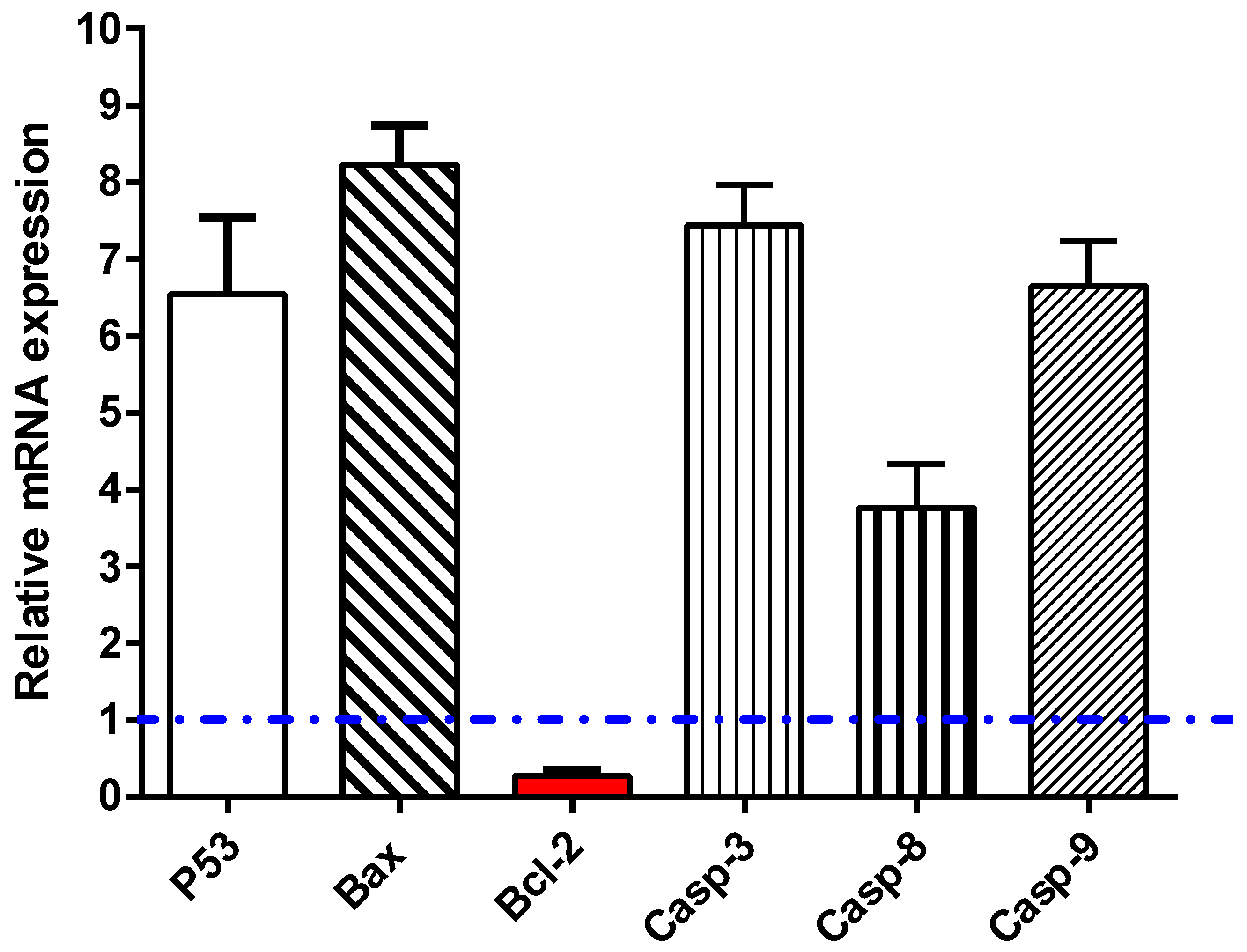
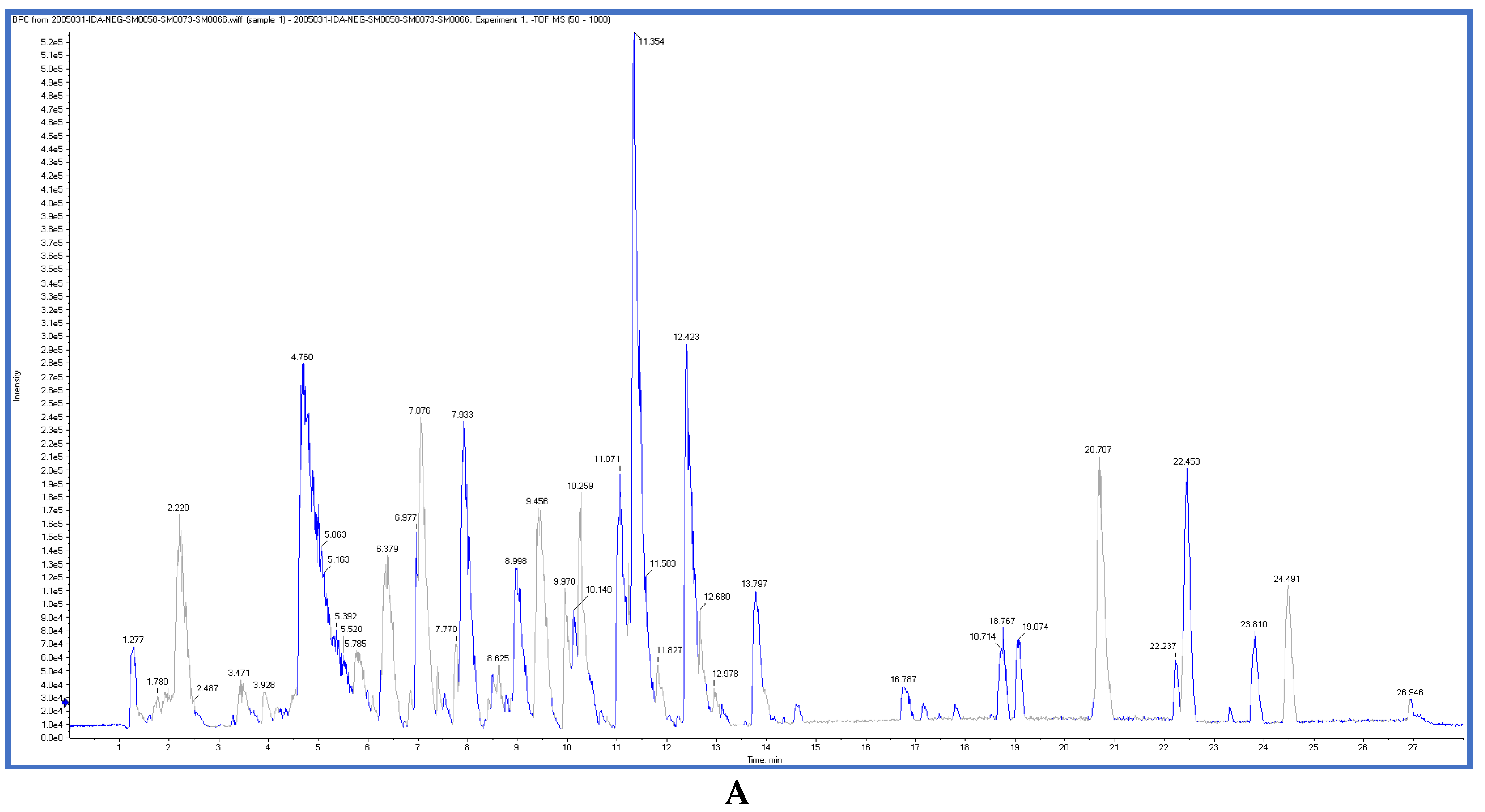
| Tested Samples | IC50 (μg/mL) * | |||
|---|---|---|---|---|
| A549 | HepG2 | MDA-MB-231 | MCF-7 | |
| Crude extract | 13.25 ± 0.46 | 18.76 ± 0.98 | 56.53 ± 1.32 | 34.65 ± 1.76 |
| Phenolic extract | ≥50 | 13.51 ± 0.54 | ≥50 | 16.67 ± 0.87 |
| 5-FU | 7.47 ± 0.35 | 15.8 ± 0.86 | 20.76 ± 0.76 | 26.98 ± 0.43 |
| Compound | RT (min) | Measured m/z [M−H]− | Expected m/z [M−H]− | Relative Error * | Molecular Formula | Fragments |
|---|---|---|---|---|---|---|
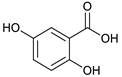 gentisic acid [41] | 1.31 | 153.0155 | 153.0188 | −21.56 | C7H6O4 | 153, 108 |
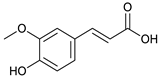 ferulic acid [42] | 2.06 | 193.0528 | 193.0501 | 13.98 | C10H10O4 | 178, 134 |
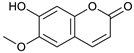 Scopoletin [42] | 5.01 | 191.0332 | 191.0344 | −6.28 | C10H8O4 | 176, 147 |
 Isookanin-7-glucoside [43] | 5.23 | 449.1062 | 449.1084 | −4.89 | C21H22O11 | 287, 151, 135, |
 (+)-epicatechin [44] | 7.06 | 289.0716 | 289.0712 | 1.38 | C15H14O6 | 125, 123 |
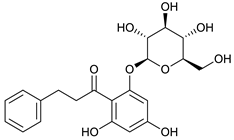 4-deoxyphloridzin [45] | 7.85 | 419.1382 | 419.1342 | 9.54 | C21H24O9 | 419, 257 |
 (isorhapontin) [44] | 9.95 | 419.1328 | 419.1342 | −3.34 | C21H24O9 | 281,257 |
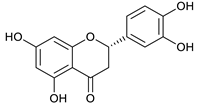 (+)-eriodictyol [46] | 9.97 | 287.0565 | 287.0556 | 3.13 | C15H12O6 | 151, 135 |
 3, 5, 7-trihydroxy-4’-methoxyflavone [47] | 11.74 | 299.0541 | 299.0556 | −5.01 | C16H12O6 | 299, 284, 256 |
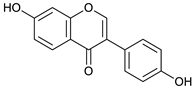 Daidzein [48] | 18.78 | 253.0524 | 253.0501 | 9.08 | C15H10O4 | 225, 224 |
| Compound | RT (min) | Measured m/z [M−H]− | Expected m/z [M−H]− | Relative Error * | Molecular Formula | Fragments |
|---|---|---|---|---|---|---|
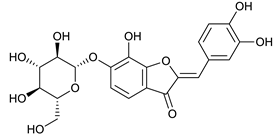 Maritimetin-6-O-glucoside [50] | 6.50 | 449.1066 | 449.1084 | −4.00 | C21H20O11 | 449, 287 |
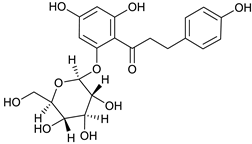 Phlorizin [51] | 6.78 | 437.1446 | 437.1448 | −0.45 | C21H24O10 | 437, 275 |
 3,4-dimethoxycinnamic acid [52] | 6.91 | 209.081 | 209.0814 | −1.91 | C11H12O4 | 209, 191, 163 |
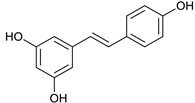 Resveratrol [53] | 7.39 | 229.0853 | 229.0865 | −5.23 | C14H12O3 | 229, 185, 157 |
 trans ortho Coumaric acid [54] | 7.45 | 165.054 | 165.0552 | −7.27 | C9H8O3 | 165, 147, 91 |
 (-)-Epicatechin [55] | 7.46 | 291.0873 | 291.0869 | 1.37 | C15H14O6 | 165, 123 |
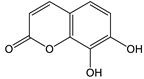 Daphnetin [56] | 8.08 | 179.0342 | 179.0344 | −1.11 | C9H6O4 | 179, 133 |
| Compound | Ligand-Receptor Interactions (HB and Van Der Waals Interactions) | |||
|---|---|---|---|---|
| Thonningianin-A | Arg 66 Ala 59 Gly 104 Tyr 161 Asn 102 | 1 HB-Acceptor 1 HB-donor 1 HB-Acceptor 1 HB-Acceptor 1 HB-Acceptor | ||
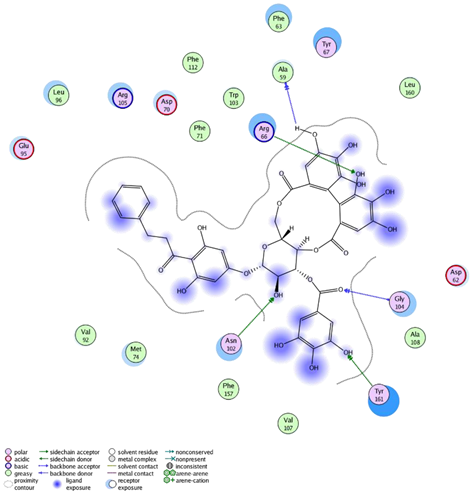 | ||||
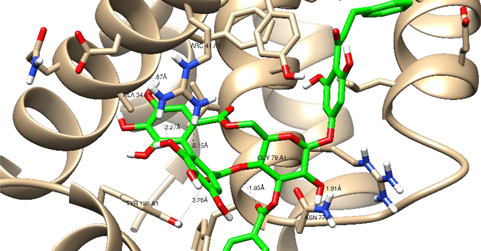 | ||||
| Thonningianin-B | Arg 105 Gly 104 Asp 70 Glu 95 | 1 HB-Acceptor 1 HB-Donor 1 HB-Donor 1 HB-Donor | 1 arene-cation with Arg 66 1 arene-arene with Tyr 161 | |
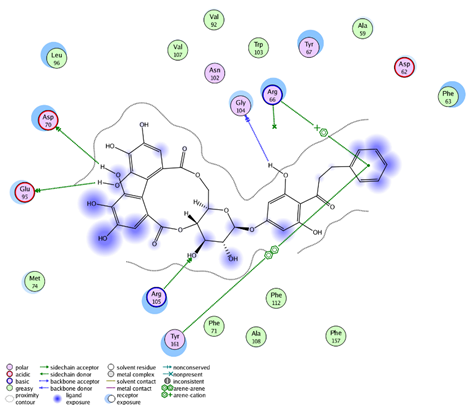 | ||||
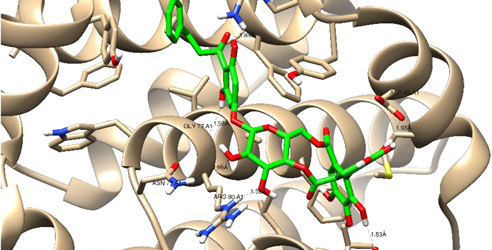 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhameed, R.F.A.; Elhady, S.S.; Sirwi, A.; Samir, H.; Ibrahim, E.A.; Thomford, A.K.; El Gindy, A.; Hadad, G.M.; Badr, J.M.; Nafie, M.S. Thonningia sanguinea Extract: Antioxidant and Cytotoxic Activities Supported by Chemical Composition and Molecular Docking Simulations. Plants 2021, 10, 2156. https://doi.org/10.3390/plants10102156
Abdelhameed RFA, Elhady SS, Sirwi A, Samir H, Ibrahim EA, Thomford AK, El Gindy A, Hadad GM, Badr JM, Nafie MS. Thonningia sanguinea Extract: Antioxidant and Cytotoxic Activities Supported by Chemical Composition and Molecular Docking Simulations. Plants. 2021; 10(10):2156. https://doi.org/10.3390/plants10102156
Chicago/Turabian StyleAbdelhameed, Reda F. A., Sameh S. Elhady, Alaa Sirwi, Hanan Samir, Elsayed A. Ibrahim, Ama Kyeraa Thomford, Alaa El Gindy, Ghada M. Hadad, Jihan M. Badr, and Mohamed S. Nafie. 2021. "Thonningia sanguinea Extract: Antioxidant and Cytotoxic Activities Supported by Chemical Composition and Molecular Docking Simulations" Plants 10, no. 10: 2156. https://doi.org/10.3390/plants10102156
APA StyleAbdelhameed, R. F. A., Elhady, S. S., Sirwi, A., Samir, H., Ibrahim, E. A., Thomford, A. K., El Gindy, A., Hadad, G. M., Badr, J. M., & Nafie, M. S. (2021). Thonningia sanguinea Extract: Antioxidant and Cytotoxic Activities Supported by Chemical Composition and Molecular Docking Simulations. Plants, 10(10), 2156. https://doi.org/10.3390/plants10102156









