Woody and Foliage Biomass, Foliage Traits and Growth Efficiency in Young Trees of Four Broadleaved Tree Species in a Temperate Forest
Abstract
:1. Introduction
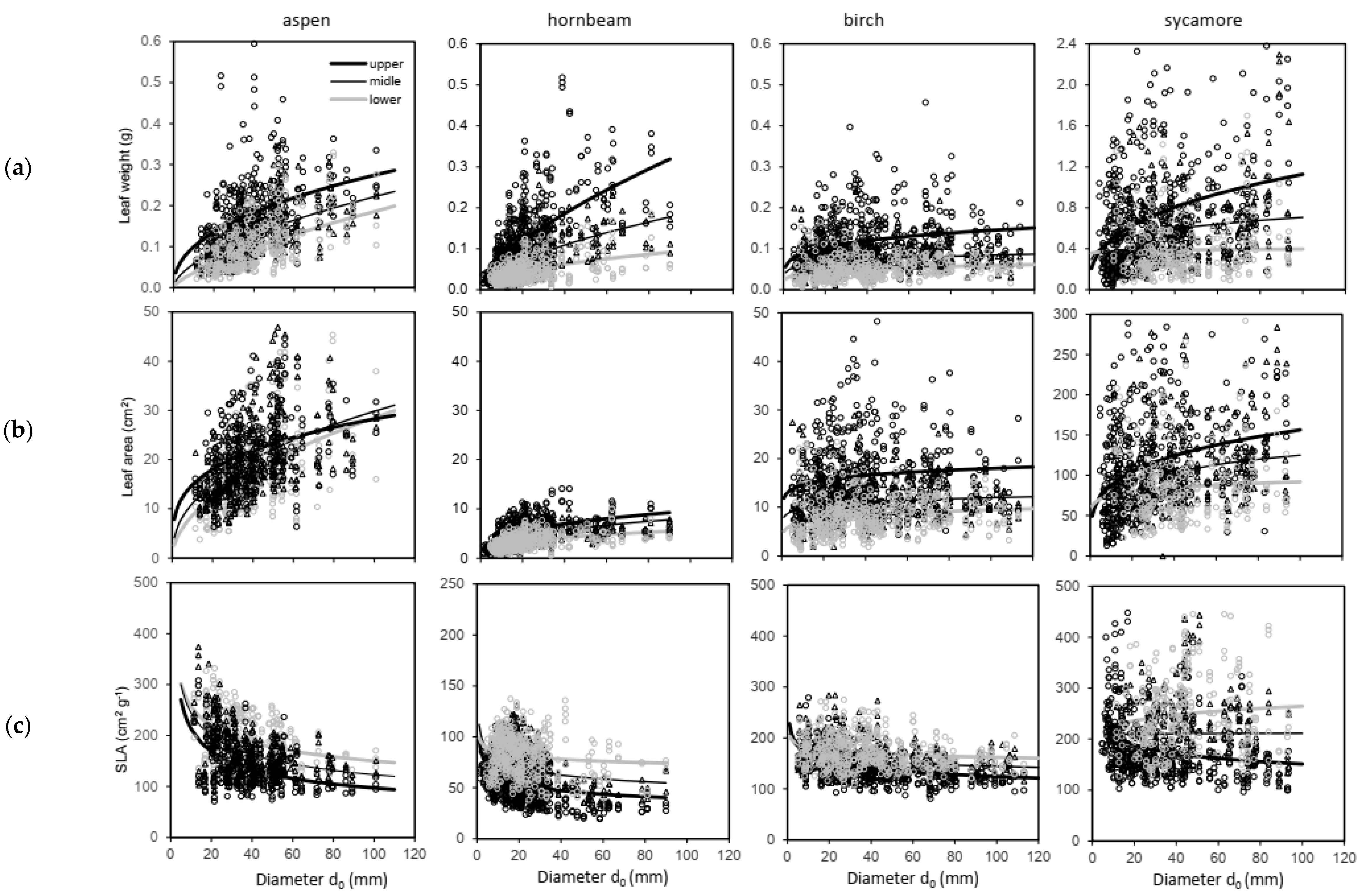
2. Results
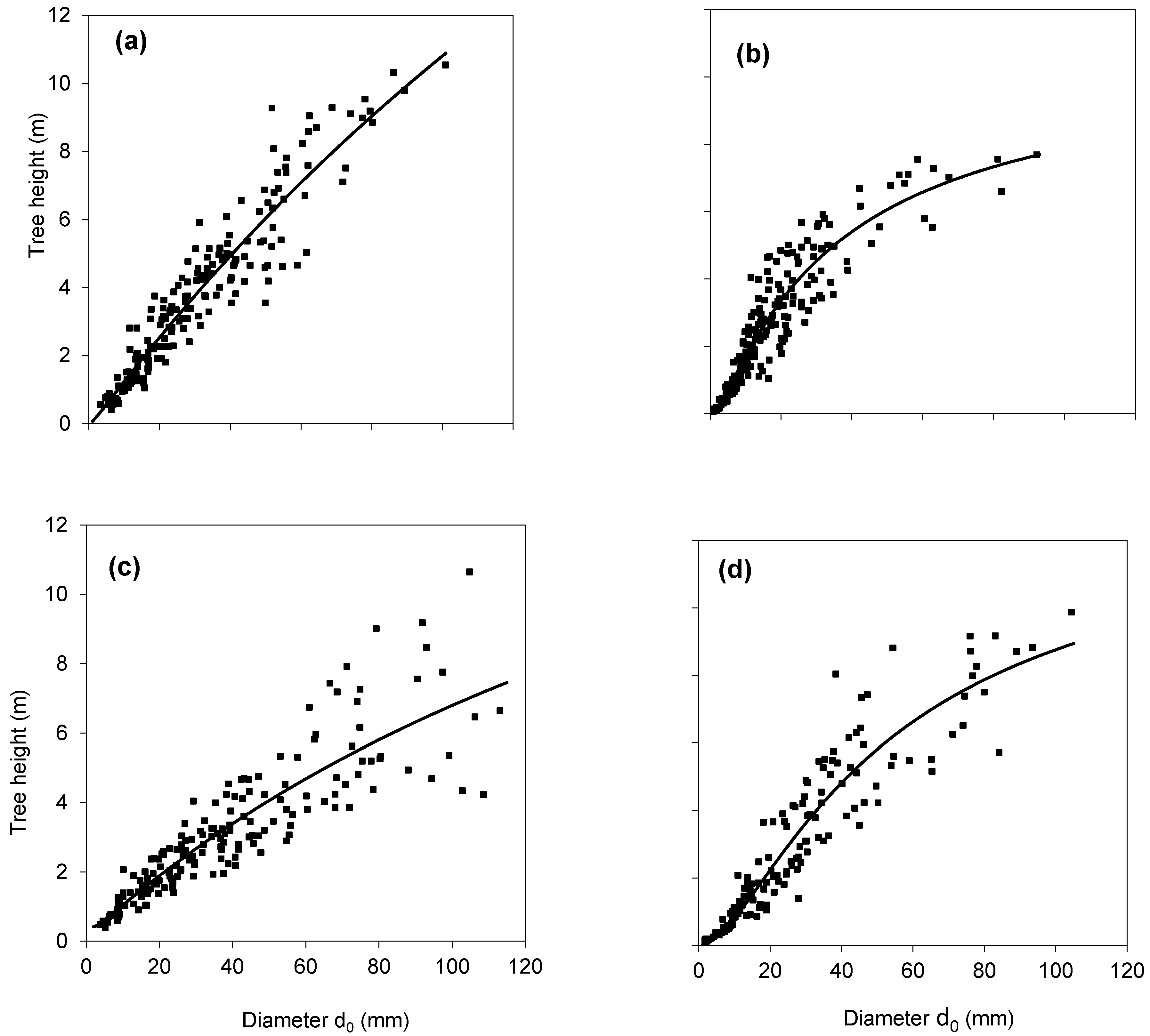
3. Discussion
4. Materials and Methods
4.1. Sampling and Data Collection
4.2. Data Analyses and Modelling
- -
- A level of individual leaves (for leaf mass weight wf expressed in g, leaf area LA in cm2 and SLA in cm per g);
- -
- A tree level (leaf mass weight wf expressed in kg, mass weight of woody parts wwp in kg, tree mass weight ww in kg, LA in m2, LAR in m2 of leaf area per kg of woody parts, LMR in kg of leaves per kg of woody parts).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Common Aspen | |||||||||
| Leaf Trait | Crown Part | b0 | S.E. | P | b1 | S.E. | P | R2 | MSE |
| Weight (g) | Lower | 0.007 | 0.002 | <0.001 | 0.712 | 0.056 | <0.001 | 0.342 | 0.002 |
| Middle | 0.015 | 0.003 | <0.001 | 0.585 | 0.048 | <0.001 | 0.332 | 0.003 | |
| Upper | 0.036 | 0.008 | <0.001 | 0.441 | 0.059 | <0.001 | 0.161 | 0.008 | |
| Area (cm2) | Lower | 2.611 | 0.470 | <0.001 | 0.519 | 0.047 | <0.001 | 0.285 | 45.98 |
| Middle | 4.113 | 0.640 | <0.001 | 0.430 | 0.041 | <0.001 | 0.265 | 44.10 | |
| Upper | 8.869 | 1.562 | <0.001 | 0.255 | 0.047 | <0.001 | 0.090 | 74.35 | |
| SLA (cm2 g−1) | Lower | 439.320 | 45.321 | <0.001 | −0.233 | 0.029 | <0.001 | 0.167 | 2077 |
| Middle | 484.466 | 51.303 | <0.001 | −0.297 | 0.030 | <0.001 | 0.225 | 1717 | |
| Upper | 469.014 | 52.938 | <0.001 | −0.342 | 0.032 | <0.001 | 0.249 | 1354 | |
| European Hornbeam | |||||||||
| Leaf trait | Crown part | b0 | S.E. | P | b1 | S.E. | P | R2 | MSE |
| Weight (g) | Lower | 0.011 | 0.0015 | <0.001 | 0.471 | 0.040 | <0.001 | 0.226 | 0.0006 |
| Middle | 0.012 | 0.0015 | <0.001 | 0.599 | 0.038 | <0.001 | 0.346 | 0.0015 | |
| Upper | 0.016 | 0.0020 | <0.001 | 0.665 | 0.036 | <0.001 | 0.434 | 0.0043 | |
| Area (cm2) | Lower | 1.265 | 0.119 | <0.001 | 0.327 | 0.029 | <0.001 | 0.222 | 1.699 |
| Middle | 1.477 | 0.124 | <0.001 | 0.369 | 0.026 | <0.001 | 0.307 | 2.364 | |
| Upper | 1.935 | 0.156 | <0.001 | 0.347 | 0.025 | <0.001 | 0.314 | 3.974 | |
| SLA (cm2 g−1) | Lower | 98.892 | 5.507 | <0.001 | −0.064 | 0.019 | <0.001 | 0.027 | 362 |
| Middle | 112.295 | 7.540 | <0.001 | −0.159 | 0.023 | <0.001 | 0.107 | 397 | |
| Upper | 97.470 | 5.718 | <0.001 | −0.196 | 0.021 | <0.001 | 0.161 | 388 | |
| Silver Birch | |||||||||
| Crown part | b0 | S.E. | P | b1 | S.E. | P | R2 | MSE | |
| Weight (g) | Lower | 0.026 | 0.0035 | <0.001 | 0.180 | 0.036 | <0.001 | 0.052 | 0.0007 |
| Middle | 0.042 | 0.0048 | <0.001 | 0.152 | 0.031 | <0.001 | 0.051 | 0.0010 | |
| Upper | 0.056 | 0.0074 | <0.001 | 0.207 | 0.035 | <0.001 | 0.077 | 0.0034 | |
| Area (cm2) | Lower | 5.096 | 0.589 | <0.001 | 0.134 | 0.031 | <0.001 | 0.039 | 14.07 |
| Middle | 7.940 | 0.779 | <0.001 | 0.089 | 0.027 | 0.001 | 0.023 | 18.75 | |
| Upper | 11.878 | 1.376 | <0.001 | 0.090 | 0.032 | 0.005 | 0.018 | 58.59 | |
| SLA (cm2 g−1) | Lower | 203.850 | 8.148 | <0.001 | −0.050 | 0.011 | <0.001 | 0.038 | 895 |
| Middle | 207.530 | 8.800 | <0.001 | −0.079 | 0.012 | <0.001 | 0.079 | 885 | |
| Upper | 228.310 | 8.925 | <0.001 | −0.132 | 0.011 | <0.001 | 0.214 | 668 | |
| Sycamore | |||||||||
| Leaf trait | Crown part | b0 | S.E. | P | b1 | S.E. | P | R2 | MSE |
| Weight (g) | Lower | 0.356 | 0.122 | 0.004 | 0.024 | 0.092 | 0.796 | 0.226 | 0.0002 |
| Middle | 0.296 | 0.080 | <0.001 | 0.189 | 0.071 | 0.008 | 0.022 | 0.113 | |
| Upper | 0.209 | 0.031 | <0.001 | 0.366 | 0.041 | <0.001 | 0.156 | 0.188 | |
| Area (cm2) | Lower | 61.590 | 17.276 | <0.001 | 0.087 | 0.075 | 0.247 | 0.005 | 2241 |
| Middle | 66.233 | 13.586 | <0.001 | 0.145 | 0.054 | 0.008 | 0.025 | 2380 | |
| Upper | 49.257 | 5.536 | <0.001 | 0.251 | 0.032 | <0.001 | 0.121 | 3092 | |
| SLA (cm2 g−1) | Lower | 190.634 | 28.250 | <0.001 | 0.071 | 0.039 | 0.073 | 0.014 | 5375 |
| Middle | 207.066 | 28.783 | <0.001 | 0.0045 | 0.037 | 0.904 | 0.0001 | 4092 | |
| Upper | 286.219 | 18.400 | <0.001 | −0.139 | 0.021 | <0.001 | 0.088 | 3556 | |
References
- West, P.W. Tree and Forest Measurement, 2nd ed.; Springer: Dordrecht, The Netherlands, 2009; p. 192. [Google Scholar]
- Enquist, B.J.; Niklas, K.J. Global Allocation Rules for Patterns of Biomass Partitioning in Seed Plants. Science 2002, 195, 1517–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowell, R.C.; Gibbins, D.; Rhoads, J.R.; Pallardy, S.G. Biomass production physiology and soil carbon dynamics in short-rotation-grown Populus deltoides and P. deltoides × P. nigra hybrids. For. Ecol. Manag. 2009, 257, 134–142. [Google Scholar] [CrossRef]
- Chen, R.; Ran, J.; Hu, W.; Dong, L.; Ji, M.; Jia, X.; Lu, J.; Gong, H.; Aqeel, S.; Yao, S.; et al. Effects of biotic and abiotic factors on forest biomass fractions. Natl. Sci. Rev. 2021, 1, 1–10. [Google Scholar]
- Konôpka, B.; Pajtík, J.; Moravčík, M.; Lukac, M. Biomass partitioning and growth efficiency in four naturally regenerated forest tree species. Basic Appl. Ecol. 2011, 11, 234–243. [Google Scholar] [CrossRef] [Green Version]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Pallardy, S.G. Physiology of Woody Plants; Academic Press: San Diego, CA, USA, 1997; p. 411. [Google Scholar]
- Härkonen, S.; Pulôkkinen, M.; Duursma, R.; Mäkelä, A. Estimating annual GPP, NPP and stem growth in Finland using summary models. For. Ecol. Manag. 2010, 259, 524–533. [Google Scholar] [CrossRef]
- Konôpka, B. Foliage and fine root litter: A comparative study in young, natural regenerated stands of European beech and Norway spruce. Austrian J. For. Sci. 2017, 134, 99–118. [Google Scholar]
- Thornley, J.H.M.; Cannell, M.G.R. Managing forests for wood yield and carbon storage: A theoretical study. Tree Physiol. 2020, 20, 477–484. [Google Scholar] [CrossRef]
- Barna, M. Adaptation of European beech (Fagus sylvatica L.) to different ecological conditions: Leaf area size variation. Polish J. Ecol. 2004, 52, 35–45. [Google Scholar]
- Closa, I.; Irigoyen, J.J.; Goicoechea, N. Microclimatic conditions determined by stem density influence leaf anatomy and leaf physiology of beech (Fagus sylvatica L.) growing within stands that naturally regenerate from clear-cutting. Trees 2012, 24, 1029–1043. [Google Scholar] [CrossRef]
- Konôpka, B.; Pajtík, J.; Šebeň, V.; Merganičová, K.; Surový, P. Silver birch aboveground biomass allocation pattern, stem and foliage traits with regard to intraspecific crown competition. Cent. Eur. For. J. 2020, 66, 159–169. [Google Scholar]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S.; Vose, J.M.; Volin, J.C.; Gresham, C.; Bowman, W.D. Relationships of leaf dark respiration to leaf nitrogen, specific leaf area and leaf life-span: A test across biomes and functional groups. Oecologia 1998, 114, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Shipley, B. Net assimilation rate, specific leaf area and leaf mass ratio: Which is most closely correlated with relative growth rate? A meta-analysis. Funct. Ecol. 2006, 20, 565–574. [Google Scholar] [CrossRef]
- Milla, R.; Reich, P.B.; Niinemets, U.; Castro-Díez, P. Environmental and developmental controls on specific leaf area are little modified by leaf allometry. Funct. Ecol. 2008, 22, 565–576. [Google Scholar] [CrossRef]
- Gersonde, R.F.; O’Hara, K.L. Comparative tree growth efficiency in Sierra Nevada mixed-conifer forests. For. Ecol. Manag. 2005, 219, 95–108. [Google Scholar] [CrossRef]
- Jack, B.S.; Sheffield, M.C.P.; McConville, D.J. Comparison of growth efficiency of mature long leaf and slash pine trees. Gen. Tech. Rep. 2002, 48, 81–85. [Google Scholar]
- Binkley, D.; Campoe, O.C.; Gspaltl, M.; Forrester, D.I. Light absorption and use efficiency in forests: Why patterns differ for trees and stands. For. Ecol. Manag. 2013, 288, 5–13. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Kunca, A.; Zúbrik, M.; Galko, J.; Vakula, J.; Leotovyč, R.; Konôpka, B.; Nikolov, C.; Gubka, J.; Longauerová, V.; Maľová, M.; et al. Salvage felling in the Slovak Republic’s forests during the last twenty years (1998–2017). Cent. Eur. For. J. 2019, 65, 3–11. [Google Scholar]
- Annighöfer, P.; Ameztegui, A.; Ammer, C.; Balandier, P.; Bartsch, N.; Bolte, A.; Coll, L.; Collet, C.; Ewald, J.; Mund, M.; et al. Species-specific and generic biomass equations for seedlings and saplings of European tree species. Eur. J. For. Res. 2015, 135, 313–329. [Google Scholar] [CrossRef] [Green Version]
- Mäkelä, A.; Valentine, H.T. The ratio of NPP to GPP: Evidence of change over the course of stand development. Tree Physiol. 2010, 21, 1015–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mette, T.; Brandl, S.; Kölling, C. Climate Analogues for Temperate European Forests to Raise Silvicultural Evidence Using Twin Regions. Sustainability 2010, 13, 6522. [Google Scholar] [CrossRef]
- Simon, J.; Dörken, V.M.; Arnold, A.L.M.; Adamczyk, B. Environmental Conditions and Species Identity Drive Metabolite Levels in Green Leaves and Leaf Litter of 14 Temperate Woody Species. Forests 2018, 9, 775. [Google Scholar] [CrossRef] [Green Version]
- Stiegel, S.; Mantilla-Contreraras, J. Experimental study of environmental effects: Leaf traits of juvenile Fagus sylvatica, Acer pseudoplatanus, and Carpinus betulus are comparable to leaves of mature trees in upper canopies. Int. J. Ecol. 2018, 2018, 3710128. [Google Scholar] [CrossRef] [Green Version]
- San-Miguel-Ayanz, J.; de Rigo, D.; Caudulllo, G.; Houston Durant, T.; Mauri, A.; Tinner, W.; Ballian, D.; Beck, P.; Birks, H.J.B.; Eaton, E.; et al. European Atlas of Forest Tree Species; European Commission: Luxembourg, 2016; p. 200. [Google Scholar]
- Blumenthal, B. Studies into the occurrence and properties of aspen in Finland. Silva Fenn. 1942, 56, 1–63. [Google Scholar]
- Jarvis, P.G.; Jarvis, M.S. Growth rates of woody plants. Physiol. Plantarum 1964, 17, 654–666. [Google Scholar] [CrossRef]
- Possen, J.H.M.; Oksanen, E.; Rousi, M.; Ruhanen, H.; Ahonen, V.; Tervahauta, A.; Heinonen, J.; Heiskanen, J.; Kärenlampi, S.; Vapaavouri, E. Adaptability of birch (Betula pendula Roth) and aspen (Populus tremula L.) genotypes to different soil moisture conditions. For. Ecol. Manag. 2011, 262, 1387–1399. [Google Scholar] [CrossRef]
- Worrell, R. European aspen (Populus tremula L.)—A review with particular reference to Scotland. 1 Distribution, ecology and genetic variation. Forestry 1995, 68, 93–105. [Google Scholar] [CrossRef]
- MacKenzie, N.A. Ecology, Conservation and Management of Aspen: A Literature Review; Scottish Native Woods: Aberfeldy, UK, 2010; p. 40. [Google Scholar]
- Atkinson, M.D. Betula pendula Roth (B. verrucosa Ehrh.) and B. pubescence Ehrh. J. Ecol. 1992, 80, 837–870. [Google Scholar] [CrossRef]
- Hein, S.; Collet, C.; Ammer, C.; Noel, G.; Skovsgaard, J.P.; Savill, P. A review of growth and stand dynamics of Acer pseudoplatanus L. in Europe: Implications for silviculture. Forestry 2009, 82, 361–385. [Google Scholar] [CrossRef] [Green Version]
- Hölscher, D.; Koch, O.; Korn, S.; Leuschner, C. Sap flux of five co-occurring tree species in a temperate broad-leaved forest during seasonal soil drought. Trees 2005, 19, 628–637. [Google Scholar] [CrossRef]
- Bouriaud, O.; Soudani, K.; Bréda, N. Leaf area index from litter collection: Impact of specific leaf area variability within a beech stand. Can. J. Remote Sens. 2003, 29, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Leuschner, C.; Voss, S.; Foetzki, A.; Clases, Y. Variation in leaf area index and stand leaf mass of European beech across gradients of soil acidity and precipitation. Plant Ecol. 2006, 186, 247–258. [Google Scholar] [CrossRef]
- Howard, S. Leaf Litter Flammability of Different UK Woodland Tree Species in Future Climate. Ph.D. Thesis, University of Bangor, Bangor, UK, 2017; p. 44. [Google Scholar]
- Petritan, A.M.; von Lupke, B.; Petritan, I.C. Influence of light availability on growth, leaf morphology and plant architecture of beech (Fagus sylvatica L.), maple (Acer pseudoplatanus L.) and ash (Fraxinus excelsior L.) saplings. Eur. J. For. Res. 2009, 128, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Konôpka, B.; Pajtík, J. Similar foliage but contrasting foliage biomass between young beech and spruce stands. Lesn. Cas. For. J. 2014, 60, 205–213. [Google Scholar]
- Kinney, K.K.; Lindroth, R.L. Responses of three deciduous tree species to atmospheric CO2 and soil NO3 availability. Can. J. For. Res. 1996, 27, 1–10. [Google Scholar] [CrossRef]
- Thomas, F.M.; Bogelein, R.; Werner, W. Interaction between Douglas fir and European beech—Investigations in pure and mixed stands. Forstarchiv 2014, 86, 83–91. [Google Scholar]
- Thomas, S.C.; Winner, W.E. Photosynthetic differences between sapling and adult trees: An integration of field results by meta-analyses. Tree Physiol. 2020, 22, 117–127. [Google Scholar] [CrossRef]
- Wright, I.J.; Leishman, M.R.; Read, C.; Westoby, M. Gradients of light availability and leaf traits with leaf age and canopy position in 28 Australian shrubs and trees. Funct. Plant Biom. 2006, 33, 407–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waring, R.H. Estimating forest growth and efficiency in relation to canopy leaf area. Adv. Ecol. Res. 1983, 13, 327–354. [Google Scholar]
- Xu, C.-Y.; Turnbull, M.H.; Tissue, D.T.; Lewis, J.D.; Carson, R.; Schuster, W.S.F.; Whitehead, D.; Walcroft, A.S.; Li, J.; Griffin, K.L. Age-realted decline of stand biomass accumulation is primary due to mortality and not to reduction in NPP associated with individual tree physiology, tree growth or stand structure in a Quercus-dominated forest. J. Ecol. 2012, 100, 428–440. [Google Scholar] [CrossRef] [Green Version]
- Iberle, B.G.; Van Pelt, R.; Sillet, S.C. Development of mature second-growth Sequoia sempervirens forests. For. Ecol. Manag. 2020, 459, 117816. [Google Scholar] [CrossRef]
- Kramer, K.; Leinonen, I.; Bartelink, H.H.; Berbigier, P.; Borghetti, M.; Bernhofer, C.; Cienciala, E.; Dolman, A.J.; Froer, O.; Garcia, A.; et al. Evaluation of six process-based forest growth models using eddy-covariance measurements of CO2 and H2O fluxes at six forest sites in Europe. Glob. Chang. Biol. 2002, 8, 213–230. [Google Scholar] [CrossRef]
- Bravo, F.; Fabrika, M.; Ammer, C.; Barreiro, S.; Bielak, K.; Coll, L.; Fonseca, T.; Kangur, A.; Löf, M.; Merganičová, K.; et al. Modelling approaches for mixed forests dynamics prognosis. Research gaps and opportunities. For. Syst. 2019, 28, 1–18. [Google Scholar]
- Hlásny, T.; Mokroš, M.; Dobor, L.; Merganičová, K.; Lukac, M. Fine-Scale Variation in Projected Climate Change Presents Opportunities for Biodiversity Conservation in Europe. Available online: https://www.researchsquare.com/article/rs-322150/v1 (accessed on 19 March 2021).
- Sohngen, B. Climate Change and Forests. Annu. Rev. Resour. Econ. 2020, 12, 23–43. [Google Scholar] [CrossRef]
- Takolander, A.; Hickler, T.; Meller, L.; Cabeza, M. Comparing future shifts in tree species distributions across Europe projected by statistical and dynamic process-based models. Reg. Environ. Chang. 2019, 19, 251–266. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.; Pedlar, J.; Peters, J.; McKenney, D.; Iverson, L.; Matthews, S.; Adams, B. Combining US and Canada forest inventories to assess habitat suitability and migration potential of 25 species under climate change. Divers. Distrib. 2020, 26, 1142–1159. [Google Scholar] [CrossRef]
- Vaško, M.; Garčár, M. Lesnícka typológia a jej význam pri plánovaní drevinového zloženia lesov Slovenska. In Výstupy NLC Pre Lesnícku Prax III; National Forest Centre: Zvolen, Slovakia, 2021; pp. 56–70. (In Slovak) [Google Scholar]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef]
- Pajtík, J.; Konôpka, B.; Šebeň, V. Mathematical Biomass Models for Young Individuals of Forest Tree Species in the Region of the Western Carpathians; National Forest Centre: Zvolen, Slovakia, 2018; p. 89. [Google Scholar]
- Marklund, L.G. Biomass Functions for Norway Spruce (Picea Abies L. Karst.) in Sweden; SLU, Department of Forest Survey: Umea, Sweden, 1987; p. 127. [Google Scholar]
- Ledermann, T.; Neuman, M. Biomass equations from data of old long-term experimental plots. Austrian J. For. Sci. 2006, 123, 447–464. [Google Scholar]






| Tree Species | b0 | S.E. | P | b1 | S.E. | P | b2 | S.E. | P | R2 | MSE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Common aspen | 8.221 | 11.812 | 0.487 | 7.077 | 0.693 | <0.001 | 0.021 | 0.009 | 0.017 | 0.897 | 0.612 |
| European hornbeam | 16.924 | 6.036 | 0.006 | 3.254 | 0.527 | <0.001 | 0.093 | 0.009 | <0.001 | 0.864 | 0.485 |
| Silver birch | −11.136 | 20.249 | 0.583 | 10.251 | 1.214 | <0.001 | 0.046 | 0.014 | <0.001 | 0.776 | 0.840 |
| Sycamore | 84.237 | 25.897 | 0.001 | 3.273 | 1.311 | 0.014 | 0.073 | 0.014 | <0.001 | 0.882 | 0.749 |
| Leaf Trait | Tree Species | b0 | S.E. | P | b1 | S.E. | P | R2 | MSE |
|---|---|---|---|---|---|---|---|---|---|
| Weight (g) | Aspen | 0.018 | 0.003 | <0.001 | 0.552 | 0.034 | <0.001 | 0.199 | 0.005 |
| Hornbeam | 0.013 | 0.001 | <0.001 | 0.606 | 0.029 | <0.001 | 0.232 | 0.003 | |
| Birch | 0.042 | 0.004 | <0.001 | 0.184 | 0.025 | <0.001 | 0.197 | 0.003 | |
| Sycamore | 0.304 | 0.039 | <0.001 | 0.189 | 0.035 | <0.001 | 0.029 | 0.168 | |
| Area (cm2) | Aspen | 4.790 | 0.495 | <0.001 | 0.390 | 0.027 | <0.001 | 0.183 | 59.3 |
| Hornbeam | 1.588 | 0.091 | <0.001 | 0.342 | 0.018 | <0.001 | 0.222 | 3.48 | |
| Birch | 8.304 | 0.651 | <0.001 | 0.099 | 0.021 | <0.001 | 0.125 | 41.7 | |
| Sycamore | 60.791 | 5.707 | <0.001 | 0.157 | 0.026 | <0.001 | 0.037 | 2921 | |
| SLA (cm2 g−1) | Aspen | 457.011 | 31.852 | <0.001 | −0.284 | 0.020 | <0.001 | 0.172 | 2174 |
| Hornbeam | 98.600 | 3.904 | <0.001 | −0.122 | 0.014 | <0.001 | 0.060 | 506 | |
| Birch | 211.803 | 5.334 | <0.001 | −0.084 | 0.007 | <0.001 | 0.291 | 943 | |
| Sycamore | 205.480 | 11.766 | <0.001 | 0.004 | 0.026 | 0.822 | 0.000 | 4992 |
| Tree Species | Compartment | b0 | S.E. | P | b2 | S.E. | P | R2 | MSE | λ | S.D. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Common aspen | Leaves | −2.907 | 0.225 | <0.001 | 2.020 | 0.069 | <0.001 | 0.829 | 0.429 | 1.191 | 0.634 |
| Woody parts | −2.760 | 0.093 | <0.001 | 2.699 | 0.028 | <0.001 | 0.982 | 0.071 | 1.036 | 0.293 | |
| Whole tree | −2.379 | 0.092 | <0.001 | 2.618 | 0.028 | <0.001 | 0.981 | 0.070 | 1.030 | 0.296 | |
| European hornbeam | Leaves | −4.127 | 0.165 | <0.001 | 2.354 | 0.061 | <0.001 | 0.884 | 0.497 | 1.225 | 0.700 |
| Woody parts | −2.604 | 0.064 | <0.001 | 2.680 | 0.024 | <0.001 | 0.985 | 0.072 | 1.037 | 0.391 | |
| Whole tree | −2.389 | 0.062 | <0.001 | 2.640 | 0.023 | <0.001 | 0.986 | 0.068 | 1.034 | 0.277 | |
| Silver birch | Leaves | −2.404 | 0.154 | <0.001 | 1.904 | 0.044 | <0.001 | 0.913 | 0.191 | 1.089 | 0.422 |
| Woody parts | −2.332 | 0.116 | <0.001 | 2.449 | 0.033 | <0.001 | 0.970 | 0.106 | 1.053 | 0.345 | |
| Whole tree | −2.715 | 0.128 | <0.001 | 2.742 | 0.029 | <0.001 | 0.973 | 0.122 | 1.034 | 0.370 | |
| Sycamore | Leaves | −2.468 | 0.135 | <0.001 | 1.894 | 0.043 | <0.001 | 0.928 | 0.247 | 1.118 | 0.509 |
| Woody parts | −3.322 | 0.081 | <0.001 | 2.816 | 0.028 | <0.001 | 0.986 | 0.101 | 1.052 | 0.347 | |
| Whole tree | −2.558 | 0.073 | <0.001 | 2.627 | 0.023 | <0.001 | 0.988 | 0.072 | 1.037 | 0.293 |
| Tree Species | b1 | S.E. | P | b2 | S.E. | P | R2 | MSE |
|---|---|---|---|---|---|---|---|---|
| Common aspen | 0.0031 | 0.0012 | 0.010 | 1.723 | 0.093 | <0.001 | 0.734 | 0.834 |
| European hornbeam | 0.0003 | 0.0001 | <0.001 | 2.060 | 0.063 | <0.001 | 0.889 | 0.014 |
| Silver birch | 0.0015 | 0.0006 | 0.011 | 1.883 | 0.088 | <0.001 | 0.821 | 1.236 |
| Sycamore | 0.0018 | 0.0007 | 0.011 | 1.884 | 0.090 | <0.001 | 0.838 | 0.807 |
| Indicator | Tree Species | b1 | S.E. | P | b2 | S.E. | P | R2 | MSE |
|---|---|---|---|---|---|---|---|---|---|
| Leaf mass ratio (LMR) | Aspen | 0.657 | 0.083 | <0.001 | −0.585 | 0.048 | <0.001 | 0.458 | 0.0031 |
| Hornbeam | 0.228 | 0.020 | <0.001 | −0.319 | 0.038 | <0.001 | 0.221 | 0.0030 | |
| Birch | 0.591 | 0.059 | <0.001 | −0.433 | 0.034 | <0.001 | 0.477 | 0.0028 | |
| Sycamore | 0.890 | 0.032 | <0.001 | −0.623 | 0.019 | <0.001 | 0.865 | 0.0026 | |
| Leaf area ratio (LAR) | Aspen | 308.403 | 37.187 | <0.001 | −0.869 | 0.051 | <0.001 | 0.634 | 165.93 |
| Hornbeam | 26.542 | 1.811 | <0.001 | −0.497 | 0.034 | <0.001 | 0.444 | 18.39 | |
| Birch | 122.745 | 11.885 | <0.001 | −0.512 | 0.034 | <0.001 | 0.560 | 75.71 | |
| Sycamore | 184.313 | 6.751 | <0.001 | −0.618 | 0.019 | <0.001 | 0.862 | 112.20 |
| Tree Species | Range of Altitudes | Number of | Mean Ages * of | Number of | Number of | Mean Value and (Standard Deviation) of | |
|---|---|---|---|---|---|---|---|
| (m a.s.l.) | Stands | Stands | Sampled Trees | Scanned Leaves | Tree Height (m) | Diameter d0 (mm) | |
| Common aspen | 335–870 | 8 | 2–12 | 185 | 980 | 3.81 (2.42) | 31.39 (20.00) |
| European hornbeam | 295–570 | 8 | 1–10 | 200 | 1392 | 2.68 (1.83) | 17.84 (13.94) |
| Silver birch | 260–950 | 8 | 1–12 | 178 | 1476 | 3.12 (1.93) | 38.49 (25.69) |
| Sycamore | 415–970 | 8 | 2–11 | 150 | 1009 | 3.00 (2.30) | 27.73 (22.18) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konôpka, B.; Pajtík, J.; Šebeň, V.; Surový, P.; Merganičová, K. Woody and Foliage Biomass, Foliage Traits and Growth Efficiency in Young Trees of Four Broadleaved Tree Species in a Temperate Forest. Plants 2021, 10, 2155. https://doi.org/10.3390/plants10102155
Konôpka B, Pajtík J, Šebeň V, Surový P, Merganičová K. Woody and Foliage Biomass, Foliage Traits and Growth Efficiency in Young Trees of Four Broadleaved Tree Species in a Temperate Forest. Plants. 2021; 10(10):2155. https://doi.org/10.3390/plants10102155
Chicago/Turabian StyleKonôpka, Bohdan, Jozef Pajtík, Vladimír Šebeň, Peter Surový, and Katarína Merganičová. 2021. "Woody and Foliage Biomass, Foliage Traits and Growth Efficiency in Young Trees of Four Broadleaved Tree Species in a Temperate Forest" Plants 10, no. 10: 2155. https://doi.org/10.3390/plants10102155
APA StyleKonôpka, B., Pajtík, J., Šebeň, V., Surový, P., & Merganičová, K. (2021). Woody and Foliage Biomass, Foliage Traits and Growth Efficiency in Young Trees of Four Broadleaved Tree Species in a Temperate Forest. Plants, 10(10), 2155. https://doi.org/10.3390/plants10102155







