Genotype-Dependent Variation of Nutritional Quality-Related Traits in Quinoa Seeds
Abstract
:1. Introduction
2. Results
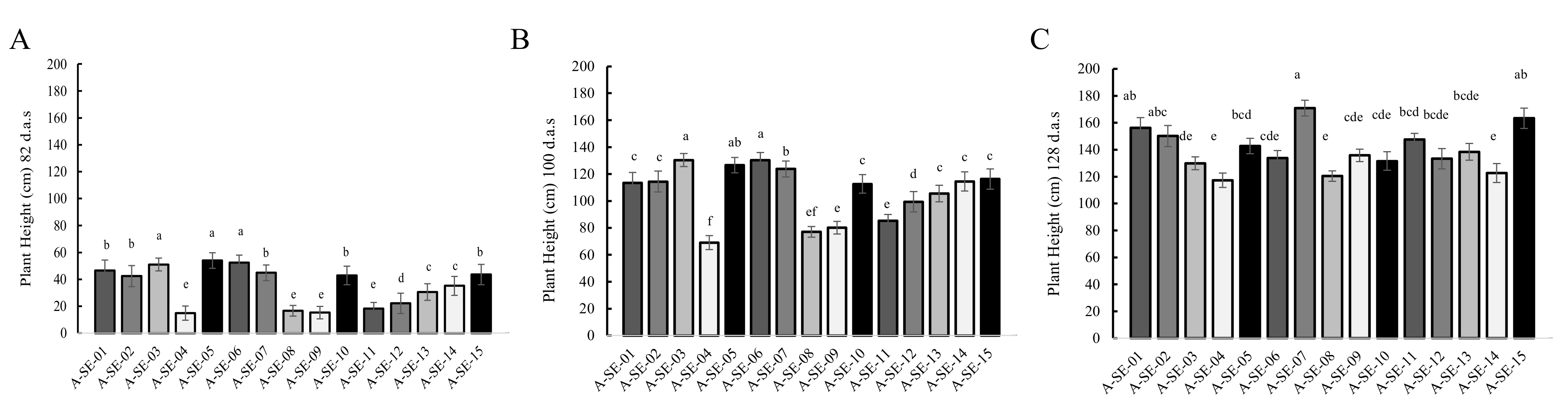
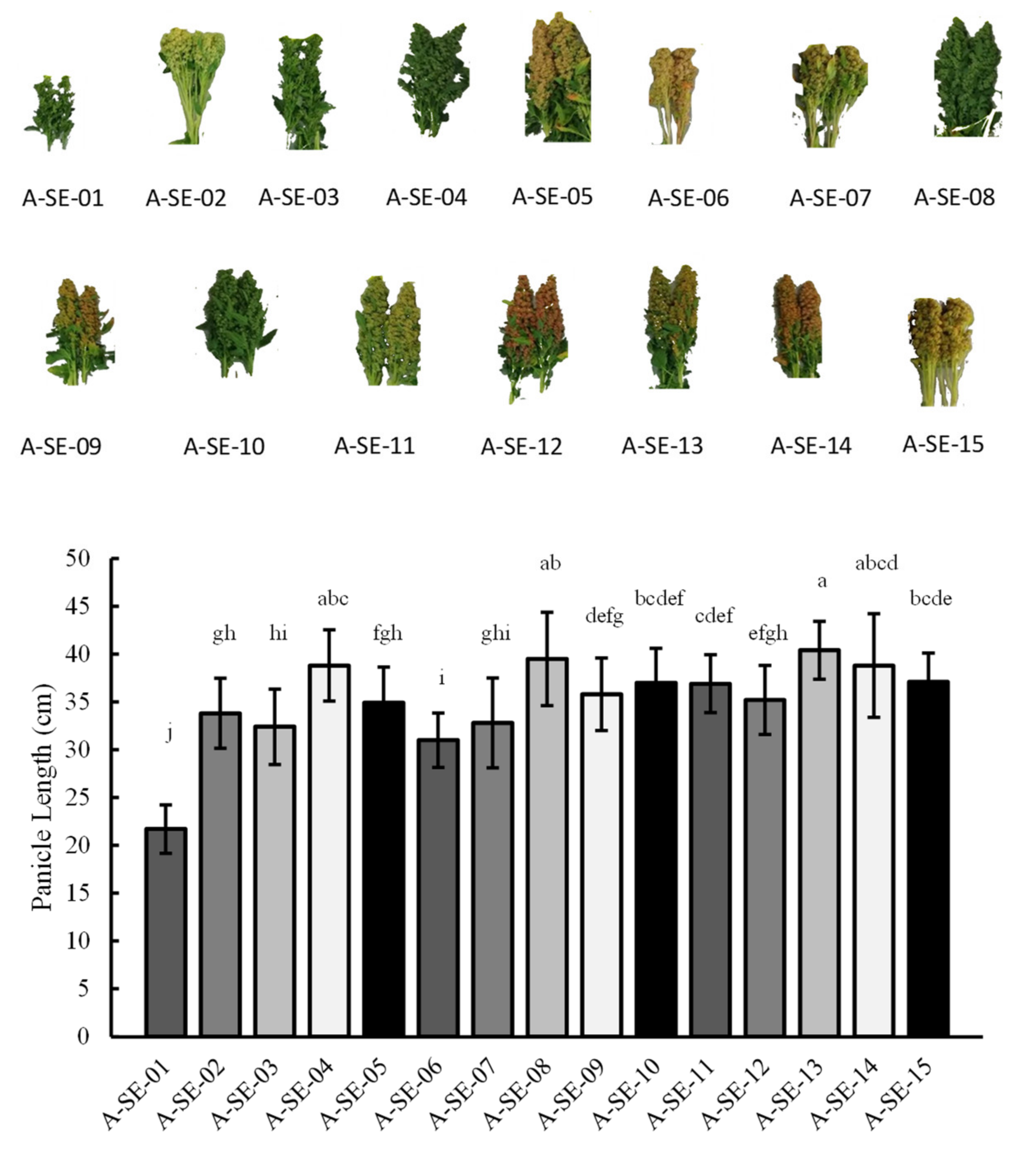
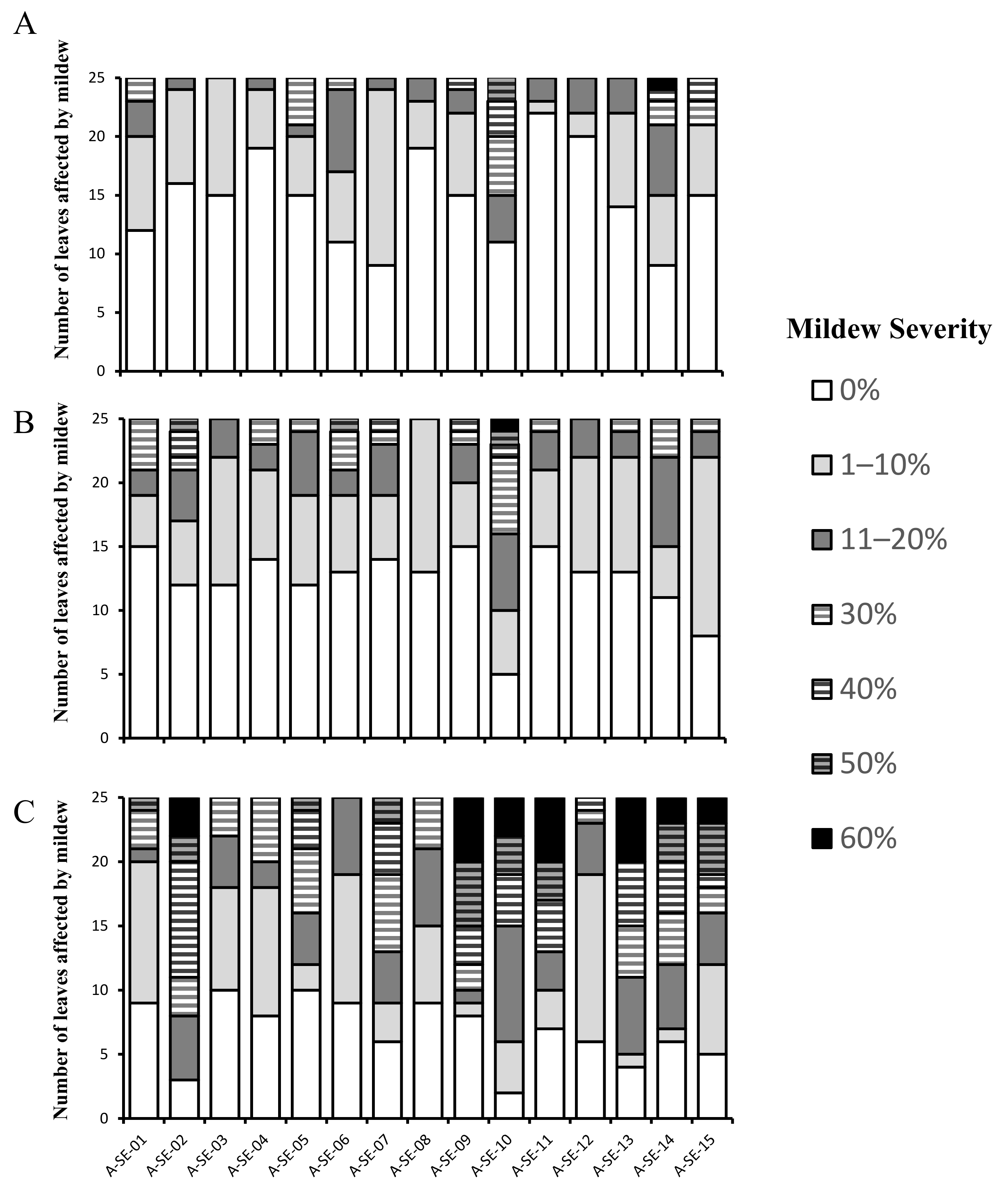
2.1. Plant Performance and Physiological and Agronomical Traits
2.2. Germination Rates and Seed Viability
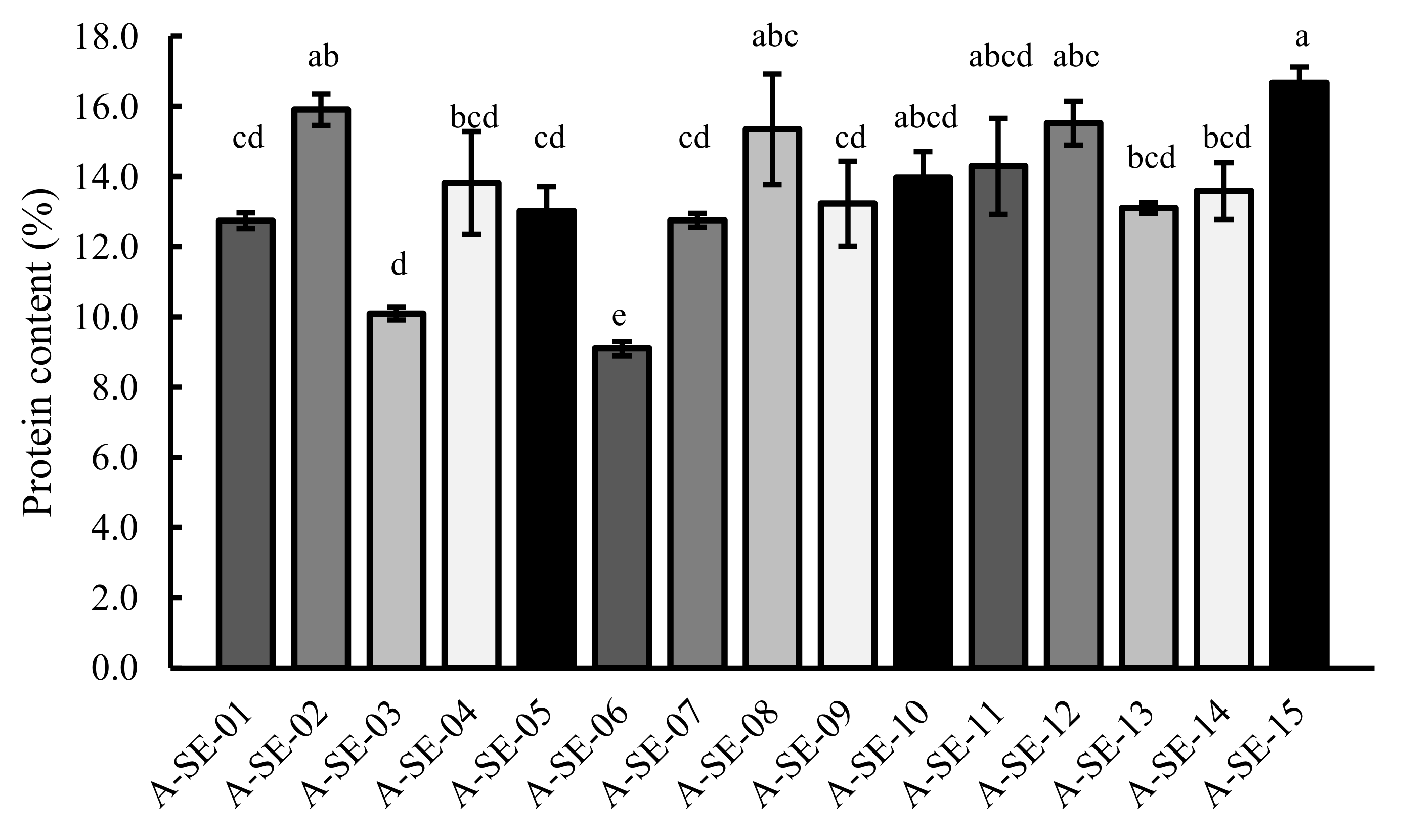
2.3. Protein Content
2.4. Mineral Content
2.5. Antioxidant Capacity
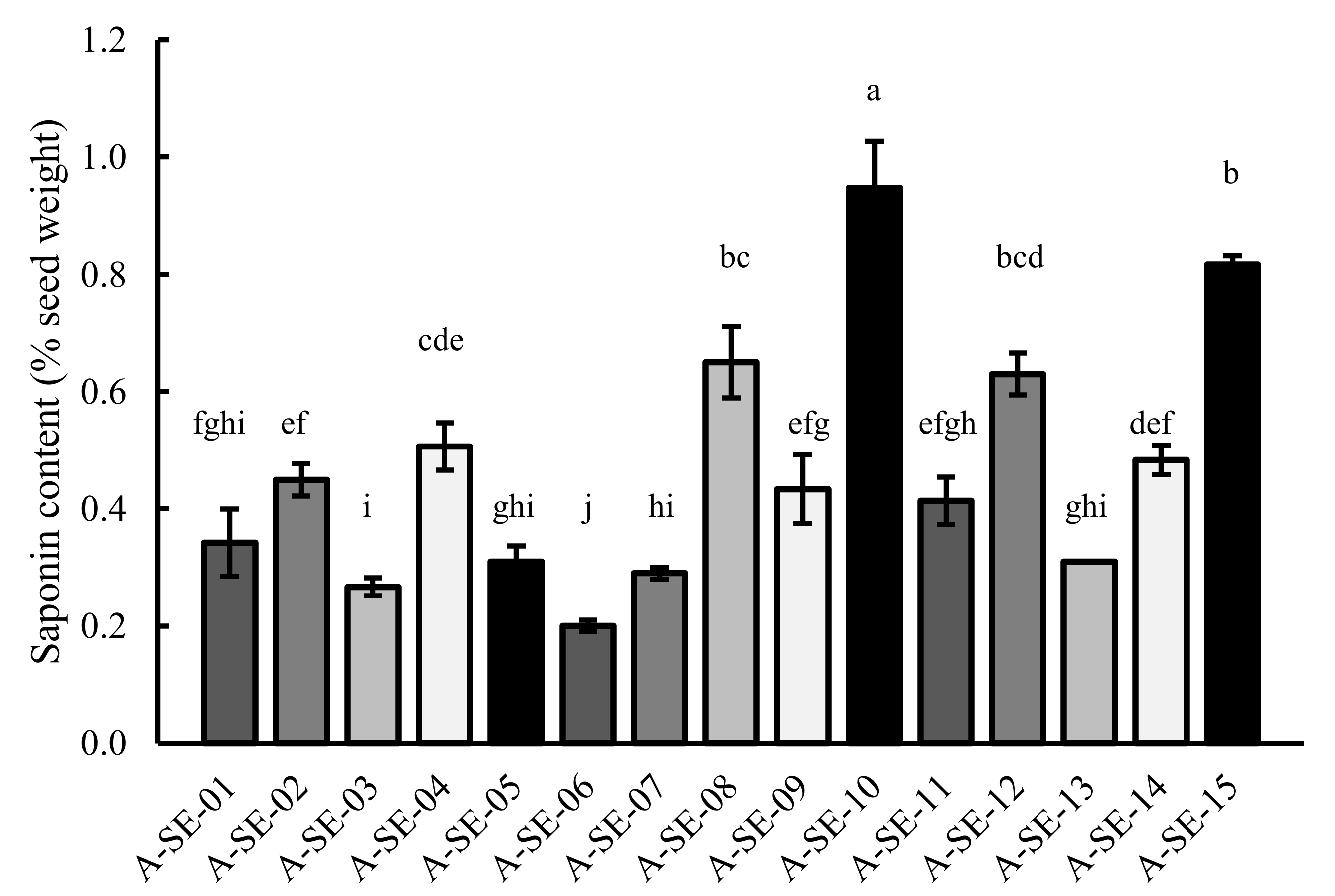
2.6. Saponin Content
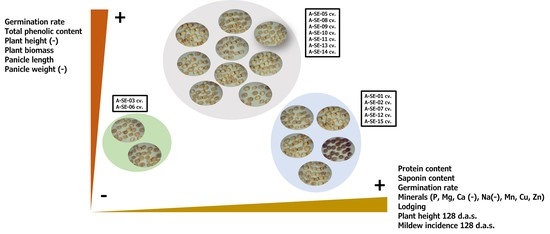
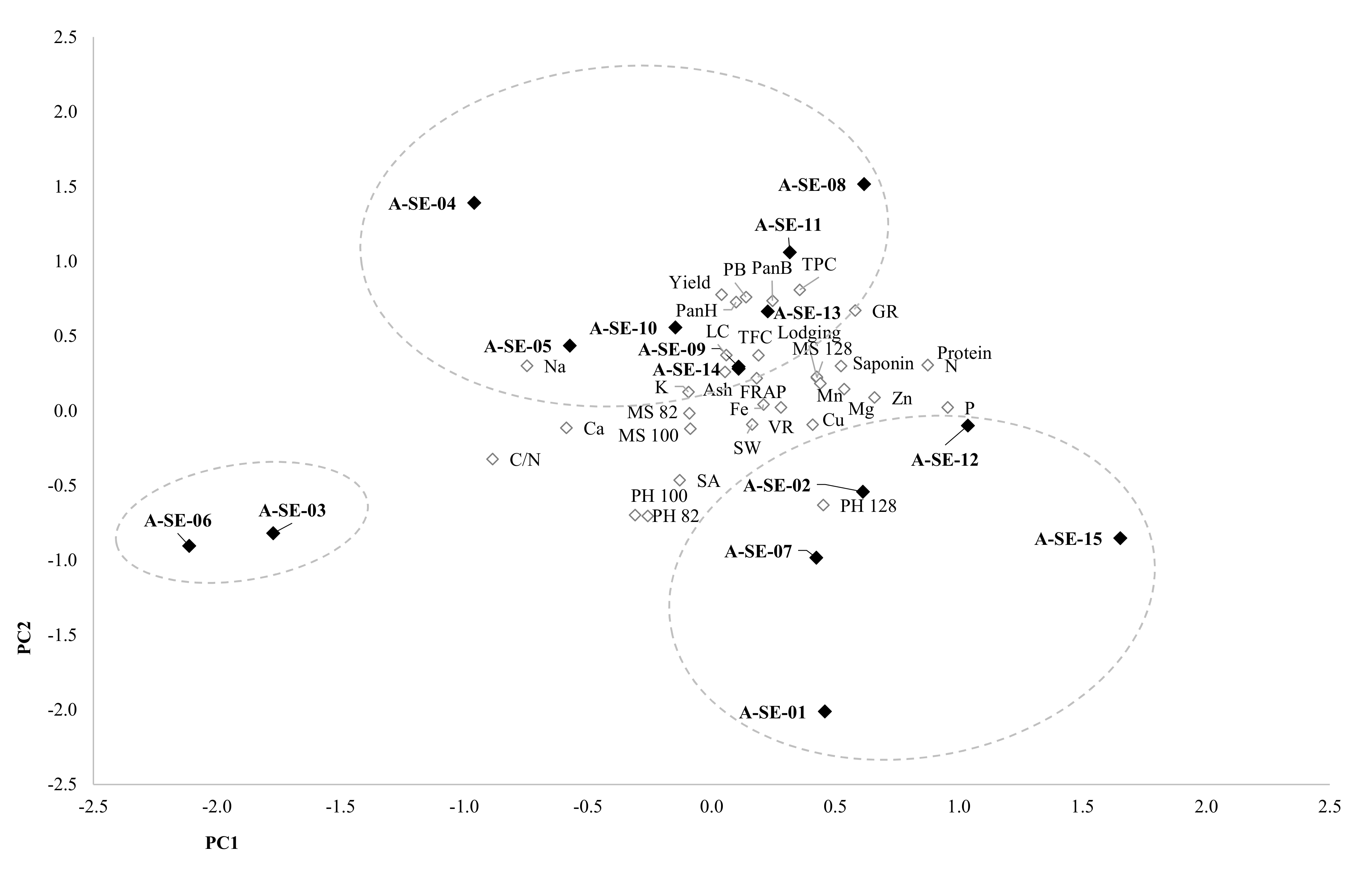
2.7. Principal Components Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Plant Material, Experimental Design, and Location
4.2. Seed Weight and Area
4.3. Seed Germination Rate
4.4. Seed Viability
4.5. Saponin Content
4.6. Protein Content
4.7. Mineral Content
4.8. Ferric Reducing Antioxidant Power (FRAP) Assay
4.9. Total Phenol Content (TPC)
4.10. Total Flavonoid Content (TFC)
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, C.; Chen, A.; Xiao, L.; Muller, H.M.; Ache, P.; Haberer, G.; Zhang, M.; Jia, W.; Deng, P.; Huang, R.; et al. A high-quality genome assembly of quinoa provides insights into the molecular basis of salt bladder-based salinity tolerance and the exceptional nutritional value. Cell Res. 2017, 27, 1327–1340. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; et al. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 2014, 34, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Bai, X.; Wu, X.; Xiang, D.; Wan, Y.; Luo, Y.; Shi, X.; Li, Q.; Zhao, J.; Qin, P.; et al. Transcriptome profiling identifies transcription factors and key homologs involved in seed dormancy and germination regulation of Chenopodium quinoa. Plant Physiol. Biochem. 2020, 151, 443–456. [Google Scholar] [CrossRef]
- Alandia, G.; Rodriguez, J.P.; Jacobsen, S.-E.; Bazile, D.; Condori, B. Global expansion of quinoa and challenges for the Andean region. Glob. Food Sec. 2020, 26, 100429. [Google Scholar] [CrossRef]
- Bazile, D.; Jacobsen, S.E.; Verniau, A. The Global Expansion of Quinoa: Trends and Limits. Front. Plant Sci. 2016, 7, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, W.; Pinto, M.; Alanoca, C.; Gómez-Pando, L.R.; León-Lobos, P.; Alercia, A.; Diulgheroff, S.; Padulosi, S.; Bazile, D. Quinoa genetic resources and ex situ conservation. In State of the Art Report of Quinoa in the World in 2013; Bazile, D., Bertero, H.D., Nieto, C., Eds.; FAO & CIRAD: Rome, Italy, 2015; pp. 56–82. [Google Scholar]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.E. Nutritional value and use of the andean crops quinoa (Chenopodium quinoa) and kañiwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Jarvis, D.E.; Ho, Y.S.; Lightfoot, D.J.; Schmöckel, S.M.; Li, B.; Borm, T.J.A.; Ohyanagi, H.; Mineta, K.; Michell, C.T.; Saber, N.; et al. The genome of Chenopodium quinoa. Nature 2017, 542, 307–312. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Kong, Z.; Liu, J.; Zhang, P.; Wang, Q.; Huan, X.; Li, L.; Qin, P. Non-targeted metabolomics of quinoa seed filling period based on liquid chromatography-mass spectrometry. Food Res. Int. 2020, 137, 109743. [Google Scholar] [CrossRef]
- Asher, A.; Galili, S.; Whitney, T.; Rubinovich, L. The potential of quinoa (Chenopodium quinoa) cultivation in Israel as a dual-purpose crop for grain production and livestock feed. Sci. Hortic. 2020, 272, 109534. [Google Scholar] [CrossRef]
- Rodríguez Gómez, M.J.; Matías Prieto, J.; Cruz Sobrado, V.; Calvo Magro, P. Nutritional characterization of six quinoa (Chenopodium quinoa Willd) varieties cultivated in Southern Europe. J. Food Compos. Anal. 2021, 99, 1–7. [Google Scholar] [CrossRef]
- Tang, Y.; Tsao, R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: A review. Mol. Nutr. Food Res. 2017, 61, 1600767. [Google Scholar] [CrossRef] [PubMed]
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, Y.J.; Kim, Y.H.; Yoon, K.S. Antioxidant and antimicrobial activities of Quinoa (Chenopodium quinoa Willd.) Seeds Cultivated in Korea. Prev. Nutr. Food Sci. 2017, 22, 195–202. [Google Scholar] [CrossRef]
- Muziri, T.; Chaibva, P.; Chofamba, A.; Madanzi, T.; Mangeru, P.; Mudada, N.; Manhokwe, S.; Mugari, A.; Matsvange, D.; Murewi, C.T.F.; et al. Using principal component analysis to explore consumers’ perception toward quinoa health and nutritional claims in Gweru, Zimbabwe. Food Sci. Nutr. 2021, 9, 1025–1033. [Google Scholar] [CrossRef]
- Groeniger, J.O.; van Lenthe, F.J.; Beenackers, M.A.; Kamphuis, C.B.M. Does social distinction contribute to socioeconomic inequalities in diet: The case of ‘superfoods’ consumption. Int. J. Behav. Nutr. Phys. Act. 2017, 14. [Google Scholar] [CrossRef] [Green Version]
- Bedoya-Perales, N.S.; Pumi, G.; Talamini, E.; Padula, A.D. The quinoa boom in Peru: Will land competition threaten sustainability in one of the cradles of agriculture? Land Use Policy 2018, 79, 475–480. [Google Scholar] [CrossRef]
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.-P.; Iglesias, A.; Lange, M.A.; Lionello, P.; Llasat, M.C.; Paz, S.; et al. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Chang. 2018, 8, 972–980. [Google Scholar] [CrossRef] [Green Version]
- El Estado Mundial de la Agricultura y la Alimentación, 1994. Available online: http://www.fao.org/3/T4450S/t4450s05.htm826 (accessed on 26 July 2021).
- Aloisi, I.; Parrotta, L.; Ruiz, K.B.; Landi, C.; Bini, L.; Cai, G.; Biondi, S.; del Duca, S. New Insight into Quinoa Seed Quality under Salinity: Changes in Proteomic and Amino Acid Profiles, Phenolic Content, and Antioxidant Activity of Protein Extracts. Front. Plant Sci. 2016, 7, 656. [Google Scholar] [CrossRef] [Green Version]
- Reguera, M.; Conesa, C.M.; Gil-Gómez, A.; Haros, C.M.; Pérez-Casas, M.Á.; Briones-Labarca, V.; Bolaños, L.; Bonilla, I.; Álvarez, R.; Pinto, K.; et al. The impact of different agroecological conditions on the nutritional composition of quinoa seeds. PeerJ 2018, 6, e4442. [Google Scholar] [CrossRef]
- Gonzalez, J.A.; Konishi, Y.; Bruno, M.; Valoy, M.; Prado, F.E. Interrelationships among seed yield, total protein and amino acid composition of ten quinoa (Chenopodium quinoa) cultivars from two different agroecological regions. J. Sci. Food Agric. 2011, 92, 1222–1229. [Google Scholar] [CrossRef]
- Bertero, H.D.; De La Vega, A.J.; Correa, G.; Jacobsen, S.E.; Mujica, A. Genotype and genotype-by-environment interaction effects for grain yield and grain size of quinoa (Chenopodium quinoa Willd.) as revealed by pattern analysis of international multi-environment trials. F. Crop. Res. 2004, 89, 299–318. [Google Scholar] [CrossRef]
- Matías, J.; Rodríguez, M.J.; Cruz, V.; Calvo, P.; Reguera, M. Heat stress lowers yields, alters nutrient uptake and changes seed quality in quinoa grown under Mediterranean field conditions. J. Agron. Crop Sci. 2021, 207, 481–491. [Google Scholar] [CrossRef]
- Granado-Rodríguez, S.; Aparicio, N.; Matías, J.; Pérez-Romero, L.F.; Maestro, I.; Garcés, I.; Pedroche, J.J.; Haros, C.M.; Fernández-García, N.; Navarro del Hierro, J.; et al. Studying the Impact of Different Field Environmental Conditions on Seed Quality of Quinoa: The Case of Three Different Years Changing Seed Nutritional Traits in Southern Europe. Front. Plant Sci. 2021, 12, 649132. [Google Scholar] [CrossRef]
- Hussain, M.I.; Muscolo, A.; Ahmed, M.; Asghar, M.A.; Al-Dakheel, A.J. Agro-Morphological, Yield and Quality Traits and Interrelationship with Yield Stability in Quinoa (Chenopodium quinoa Willd.) Genotypes under Saline Marginal Environment. Plants 2020, 9, 1763. [Google Scholar] [CrossRef] [PubMed]
- Zurita-Silva, A.; Fuentes, F.; Zamora, P.; Jacobsen, S.E.; Schwember, A.R. Breeding quinoa (Chenopodium quinoa Willd.): Potential and perspectives. Mol. Breed. 2014, 34, 13–30. [Google Scholar] [CrossRef]
- Koziol, M.J. Afrosimetric estimation of threshold saponin concentration for bitterness in quinoa (Chenopodium quinoa Willd). J. Sci. Food Agric. 1991, 54, 211–219. [Google Scholar] [CrossRef]
- Medina-Meza, I.G.; Aluwi, N.A.; Saunders, S.R.; Ganjyal, G.M. GC-MS Profiling of Triterpenoid Saponins from 28 Quinoa Varieties (Chenopodium quinoa Willd.) Grown in Washington State. J. Agric. Food Chem. 2016, 64, 8583–8591. [Google Scholar] [CrossRef]
- Machado Alencar, N.M.; de Carvalho Oliveira, L. Advances in Pseudocereals: Crop Cultivation, Food Application, and Consumer Perception. In Bioactive Molecules in Food. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2019; pp. 1695–1713. [Google Scholar]
- Abugoch James, L.E. Quinoa (Chenopodium quinoa Willd.): Composition, Chemistry, Nutritional, and Functional Properties. In Advances in Food and Nutrition Research; Elsevier Inc.: Santiago, Chile, 2009; Volume 58, pp. 1–31. [Google Scholar]
- Fuentes, F.; Bhargava, A. Morphological Analysis of Quinoa Germplasm Grown Under Lowland Desert Conditions. J. Agron. Crop Sci. 2011, 197, 124–134. [Google Scholar] [CrossRef]
- Miranda, M.; Vega-Gálvez, A.; Martinez, E.; López, J.; Rodríguez, M.J.; Henríquez, K.; Fuentes, F. Genetic diversity and comparison of physicochemical and nutritional characteristics of six quinoa (Chenopodium quinoa willd.) genotypes cultivated in Chile. Food Sci. Technol. 2012, 32, 835–843. [Google Scholar] [CrossRef] [Green Version]
- Benlhabib, O.; Boujartani, N.; Maughan, P.J.; Jacobsen, S.E.; Jellen, E.N. Elevated genetic diversity in an F2:6 population of quinoa (Chenopodium quinoa) developed through an inter-ecotype cross. Front. Plant Sci. 2016, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pulvento, C.; Riccardi, M.; Lavini, A.; D’Andria, R.; Iafelice, G.; Marconi, E. Field Trial Evaluation of Two Chenopodium quinoa Genotypes Grown Under Rain-Fed Conditions in a Typical Mediterranean Environment in South Italy. J. Agron. Crop Sci. 2010, 196, 407–411. [Google Scholar] [CrossRef]
- Gómez, M.B.; Castro, P.A.; Mignone, C.; Bertero, H.D. Can yield potential be increased by manipulation of reproductive partitioning in quinoa (Chenopodium quinoa)? Evidence from gibberellic acid synthesis inhibition using Paclobutrazol. Funct. Plant Biol. 2011, 38, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Matsuoka, M. Generating high-yielding varieties by genetic manipulation of plant architecture. Curr. Opin. Biotechnol. 2004, 15, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Maliro, M.F.A.; Guwela, V.F.; Nyaika, J.; Murphy, K.M. Preliminary studies of the performance of quinoa (Chenopodium quinoa Willd.) genotypes under irrigated and rainfed conditions of central Malawi. Front. Plant Sci. 2017, 8, 227. [Google Scholar] [CrossRef] [Green Version]
- Curti, R.N.; Sanahuja, M.d.C.; Vidueiros, S.M.; Pallaro, A.N.; Bertero, H.D. Trade-off between seed yield components and seed composition traits in sea level quinoa in response to sowing dates. Cereal Chem. 2018, 95, 734–741. [Google Scholar] [CrossRef]
- Gandarillas, A.; Rojas, W.; Ojeda, N. Quinoa in Bolivia: The Proinpa foundation’s perspective. In State of the Art Report on Quinoa around the World in 2013; Bazile, D., Daniel Bertero, H., Nieto, C., Eds.; FAO & CIRAD: Rome, Italy, 2015; pp. 344–361. [Google Scholar]
- Danielsen, S.; Munk, L. Evaluation of disease assessment methods in quinoa for their ability to predict yield loss caused by downy mildew. Crop Prot. 2004, 23, 219–228. [Google Scholar] [CrossRef]
- Danielsen, S.; Bonifacio, A.; Ames, T. Diseases of quinoa (Chenopodhim quinoa). Food Rev. Int. 2003, 19, 43–59. [Google Scholar] [CrossRef]
- Jacobsen, S.E. The scope for adaptation of quinoa in Northern Latitudes of Europe. J. Agron. Crop Sci. 2017, 203, 603–613. [Google Scholar] [CrossRef]
- Ruales, J.; Nair, B.M. Content of fat, vitamins and minerals in quinoa (Chenopodium quinoa, Willd) seeds. Food Chem. 1993, 48, 131–136. [Google Scholar] [CrossRef]
- Wahli, C. Quinua: Hacia su Cultivo Comercial; Wahli, C., Ed.; Latinreco S. A.: Quito, Ecuador, 1990; ISBN 9978990135. [Google Scholar]
- Mastebroek, H.D.; Limburg, H.; Gilles, T.; Marvin, H.J.P. Occurrence of sapogenins in leaves and seeds of quinoa (Chenopodium quinoa Willd). J. Sci. Food Agric. 2000, 80, 152–156. [Google Scholar] [CrossRef]
- Khalifa, W.; Thabet, M. Variation in downy mildew (Peronospora variabilis Gäum) resistance of some quinoa ( Chenopodium quinoa Willd ) cultivars under Egyptian conditions. Middle East J. Agric. Res. 2018, 7, 671–682. [Google Scholar]
- Woldemichael, G.M.; Wink, M. Identification and biological activities of triterpenoid saponins from Chenopodium quinoa. J. Agric. Food Chem. 2001, 49, 2327–2332. [Google Scholar] [CrossRef]
- Colque-Little, C.; Abondano, M.C.; Lund, O.S.; Amby, D.B.; Piepho, H.P.; Andreasen, C.; Schmöckel, S.; Schmid, K. Genetic variation for tolerance to the downy mildew pathogen Peronospora variabilis in genetic resources of quinoa (Chenopodium quinoa). BMC Plant Biol. 2021, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- De Santis, G.; Maddaluno, C.; D’Ambrosio, T.; Rascio, A.; Rinaldi, M.; Troisi, J. Characterisation of quinoa (Chenopodium quinoa Willd.) accessions for the saponin content in Mediterranean environment. Ital. J. Agron. 2016, 11, 277–281. [Google Scholar] [CrossRef] [Green Version]
- Escribano, J.; Cabanes, J.; Jiménez-Atiénzar, M.; Ibañez-Tremolada, M.; Gómez-Pando, L.R.; García-Carmona, F.; Gandía-Herrero, F. Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem. 2017, 234, 285–294. [Google Scholar] [CrossRef]
- Präger, A.; Munz, S.; Nkebiwe, P.M.; Mast, B.; Graeff-Hönninger, S. Yield and quality characteristics of different quinoa (Chenopodium quinoa willd.) cultivars grown under field conditions in southwestern Germany. Agronomy 2018, 8, 197. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Caravaca, A.M.; Iafelice, G.; Lavini, A.; Pulvento, C.; Caboni, M.F.; Marconi, E. Phenolic compounds and saponins in quinoa samples (Chenopodium quinoa Willd.) grown under different saline and nonsaline irrigation regimens. J. Agric. Food Chem. 2012, 60, 4620–4627. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Souto, X.C.; Gonz´lez, L. Effect of phenolic compounds on the germination of six weeds species. Plant Growth Regul. 1999, 28, 83–88. [Google Scholar] [CrossRef]
- Konishi, Y.; Hirano, S.; Tsuboi, H.; Wada, M. Distribution of minerals in quinoa (Chenopodium quinoa Willd.) seeds. Biosci. Biotechnol. Biochem. 2004, 68, 231–234. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; Becker, K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010, 120, 945–959. [Google Scholar] [CrossRef]
- Holm, P.B.; Kristiansen, K.N.; Pedersen, H.B. Transgenic approaches in commonly consumed cereals to improve iron and zinc content and bioavailability. J. Nutr. 2002, 132, 514–516. [Google Scholar] [CrossRef]
- Honke, J.; Kozłowska, H.; Vidal-Valverde, C.; Frias, J.; Górecki, R. Changes in quantities of inositol phosphates during maturation and germination of legume seeds. Eur. Food Res. Technol. 1998, 206, 279–283. [Google Scholar] [CrossRef]
- Hall, J.R.; Hodges, T.K. Phosphorus Metabolism of Germinating Oat Seeds. Plant Physiol. 1966, 41, 1459–1464. [Google Scholar] [CrossRef] [Green Version]
- White, P.J.; Veneklaas, E.J. Nature and nurture: The importance of seed phosphorus content. Plant Soil 2012, 357, 1–8. [Google Scholar] [CrossRef]
- Nadeem, M.; Mollier, A.; Morel, C.; Vives, A.; Prud’homme, L.; Pellerin, S. Relative contribution of seed phosphorus reserves and exogenous phosphorus uptake to maize (Zea mays L.) nutrition during early growth stages. Plant Soil 2011, 346, 231–244. [Google Scholar] [CrossRef]
- Grant, C.A.; Flaten, D.N.; Tomasiewicz, D.J.; Sheppard, S.C. The importance of early season phosphorus nutrition. Can. J. Plant Sci. 2001, 81, 211–224. [Google Scholar] [CrossRef]
- Koyro, H.W.; Eisa, S.S. Effect of salinity on composition, viability and germination of seeds of Chenopodium quinoa Willd. Plant Soil 2008, 302, 79–90. [Google Scholar] [CrossRef]
- Pilco-Quesada, S.; Tian, Y.; Yang, B.; Repo-Carrasco-Valencia, R.; Suomela, J.P. Effects of germination and kilning on the phenolic compounds and nutritional properties of quinoa (Chenopodium quinoa) and kiwicha (Amaranthus caudatus). J. Cereal Sci. 2020, 94, 102996. [Google Scholar] [CrossRef]
- Koziol, M.J. Chemical Composition and Nutritional Evaluation of Quinoa (Chenopodium quinoa Willd.). J. Food Compos. Anal. 1992, 5, 35–68. [Google Scholar] [CrossRef]
- Maradini Filho, A.M.; Ribeiro Pirozi, M.; da Silva Borges, J.T.; Pinheiro Sant’Ana, H.M.; Paes Chaves, J.B.; Dos Reis Coimbra, J.S. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization and United Nations University; FAO. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; WHO: Geneva, Switzerland, 2004; ISBN 92-4-154612-3. [Google Scholar]
- Ismail, H.; Dragišic Maksimovic, J.; Maksimovic, V.; Shabala, L.; Branka, D.Ž.; Tian, Y.; Jacobsen, S.; Shabala, S. Rutin, a fl avonoid with antioxidant activity, improves plant salinity tolerance by regulating K+ retention and Na+ exclusion from leaf mesophyll in quinoa and broad beans. Funct. Plant Biol. 2016, 43, 75–86. [Google Scholar] [CrossRef]
- Abderrahim, F.; Huanatico, E.; Segura, R.; Arribas, S.; Gonzalez, M.C.; Condezo-Hoyos, L. Physical features, phenolic compounds, betalains and total antioxidant capacity of coloured quinoa seeds (Chenopodium quinoa Willd.) from Peruvian Altiplano. Food Chem. 2015, 183, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Bascuñán-Godoy, L.; Reguera, M.; Abdel-tawab, Y.M.; Blumwald, E. Water deficit stress-induced changes in carbon and nitrogen partitioning in Chenopodium quinoa Willd. Planta 2015, 243, 591–603. [Google Scholar] [CrossRef]
- Lozano, M.; Tícona, E.; Carrasco, C.; Flores, Y.; Almanza, G.R. Cuantificación de saponinas en residuos de quinua real chenopodium quinoa willd. Rev. Boliv. Química 2012, 29, 131–138. [Google Scholar]
- Proceedings of the AOAC International Annual Meeting & Exposition—AOAC International, Denver, CO, USA, 6–12 September 2019.
- Nascimento, A.C.; Mota, C.; Coelho, I.; Gueifão, S.; Santos, M.; Matos, A.S.; Gimenez, A.; Lobo, M.; Samman, N.; Castanheira, I. Characterisation of nutrient profile of quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus), and purple corn (Zea mays L.) consumed in the North of Argentina: Proximates, minerals and trace elements. Food Chem. 2014, 148, 420–426. [Google Scholar] [CrossRef] [PubMed]
- MAPA Ministerio de Agricultura, Pesca y Alimentación. Métodos Oficiales de Análisis. Tomo III; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1995; ISBN 84-491-0003-0008. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Valenzuela Bustamante, P.D. Evaluación de la Actividad Antioxidante y Determinación del Contenido de Fenoles Totales y Flavonoides de Hojas de Diferentes Genotipos de Ugni Molinae Turcz. Bachelor’s Thesis, University of Chile, Santiago, Chile, 2015; p. 65. [Google Scholar]
- Mohammadi, S.A.; Prasanna, B.M. Analysis of Genetic Diversity in Crop Plants—Salient Statistical Tools and Considerations. Crop Sci. 2003, 43, 1235–1248. [Google Scholar] [CrossRef] [Green Version]
- Martínez, E.A. Quinoa: Nutritional aspects of the rice of the Incas. In State of the Art Report of Quinoa in the World in 2013; Bazile, D., Bertero, H.D., Nieto, C., Eds.; FAO & CIRAD: Rome, Italy, 2015; pp. 278–285. [Google Scholar]
- Ruales, J.; Nair, B.M. Saponins, phytic acid, tannins and protease inhibitors in quinoa (Chenopodium quinoa Willd) seeds. Food Chem. 1993, 48, 137–143. [Google Scholar] [CrossRef]
- Pulvento, C.; Riccardi, M.; Lavini, A.; Iafelice, G.; Marconi, E.; D’Andria, R. Yield and Quality Characteristics of Quinoa Grown in Open Field Under Different Saline and Non-Saline Irrigation Regimes. J. Agron. Crop Sci. 2012, 198, 254–263. [Google Scholar] [CrossRef]



| Ash (%) | Nitrogen (%) | C/N ratio | P (%) | K (%) | Ca (%) | Mg (%) | Na (ppm) | Fe (ppm) | Mn (ppm) | Cu (ppm) | Zn (ppm) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A-SE-01 | 3.48 ± 0.02 | 2.04 ± 0.04 | 18.45 ± 0.35 | 0.29 ± 0.00 | 1.14 ± 0.01 | 0.30 ± 0.02 | 0.21 ± 0.00 | 146.98 ± 5.99 | 40.41 ± 0.27 | 19.57 ± 0.30 | 9.30 ± 1.42 | 29.18 ± 0.34 |
| b | cd | bc | bc | ab | abc | - | bcdefg | b | bcdef | bcde | efg | |
| A-SE-02 | 3.22 ± 0.10 | 2.55 ± 0.07 | 14.81 ± 0.56 | 0.27 ± 0.00 | 1.03 ± 0.01 | 0.28 ± 0.01 | 0.19 ± 0.01 | 116.63 ± 7.66 | 38.98 ± 1.72 | 11.69 ± 0.05 | 11.70 ± 1.73 | 29.56 ± 0.44 |
| abc | ab | de | bc | b | bc | - | gh | b | f | bc | ef | |
| A-SE-03 | 3.47 ± 0.07 | 1.62 ± 0.03 | 22.59 ± 0.28 | 0.17 ± 0.00 | 1.26 ± 0.01 | 0.38 ± 0.02 | 0.19 ± 0.00 | 233.42 ± 55.08 | 36.05 ± 1.35 | 16.65 ± 0.99 | 7.54 ± 0.56 | 23.93 ± 0.38 |
| abc | d | b | d | ab | a | - | ab | b | cdef | cdef | i | |
| A-SE-04 | 3.03 ± 0.33 | 2.21 ± 0.23 | 17.47 ± 1.56 | 0.22 ± 0.02 | 0.98 ± 0.12 | 0.35 ± 0.02 | 0.20 ± 0.02 | 279.7 ± 37.63 | 32.85 ± 3.45 | 13.83 ± 0.95 | 7.86 ± 1.21 | 29.95 ± 0.52 |
| abc | bcd | bcd | cd | ab | ab | - | a | bc | ef | bcdef | def | |
| A-SE-05 | 3.66 ± 0.27 | 2.08 ± 0.11 | 18.21 ± 0.92 | 0.27 ± 0.02 | 1.24 ± 0.10 | 0.35 ± 0.04 | 0.18 ± 0.01 | 173.49 ± 21.92 | 29.73 ± 2.74 | 13.42 ± 0.72 | 9.34 ± 0.80 | 31.60 ± 0.74 |
| abc | cd | bcd | bcd | ab | abcd | - | abcdef | bc | def | bcde | bcd | |
| A-SE-06 | 3.11 ± 0.17 | 1.46 ± 0.03 | 25.34 ± 0.44 | 0.15 ± 0.00 | 1.13 ± 0.03 | 0.36 ± 0.12 | 0.17 ± 0.02 | 194.52 ± 59.36 | 35.08 ± 15.60 | 14.32 ± 6.60 | 9.40 ± 3.17 | 25.53 ± 1.23 |
| abc | e | a | e | ab | abcdef | - | abcde | bc | cdef | bcdef | ghi | |
| A-SE-07 | 3.18 ± 0.09 | 2.04 ± 0.03 | 18.40 ± 0.45 | 0.26 ± 0.01 | 1.10 ± 0.02 | 0.18 ± 0.02 | 0.19 ± 0.00 | 125.43 ± 14.68 | 42.71 ± 3.77 | 19.92 ± 0.18 | 10.28 ± 0.31 | 30.85 ± 0.22 |
| bc | cd | bc | bcd | ab | def | - | efgh | abc | bcde | bc | cde | |
| A-SE-08 | 3.43 ± 0.36 | 2.46 ± 0.25 | 15.65 ± 1.65 | 0.28 ± 0.03 | 1.15 ± 0.12 | 0.21 ± 0.02 | 0.22 ± 0.02 | 141.79 ± 12.98 | 43.18 ± 3.60 | 22.90 ± 0.38 | 10.32 ± 0.44 | 31.28 ± 0.80 |
| abc | abc | bcde | bc | ab | cde | - | cdefgh | abc | ab | bcd | bcd | |
| A-SE-09 | 3.50 ± 0.47 | 2.12 ± 0.19 | 17.90 ± 1.50 | 0.26 ± 0.03 | 1.16 ± 0.16 | 0.27 ± 0.01 | 0.18 ± 0.02 | 153.07 ± 32.93 | 39.54 ± 4.68 | 22.19 ± 1.50 | 7.08 ± 0.53 | 23.80 ± 0.25 |
| abc | cd | bcd | bcd | ab | bcde | - | bcdefgh | abc | ab | def | i | |
| A-SE-10 | 3.68 ± 0.34 | 2.24 ± 0.12 | 16.95 ± 0.99 | 0.24 ± 0.04 | 1.22 ± 0.17 | 0.25 ± 0.04 | 0.19 ± 0.03 | 191.24 ± 21.87 | 47.12 ± 8.56 | 20.77 ± 2.11 | 6.41 ± 1.01 | 25.32 ± 1.36 |
| abc | abcd | bcde | bcd | ab | abcdef | - | abcd | abc | abcd | ef | hi | |
| A-SE-11 | 3.62 ± 0.63 | 2.29 ± 0.22 | 16.74 ± 1.57 | 0.28 ± 0.03 | 1.23 ± 0.21 | 0.24 ± 0.03 | 0.21 ± 0.03 | 194.76 ± 22.11 | 39.56 ± 6.24 | 20.05 ± 1.63 | 6.55 ± 0.68 | 28.76 ± 0.30 |
| abc | abcd | bcde | bcd | ab | abcdef | - | abc | abc | abcd | f | fgh | |
| A-SE-12 | 3.26 ± 0.03 | 2.48 ± 0.10 | 15.13 ± 0.59 | 0.31 ± 0.01 | 1.12 ± 0.01 | 0.36 ± 0.01 | 0.19 ± 0.00 | 139.22 ± 16.03 | 56.39 ± 1.56 | 17.62 ± 0.51 | 8.98 ± 0.76 | 35.23 ± 0.21 |
| c | abc | cde | b | ab | a | - | defgh | a | cdef | bcdef | a | |
| A-SE-13 | 3.73 ± 0.03 | 2.10 ± 0.02 | 17.93 ± 0.28 | 0.27 ± 0.01 | 1.39 ± 0.03 | 0.10 ± 0.03 | 0.18 ± 0.01 | 121.64 ± 15.62 | 35.17 ± 3.55 | 22.17 ± 1.15 | 19.52 ± 0.60 | 32.45 ± 0.58 |
| a | bcd | bcd | bcd | a | ef | - | fgh | bc | abc | a | bc | |
| A-SE-14 | 3.34 ± 0.21 | 2.17 ± 0.13 | 17.52 ± 1.00 | 0.25 ± 0.01 | 1.16 ± 0.06 | 0.10 ± 0.02 | 0.20 ± 0.01 | 125.35 ± 15.11 | 33.19 ± 2.41 | 23.16 ± 1.16 | 18.46 ± 0.88 | 29.37 ± 0.97 |
| abc | bcd | bcd | bcd | ab | f | - | fgh | bc | a | ab | efg | |
| A-SE-15 | 3.19 ± 0.08 | 2.67 ± 0.07 | 14.39 ± 0.48 | 0.35 ± 0.01 | 1.12 ± 0.02 | 0.09 ± 0.01 | 0.21 ± 0.01 | 100.87 ± 8.96 | 25.10 ± 1.29 | 20.34 ± 0.50 | 19.75 ± 0.96 | 34.09 ± 0.81 |
| bc | a | e | a | ab | f | - | h | c | abcd | a | b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granado-Rodríguez, S.; Vilariño-Rodríguez, S.; Maestro-Gaitán, I.; Matías, J.; Rodríguez, M.J.; Calvo, P.; Cruz, V.; Bolaños, L.; Reguera, M. Genotype-Dependent Variation of Nutritional Quality-Related Traits in Quinoa Seeds. Plants 2021, 10, 2128. https://doi.org/10.3390/plants10102128
Granado-Rodríguez S, Vilariño-Rodríguez S, Maestro-Gaitán I, Matías J, Rodríguez MJ, Calvo P, Cruz V, Bolaños L, Reguera M. Genotype-Dependent Variation of Nutritional Quality-Related Traits in Quinoa Seeds. Plants. 2021; 10(10):2128. https://doi.org/10.3390/plants10102128
Chicago/Turabian StyleGranado-Rodríguez, Sara, Susana Vilariño-Rodríguez, Isaac Maestro-Gaitán, Javier Matías, María José Rodríguez, Patricia Calvo, Verónica Cruz, Luis Bolaños, and María Reguera. 2021. "Genotype-Dependent Variation of Nutritional Quality-Related Traits in Quinoa Seeds" Plants 10, no. 10: 2128. https://doi.org/10.3390/plants10102128
APA StyleGranado-Rodríguez, S., Vilariño-Rodríguez, S., Maestro-Gaitán, I., Matías, J., Rodríguez, M. J., Calvo, P., Cruz, V., Bolaños, L., & Reguera, M. (2021). Genotype-Dependent Variation of Nutritional Quality-Related Traits in Quinoa Seeds. Plants, 10(10), 2128. https://doi.org/10.3390/plants10102128