In Vitro Anti-HIV-1 Reverse Transcriptase and Integrase Properties of Punica granatum L. Leaves, Bark, and Peel Extracts and Their Main Compounds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Anti-HIV-1 Activity of Ethanolic Extracts Obtained from P. granatum Bark (PGB), Leaves (PGL), and Fruit Peels (PGP)
2.2. Phytochemical Study of Punica granatum Extracts
2.3. Anti-HIV-1 Activity of Pure Compounds Detected in PGB, PGL, and PGP
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Extraction and Detection of Active Compounds
3.4. Expression and Purification of Recombinant HIV-1 RT
3.5. Expression and Purification of Recombinant HIV-1 IN and LEDGF/p75 Proteins
3.6. RNase H Polymerase Independent Cleavage Assay
3.7. Homogeneous Time-Resolved Fluorescence (HTRF) IN LEDGF-Dependent Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012, 143, 397–405. [Google Scholar] [CrossRef]
- Kandylis, P.; Kokkinomagoulos, E. Food applications and potential health benefits of pomegranate and its derivatives. Foods 2020, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.; Duo, L.; Wang, J.; GegenZhula; Yang, J.; Li, Z.; Tu, Y. A unique understanding of traditional medicine of pomegranate, Punica granatum L. and its current research status. J. Ethnopharmacol. 2021, 271, 113877. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ding, Y.; Liu, R.; Xiang, L.; Du, L. Pomegranate: Constituents, bioactivities and pharmacokinetics. Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 77–87. [Google Scholar]
- Orgil, O.; Schwartz, E.; Baruch, L.; Matityahu, I.; Mahajna, J.; Amir, R. The antioxidative and anti-proliferative potential of non-edible organs of the pomegranate fruit and tree. LWT-Food Sci. Technol. 2014, 58, 571–577. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Caputo, L.; De Martino, L.; Sakr, S.H.; De Feo, V.; Camele, I. Study of bio-pharmaceutical and antimicrobial properties of pomegranate (Punica granatum L.) leathery exocarp extract. Plants 2021, 10, 1–9. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Tomasi, P.; Marincich, L.; Poli, F. Plant secondary metabolites: An opportunity for circular economy. Molecules 2021, 26, 495. [Google Scholar] [CrossRef]
- Howell, A.B.; D’Souza, D.H. The pomegranate: Effects on bacteria and viruses that influence human health. Evid. -Based Complementary Altern. Med. 2013, 606212. [Google Scholar] [CrossRef] [Green Version]
- Balli, D.; Cecchi, L.; Khatib, M.; Bellumori, M.; Cairone, F.; Carradori, S.; Zengin, G.; Cesa, S.; Innocenti, M.; Mulinacci, N. Characterization of arils juice and peel decoction of fifteen varieties of Punica granatum L.: A focus on anthocyanins, ellagitannins and polysaccharides. Antioxidants 2020, 9, 238. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nonaka, G.-I.; Nishioka, I. Tannins and related compounds. XLI. Isolation and characterization of novel ellagitannins, punicacorteins A, B, C and D, and punigluconin from the bark of Punica granatum L. Chem. Pharm. Bull. 1986, 34, 656–663. [Google Scholar] [CrossRef]
- Tantray, M.A.; Akbar, S.; Khan, R.; Tariq, K.A.; Shawl, A.S. Humarain: A new dimeric gallic acid glycoside from Punica granatum L. bark. Fitoterapia 2009, 80, 223–225. [Google Scholar] [CrossRef]
- El-Toumy, S.A.A.; Rauwald, H.W. Two ellagitannins from Punica granatum heartwood. Phytochemistry 2002, 61, 971–974. [Google Scholar] [CrossRef]
- Acquadro, S.; Civra, A.; Cagliero, C.; Marengo, A.; Rittà, M.; Francese, R.; Sanna, C.; Bertea, C.; Sgorbini, B.; Lembo, D.; et al. Punica granatum leaf ethanolic extract and ellagic acid as inhibitors of zika virus infection. Planta Med. 2020, 86, 1363–1374. [Google Scholar] [CrossRef]
- Wu, S.; Tian, L. Diverse phytochemicals and bioactivities in the ancient fruit and modern functional food pomegranate (Punica granatum). Molecules 2017, 22, 10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Gao, Y.; Zhang, Y.; Liu, J.; Yu, J. Changes in bioactive compounds and antioxidant activities in pomegranate leaves. Sci. Hortic. 2010, 123, 543–546. [Google Scholar] [CrossRef]
- Marques, L.C.F.; Pinheiro, A.J.M.C.R.; Araújo, J.G.G.; De Oliveira, R.A.G.; Silva, S.N.; Abreu, I.C.; De Sousa, E.M.; Fernandes, E.S.; Luchessi, A.D.; Silbiger, V.N.; et al. Anti-inflammatory effects of a pomegranate leaf extract in LPS-induced peritonitis. Planta Med. 2016, 82, 1463–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salwe, K.J.; Sachdev, D.O.; Bahurupi, Y.; Kumarappan, M. Evaluation of antidiabetic, hypolipedimic and antioxidant activity of hydroalcoholic extract of leaves and fruit peel of Punica granatum in male Wistar albino rats. J. Nat. Sci. Biol. Med. 2015, 6, 56–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šavikin, K.; Živković, J.; Alimpić, A.; Zdunić, G.; Janković, T.; Duletić-Laušević, S.; Menković, N. Activity guided fractionation of pomegranate extract and its antioxidant, antidiabetic and antineurodegenerative properties. Ind. Crops Prod. 2018, 113, 142–149. [Google Scholar] [CrossRef]
- Trabelsi, A.; El Kaibi, M.A.; Abbassi, A.; Horchani, A.; Chekir-Ghedira, L.; Ghedira, K. Phytochemical study and antibacterial and antibiotic modulation activity of Punica granatum (pomegranate) leaves. Scientifica 2020, 8271203. [Google Scholar] [CrossRef] [Green Version]
- Khwairakpam, A.D.; Bordoloi, D.; Thakur, K.K.; Monisha, J.; Arfuso, F.; Sethi, G.; Mishra, S.; Kumar, A.P.; Kunnumakkara, A.B. Possible use of Punica granatum (Pomegranate) in cancer therapy. Pharmacol. Res. 2018, 133, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Angamuthu, D.; Purushothaman, I.; Kothandan, S.; Swaminathan, R. Antiviral study on Punica granatum L., Momordica charantia L., Andrographis paniculata Nees, and Melia azedarach L., to human herpes virus-3. Eur. J. Integr. Med. 2019, 28, 98–108. [Google Scholar] [CrossRef]
- Arunkumar, J.; Rajarajan, S. Study on antiviral activities, drug-likeness and molecular docking of bioactive compounds of Punica granatum L. to Herpes simplex virus-2 (HSV-2). Microb. Pathog. 2018, 118, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Haidari, M.; Ali, M.; Ward Casscells, S.; Madjid, M. Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir. Phytomedicine 2009, 16, 1127–1136. [Google Scholar] [CrossRef]
- Yang, Y.; Xiu, J.; Zhang, L.; Qin, C.; Liu, J. Antiviral activity of punicalagin toward human enterovirus 71 in vitro and in vivo. Phytomedicine 2012, 20, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Neurath, A.R.; Strick, N.; Li, Y.Y.; Debnath, A.K. Punica granatum (Pomegranate) juice provides an HIV-1 entry inhibitor and candidate topical microbicide. BMC Infect. Dis. 2004, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Esposito, F.; Ambrosio, F.A.; Maleddu, R.; Costa, G.; Rocca, R.; Maccioni, E.; Catalano, R.; Romeo, I.; Eleftheriou, P.; Karia, D.C.; et al. Chromenone derivatives as a versatile scaffold with dual mode of inhibition of HIV-1 reverse transcriptase-associated Ribonuclease H function and integrase activity. Eur. J. Med. Chem. 2019, 182. [Google Scholar] [CrossRef] [PubMed]
- Sanna, C.; Scognamiglio, M.; Fiorentino, A.; Corona, A.; Graziani, V.; Caredda, A.; Cortis, P.; Montisci, M.; Ceresola, E.R.; Canducci, F.; et al. Prenylated phloroglucinols from Hypericum scruglii, an endemic species of Sardinia (Italy), as new dual HIV-1 inhibitors effective on HIV-1 replication. PLoS ONE 2018, 13, e0195168. [Google Scholar] [CrossRef] [Green Version]
- Esposito, F.; Tramontano, E. Past and future. Current drugs targeting HIV-1 integrase and reverse transcriptase-associated ribonuclease H activity: Single and dual active site inhibitors. Antivir. Chem. Chemother. 2014, 23, 129–144. [Google Scholar] [CrossRef] [Green Version]
- Carcelli, M.; Rogolino, D.; Gatti, A.; Pala, N.; Corona, A.; Caredda, A.; Tramontano, E.; Pannecouque, C.; Naesens, L.; Esposito, F. Chelation motifs affecting metal-dependent viral enzymes: N0-acylhydrazone ligands as dual target inhibitors of HIV-1 integrase and reverse transcriptase ribonuclease h domain. Front. Microbiol. 2017, 8, 440. [Google Scholar] [CrossRef] [PubMed]
- Corona, A.; Esposito, F.; Tramontano, E. Can the ever-promising target HIV reverse transcriptase-associated RNase H become a success story for drug development? Future Virol. 2014, 9, 445–448. [Google Scholar] [CrossRef]
- Christ, F.; Debyser, Z. The LEDGF/p75 integrase interaction, a novel target for anti-HIV therapy. Virology 2013, 435, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Cherepanov, P.; Maertens, G.; Proost, P.; Devreese, B.; Van Beeumen, J.; Engelborghs, Y.; De Clercq, E.; Debyser, Z. HIV-1 integrase forms stable tetramers and associates with LEDGF/p75 protein in human cells. J. Biol. Chem. 2003, 278, 372–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bicchi, C.; Rubiolo, P.; Ballero, M.; Sanna, C.; Matteodo, M.; Esposito, F.; Zinzula, L.; Tramontano, E. HIV-1-inhibiting activity of the essential oil of ridolfia segetum and oenanthe crocata. Planta Med. 2009, 75, 1331–1335. [Google Scholar] [CrossRef]
- Esposito, F.; Sanna, C.; Del Vecchio, C.; Cannas, V.; Venditti, A.; Corona, A.; Bianco, A.; Serrilli, A.M.; Guarcini, L.; Parolin, C.; et al. Hypericum hircinum L. Components as new single-molecule inhibitors of both HIV-1 reverse transcriptase-associated DNA polymerase and ribonuclease H activities. Pathog. Dis. 2013, 68, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Esposito, F.; Zinzula, L.; Maxia, A.; Tramontano, E.; Sanna, C. Inhibition of HIV-1 reverse transcriptase associated activities by the hydroalcoholic extract of Casimiroa edulis seeds. Nat. Prod. Res. 2011, 25, 1067–1073. [Google Scholar] [CrossRef]
- Guzzo, F.; Russo, R.; Sanna, C.; Celaj, O.; Caredda, A.; Corona, A.; Tramontano, E.; Fiorentino, A.; Esposito, F.; D’abrosca, B. Chemical characterization and anti-HIV-1 activity assessment of iridoids and flavonols from Scrophularia trifoliata. Molecules 2021, 26, 4777. [Google Scholar] [CrossRef]
- Sanna, C.; Rigano, D.; Cortis, P.; Corona, A.; Ballero, M.; Parolin, C.; Del Vecchio, C.; Chianese, G.; Saccon, E.; Formisano, C.; et al. Onopordum illyricum L., a Mediterranean plant, as a source of anti HIV-1 compounds. Plant. Biosyst. 2018, 152, 1274–1281. [Google Scholar] [CrossRef]
- Sanna, C.; Rigano, D.; Corona, A.; Piano, D.; Formisano, C.; Farci, D.; Franzini, G.; Ballero, M.; Chianese, G.; Tramontano, E.; et al. Dual HIV-1 reverse transcriptase and integrase inhibitors from Limonium morisianum Arrigoni, an endemic species of Sardinia (Italy). Nat. Prod. Res. 2019, 33, 1798–1803. [Google Scholar] [CrossRef]
- Cuzzucoli Crucitti, G.; Métifiot, M.; Pescatori, L.; Messore, A.; Madia, V.N.; Pupo, G.; Saccoliti, F.; Scipione, L.; Tortorella, S.; Esposito, F.; et al. Structure-activity relationship of pyrrolyl diketo acid derivatives as dual inhibitors of HIV-1 integrase and reverse transcriptase ribonuclease H domain. J. Med. Chem. 2015, 58, 1915–1928. [Google Scholar] [CrossRef]
- Martini, R.; Esposito, F.; Corona, A.; Ferrarese, R.; Ceresola, E.R.; Visconti, L.; Tintori, C.; Barbieri, A.; Calcaterra, A.; Iovine, V.; et al. Natural product Kuwanon-L inhibits HIV-1 replication through multiple target binding. ChemBioChem 2017, 18, 374–377. [Google Scholar] [CrossRef]
- Romani, A.; Campo, M.; Pinelli, P. HPLC/DAD/ESI-MS analyses and anti-radical activity of hydrolyzable tannins from different vegetal species. Food Chem. 2012, 130, 214–221. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Tsiaka, T.; Proestos, C.; Zoumpoulakis, P. Total phenolic content, antioxidant capacity and phytochemical profiling of grape and pomegranate wines. RSC Adv. 2015, 5, 101683–101692. [Google Scholar] [CrossRef]
- Wyrepkowski, C.C.; Da Costa, D.L.M.G.; Sinhorin, A.P.; Vilegas, W.; De Grandis, R.A.; Resende, F.A.; Varanda, E.A.; Dos Santos, L.C. Characterization and quantification of the compounds of the ethanolic extract from Caesalpinia ferrea stem bark and evaluation of their mutagenic activity. Molecules 2014, 19, 16039–16057. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Caravaca, A.M.; Verardo, V.; Toselli, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Caboni, M.F. Determination of the major phenolic compounds in pomegranate juices by HPLC-DAD-ESI-MS. J. Agric. Food Chem. 2013, 61, 5328–5337. [Google Scholar] [CrossRef]
- García-Villalba, R.; Espín, J.C.; Aaby, K.; Alasalvar, C.; Heinonen, M.; Jacobs, G.; Voorspoels, S.; Koivumäki, T.; Kroon, P.A.; Pelvan, E.; et al. Validated method for the characterization and quantification of extractable and nonextractable ellagitannins after acid hydrolysis in pomegranate fruits, juices, and extracts. J. Agric. Food Chem. 2015, 63, 6555–6566. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis chemical analysis working group (CAWG) metabolomics standards initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swilam, N.; Nematallah, K.A. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci. Rep. 2020, 10, 14851. [Google Scholar] [CrossRef] [PubMed]
- Siwe-Noundou, X.; Ndinteh, D.T.; Olivier, D.K.; Mnkandhla, D.; Isaacs, M.; Muganza, F.M.; Mbafor, J.T.; Van Vuuren, S.F.; Patnala, S.; Hoppe, H.; et al. Biological activity of plant extracts and isolated compounds from Alchornea laxiflora: Anti-HIV, antibacterial and cytotoxicity evaluation. South. African J. Bot. 2019, 122, 498–503. [Google Scholar] [CrossRef]
- Promsong, A.; Chuenchitra, T.; Saipin, K.; Tewtrakul, S.; Panichayupakaranant, P.; Satthakarn, S.; Nittayananta, W. Ellagic acid inhibits HIV-1 infection in vitro: Potential role as a novel microbicide. Oral Dis. 2018, 24, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Nutan; Modi, M.; Goelb, T.; Das, T.; Malik, S.; Suri, S.; Singh Rawat, A.K.; Srivastava, S.K.; Tuli, R.; Malhotra, S.; et al. Ellagic acid & gallic acid from Lagerstroemia speciosa L. inhibit HIV-1 infection through inhibition of HIV-1 protease & reverse transcriptase activity. Indian J. Med. Res. 2013, 137, 540–548. [Google Scholar]
- Kuzikov, M.; Costanzi, E.; Reinshagen, J.; Esposito, F.; Vangeel, L.; Wolf, M.; Ellinger, B.; Claussen, C.; Geisslinger, G.; Corona, A.; et al. Identification of inhibitors of SARS-CoV-2 3CL-pro enzymatic activity using a small molecule in vitro repurposing screen. ACS Pharmacol. Transl. Sci. 2021, 4, 1096–1110. [Google Scholar] [CrossRef]
- Asres, K.; Seyoum, A.; Veeresham, C.; Bucar, F.; Gibbons, S. Naturally derived anti-HIV agents. Phyther. Res. 2005, 19, 557–581. [Google Scholar] [CrossRef]
- Mohan, S.; Elhassan Taha, M.M.; Makeen, H.A.; Alhazmi, H.A.; Al Bratty, M.; Sultana, S.; Ahsan, W.; Najmi, A.; Khalid, A. Bioactive natural antivirals: An updated review of the available plants and isolated molecules. Molecules 2020, 25, 4878. [Google Scholar] [CrossRef]
- Mehla, R.; Bivalkar-Mehla, S.; Chauhan, A. A flavonoid, luteolin, cripples HIV-1 by abrogation of Tat function. PLoS ONE 2011, 6, e27915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.W.; Zhang, F.H.; Serrao, E.; Chen, H.; Sanchez, T.W.; Yang, L.M.; Neamati, N.; Zheng, Y.T.; Wang, H.; Long, Y.Q. Design and discovery of flavonoid-based HIV-1 integrase inhibitors targeting both the active site and the interaction with LEDGF/p75. Bioorganic Med. Chem. 2014, 22, 3146–3158. [Google Scholar] [CrossRef]
- Lan, P.; Chen, W.N.; Huang, Z.J.; Sun, P.H.; Chen, W.M. Understanding the structure-activity relationship of betulinic acid derivatives as anti-HIV-1 agents by using 3D-QSAR and docking. J. Mol. Model. 2011, 17, 1643–1659. [Google Scholar] [CrossRef]
- Aiken, C.; Chen, C.H. Betulinic acid derivatives as HIV-1 antivirals. Trends Mol. Med. 2005, 11, 31–36. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Wang, H.K.; Nagao, T.; Kitanaka, S.; Yasuda, I.; Fujioka, T.; Yamagishi, T.; Cosentino, L.M.; Kozuka, M.; Okabe, H.; et al. Anti-AIDS agents, 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J. Nat. Prod. 1998, 61, 1090–1095. [Google Scholar] [CrossRef]
- Lee, J.S.; Miyashiro, H.; Nakamura, N.; Hattori, M. Two new triterpenes from the rhizome of Dryopteris crassirhizoma, and inhibitory activities of its constituents on human immunodeficiency virus-1 protease. Chem. Pharm. Bull. 2008, 56, 711–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina-O’donnell, M.; Rivas, F.; Reyes-Zurita, F.J.; Cano-Muñoz, M.; Martinez, A.; Lupiañez, J.A.; Parra, A. Oleanolic acid derivatives as potential inhibitors of HIV-1 protease. J. Nat. Prod. 2019, 82, 2886–2896. [Google Scholar] [CrossRef]
- Nonaka, G.I.; Nishioka, I.; Nishizawa, M.; Yamagishi, T.; Kashiwada, Y.; Lee, K.H.; Dutschman, G.E.; Cheng, Y.C.; Bodner, A.J.; Kilkuskie, R.E. Anti-aids agents, 21: Inhibitory effects of tannins on HIV reverse transcriptase and HIV replication in H9 lymphocyte cells. J. Nat. Prod. 1990, 53, 587–595. [Google Scholar] [CrossRef]
- Martino, V.; Morales, J.; Martínez-Irujo, J.J.; Font, M.; Monge, A.; Coussio, J. Two ellagitannins from the leaves of Terminalia triflora with inhibitory activity on HIV-1 reverse transcriptase. Phyther. Res. 2004, 18, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.L.; Pine, P.S.; Dutschman, G.; Cheng, Y.C.; Lee, K.H.; Aszalos, A. Prevention of binding of rgp120 by anti-HIV active tannins. Biochem. Pharmacol. 1992, 43, 2479–2480. [Google Scholar] [CrossRef]
- Rubiolo, P.; Casetta, C.; Cagliero, C.; Brevard, H.; Sgorbini, B.; Bicchi, C. Populus nigra L. bud absolute: A case study for a strategy of analysis of natural complex substances. Anal. Bioanal. Chem. 2013, 405, 1223–1235. [Google Scholar] [CrossRef]
- Corona, A.; Onnis, V.; Deplano, A.; Bianco, G.; Demurtas, M.; Distinto, S.; Cheng, Y.-C.; Alcaro, S.; Esposito, F.; Tramontano, E. Design, synthesis and antiviral evaluation of novel heteroarylcarbothioamide derivatives as dual inhibitors of HIV-1 reverse transcriptase-associated RNase H and RDDP functions. Pathog. Dis. 2017, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tintori, C.; Corona, A.; Esposito, F.; Brai, A.; Grandi, N.; Ceresola, E.R.; Clementi, M.; Canducci, F.; Tramontano, E.; Botta, M. Inhibition of HIV-1 reverse transcriptase dimerization by small molecules. ChemBioChem 2016, 17, 683–688. [Google Scholar] [CrossRef]
- Esposito, F.; Sechi, M.; Pala, N.; Sanna, A.; Koneru, P.C.; Kvaratskhelia, M.; Naesens, L.; Corona, A.; Grandi, N.; di Santo, R.; et al. Discovery of dihydroxyindole-2-carboxylic acid derivatives as dual allosteric HIV-1 integrase and reverse transcriptase associated ribonuclease H inhibitors. Antiviral Res. 2020, 174, 104671. [Google Scholar] [CrossRef]
- Corona, A.; Meleddu, R.; Esposito, F.; Distinto, S.; Bianco, G.; Masaoka, T.; Maccioni, E.; Menéndez-Arias, L.; Alcaro, S.; Le Grice, S.F.J.; et al. Ribonuclease H/DNA polymerase HIV-1 reverse transcriptase dual inhibitor: Mechanistic studies on the allosteric mode of action of isatin-based compound RMNC6. PLoS ONE 2016, 11, e014722. [Google Scholar] [CrossRef] [Green Version]
- Tintori, C.; Esposito, F.; Morreale, F.; Martini, R.; Tramontano, E.; Botta, M. Investigation on the sucrose binding pocket of HIV-1 Integrase by molecular dynamics and synergy experiments. Bioorganic Med. Chem. Lett. 2015, 25, 3013–3016. [Google Scholar] [CrossRef] [PubMed]
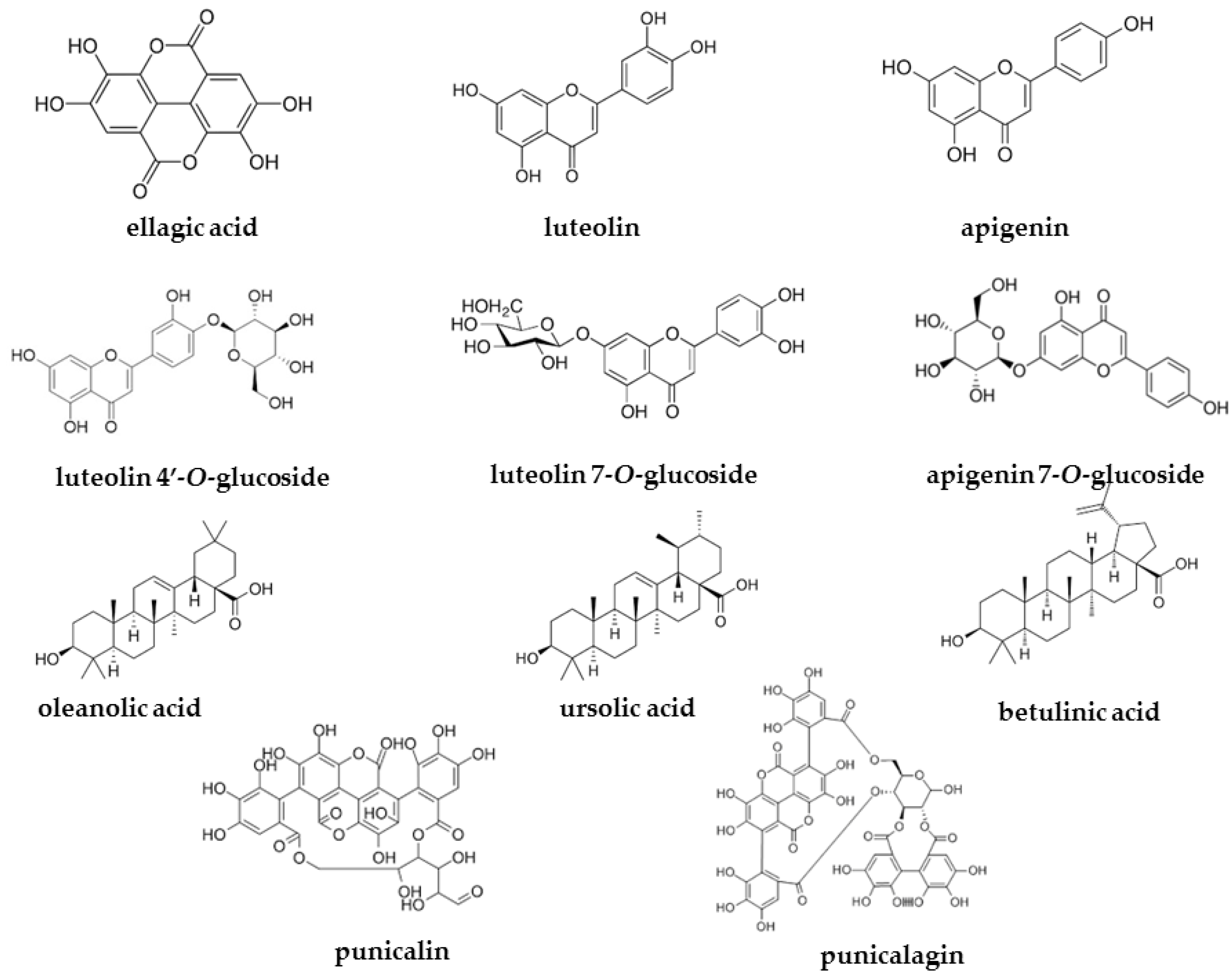
| Extracts/Pure Compounds | HIV-1 RT-Associated RNase H IC50 (µg/mL) a | HIV-1 Integrase LEDGF-Dependent IC50 (µg/mL) b |
|---|---|---|
| Punica granatum leaves extract (PGL) | 0.61 ± 0.02 | 0.12 ± 0.065 |
| Punica granatum bark extract (PGB) | 0.22 ± 0.04 | 0.18 ± 0.02 |
| Punica granatum peel extract (PGP) | 0.85 ± 0,01 | 0.5 ± 0.035 |
| RDS1759 | 10.7 ± 0.9c | - |
| Raltegravir | - | 0.058 ± 0.01 c |
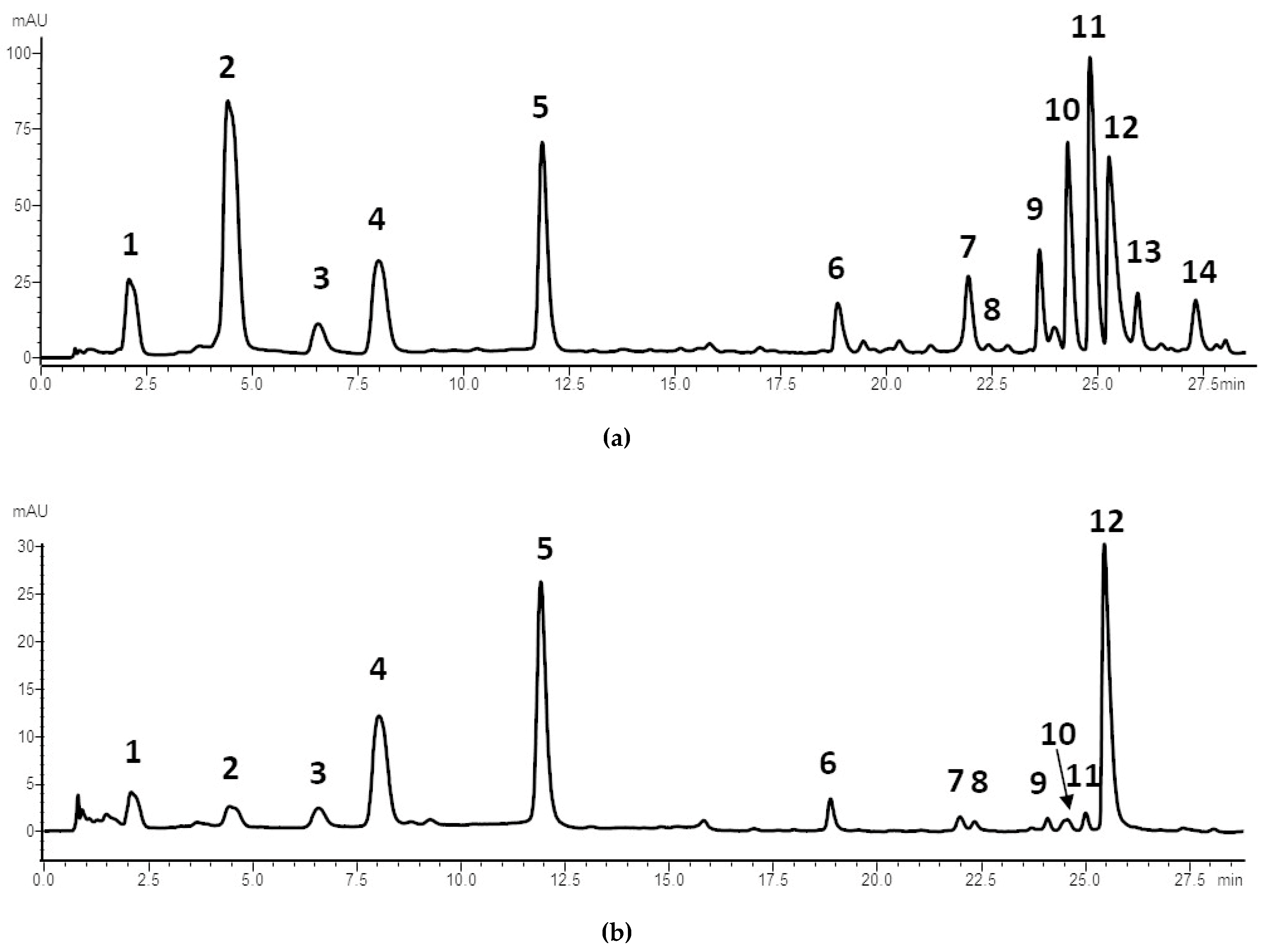
| N° | RT | λmax (nm) | Molecular Formula | [M+H]+ | [M−H]− | Supposed MW | MS2+ m/z | MS2− m/z | Compound Name | Identif. Confidence a | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.076 | 257/377 | C34H22O22 | 783 | 781 | 782 | 603 | 601 | Punicalin α + β | 1 | [42] |
| 2 | 4.414 | 258/376 | C48H28O30 | 1085 | 1083 | 1084 | 603 | 601 | Punicalagin isomer | 2 | [43] |
| 3 | 6.553 | 257/370 | C21H10O13 | 471 | 469 | 470 | 453, 407, 151, 363 | 451, 425, 353, 341 | Valoneic acid dilactone | 2 | [44] |
| 4 | 7.984 | 257/376 | C48H28O30 | 1085 | 1083 | 1084 | 621, 603 | 601 | Punicalagin α | 1 | [42] |
| 5 | 11.853 | 257/380 | C48H28O30 | 1085 | 1083 | 1084 | 765, 621, 603 | 781, 721, 301 | Punicalagin β | 1 | [42] |
| 6 | 18.842 | 252/360 | C20H16O13 | 465 | 463 | 464 | 345, 315, 303, 285, 223 | 301, 283 | Ellagic acid glucoside | 2 | [45] |
| 7 | 21.931 | 254/364 | C21H10O13 | 471 | 469 | 470 | 407, 303, 168, 139 | 301, 271, 227, 201, 171 | Sanguisorbic acid dilactone | 2 | [46] |
| 8 | 22.859 | 252/364 | 435 | 433 | 434 | 303, 285, 275 | 301 | Ellagic acid derivative | 3 | ||
| 9 | 23.613 | 251/360 | C19H14O12 | 435 | 433 | 434 | 303, 285 | 301, 283 | Ellagic acid-pentoside | 2 | [42] |
| 10 | 24.279 | 251/360 | C20H16O12 | 449 | 447 | 448 | 303, 285, 273 | 301, 257, 229 | Ellagic acid deoxyhexoside | 2 | [45] |
| 11 | 25.409 | 255/373 | 1067 | 1065 | 1066 | 603, 575 | / | Ellagitannin | 3 | ||
| 12 | 25.658 | 252/366 | C14H6O8 | 303 | 301 | 302 | / | 284, 229, 185 | Ellagic acid | 1 | [42] |
| 13 | 25.934 | 255/372 | 301 | 275, 256, 127 | Ellagitannin | 3 | |||||
| 14 | 27.309 | 256/379 | C28H10O16 | 601 | 101 | Gallagic acid dilactone | 2 | [46] |
| Pure Compounds | HIV-1 RT-Associated RNase HIC50 (µM) a | HIV-1 Integrase LEDGF-Dependent IC50 (µM) b |
|---|---|---|
| Ellagic acid | 1.4 ± 0.11 | 0.075 ± 0.0005 |
| Luteolin | 3.7 ± 0.5 | 6.5 ± 0.5 |
| Apigenin | 16.1 ± 0.6 | 22 ± 3.5 |
| Luteolin 4′-O-glucoside | >100 (100%) c | >100 (100%) c |
| Luteolin 7-O-glucoside | >100 (100%) c | 8.5 ± 1.4 |
| Apigenin 7-O-glucoside | >100 (100%) c | >100 (100%) c |
| Oleanolic acid | 6.7 ± 0.4 | >100 (74%) c |
| Ursolic acid | 5.7 ± 0.1 | >100 (68%) c |
| Betulinic acid | 2.0 ± 0.2 | 96.5 ± 3.5 |
| Punicalins | 0.18 ± 0.03 | 0.09 ± 0.01 |
| Punicalagins | 0.12 ± 0.00 | 0.065 ± 0.00 |
| Compounds | PGL | PGB | PGP | |||
|---|---|---|---|---|---|---|
| mg/g 1 | RSD% 2 | mg/g | RSD% | mg/g | RSD% | |
| Ellagic acid | 31.06 | 0.37 | 22.65 | 2.67 | 16.81 | 0.21 |
| Luteolin | Tr 3 | / | / | / | / | / |
| Apigenin | Tr 3 | / | / | / | / | / |
| Punicalin α + β | / | / | 15.80 | 0.78 | 2.51 | 0.24 |
| Punicalagin α + β | / | / | 76.06 | 0.75 | 29.51 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanna, C.; Marengo, A.; Acquadro, S.; Caredda, A.; Lai, R.; Corona, A.; Tramontano, E.; Rubiolo, P.; Esposito, F. In Vitro Anti-HIV-1 Reverse Transcriptase and Integrase Properties of Punica granatum L. Leaves, Bark, and Peel Extracts and Their Main Compounds. Plants 2021, 10, 2124. https://doi.org/10.3390/plants10102124
Sanna C, Marengo A, Acquadro S, Caredda A, Lai R, Corona A, Tramontano E, Rubiolo P, Esposito F. In Vitro Anti-HIV-1 Reverse Transcriptase and Integrase Properties of Punica granatum L. Leaves, Bark, and Peel Extracts and Their Main Compounds. Plants. 2021; 10(10):2124. https://doi.org/10.3390/plants10102124
Chicago/Turabian StyleSanna, Cinzia, Arianna Marengo, Stefano Acquadro, Alessia Caredda, Roberta Lai, Angela Corona, Enzo Tramontano, Patrizia Rubiolo, and Francesca Esposito. 2021. "In Vitro Anti-HIV-1 Reverse Transcriptase and Integrase Properties of Punica granatum L. Leaves, Bark, and Peel Extracts and Their Main Compounds" Plants 10, no. 10: 2124. https://doi.org/10.3390/plants10102124
APA StyleSanna, C., Marengo, A., Acquadro, S., Caredda, A., Lai, R., Corona, A., Tramontano, E., Rubiolo, P., & Esposito, F. (2021). In Vitro Anti-HIV-1 Reverse Transcriptase and Integrase Properties of Punica granatum L. Leaves, Bark, and Peel Extracts and Their Main Compounds. Plants, 10(10), 2124. https://doi.org/10.3390/plants10102124












