DNA-Based Herbal Teas’ Authentication: An ITS2 and psbA-trnH Multi-Marker DNA Metabarcoding Approach
Abstract
:1. Introduction
2. Results
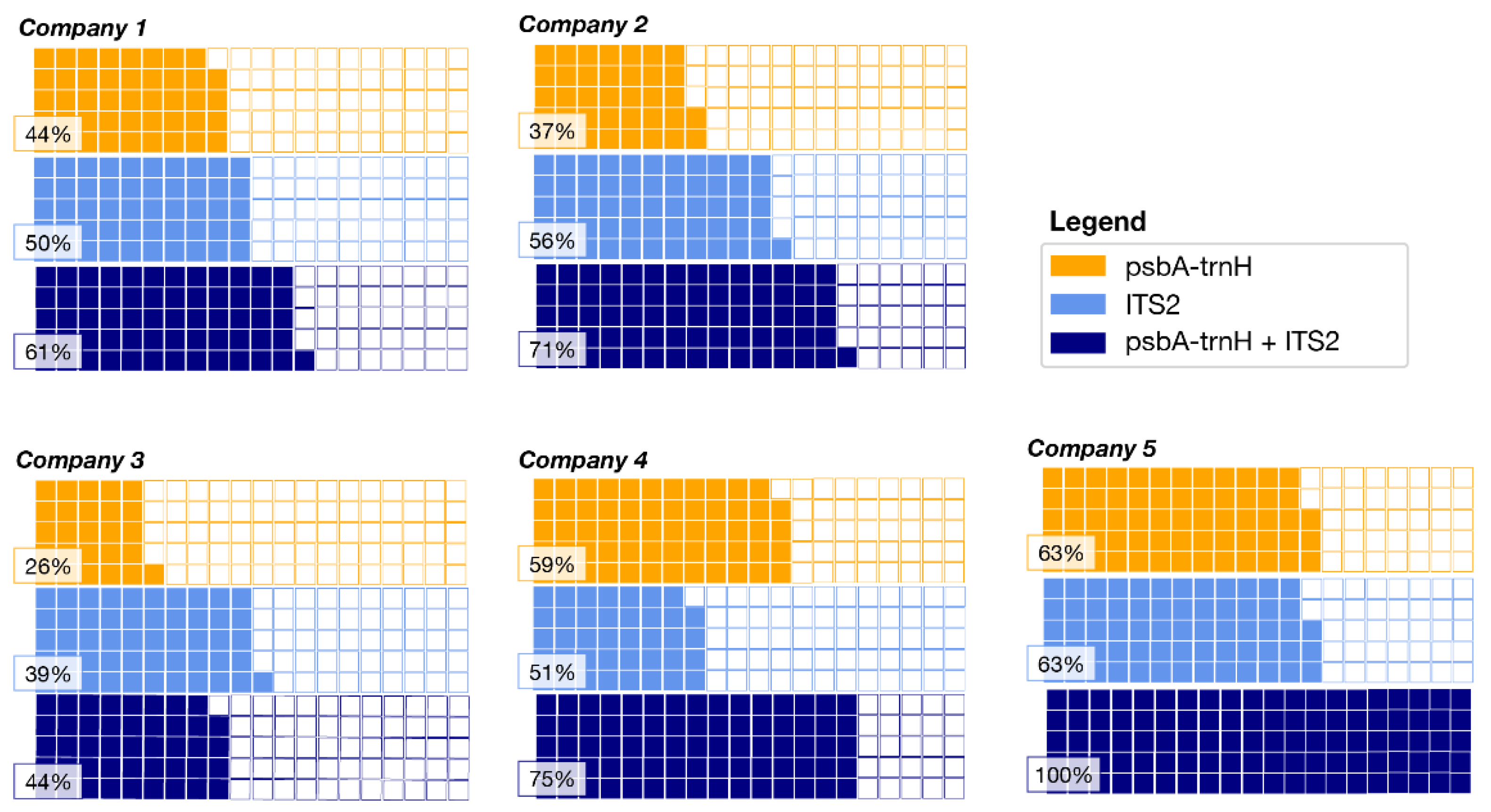
2.1. DNA Metabarcoding Characterization of Commercial Herbal Teas
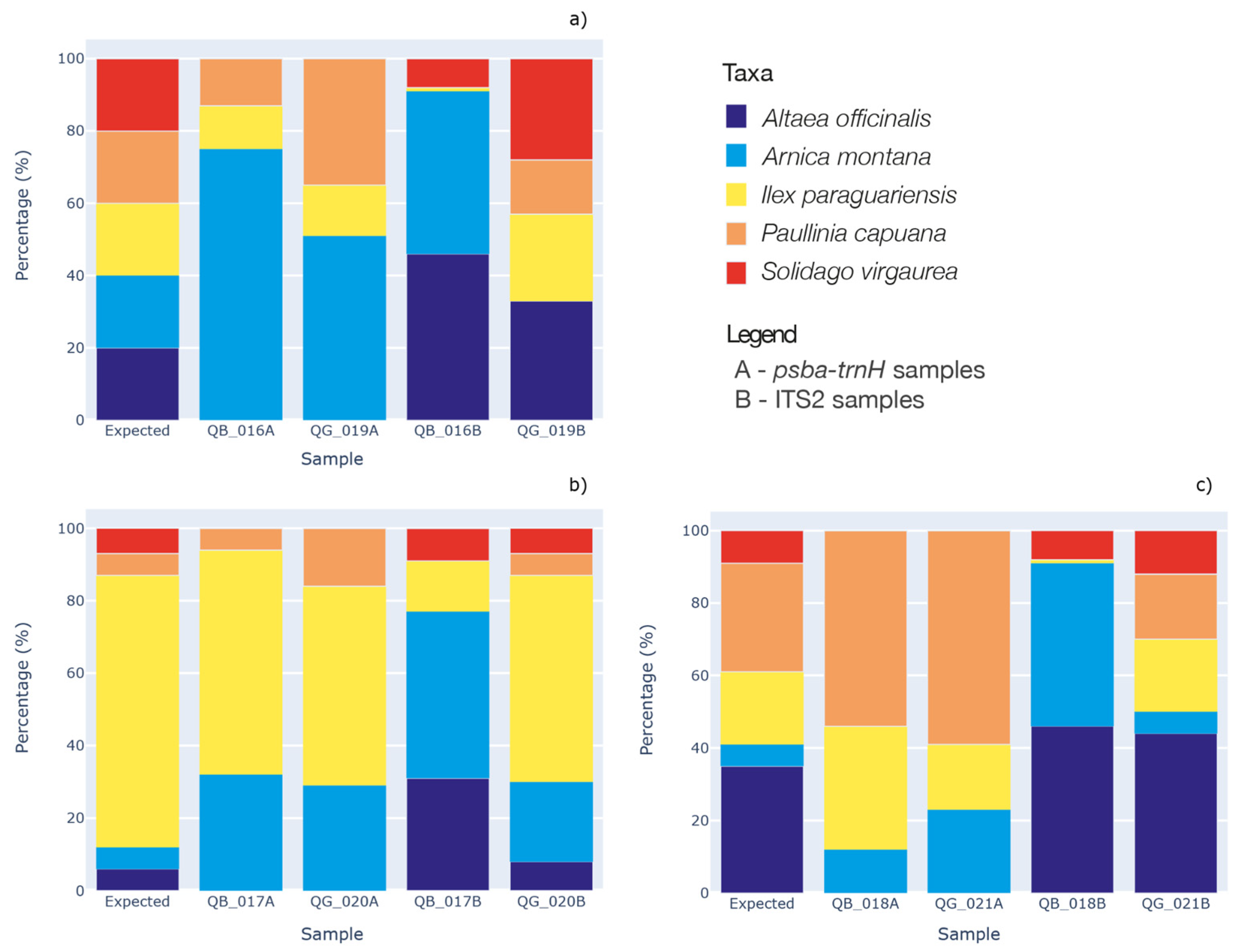
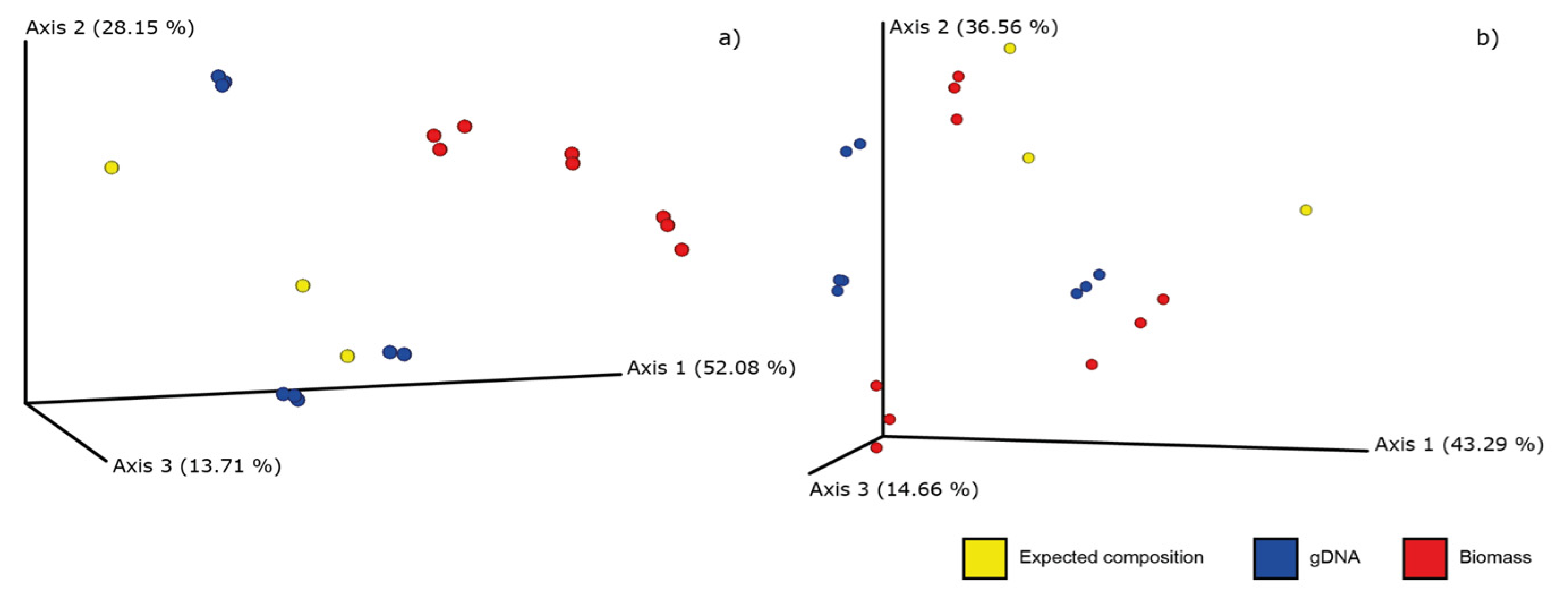
2.2. DNA Metabarcoding for Mock Mixtures’ Quantification
3. Discussion
3.1. A Multi-Marker Approach
3.2. Quantitative Ability of High-Throughput DNA-Sequencing
4. Materials and Methods
4.1. Sampling of Herbal Teas and Assembling of Mock Mixtures
4.2. DNA Extraction and Quantification
4.3. Libraries’ Preparation and Sequencing
4.4. Bioinformatic Analysis and Data Visualization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Z.; Rubinsky, M.; Babajanian, S.; Zhang, Y.; Chang, P.; Swanson, G. Visualization of DNA in highly processed botanical materials. Food Chem. 2018, 245, 1042–1051. [Google Scholar] [CrossRef]
- Smith, T.; Kawa, K.; Eckl, V.; Morton, C.; Stredney, R. Herbal Supplement Sales in US Increase 7.7% in 2016. HerbalGram 2017, 115, 56–65. [Google Scholar]
- Marieschi, M.; Torelli, A.; Poli, F.; Sacchetti, G.; Bruni, R. RAPD-based method for the quality control of Mediter-ranean oregano and its contribution to pharmacognostic techniques. J. Agric. Food Chem. 2009, 57, 1835–1840. [Google Scholar] [CrossRef]
- Lupien, J.R. Food Quality and Safety: Traceability and Labeling. Crit. Rev. Food Sci. Nutr. 2005, 45, 119–123. [Google Scholar] [CrossRef]
- Anthoons, B.; Karamichali, I.; Schrøder-Nielsen, A.; Drouzas, A.D.; de Boer, H.; Madesis, P. Metabarcoding reveals low fidelity and presence of toxic species in short chain-of-commercialization of herbal products. J. Food Compos. Anal. 2021, 97, 103767. [Google Scholar] [CrossRef]
- Nithaniyal, S.; Vassou, S.L.; Poovitha, S.; Raju, B.; Parani, M. Identification of species adulteration in traded medicinal plant raw drugs using DNA barcoding. Genome 2017, 60, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosa, K.A.; Soliman, S.; El-Keblawy, A.; Ali, M.A.; Hassan, H.A.; Tamim, A.A.; Al-Ali, M.M. Using DNA bar-coding to detect adulteration in different herb-al plant-based products in the United Arab Emirates: Proof of concept and validation. Recent Pat. Food Nutr. Agric. 2018, 9, 55–64. [Google Scholar] [CrossRef]
- Grazina, L.; Amaral, J.S.; Mafra, I. Botanical origin authentication of die-tary supplements by DNA-based approaches. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1080–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornara, L.; Smeriglio, A.; Frigerio, J.; Labra, M.; Di Gristina, E.; Denaro, M.; Mora, E.; Trombetta, D. The problem of misi-dentification between edible and poisonous wild plants: Reports from the Mediterranean area. Food Chem. Toxicol. 2018, 119, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Garzo, C.F.; Gómez, P.P.; Barrasa, A.B.; Martínez, R.A.; Ramírez, R.F.; Ramón, F.R. Cases of neurological symptoms associated with star anise consump-tion used as a carminative. An. Esp. Pediatr. 2002, 57, 290–294. [Google Scholar]
- Wiedenfeld, H. Plants containing pyrrolizidine alkaloids: Toxicity and problems. Food Addit. Contam. Part A 2011, 28, 282–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raclariu, A.C.; Paltinean, R.; Vlase, L.; Labarre, A.; Manzanilla, V.; Ichim, M.C.; de Boer, H. Comparative au-thentication of Hypericum perforatum herbal products using DNA metabarcoding, TLC and HPLC-MS. Sci. Rep. 2017, 7, 1291. [Google Scholar] [CrossRef] [PubMed]
- Raclariu, A.C.; Mocan, A.; Popa, M.O.; Vlase, L.; Ichim, M.C.; Crisan, G.; de Boer, H. Veronica officinalis prod-uct authentication using DNA metabarcoding and HPLC-MS reveals widespread adulteration with Veronica chamaedrys. Front. Pharmacol. 2017, 8, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, Y.-T.; Shaw, P.-C. DNA-based techniques for authentication of processed food and food supplements. Food Chem. 2018, 240, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, A.; Casiraghi, M.; Bruni, I.; Guzzetti, L.; Cortis, P.; Berterame, N.M.; Labra, M. From DNA barcoding to personalized nutrition: The evolution of food traceability. Curr. Opin. Food Sci. 2019, 28, 41–48. [Google Scholar] [CrossRef]
- Galimberti, A.; Labra, M.; Sandionigi, A.; Bruno, A.; Mezzasalma, V.; De Mattia, F. DNA Barcoding for Minor Crops and Food Traceability. Adv. Agric. 2014, 2014, 831875. [Google Scholar] [CrossRef] [Green Version]
- Hellberg, R.S.; Hernandez, B.C.; Hernandez, E.L. Identification of meat and poultry species in food products using DNA barcoding. Food Control 2017, 80, 23–28. [Google Scholar] [CrossRef] [Green Version]
- De Mattia, F.; Bruni, I.; Galimberti, A.; Cattaneo, F.; Casiraghi, M.; Labra, M. A comparative study of different DNA barcoding markers for the iden-tification of some members of Lamiacaea. Food Res. Int. 2011, 44, 693–702. [Google Scholar] [CrossRef]
- Bruno, A.; Sandionigi, A.; Agostinetto, G.; Bernabovi, L.; Frigerio, J.; Casiraghi, M.; Labra, M. Food Tracking Perspective: DNA Metabarcoding to Identify Plant Composition in Complex and Processed Food Products. Genes 2019, 10, 248. [Google Scholar] [CrossRef] [Green Version]
- Haynes, E.; Jimenez, E.; Pardo, M.A.; Helyar, S.J. The future of NGS (Next Generation Sequencing) analysis in testing food authenticity. Food Control 2019, 101, 134–143. [Google Scholar] [CrossRef]
- Shokralla, S.; Spall, J.L.; Gibson, J.F.; Hajibabaei, M. Next-generation sequencing technologies for environmental DNA research. Mol. Ecol. 2012, 21, 1794–1805. [Google Scholar] [CrossRef]
- Elbrecht, V.; Leese, F. Can DNA-based ecosystem assessments quantify species abundance? Testing primer bias and biomass—Sequence relationships with an innovative metabarcoding protocol. PLoS ONE 2015, 10, e0130324. [Google Scholar] [CrossRef]
- Frigerio, J.; Pellesi, R.; Mezzasalma, V.; De Mattia, F.; Galimberti, A.; Lambertini, F.; Suman, M.; Zanardi, S.; Leporati, A.; Labra, M. Development of a DNA Barcoding-Like Approach to Detect Mustard Allergens in Wheat Flours. Genes 2019, 10, 234. [Google Scholar] [CrossRef] [Green Version]
- Staats, M.; Arulandhu, A.J.; Gravendeel, B.; Holst-Jensen, A.; Scholtens, I.; Peelen, T.; Prins, T.W.; Kok, E. Advances in DNA metabarcoding for food and wildlife forensic species identification. Anal. Bioanal. Chem. 2016, 408, 4615–4630. [Google Scholar] [CrossRef] [Green Version]
- CBOL Plant Working Group; Hollingsworth, P.M.; Forrest, L.L.; Spouge, J.L.; Hajibabaei, M.; Ratnasingham, S.; van der Bank, M.; Chase, M.W.; Cowan, R.S.; Erickson, D.L.; et al. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Yao, H.; Han, J.; Liu, C.; Song, J.; Shi, L.; Zhu, Y.; Ma, X.; Gao, T.; Pang, X.; et al. Validation of the ITS2 Region as a Novel DNA Barcode for Identifying Medicinal Plant Species. PLoS ONE 2010, 5, e8613. [Google Scholar] [CrossRef]
- Kuzmina, M.L.; Braukmann, T.W.A.; Fazekas, A.J.; Graham, S.W.; Dewaard, S.L.; Rodrigues, A.; Bennett, B.A.; Dickinson, T.A.; Saarela, J.M.; Catling, P.M.; et al. Using herbarium-derived DNAs to assemble a large-scale DNA barcode library for the vascular plants of Canada1. Appl. Plant Sci. 2017, 5, 1700079. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Song, J.; Liu, C.; Luo, K.; Han, J.; Li, Y.; Pang, X.; Xu, H.; Zhu, Y.; Xiao, P.; et al. Use of ITS2 Region as the Universal DNA Barcode for Plants and Animals. PLoS ONE 2010, 5, e13102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kress, W.J.; Erickson, D.L. A Two-Locus Global DNA Barcode for Land Plants: The Coding rbcL Gene Comple-ments the Non-Coding trnH-psbA Spacer Region. PLoS ONE 2007, 2, e508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanni, S.; Stanković, D.; Borme, D.; De Olazabal, A.; Juretić, T.; Pallavicini, A.; Tirelli, V. Multi-marker metabarcoding ap-proach to study mesozooplankton at basin scale. Sci. Rep. 2018, 8, 12085. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, L.P.; Mata, V.A.; Lopes, P.B.; Pereira, P.; Jarman, S.N.; Lopes, R.J.; Beja, P. Advancing the integration of multi-marker metabarcoding data in dietary analysis of trophic generalists. Mol. Ecol. Resour. 2019, 19, 1420–1432. [Google Scholar] [CrossRef] [Green Version]
- Arulandhu, A.J.; Staats, M.; Hagelaar, R.; Peelen, T.; Kok, E.J. The application of multi-locus DNA metabarcoding in traditional medicines. J. Food Compos. Anal. 2019, 79, 87–94. [Google Scholar] [CrossRef]
- Deagle, B.E.; Jarman, S.N.; Coissac, E.; Pompanon, F.; Taberlet, P. DNA metabarcoding and the cytochrome c oxi-dase subunit I marker: Not a perfect match. Biol. Lett. 2014, 10, 20140562. [Google Scholar] [CrossRef] [Green Version]
- Piñol, J.; Senar, M.A.; Symondson, W.O. The choice of universal primers and the characteristics of the species mixture determine when DNA metabarcoding can be quantitative. Mol. Ecol. 2019, 28, 407–419. [Google Scholar] [CrossRef]
- Lv, Y.-N.; Yang, C.-Y.; Shi, L.-C.; Zhang, Z.-L.; Xu, A.-S.; Zhang, L.-X.; Li, X.-L.; Li, H.-T. Identification of medicinal plants within the Apocynaceae family using ITS2 and psbA-trnH barcodes. Chin. J. Nat. Med. 2020, 18, 594–605. [Google Scholar] [CrossRef]
- Lamb, P.D.; Hunter, E.; Pinnegar, J.; Creer, S.; Davies, R.G.; Taylor, M.I. How quantitative is metabarcoding: A meta-analytical approach. Mol. Ecol. 2018, 28, 420–430. [Google Scholar] [CrossRef]
- Krehenwinkel, H.; Kennedy, S.R.; Rueda, A.; Lam, A.; Gillespie, R.G. Scaling up DNA barcoding—Primer sets for simple and cost efficient arthropod systematics by multiplex PCR and Illumina amplicon sequencing. Methods Ecol. Evol. 2018, 9, 2181–2193. [Google Scholar] [CrossRef]
- Kelly, R.P.; Shelton, A.O.; Gallego, R. Understanding PCR processes to draw meaningful conclusions from en-vironmental DNA studies. Sci. Rep. 2019, 9, 12133. [Google Scholar] [CrossRef] [Green Version]
- Paranaiba, R.T.; Carvalho, C.B.; Paiva, R.S.; Trindade, B.R.; Barros, M.G.; Souza, E.P.; Gontijo, A.B.; Silveira, D. DNA from wood—A simple approach facing a challenging matrix—A preliminary study. Forensic Sci. Int. 2020, 314, 110371. [Google Scholar] [CrossRef]
- Lu, Y.; Jiao, L.; He, T.; Zhang, Y.; Jiang, X.; Yin, Y. An optimized DNA extraction protocol for wood DNA barcoding of Pterocarpus erinaceus. IAWA J. 2020, 41, 644–659. [Google Scholar] [CrossRef]
- Biella, P.; Tommasi, N.; Akter, A.; Guzzetti, L.; Klecka, J.; Sandionigi, A.; Labra, M.; Galimberti, A. Foraging strategies are maintained despite workforce reduction: A multidisciplinary survey on the pollen collected by a social pollinator. PLoS ONE 2019, 14, e0224037. [Google Scholar]
- Richardson, R.T.; Lin, C.H.; Sponsler, D.B.; Quijia, J.O.; Goodell, K.; Johnson, R.M. Application of ITS2 metabar-coding to determine the provenance of pollen collected by honey bees in an agroecosystem. Appl. Plant Sci. 2015, 3, 1400066. [Google Scholar] [CrossRef] [PubMed]
- Bolson, M.; Smidt ED, C.; Brotto, M.L.; Silva-Pereira, V. ITS and trnH-psbA as efficient DNA barcodes to identify threatened commercial woody angiosperms from southern Brazilian Atlantic rainforests. PLoS ONE 2015, 10, e0143049. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An effective distance metric for microbial community comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar]
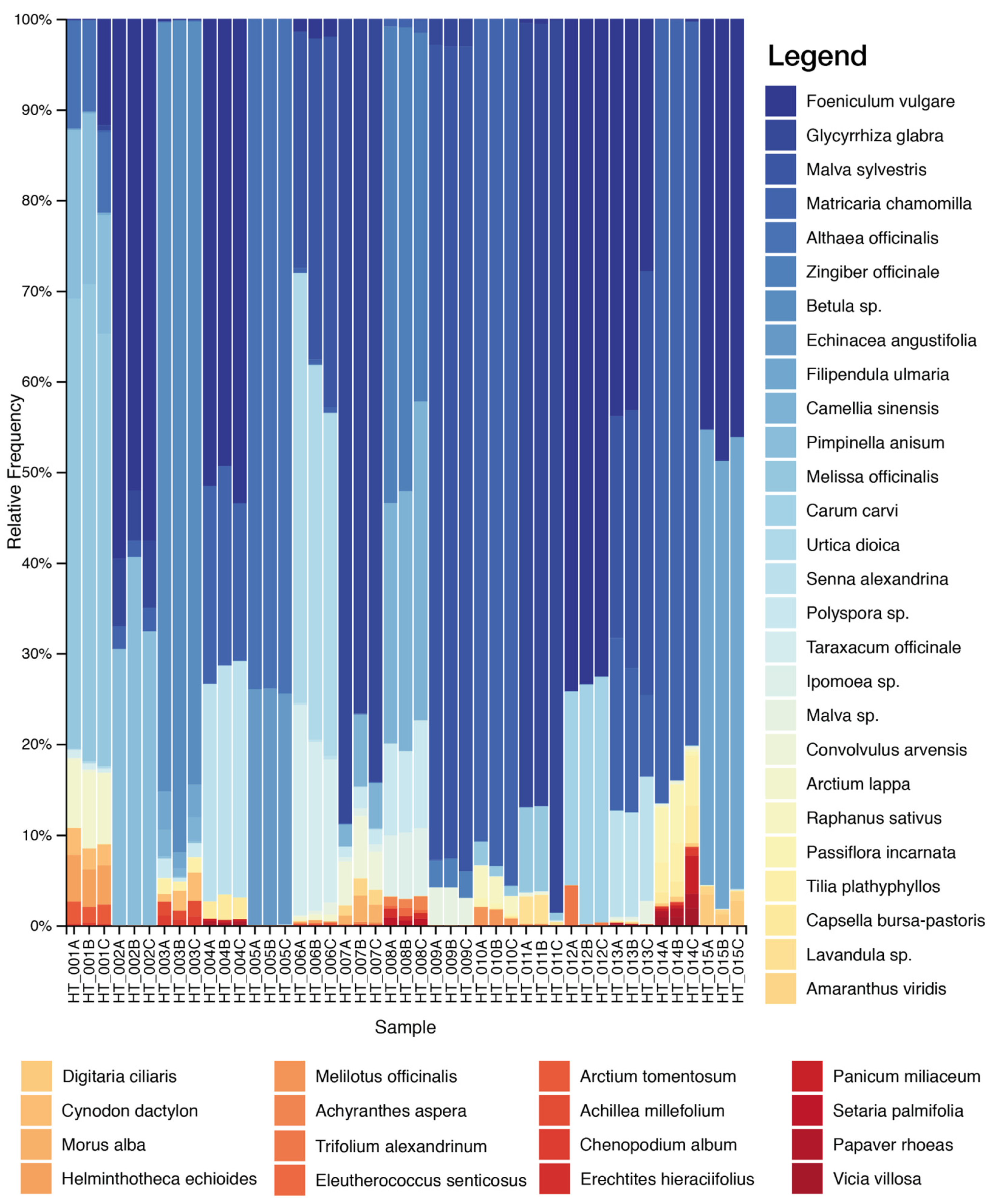
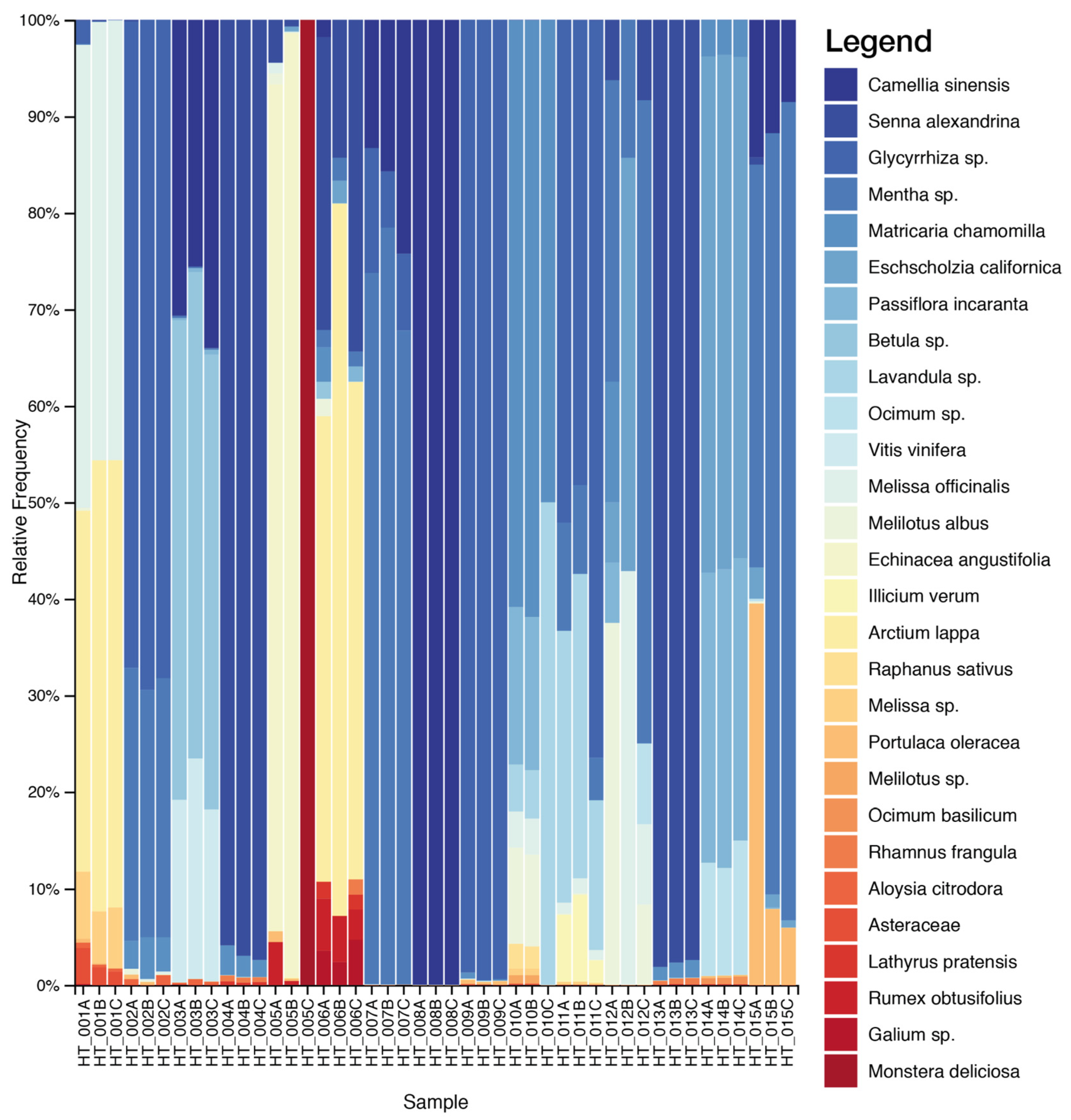
| ID LAB | Declared Species | Assigned Species (psbA-trnH) | Assigned Species (ITS2) |
|---|---|---|---|
| HT_001 | Agropyron repens Beauv. 20%, Taraxacum officinale Weber 20%, Arctium Iappa 15%, Cichorium intybus 15%, Melissa officinalis 15%, Cynara scolymus 15% | Melissa officinalis 52%, Arctium lappa 46%, Reichardia ligulata 2% | Melissa officinalis 51%, Pimpinella anisum 17%, Althaea officinalis 10%, Arctium lappa 8%, Helminthotheca echioides 4%, Arctium tomentosum 2%, Cynodon dactylon 2%, Taraxacum officinale 3%, Asteraceae 2% |
| HT_002 | Foeniculum vulgare 20%, Glycyrrhiza glabra 20%, Pimpinella anisum 20%, Mentha piperita 20%, Citrus sinensis var. dulcis 15%, Matricaria chamomilla 5% | Glycyrrhiza sp. 69%, Mentha sp. 26%, Matricaria chamomilla 4%, Aloysia citrodora 1% | Foeniculum vulgare 54%, Pimpinella anisum 37%, Glycyrrhiza glabra 7%, Matricaria chamomilla 2% |
| HT_003 | Camelia sinensis 20%, Prunus cerasus 20%, Citrus limon 20%, Betula pendula 15%, Agropyron Repens 15%, Vitis vinifera 10% | Betula sp. 49%, Camellia sinensis 30%, Vitis vinifera 21% | Betula sp. 89%, Camellia sinensis 2%, Chenopodium album 1%, Cynodon dactylon 2%, Filipendula ulmaria 3%, Achillea millefolium 1%, Polyspora axillaris 1%, Tilia platyphyllos 1% |
| HT_004 | Senna alexandrina 40%, Rhamnus frangula 20%, Matricaria chamomilla, Foeniculum vulgare | Senna alexandrina 97%, Rhamnus frangula 1%, Matricaria chamomilla 2% | Foeniculum vulgare 52%, Matricaria chamomilla 22%, Senna alexandrina 24%, Capsella bursa-pastoris 2% |
| HT_005 | Echinacea angustifolia 30%, Citrus x limon, Althaea officinalis, Rosa canina, Hibiscus sabdariffa, Sambucus nigra 10% | Echinacea angustifolia 94%, Monstera deliciosa 4%, Portulaca oleracea 1%, Rumex obtusifolius 1% | Althaea officinalis 74%, Echinacea angustifolia 26% |
| HT_006 | Urtica dioica 30%, Arctium lappa 20%, Taraxacum officinale, Citrus x limon, Malva officinalis | Arctium lappa 62%, Senna alexandrina 24%, Galium sp. 4%, Lathyrus pratensis 2%, Mentha sp. 4%, Rumex obtusifolius 4% | Urtica dioica 43%, Malva sp. 35%, Taraxacum officinale 19%, Foeniculum vulgare 2%, Matricaria chamomilla 1% |
| HT_007 | Camellia sinensis 51%, Mentha 29%, Glycyrrhiza glabra 8.25%, Mentha piperita 3.9%, Aloe vera | Mentha sp. 74%, Camellia sinensis 17%, Glycyrrhiza sp. 9% | Glycyrrhiza glabra 84%, Amaranthus viridis 2%, Camellia sinensis 5%, Convolvulus arvensis 5%, Ipomoea sp. 1%, Morus alba 2%, Polyspora axillaris 1% |
| HT_008 | Camellia sinensis 62.9%, Zingiber officinalis 22%, Peach 1%, Ginseng 1%, Aloe vera | Camelia sinensis 100% | Camellia sinensis 30%, Zingiber officinale 23%, Eleutherococcus senticosus 1%, Ocimum sp. 25%, Polyspora sp. 10%, Ipomoea sp. 7%, Achyranthes aspera 1%, Erechtites hieraciifolius 1%, Setaria palmifolia 1% |
| HT_009 | Zingiber officinale, Citrus limon, Malva sylvestris, Cymbopogon citratus, Glycyrrhiza glabra | Glycyrrhiza sp. 100% | Malva sylvestris 94%, Glycyrrhiza glabra 3%, Ocimum sp. 2%, Zingiber officinale 1% |
| HT_010 | Matricaria chamomilla 44.4%, Melissa officinalis 22.2%, Betula pendula/pubescens, Passiflora incarnata, Lavandula officinalis 5.6% | Matricaria chamomilla 51%, Lavandula sp. 14%, Melilotus sp. 11%, Melissa officinalis 6%, Passiflora incarnata 16%, Raphanus sativus 2% | Matricaria chamomilla 93%, Melilotus officinalis 1%, Melissa officinalis 2%, Passiflora incarnata 1%, Raphanus sativus 3% |
| HT_011 | Illicium verum 27%, Mentha piperita 25%, Melissa officinalis, Glycyrrhiza glabra, Lavandula officinalis, Cinchona officinalis, Gentiana lutea 2%. | Glycyrrhiza sp. 60%, Lavandula sp. 25%, Mentha sp. 7%, Illicium verum 7%, Melissa officinalis 1% | Glycyrrhiza glabra 90%, Melissa officinalis 8%, Lavandula sp. 2% |
| HT_012 | Foeniculum vulgare 40%, Illicium verum 40%, Carum carvi, Mentha piperita 9% | Mentha sp. 41%, Eschscholzia californica 20%, Melilotus sp. 21%, Melissa officinalis 18% | Foeniculum vulgare 72%, Carum carvi 27%, Trifolium alexandrinum 1% |
| HT_013 | Senna alexandrina 40%, Rhamnus frangula 15%, Matricaria chamomilla 15%, Foeniculum vulgare 15%, Malva officinalis 15% | Senna alexandrina 98%, Rhamnus frangula 1%, Matricaria chamomilla 1% | Foeniculum vulgare 38%, Malva sp. 34%, Matricaria chamomilla 16%, Senna alexandrina 12% |
| HT_014 | Passiflora incarnata, Eschscholzia californica Cham., Matricaria chamomilla, Tilia platyphyllos, Ocimum basilicum | Eschscholzia californica 53%, Passiflora incarnata 30%, Matricaria chamomilla 4%, Ocimum sp. 13% | Matricaria chamomilla 83%, Passiflora incarnata 7%, Panicum miliaceum 2%, Papaver rhoeas 1%, Tilia sp. 4%, Vicia villosa 1%, Capsella bursa-pastoris 2% |
| HT_015 | Camellia sinensis, Filipendula ulmaria, Foeniculum vulgare, Mentha spicata | Mentha sp. 69%, Portulaca oleracea 17%, Camellia sinensis 12%, Eschscholzia californica 2% | Filipendula ulmaria 50%, Foeniculum vulgare 47%, Digitaria ciliaris 2% |
| ID LAB | Company | Sample Typology |
|---|---|---|
| HT_001 | Company 1 | Purifying Herbal Tea |
| HT_002 | Company 1 | Digestive Herbal Tea |
| HT_003 | Company 1 | Slimming Herbal Tea |
| HT_004 | Company 2 | Laxative Herbal Tea |
| HT_005 | Company 2 | Aromatic Herbal Tea |
| HT_006 | Company 2 | Purifying Herbal Tea |
| HT_007 | Company 3 | Aromatic Herbal Tea |
| HT_008 | Company 3 | Aromatic Herbal Tea |
| HT_009 | Company 3 | Depurative Herbal Tea |
| HT_010 | Company 4 | Relaxing Herbal Tea |
| HT_011 | Company 4 | Digestion Herbal Tea |
| HT_012 | Company 4 | Flat Stomach Herbal Tea |
| HT_013 | Company 5 | Laxative Herbal Tea |
| HT_014 | Company 5 | Sleep Herbal Tea |
| HT_015 | Company 5 | Draining Herbal Tea |
| Species | Plant Section | QB_016 | QB_017 | QB_018 | QG_019 | QG_020 | QG_021 |
|---|---|---|---|---|---|---|---|
| Althaea officinalis | Roots | 20% | 6% | 35% | 20% | 6% | 35% |
| Arnica montana | Flowers | 20% | 6% | 6% | 20% | 6% | 6% |
| Ilex paraguariensis | Leaves | 20% | 76% | 20% | 20% | 76% | 20% |
| Paullinia cupana | Seeds | 20% | 6% | 30% | 20% | 6% | 30% |
| Solidago virgaurea | Aerial parts | 20% | 6% | 9% | 20% | 6% | 9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frigerio, J.; Agostinetto, G.; Mezzasalma, V.; De Mattia, F.; Labra, M.; Bruno, A. DNA-Based Herbal Teas’ Authentication: An ITS2 and psbA-trnH Multi-Marker DNA Metabarcoding Approach. Plants 2021, 10, 2120. https://doi.org/10.3390/plants10102120
Frigerio J, Agostinetto G, Mezzasalma V, De Mattia F, Labra M, Bruno A. DNA-Based Herbal Teas’ Authentication: An ITS2 and psbA-trnH Multi-Marker DNA Metabarcoding Approach. Plants. 2021; 10(10):2120. https://doi.org/10.3390/plants10102120
Chicago/Turabian StyleFrigerio, Jessica, Giulia Agostinetto, Valerio Mezzasalma, Fabrizio De Mattia, Massimo Labra, and Antonia Bruno. 2021. "DNA-Based Herbal Teas’ Authentication: An ITS2 and psbA-trnH Multi-Marker DNA Metabarcoding Approach" Plants 10, no. 10: 2120. https://doi.org/10.3390/plants10102120
APA StyleFrigerio, J., Agostinetto, G., Mezzasalma, V., De Mattia, F., Labra, M., & Bruno, A. (2021). DNA-Based Herbal Teas’ Authentication: An ITS2 and psbA-trnH Multi-Marker DNA Metabarcoding Approach. Plants, 10(10), 2120. https://doi.org/10.3390/plants10102120








