Interaction Effect of EDTA, Salinity, and Oxide Nanoparticles on Alga Chlamydomonas reinhardtii and Chlamydomonas euryale
Abstract
:1. Introduction
2. Results and Discussion
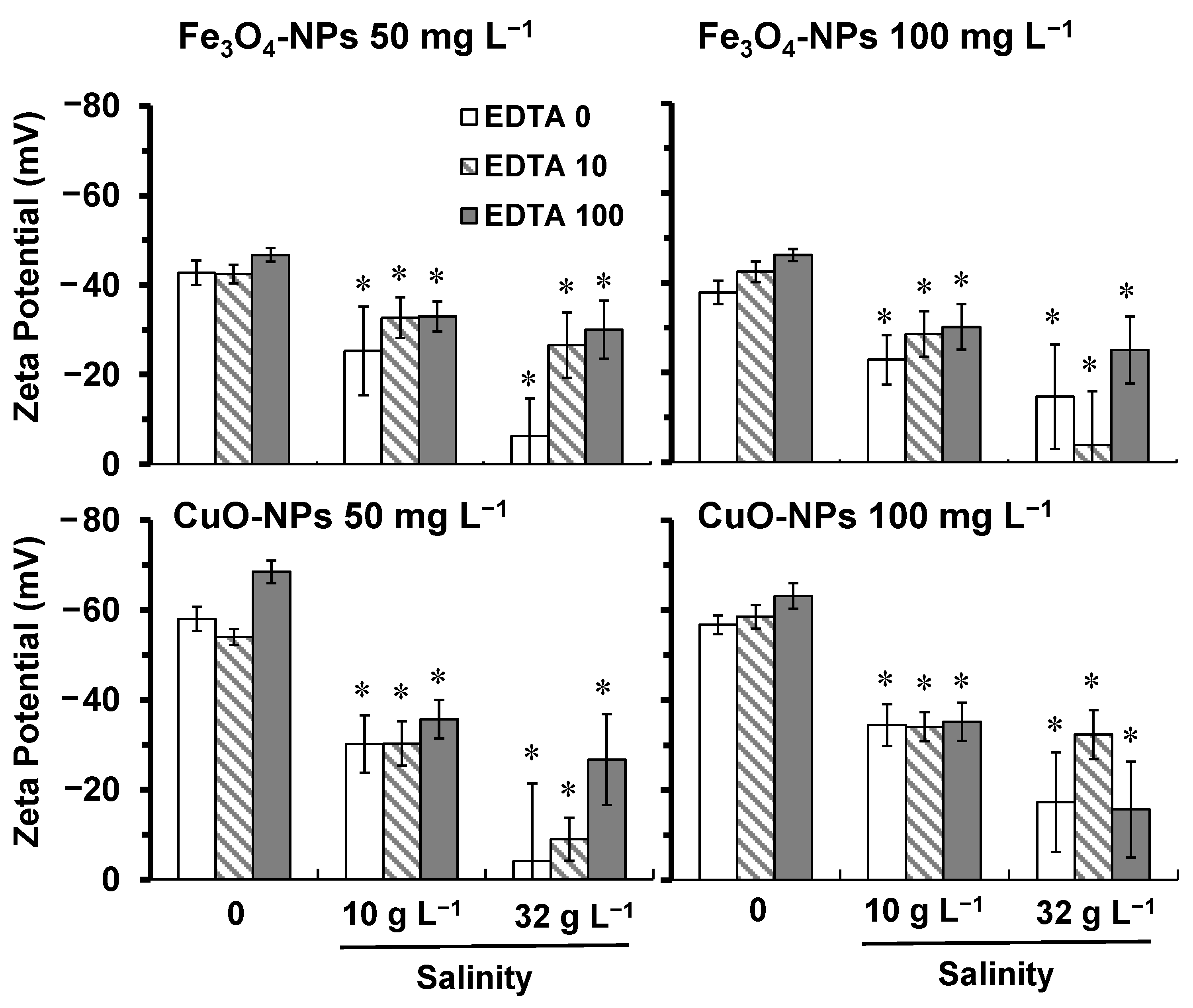
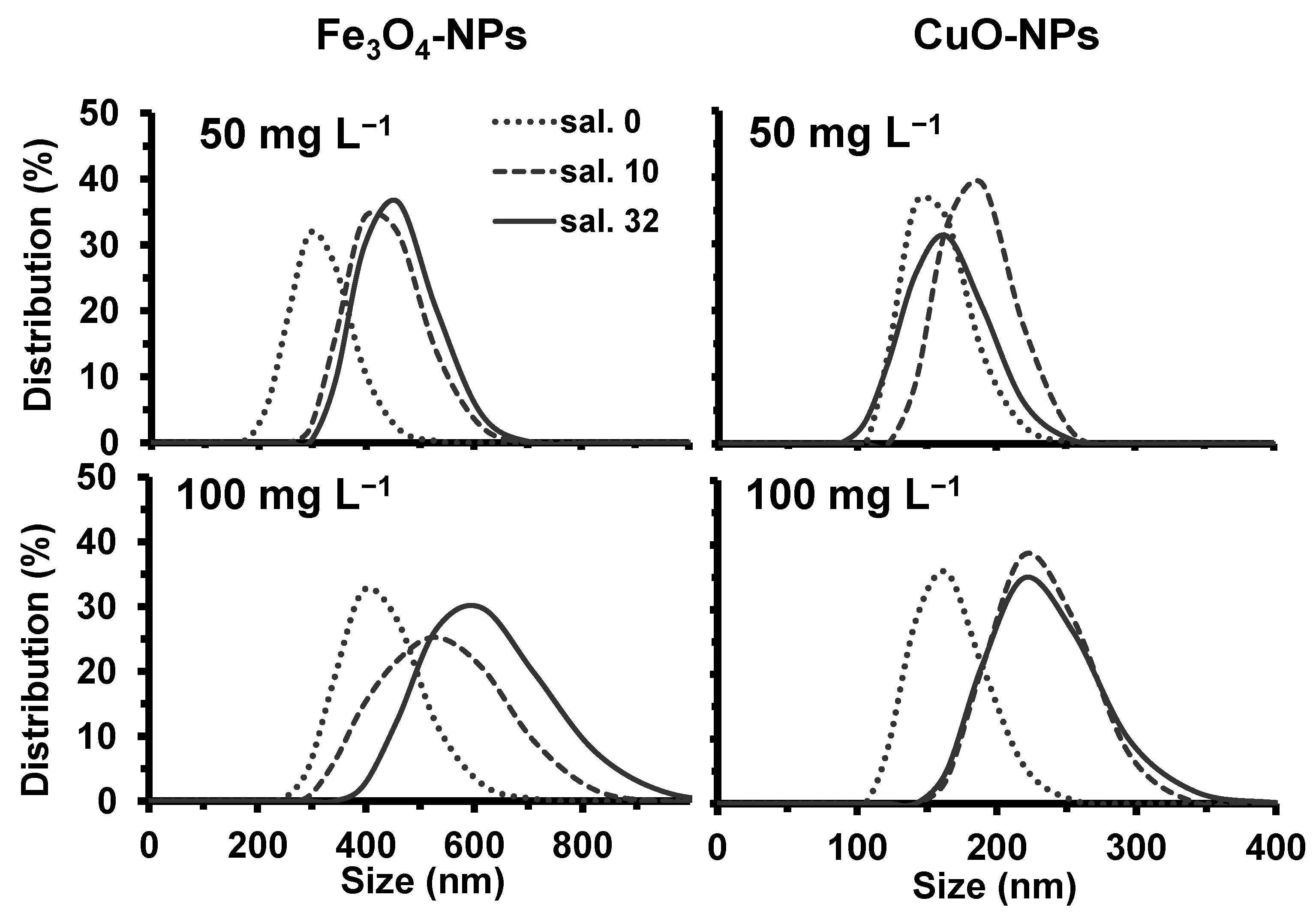
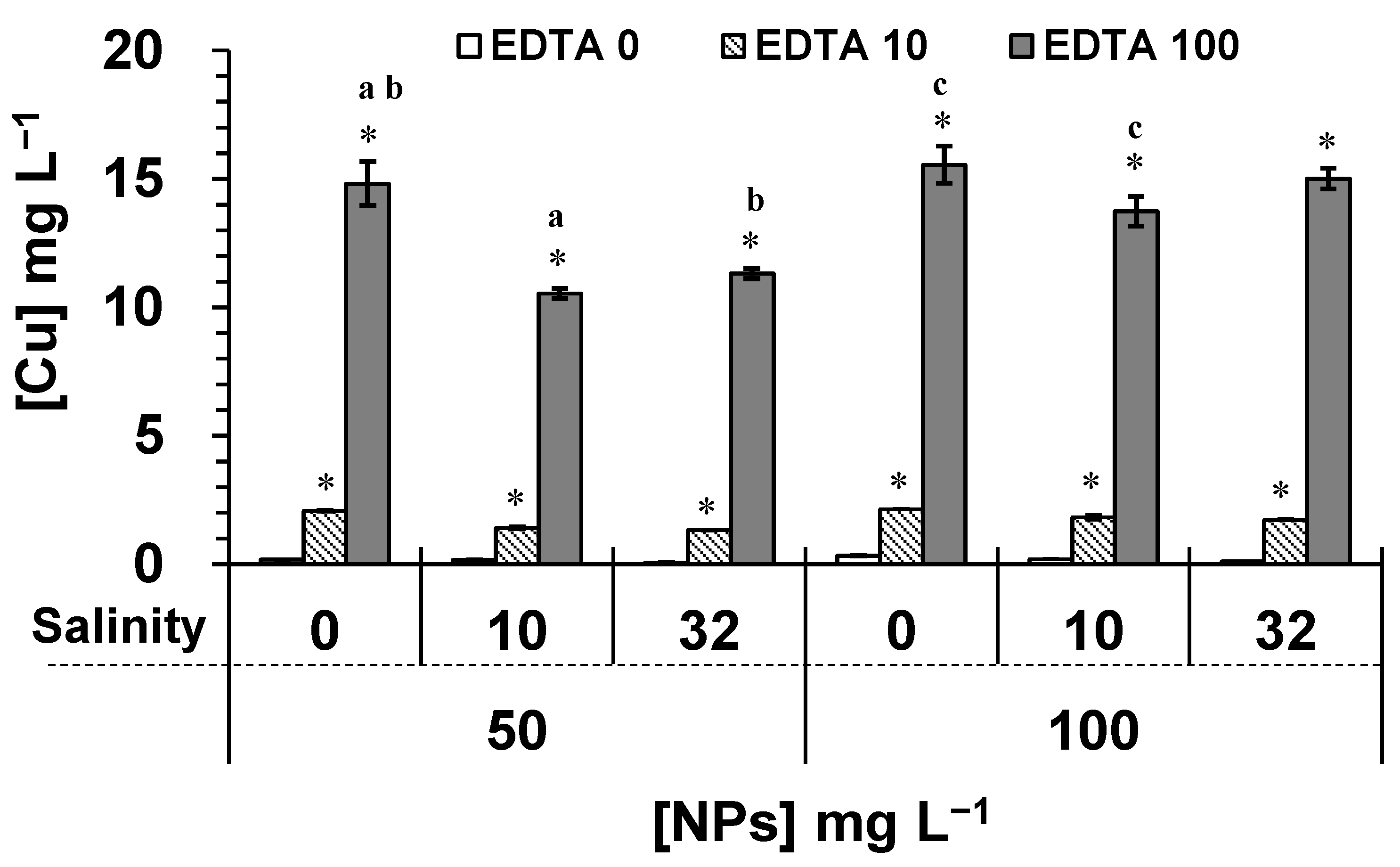
2.1. Effect of Salinity and EDTA on NPs Properties
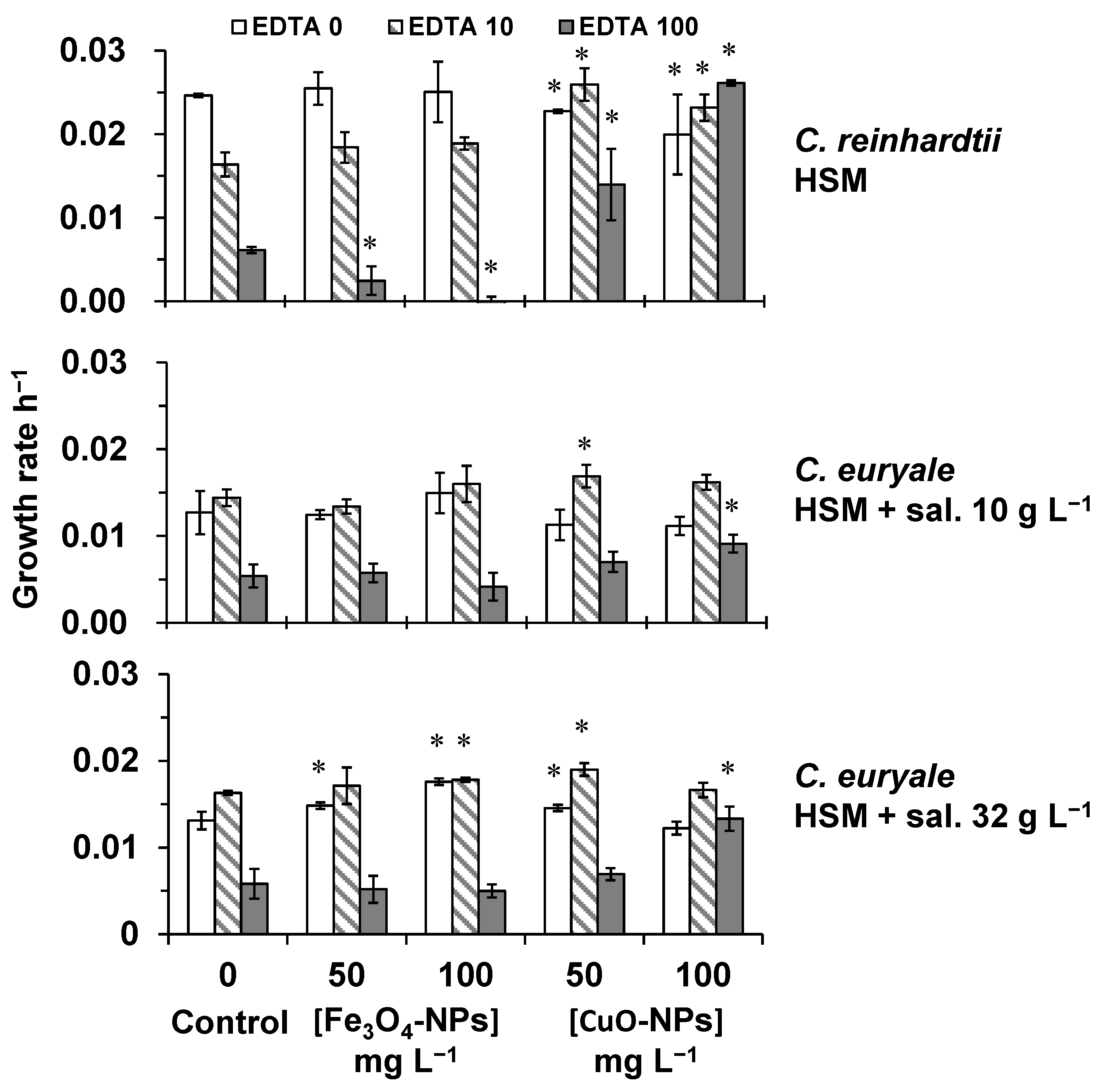
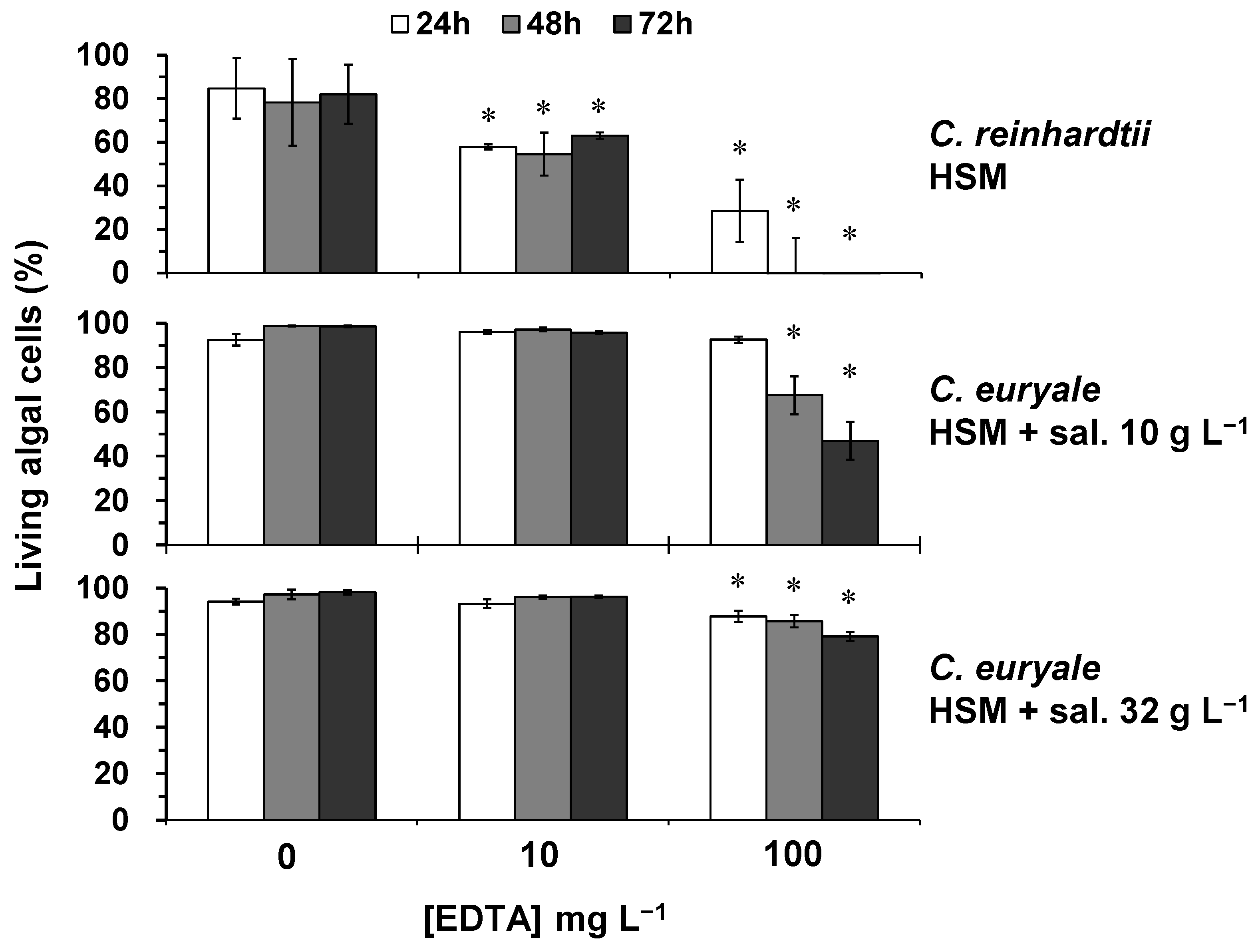
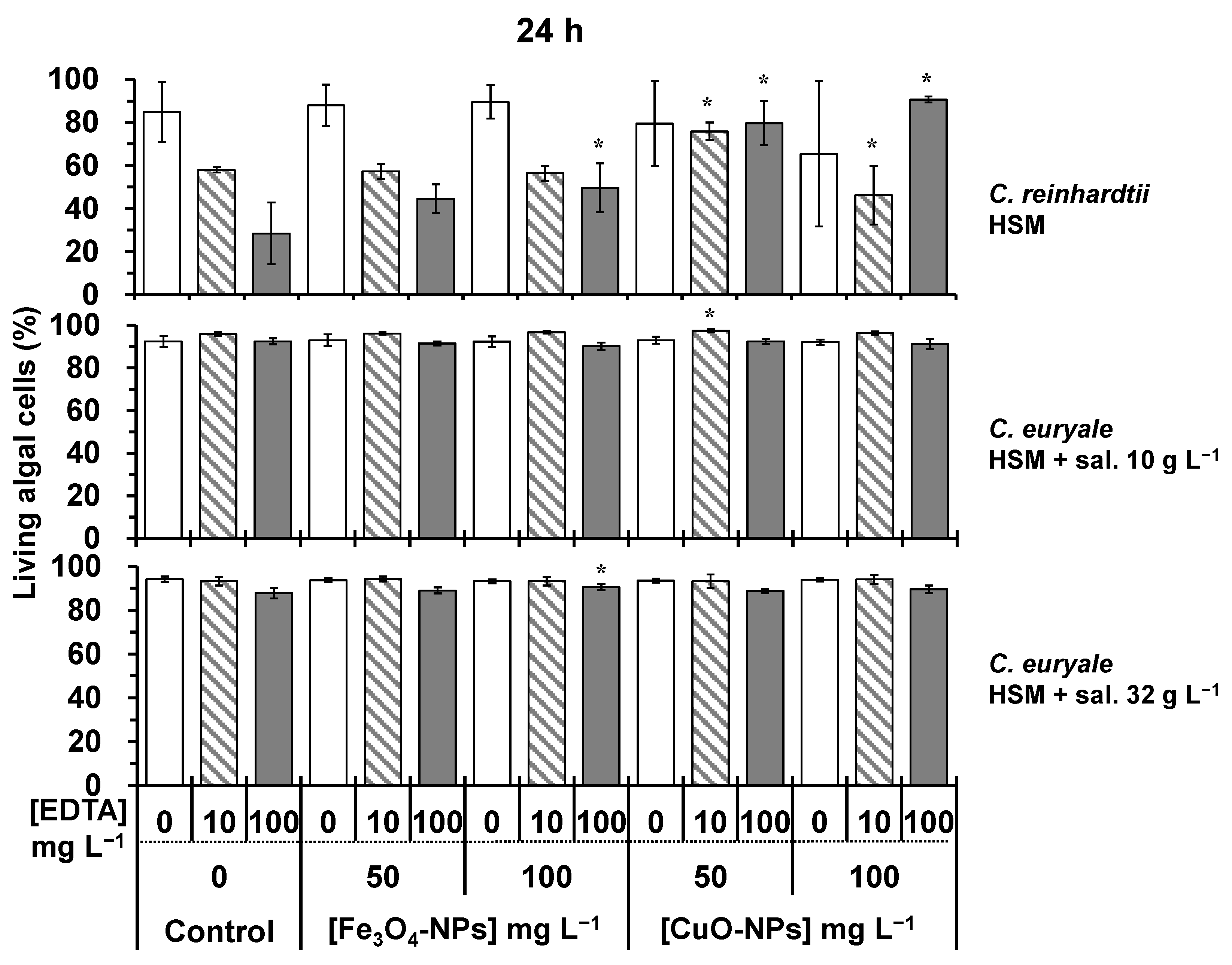
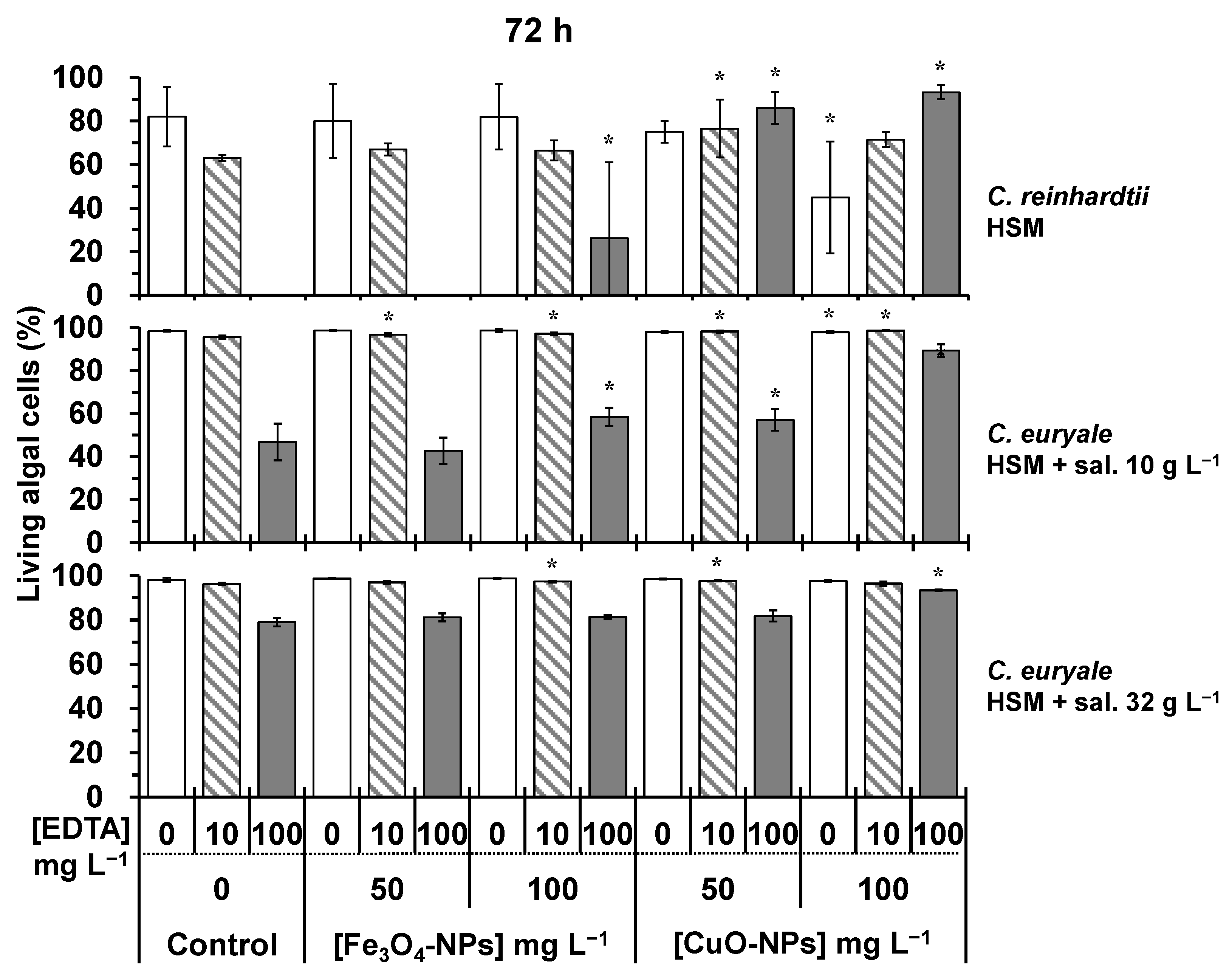
2.2. Toxicity Testing on Algal Cells
3. Conclusions
4. Materials and Methods
4.1. Algal Strains and Culture
4.2. Characterization of Nanoparticles
4.3. Nanoparticles and EDTA Treatments
4.4. Growth Rate and Cellular Viability
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D 2009, 42, 224001. [Google Scholar] [CrossRef] [Green Version]
- Panneerselvam, P.; Morad, N.; Tan, K.A. Magnetic nanoparticle (Fe3O4) impregnated onto tea waste for the removal of nickel (II) from aqueous solution. J. Hazard. Mater. 2011, 186, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Zeng, G.M.; Gong, J.L. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: A review. Sci. Total Environ. 2014, 468-469, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Keller, A.A. EDTA functionalized magnetic nanoparticle sorbents for cadmium and lead contaminated water treatment. Water Res. 2015, 80, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.; Park, H.S.; Jung, W.H. Thermal conductivity and lubrication characteristics of nanofluids. Cur. Appl. Phys. 2006, 6, 67–71. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. B 2010, 79, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Nowack, B. The release of engineered nanomaterials to the environment. J. Environ. Monitor. 2011, 13, 1145–1155. [Google Scholar] [CrossRef]
- Klaine, S.J.; Alvarez, P.J.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Bhatt, I.; Tripathi, B.N. Interaction of engineered nanoparticles with various components of the environment and possible strategies for their risk assessment. Chemosphere 2011, 82, 308–317. [Google Scholar] [CrossRef]
- Vale, G.; Mehennaoui, K.; Cambier, S.; Libralato, G.; Jomini, S.; Domingos, R.F. Manufactured nanoparticles in the aquatic environment-biochemical responses on freshwater organisms: A critical overview. Aquat. Toxicol. 2016, 170, 162–174. [Google Scholar] [CrossRef]
- Baek, Y.W.; An, Y.J. Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci. Total Environ. 2011, 409, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Aruoja, V.; Pokhrel, S.; Sihtmäe, M.; Mortimer, M.; Mädler, L.; Kahru, A. Toxicity of 12 metal-based nanoparticles to algae, bacteria and protozoa. Environ. Sci. Nano. 2015, 2, 630. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Zhao, J. Toxicity and internalization of CuO nanoparticles to prokaryotic alga Microcystis aeruginosa as affected by dissolved organic matter. Environ. Sci. Technol. 2011, 45, 6532–6540. [Google Scholar]
- Aruoja, V.; Dubourguier, H.C.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, L.; Dewez, D. Toxicity of superparamagnetic iron oxide nanoparticles on green alga Chlorella vulgaris. BioMed Res. Int. 2013, 2013, 647974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melegari, S.P.; Perreault, F.; Matias, W.G. Evaluation of toxicity and oxidative stress induced by copper oxide nanoparticles in the green alga Chlamydomonas reinhardtii. Aquat. Toxicol. 2013, 142–143, 431–440. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, X.; Liu, X.; Wang, Z.; Zhang, C.; White, J.; Xing, B. Interactions of CuO nanoparticles with the algae Chlorella pyrenoidosa: Adhesion, uptake, and toxicity. Nanotoxicology 2016, 10, 1297–1305. [Google Scholar] [CrossRef]
- Saxena, P.; Sangela, V.; Harish. Toxicity evaluation of iron oxide nanoparticles and accumulation by microalgae Coelastrella terrestris. Environ. Sci. Poll. Res. 2020, 27, 19650–19660. [Google Scholar] [CrossRef]
- Saxena, P.; Saharan, V.; Baroliya, P.K.; Gour, V.S.; Rai, M.K.; Harish. Mechanism of nanotoxicity in Chlorella vulgaris exposed to zinc and iron oxide. Toxicol. Rep. 2021, 8, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Hazeem, L.; Waheed, F.; Rashdan, S.; Bououdina, M.; Loïc, B.; Slomianny, C.; Boukherroub, R.; Elmeselmani, W. Effect of magnetic iron oxide (Fe3O4) nanoparticles on the growth and photosynthetic pigment content of Picochlorum sp. Environ. Sci. Poll. Res. Int. 2015, 22, 11728–11739. [Google Scholar] [CrossRef]
- Cheloni, G.; Marti, E.; Slaveykova, V.I. Interactive effects of copper oxide nanoparticles and light to green alga Chlamydomonas reinhardtii. Aquat. Toxicol. 2015, 170, 120–128. [Google Scholar] [CrossRef]
- Brul, S.; Coote, P. Mode of action and microbial resistance mechanisms. Int. J. Food Microbiol. 1999, 50, 1–17. [Google Scholar] [CrossRef]
- Nowack, B.; VanBriesen, J.M. Chelating agents in the environment. In Biogeochemistry of Chelating Agents; Nowack, B., Van Briesen, J.M., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2005; pp. 1–18. [Google Scholar]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Bucheli-Witschel, M.; Egli, T. Environmental fate and microbial degradation of aminopolycarboxylic acids. FEMS Microbiol. Rev. 2001, 25, 69–106. [Google Scholar] [CrossRef] [PubMed]
- Reemtsma, T.; Weiss, S.; Mueller, J.; Petrovic, M.; Gonzalez, S.; Barcelo, D.; Ventura, F.; Knepper, T.P. Polar pollutants entry into the water cycle by municipal wastewater: A European perspective. Environ. Sci. Technol. 2006, 40, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhu, W.; Wang, Z. Accumulation, assimilation and growth inhibition of copper on freshwater alga (Scenedesmus subspicatus 86.81 SAG) in the presence of EDTA and fulvic acid. Aquat. Toxicol. 2003, 63, 221–228. [Google Scholar] [CrossRef]
- Mendes, L.; Zambotti-Villela, L.; Simas, C.; Colepicolo, P. Toxicological effects of metal-EDTA/NTA complex formation in a synthetic medium on the macroalga Gracilaria domingensis. J. Appl. Phycol. 2015, 27, 1307–1314. [Google Scholar] [CrossRef]
- Pascual, G.; Sano, D.; Sakamaki, T.; Nishimura, O. Effects of chemical interaction of nutrients and EDTA on metals toxicity to Pseudokirckneriella subcapitata. Ecotox. Environ. Saf. 2020, 203, 110966. [Google Scholar] [CrossRef]
- Hsu-Kim, H.; Lau, B.L.T. Precipitation and growth of zinc sulfide nanoparticles in the presence of thiol-containing natural organic ligands. Environ. Sci. Technol. 2008, 42, 7236–7241. [Google Scholar]
- Deonarine, A.; Lau, B.L.T.; Aiken, G.R.; Ryan, J.N.; Hsu-Kim, H. Effects of humic substances on precipitation and aggregation of zinc sulfide nanoparticles. Environ. Sci. Technol. 2011, 45, 3217–3223. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.J.; Chappell, M.A.; Bednar, A.J.; Ryan, A.C.; Laird, J.G.; Stanley, J.K.; Steevens, A. Impact of organic carbon on the stability and toxicity of fresh and stored silver nanoparticles. Environ. Sci. Technol. 2012, 46, 10772–10780. [Google Scholar] [CrossRef] [PubMed]
- Hanikenne, M. Chlamydomonas reinhardtii as a eukaryotic photosynthetic model for studies of heavy metal homeostasis and tolerance. New Phytol. 2003, 159, 331–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, E.H. The Chlamydomonas Sourcebook: Introduction to Chlamydomonas and Its Laboratory Use, 2nd ed.; Academic Press: San Diego, CA, USA, 2009; p. 444. [Google Scholar]
- Nowack, B. Environmental chemistry of aminopolycarboxylate chelating agents. Environ. Sci. Technol. 2002, 36, 4009–4016. [Google Scholar] [CrossRef]
- Tardat-Henry, M.; Beaudry, J.P. Chimie Des Eaux., 2nd ed.; Le Griffon d’Argile: Sainte-Foy, QC, Canada, 1992. [Google Scholar]
- Wang, L.F.; Habibul, N.; He, D.Q.; Li, W.W.; Zhang, X.; Jiang, H.; Yu, H.Q. Copper release from copper nanoparticles in the presence of natural organic matter. Water Res. 2015, 68, 12–23. [Google Scholar] [CrossRef]
- Ward, T.J.; Rausina, G.A.; Stonebraker, P.M.; Robinson, W.E. Apparent toxicity resulting from the sequestering of nutrient trace metals during standard Selenastrum capricornutum toxicity tests. Aquat. Toxicol. 2002, 60, 1–16. [Google Scholar] [CrossRef]
- Sueoka, N.; Chiang, K.S.; Kates, J.R. Deoxyribonucleic acid replication in meiosis of Chlamydomonas reinhardtii. Isotopic transfer experiments with a strain producing eight zoospores. J. Mol. Biol. 1967, 25, 44–67. [Google Scholar] [CrossRef]
- Schecher, W.D.; McAvoy, D.C. MINEQL+: A Chemical Equilibrium Modeling System, Version 4.5 for Windows, User’s Manual, 2nd ed.; Environmental Research Software: Hallowel, ME, USA, 2003. [Google Scholar]
- Hull, M.; Kennedy, A.J.; Detzel, C.; Vikesland, P.; Chappell, M.A. Moving beyond mass: The unmet need to consider dose metrics in environmental nanotoxicology studies. Environ. Sci. Technol. 2012, 46, 10881–10882. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canuel, E.; Vaz, C.; Matias, W.G.; Dewez, D. Interaction Effect of EDTA, Salinity, and Oxide Nanoparticles on Alga Chlamydomonas reinhardtii and Chlamydomonas euryale. Plants 2021, 10, 2118. https://doi.org/10.3390/plants10102118
Canuel E, Vaz C, Matias WG, Dewez D. Interaction Effect of EDTA, Salinity, and Oxide Nanoparticles on Alga Chlamydomonas reinhardtii and Chlamydomonas euryale. Plants. 2021; 10(10):2118. https://doi.org/10.3390/plants10102118
Chicago/Turabian StyleCanuel, Emilie, Cleiton Vaz, William Gerson Matias, and David Dewez. 2021. "Interaction Effect of EDTA, Salinity, and Oxide Nanoparticles on Alga Chlamydomonas reinhardtii and Chlamydomonas euryale" Plants 10, no. 10: 2118. https://doi.org/10.3390/plants10102118
APA StyleCanuel, E., Vaz, C., Matias, W. G., & Dewez, D. (2021). Interaction Effect of EDTA, Salinity, and Oxide Nanoparticles on Alga Chlamydomonas reinhardtii and Chlamydomonas euryale. Plants, 10(10), 2118. https://doi.org/10.3390/plants10102118






