Periphytic Algae and Cyanobacteria from the Rio Doce Basin Respond Differently to Metals and Salinity, Showing Different Potential for Bioremediation
Abstract
:1. Introduction
2. Results
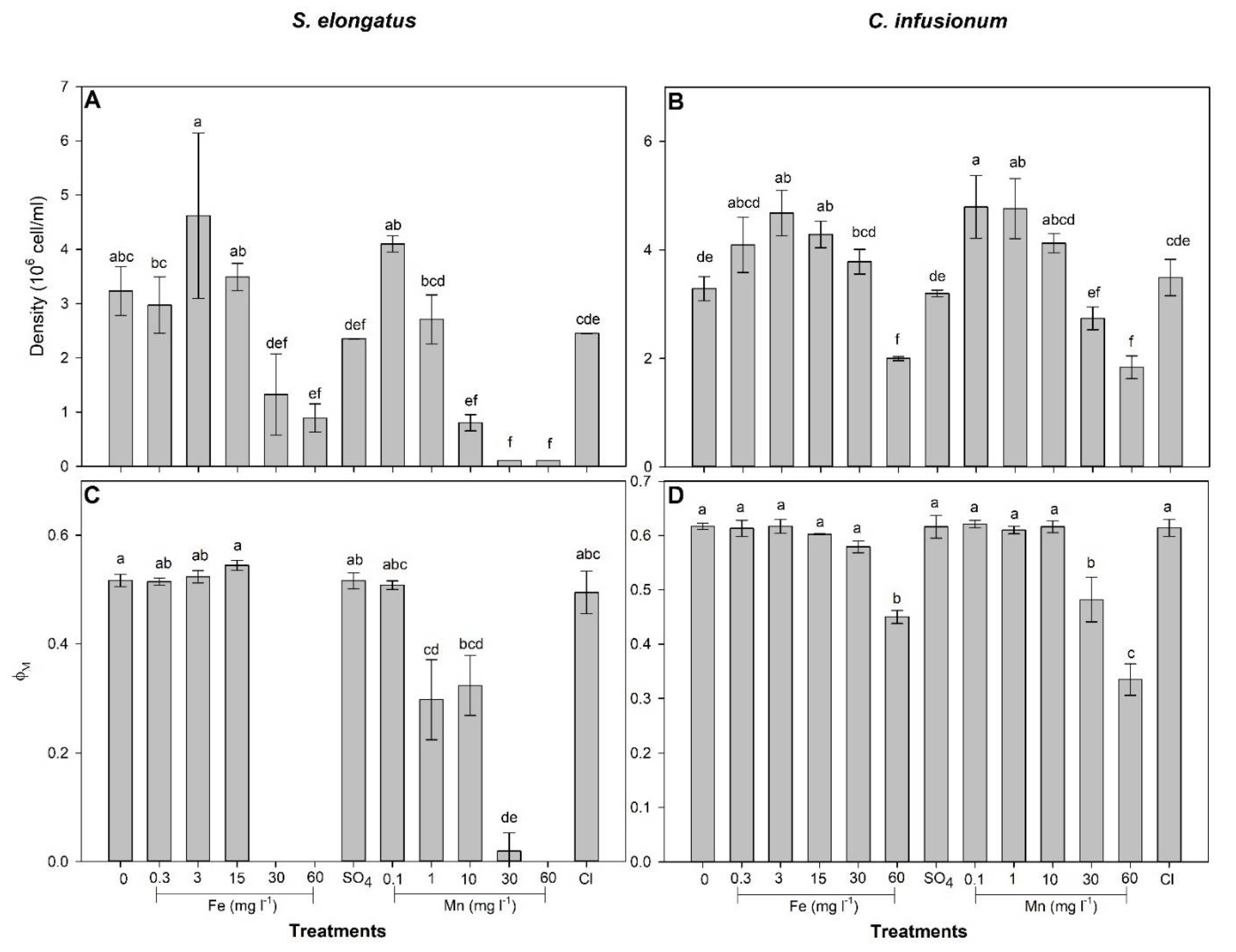
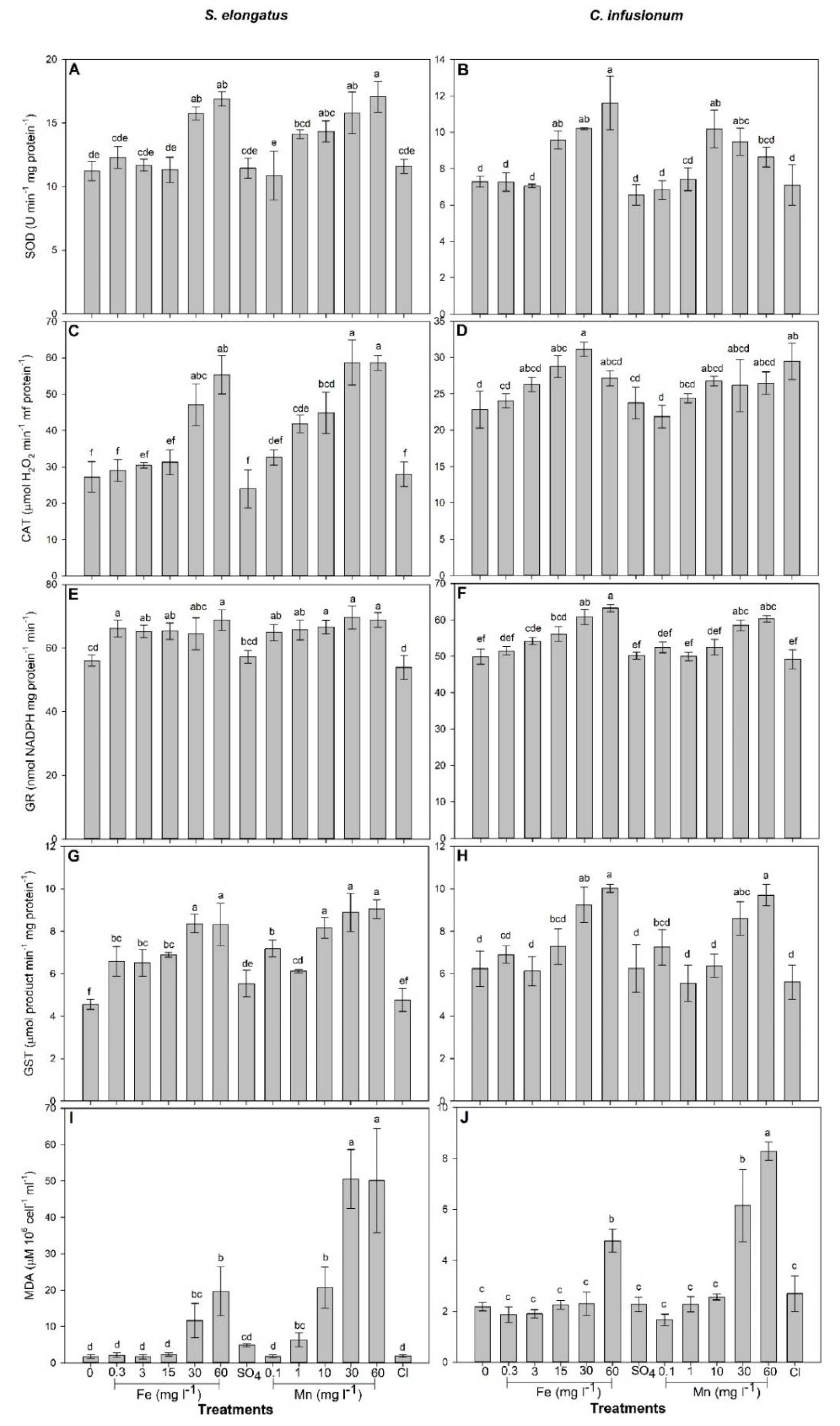
2.1. Mn and Fe Effects and Uptake in S. elongatus
2.2. Mn and Fe Effects and Uptake in C. infusionum
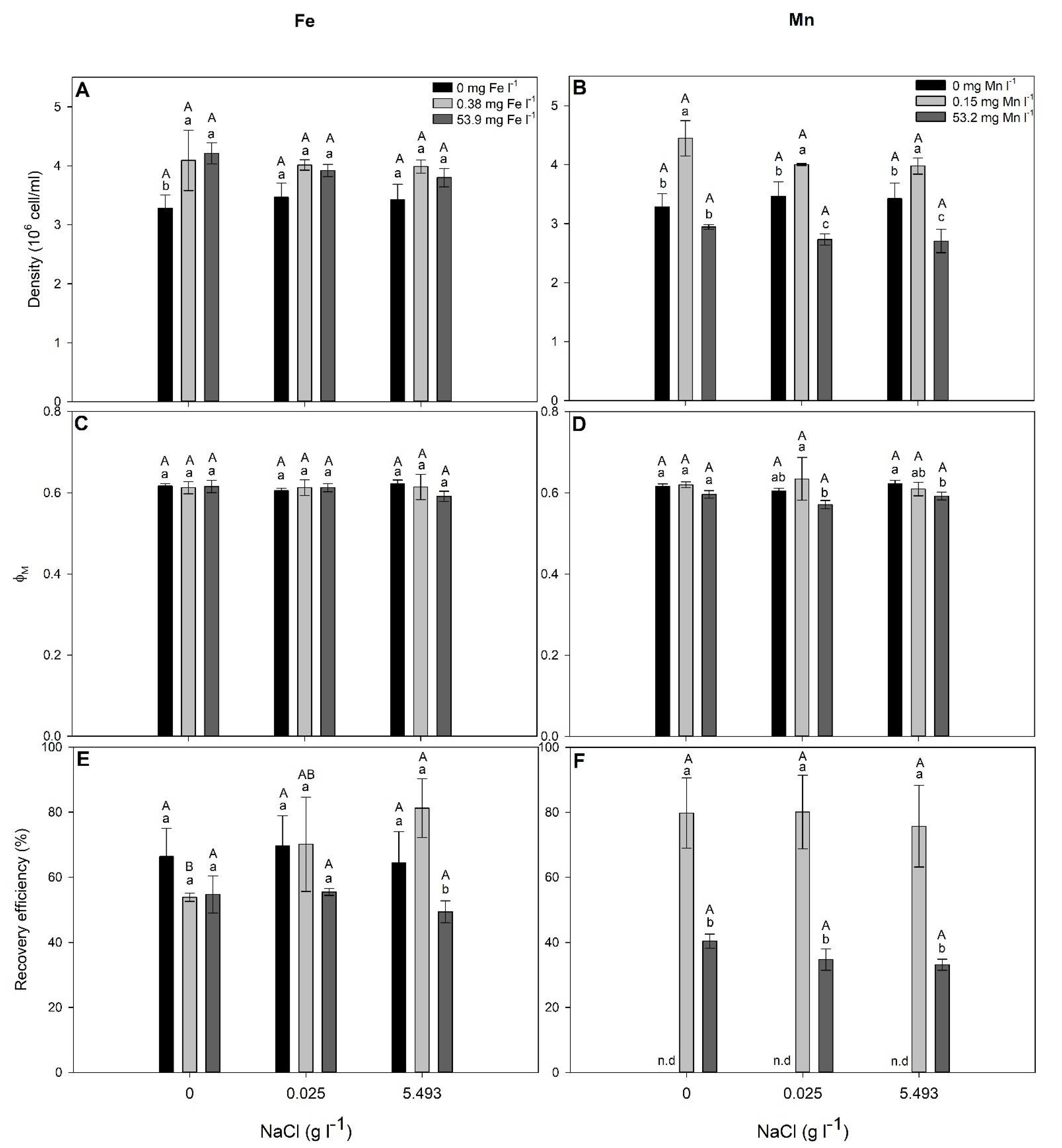
2.3. NaCl and Metal (Fe and Mn) Combined Effects on S. elongatus and C. infusionum
3. Discussion
4. Materials and Methods
4.1. Sampling and Collection
4.2. Mn and Fe Effects
4.3. NaCl and Metal (Fe and Mn) Combined Effects
4.4. Evaluation
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IBAMA. Laudo Técnico Preliminar; Diretoria de Proteção Ambiental-DIPRO, Ministério do Meio Ambiente: Brasília, Brazil, 2015.
- de Oliveira Gomes, L.E.; Correa, L.B.; Sá, F.; Neto, R.R.; Bernardino, A.F. The impacts of the Samarco mine tailing spill on the Rio Doce estuary, Eastern Brazil. Mar. Pollut. Bull. 2017, 120, 28–36. [Google Scholar] [CrossRef]
- IBAMA. Resumo das Análises Realizadas—Expedição Soloncy Moura; Instituto Chico Mendes de Conservação da Biodiversidade, Ministério do Meio Ambiente: Vitória, Brazil, 2016.
- de Carvalho, M.S.; Moreira, R.M.; Ribeiro, K.D.; de Almeida, A.M. Concentration of metals in the Doce river in Mariana, Minas Gerais, Brazil. Acta Bras. 2017, 1, 37–41. [Google Scholar] [CrossRef]
- CPRM. Monitoramento Especial da Bacia do rio Doce: Relatório 02—Geoquímica; Serviço Geológico do Brasil: Belo Horizonte, Brazil, 2015.
- Kaushal, S.S.; Likens, G.E.; Pace, M.L.; Utz, R.M.; Haq, S.; Gorman, J.; Grese, M. Freshwater salinization syndrome on a continental scale. Proc. Natl. Acad. Sci. USA 2018, 115, E574–E583. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Yao, J.; Wang, F.; Yuan, Z.; Liu, J.; Jordan, G.; Knudsen, T.Š.; Avdalović, J. Combined effects of antimony and sodium diethyldithiocarbamate on soil microbial activity and speciation change of heavy metals. Implications for contaminated lands hazardous material pollution in nonferrous metal mining areas. J. Hazard. Mater. 2018, 349, 160–167. [Google Scholar] [CrossRef]
- Santos, O.S.H.; Avellar, F.C.; Alves, M.; Trindade, R.C.; Menezes, M.B.; Ferreira, M.C.; França, G.S.; Cordeiro, J.; Sobreira, F.G.; Yoshida, I.M.; et al. Understanding the Environmental Impact of a Mine Dam Rupture in Brazil: Prospects for Remediation. J. Environ. Qual. 2019, 48, 439–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglesias, M.C.-A. A review of recent advances and future challenges in freshwater salinization. Limnetica 2020, 39, 185–211. [Google Scholar] [CrossRef]
- Gulzar, A.; Mehmood, M.A.; Chaudhary, R. Stream Periphyton community: A brief review on Ecological importance and Regulation. Int. J. Appl. Pure Sci. Agric. 2017, 3, 64–68. [Google Scholar] [CrossRef]
- Nagase, H.; Inthorn, D.; Oda, A.; Nishimura, J.; Kajiwara, Y.; Park, M.; Hirata, K.; Miyamoto, K. Improvement of selective removal of heavy metals in cyanobacteria by NaOH treatment. J. Biosci. Bioeng. 2005, 99, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.; Jetley, U.K.; Abash Khan, M.; Zutshi, S.; Fatma, T. Effect of heavy metal stress on proline, malondialdehyde, and superoxide dismutase activity in the cyanobacterium Spirulina platensis-S5. Ecotoxicol. Environ. Saf. 2007, 66, 204–209. [Google Scholar] [CrossRef]
- Moisander, P.H.; McClinton, E.; Paerl, H.W. Salinity effects on growth, photosynthetic parameters, and nitrogenase activity in estuarine planktonic cyanobacteria. Microb. Ecol. 2002, 43, 432–442. [Google Scholar] [CrossRef]
- Pilkaitytë, R.; Schoor, A.; Schubert, H. Response of phytoplankton communities to salinity changes—A mesocosm approach. Hydrobiologia 2004, 513, 27–38. [Google Scholar] [CrossRef]
- Pawlik-Skowrońska, B. When adapted to high zinc concentrations the periphytic green alga Stigeoclonium tenue produces high amounts of novel phytochelatin-related peptides. Aquat. Toxicol. 2003, 62, 155–163. [Google Scholar] [CrossRef]
- Duong, T.T.; Morin, S.; Herlory, O.; Feurtet-Mazel, A.; Coste, M.; Boudou, A. Seasonal effects of cadmium accumulation in periphytic diatom communities of freshwater biofilms. Aquat. Toxicol. 2008, 90, 19–28. [Google Scholar] [CrossRef]
- Adenan, N.S.; Yusoff, F.M.; Shariff, M. Effect of salinity and temperature on the growth of diatoms and green algae. J. Fish. Aquat. Sci. 2013, 8, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Upadhyay, A.K.; Chandra, P.; Singh, D.P. Sodium chloride incites reactive oxygen species in green algae Chlorococcum humicola and Chlorella vulgaris: Implication on lipid synthesis, mineral nutrients and antioxidant system. Bioresour. Technol. 2018, 270, 489–497. [Google Scholar] [CrossRef]
- Sood, A.; Renuka, N.; Prasanna, R.; Ahluwalia, A.S. Cyanobacteria as Potential Options for Wastewater Treatment BT. In Phytoremediation: Management of Environmental Contaminants, Volume 2; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 83–93. ISBN 978-3-319-10969-5. [Google Scholar]
- Ayya Raju, M. Impact of Heavy Metal Poisoning on Cyanobacterial Photosynthesis and Its Detoxification—A Review. Innoriginal Int. J. Sci. 2016, 3, 18–23. [Google Scholar]
- Morrissey, J.; Bowler, C. Iron utilization in marine cyanobacteria and eukaryotic algae. Front. Microbiol. 2012, 3, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kosakowska, A.; Nedzi, M.; Pempkowiak, J. Responses of the toxic cyanobacterium Microcystis aeruginosa to iron and humic substances. Plant Physiol. Biochem. 2007, 45, 365–370. [Google Scholar] [CrossRef]
- Orihel, D.M.; Schindler, D.W.; Ballard, N.C.; Wilson, L.R.; Vinebrooke, R.D. Experimental iron amendment suppresses toxic cyanobacteria in a hypereutrophic lake. Ecol. Appl. 2016, 26, 1517–1534. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Kranzler, C.; Rudolf, M.; Keren, N.; Schleif, E. Iron in cyanobacteria. In Advances in Botanical Research: Genomics of Cyanobacteria; Chauvat, F., Cassier-Chauvat, C., Eds.; Academic Press: Amsterdam, The Netherlands, 2013; pp. 57–105. [Google Scholar]
- Du, J.; Qiu, B.; Gomes, M.P.; Juneau, P.; Dai, G. Influence of light intensity on cadmium uptake and toxicity in the cyanobacteria Synechocystis sp. PCC6803. Aquat. Toxicol. 2019, 211, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Juneau, P. Different physiological and photosynthetic responses of three cyanobacterial strains to light and zinc. Aquat. Toxicol. 2016, 170, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Renova, F. Monitoramento Rio Doce; Fundação Renova: Belo Horizonte, Brazil, 2017. [Google Scholar]
- Andersson, B.; Godhe, A.; Filipsson, H.L.; Rengefors, K.; Berglund, O. Differences in metal tolerance among strains, populations, and species of marine diatoms—Importance of exponential growth for quantification. Aquat. Toxicol. 2020, 226, 105551. [Google Scholar] [CrossRef]
- Kalinowska, R.; Pawlik-Srowronska, B. Metal resistance of soil algae (Chlorophyta) occurring in post-flotation Zn/Pb- and Cu- tailing ponds. Pol. J. Ecol. 2008, 56, 415–430. [Google Scholar]
- Priyadarshini, E.; Priyadarshini, S.S.; Pradhan, N. Heavy metal resistance in algae and its application for metal nanoparticle synthesis. Appl. Microbiol. Biotechnol. 2019, 103, 3297–3316. [Google Scholar] [CrossRef] [PubMed]
- Rodgher, S.; Espíndola, E.L.G.; Simões, F.C.F.; Tonietto, A.E. Cadmium and Chrominum toxicity to Pseudokirchneriella subcapitata and Microcystis aeruginosa. Brazilian Arch. Biol. Technol. 2012, 55, 161–169. [Google Scholar] [CrossRef]
- Takamura, N.; Kasai, F.; Watanabe, M. Effects of Cu, Cd and Zn on photosysnthesis of freshwater benthic algae. J. Appl. Phycol. 1989, 1, 39–52. [Google Scholar] [CrossRef]
- Miao, A.-J.; Wang, W.-X.; Juneau, P. Comparison of Cd, Cu, and Zn toxic effects on four marine phytoplankton by pulse-amplitude-modulated fluorometry. Environ. Toxicol. Chem. 2005, 24, 2603. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Yun, Y. Biosorption of cadmium by various types of dried sludge: An equilibrium study and investigation of mechanisms. J. Hazard. Mater. 2006, 138, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Cutt, B.; Lawrence, R. Using intrinsic data skew to improve hash join performance. Inf. Syst. 2009, 34, 493–510. [Google Scholar] [CrossRef]
- Liu, J.; Tan, K.; He, L.; Qiu, Y.; Tan, W.; Guo, Y.; Wang, Z.; Sun, W. Effect of limitation of iron and manganese on microalgae growth in fresh water. Microbiology 2018, 164, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Cañedo-Argüelles, M.; Kefford, B.J.; Piscart, C.; Prat, N.; Schäfer, R.B.; Schulz, C.-J. Salinisation of rivers: An urgent ecological issue. Environ. Pollut. 2013, 173, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Figler, A.; B-Béres, V.; Dobronoki, D.; Márton, K.; Nagy, S.A.; Bácsi, I. Salt Tolerance and Desalination Abilities of Nine Common Green Microalgae Isolates. Water 2019, 11, 2527. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, K.; Ladas, N.P.; Alygizaki-Zorba, A.; Papageorgiou, G.C. Sodium Chloride-Induced Volume Changes of Freshwater Cyanobacterium Synechococcus sp. PCC 7942 Cells Can Be Probed by Chlorophyll a Fluorescence. Arch. Biochem. Biophys. 1999, 370, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Deshnium, P.; Los, D.A.; Hayashi, H.; Mustardy, L.; Murata, N. Transformation of Synechococcus with a gene for choline oxidase enhances tolerance to salt stress. Plant Mol. Biol. 1995, 29, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.A.; Kawachi, K. Traditional microalgae isolation techniques. In Algal Culturing Techniques; Andersen, R.A., Ed.; Academic Press: New York, NY, USA, 2005; pp. 83–100. [Google Scholar]
- CONAMA. RESOLUÇÃO CONAMA N° 430/2011; Ministério do Meio Ambiente: Brasília, Brazil, 2011.
- Solley, W.B.; Pierce, R.R.; Pelman, H.A. Estimated Use of Water in the United States in 1995; U.S. Geological Survey Circular: Washington, DC, USA, 1998; ISBN 0-607-90075-X.
- Novák, Z.; Harangi, S.; Baranyai, E.; Gonda, S.; B-Béres, V.; Bácsi, I. Effects of metal quantity and quality to the removal of zinc and copper by two common green microalgae (Chlorophyceae) species. Phycol. Res. 2020, 68, 227–235. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Garcin, C.; van Hille, R.P.; Harrison, S.T.L. Interference by pigment in the estimation of microalgal biomass concentration by optical density. J. Microbiol. Methods 2011, 83, 119–123. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Campbell, D.; Hurry, V.; Clarke, A.K.; Gustafsson, P.; Öquist, G. Chlorophyll Fluorescence Analysis of Cyanobacterial Photosynthesis and Acclimation. Microbiol. Mol. Biol. Rev. 1998, 62, 667–683. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Halliwell, B.; Foywe, C. Properties and physical function of a glutathione reductase purified from spinach leaves by affinity chromatography. Planta 1978, 139, 9–17. [Google Scholar] [CrossRef]
- Habig, W.; Pabst, M.; Jakiby, W. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 1, 604–611. [Google Scholar] [CrossRef]
- van der Heever, J.A.; Grobbelaar, J.U. The use of Selenastrum capricornutum growth potential as a measure of toxicity of a few selected compounds. Water SA 1996, 22, 183–191. [Google Scholar]

| . | Fe (mg L−1) | |||||
|---|---|---|---|---|---|---|
| 0 | 0.3 | 3 | 15 | 30 | 60 | |
| Ci | 8.2 × 10−4 ± 0.4 × 10−4 f | 0.31 ± 0.02 e | 3.12 ± 0.32 d | 15.01 ± 0.10 c | 31.25 ± 1.16 b | 60.01 ± 1.75 a |
| Synechococcus elongatus | ||||||
| TR | 0.00 ± 0.00 f | 0.13 ± 0.00 e | 1.09 ± 0.17 d | 4.92 ± 0.67c | 16.87 ± 0.83b | 21.85 ± 1.72 a |
| Cextra | 0.03 ± 0.00 d | 15.86 ± 4.65 cd | 90.36 ± 18.17 bc | 474.90 ± 250.64 b | 5974.99 ± 2925.35 a | 9811.92 ± 3940.66 a |
| Cintra | 0.08 ± 0.00 d | 29.64 ± 6.55 cd | 159.14 ± 49.99 bc | 967.28 ± 155.97 b | 9196.76 ± 3632.58 a | 16592.16 ± 6097.36 a |
| RE | 46.1 ± 4.5 ab | 42.1 ± 2.7 bc | 35.1 ± 5.6 c | 32.8 ± 4.4 d | 56.1 ± 2.7 a | 36.4 ± 2.8 bc |
| Chlorococcum infusionum | ||||||
| TR | 0.00 ± 0.00 f | 0.16 ± 0.00 e | 1.39 ± 0.11 d | 8.65 ± 1.20 c | 21.97 ± 0.87 b | 33.33 ± 2.50 a |
| Cextra | 0.05 ± 0.00 f | 14.37 ± 2.21 e | 109.58 ± 6.69 d | 1124.67 ± 270.27 c | 3629.25 ± 240.24 b | 10673.54 ± 1068.90 a |
| Cintra | 0.11 ± 0.02 f | 27.14 ± 4.30 e | 189.39 ± 9.01 d | 905.70 ± 100.47 c | 2201.31 ± 278.58 b | 5989.91 ± 641.00 a |
| RE | 66.3 ± 8.6 ab | 53.7 ± 1.2 bc | 44.7 ± 3.6 c | 57.6 ± 8.0 bc | 73.2 ± 2.9 a | 55.5 ± 4.1 bc |
| Mn (mg L−1) | ||||||
|---|---|---|---|---|---|---|
| 0 | 0.1 | 1 | 10 | 30 | 60 | |
| Ci | n.d | 0.11 ± 0.00 e | 1.02 ± 0.23 d | 10.06 ± 0.39 c | 30.06 ± 0.33 b | 60.02 ± 0.13 a |
| Synechococcus elongatus | ||||||
| TR | - | 0.08 ± 0.00 c | 0.76 ± 0.18 bc | 4.01 ± 0.25 a | 2.42 ± 1.56 ab | 2.15 ± 0.57 ab |
| Cextra | - | 6.97 ± 0.69 c | 102.60 ± 13.90 bc | 1394.39 ± 140.95 b | 8557.21 ± 5786.71 a | 5005.08 ± 1672.92 a |
| Cintra | - | 14.82 ± 1.46 c | 179.57 ± 40.97 bc | 3712.49 ± 875.41 ab | 1293.15 ± 8082.12 a | 14,067.02 ± 3480.82 a |
| RE | - | 79.2 ± 5.8 a | 65.7 ± 7.9 b | 46.7 ± 2.6 c | 2.3 ± 0.2 d | 1.1 ± 0.1 e |
| Chlorococcum infusionum | ||||||
| TR | - | 0.08 ± 0.00 e | 0.90 ± 0.05 d | 5.38 ± 0.22 c | 14.11 ± 1.64 b | 24.90 ± 1.20 a |
| Cextra | - | 6.84 ± 1.26 e | 73.69 ± 9.72 d | 751.05 ± 65.43 c | 3485.03 ± 789.47 b | 8831.65 ± 435.99 a |
| Cintra | - | 10.06 ± 1.19 e | 119.12 ± 21.25 d | 557.52 ± 13.13 c | 1717.64 ± 201.72 b | 4778.58 ± 692.02 a |
| RE | - | 72.5 ± 4.6 bc | 88.4 ± 4.3 a | 53.5 ± 2.4 bc | 46.9 ± 5.4 cd | 41.4 ± 2.0 d |
| Anova F Values | D.F | Cell Density | ΦM | RE | |||
|---|---|---|---|---|---|---|---|
| S. elongatus | C. infusionum | S. elongatus | C. infusionum | S. elongatus | C. infusionum | ||
| Fe | 2 | 8.11 ** | 19.46 *** | 13.14 *** | 0.69 | 28.53 *** | 13.20 *** |
| NaCl | 2 | 1.90 | 0.60 | 1.07 | 0.33 | 0.06 | 2.13 |
| Fe × NaCl | 4 | 0.87 | 1.15 | 4.11 * | 1.40 | 3.85 * | 4.60 * |
| Comparison of means $ | |||||||
| Fe (mg L−1) | |||||||
| 0 | 2.91 a | 3.39 b | 0.54 a | 0.61 | 43.57 a | 66.75 a | |
| 0.38 | 2.95 a | 4.03 a | 0.52 b | 0.61 | 44.95 a | 68.37 a | |
| EC10 $$ | 2.47 b | 3.97 a | 0.42 b | 0.60 | 28.03 b | 53.14 b | |
| NaCl (g L−1) | |||||||
| 0 | 3.03 | 3.86 | 0.53 | 0.61 | 40.85 | 58.26 | |
| 0.025 | 2.72 | 3.80 | 0.52 | 0.61 | 35.27 | 65.04 | |
| 5.493 | 2.58 | 3.73 | 0.44 | 0.60 | 40.41 | 64.97 | |
| Mn | 32.33 *** | 110.58 *** | 19.51 *** | 17.95 *** | 0.20 | 59.64 *** | |
| NaCl | 7.58 ** | 2.52 | 0.07 | 3.56 | 0.70 | 2.48 | |
| Mn × NaCl | 0.27 | 2.63 | 0.15 | 1.29 | 0.81 | 1.33 | |
| Comparison of means $ | |||||||
| Mn (mg L−1) | |||||||
| 0 | 2.91 b | 17.88 a | 0.54 a | 0.61 a | - | - | |
| 0.3 | 3.62 a | 18.22 a | 0.54 a | 0.62 a | 61.72 | 78.50 a | |
| EC1 0 $$ | 2.47 c | 5.88 b | 0.27 b | 0.58 b | 58.05 | 36.10 b | |
| NaCl (g L−1) | |||||||
| 0 | 3.30 a | 16.33 | 0.46 | 0.61 | 42.99 | 60.07 | |
| 0.025 | 2.94 ab | 10.44 | 0.45 | 0.60 | 41.24 | 57.41 | |
| 5.493 | 2.75 b | 15.22 | 0.45 | 0.60 | 35.53 | 54.41 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, M.P.; Kochi, L.Y.; Freitas, P.L.; Figueredo, C.C.; Juneau, P. Periphytic Algae and Cyanobacteria from the Rio Doce Basin Respond Differently to Metals and Salinity, Showing Different Potential for Bioremediation. Plants 2021, 10, 2349. https://doi.org/10.3390/plants10112349
Gomes MP, Kochi LY, Freitas PL, Figueredo CC, Juneau P. Periphytic Algae and Cyanobacteria from the Rio Doce Basin Respond Differently to Metals and Salinity, Showing Different Potential for Bioremediation. Plants. 2021; 10(11):2349. https://doi.org/10.3390/plants10112349
Chicago/Turabian StyleGomes, Marcelo Pedrosa, Letícia Yoshie Kochi, Patrícia Lawane Freitas, Cleber Cunha Figueredo, and Philippe Juneau. 2021. "Periphytic Algae and Cyanobacteria from the Rio Doce Basin Respond Differently to Metals and Salinity, Showing Different Potential for Bioremediation" Plants 10, no. 11: 2349. https://doi.org/10.3390/plants10112349
APA StyleGomes, M. P., Kochi, L. Y., Freitas, P. L., Figueredo, C. C., & Juneau, P. (2021). Periphytic Algae and Cyanobacteria from the Rio Doce Basin Respond Differently to Metals and Salinity, Showing Different Potential for Bioremediation. Plants, 10(11), 2349. https://doi.org/10.3390/plants10112349








