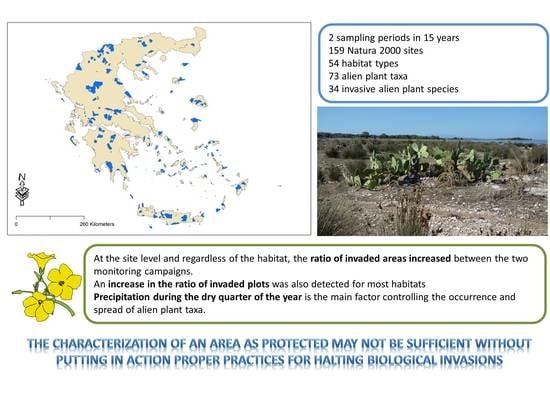
How Effective Are the Protected Areas of the Natura 2000 Network in Halting Biological Invasions? A Case Study in Greece
Abstract
:1. Introduction
2. Results
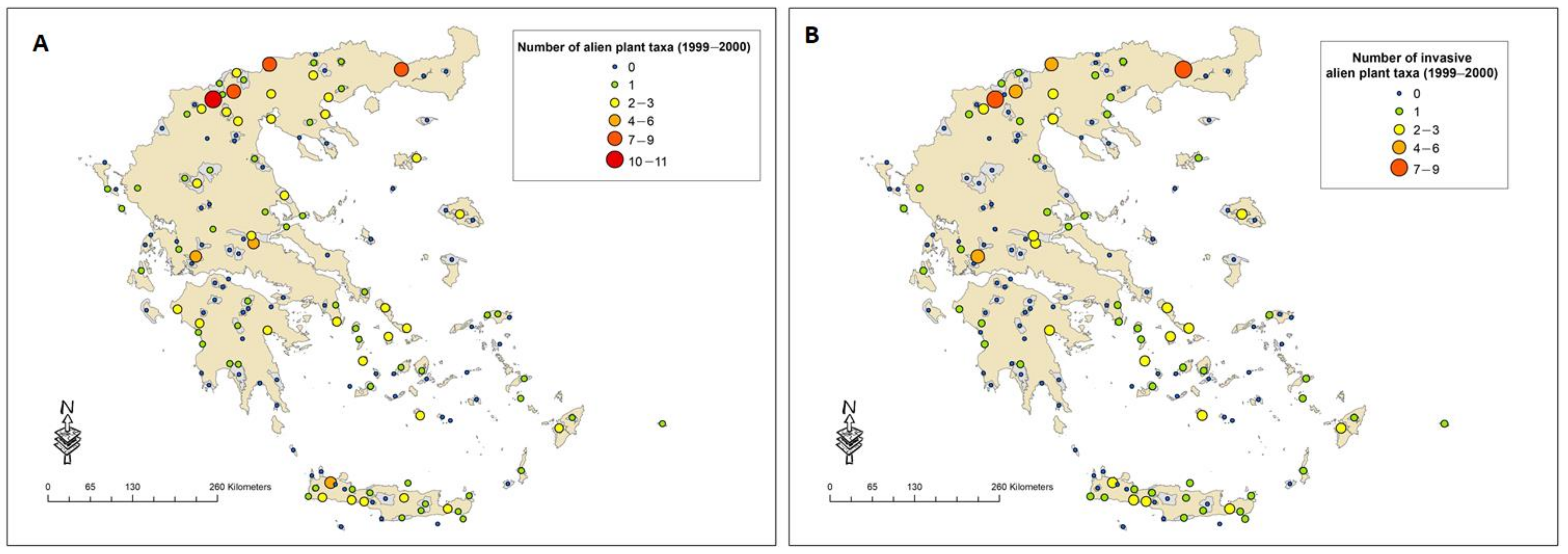
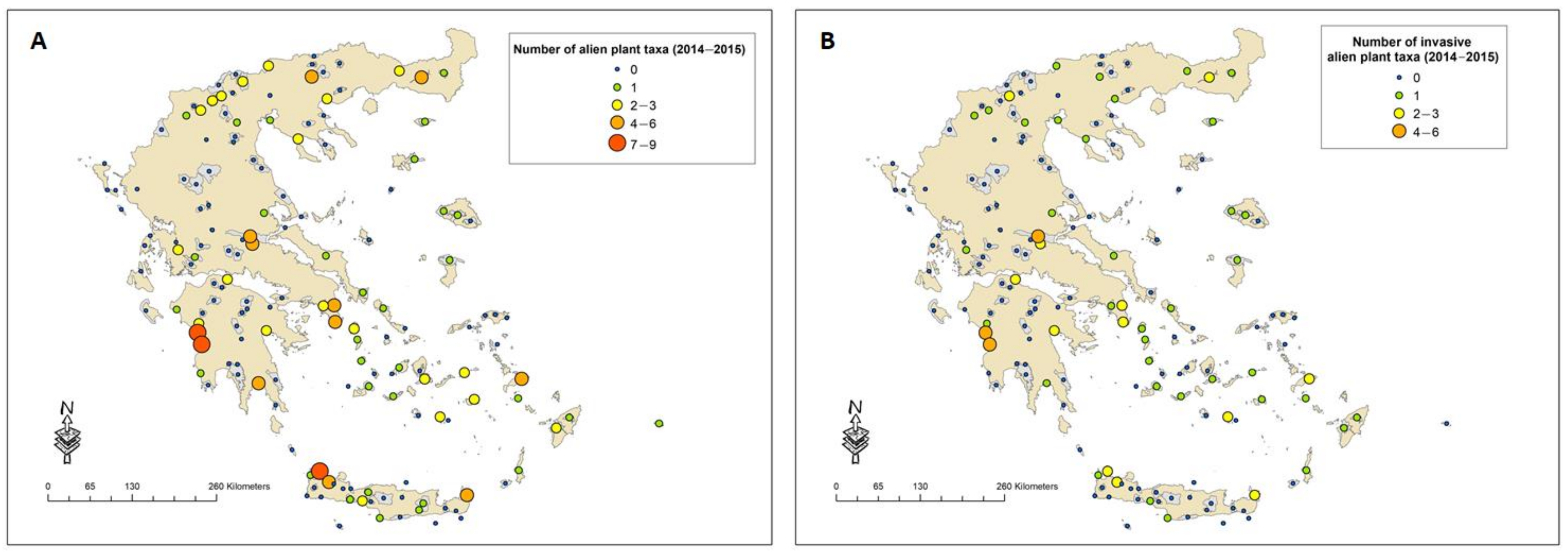
2.1. The Most Frequently Recorded Alien Plant Taxa among PAs During the Last 15 Years and Their Distribution
2.2. Spread of Alien Plant Taxa in New Sites and/or New Habitats 15 Years after the First National Monitoring Field Campaign
2.3. Factors Controlling the Occurrence and Spread of Alien Plant Taxa in the PAs
3. Discussion
4. Materials and Methods
4.1. Study Sites
4.2. Data Preparation
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millennium Ecosystem Assessment. In Ecosystems and Human Well-Being: Scenarios; Island Press: Washington, DC, USA, 2005.
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Tylianakis, J.M.; Didham, R.K.; Bascompte, J.; Wardle, D.A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 2008, 11, 1351–1363. [Google Scholar] [CrossRef]
- Scalera, R.; Genovesi, P.; Essl, F.; Rabitsch, W. The Impacts of Invasive Alien Species in Europe. EEA Technical Report/ No 16/2012; European Environment Agency: Copenhagen, Denmark, 2012. [Google Scholar]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; De Vries, W.; De Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naughton-Treves, L.M.; Holland, B.; Brandon, K. The Role of Protected Areas in Conserving Biodiversity and Sustaining Local Livelihoods. Annu. Rev. Environ. Resour. 2005, 30, 219–252. [Google Scholar] [CrossRef] [Green Version]
- Pyšek, P.; Richardson, D.M. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef] [Green Version]
- Rands, M.R.W.; Adams, W.M.; Bennun, L.; Butchart, S.H.M.; Clements, A.; Coomes, D.; Entwistle, A.; Hodge, I.; Kapos, V.; Scharlemann, J.P.W.; et al. Biodiversity conservation: Challenges beyond 2010. Science 2010, 329, 1298–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shackleton, R.T.; Foxcroft, L.C.; Pyšek, P.; Wood, L.E. Assessing biological invasions in protected areas after 30 years: Revisiting nature reserves targeted by the 1980s SCOPE programme. Biol. Conserv. 2020, 243, 108424. [Google Scholar] [CrossRef]
- Barber, C.V.; Miller, K.R.; Boness, M. Securing Protected Areas in the Face of Global Change: Issues and Strategies; IUCN: Gland/Cambridge, UK, 2004; pp. 1–236. [Google Scholar]
- Bomanowska, A.; Adamowski, W.; Kirpluk, I.; Otręba, A.; Rewicz, A. Invasive alien plants in Polish national parks—Threats to species diversity. Peer J. 2019, 7, e8034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudley, N.; Belokurov, A.; Borodin, O.; Higgins-Zogib, L.; Hockings, M.; Lacerda, L.; Stolton, S. How Effective Are Protected Areas? WWF International: Gland, Switzerland, 2004. [Google Scholar]
- Laurance, W.F.; Useche, D.C.; Rendeiro, J.; Kalka, M.; Bradshaw, C.J.; Sloan, S.P.; Laurance, S.G.; Campbell, M.; Abernethy, K.; Alvarez, P.; et al. Averting biodiversity collapse in tropical forest protected areas. Nature 2012, 489, 290. [Google Scholar] [CrossRef] [Green Version]
- Pyšek, P.; Genovesi, P.; Pergl, J.; Monaco, A.; Wild, J. Plant invasions of protected areas in Europe: An old continent facing new problems. In Plant Invasions in Protected Areas: Patterns, Problems and Challenges; Foxcroft, L.C., Pyšek, P., Richardson, D.M., Genovesi, P., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 209–240. [Google Scholar]
- Schulze, K.; Knights, K.; Coad, L.; Geldmann, J.; Leverington, F.; Eassom, A.; Marr, M.; Butchart, S.H.M.; Hockings, M.; Burgess, N.D. An assessment of threats to terrestrial protected areas. Conserv. Lett. 2018, 11, e12435. [Google Scholar] [CrossRef] [Green Version]
- Velazco, S.J.E.; Villalobos, F.; Galvão, F.; De Marco Júnior, P. A dark scenario for Cerrado plant species: Effects of future climate, land use and protected areas ineffectiveness. Divers. Distrib. 2019, 25, 660–673. [Google Scholar] [CrossRef]
- Pejchar, L.; Mooney, H.A. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; Garcia-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Winter, M.; Schweiger, O.; Klotz, S.; Nentwig, W.; Andriopoulos, P.; Arianoutsou, M.; Basnou, C.; Delipetrou, P.; Didžiulis, V.; Hejda, M.; et al. Plant extinctions and introductions lead to phylogenetic and taxonomic homogenization of the European flora. Proc. Natl. Acad. Sci. USA 2009, 106, 21721–21725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeschke, J.M.; Bacher, S.; Blackburn, T.M.; Dick, J.T.; Essl, F.; Evans, T.; Gaertner, M.; Hulme, P.E.; Kuhn, I.; Mrugała, A.; et al. Defining the impact of non-native species. Biol. Conserv. 2014, 28, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.; Penone, C.; Pennino, M.G.; Courchamp, F. Predicting future invaders and future invasions. Proc. Natl. Acad. Sci. USA 2019, 116, 7905–7910. [Google Scholar] [CrossRef] [Green Version]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Guèze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; et al. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019; p. 56.
- Kumar Rai, P.; Singh, J.S. Invasive alien plant species: Their impact on environment, ecosystem services and human health. Ecol. Indic. 2020, 111, 10062020. [Google Scholar] [CrossRef]
- Arianoutsou, M.; Delipetrou, P.; Vilà, M.; Dimitrakopoulos, P.G.; Celesti-Grapow, L.; Wardell-Johnson, G.; Henderson, L.; Fuentes, N.; Ugarte-Mendes, E.; Rundel, P.W. Comparative Patterns of Plant Invasions in the Mediterranean Biome. PLoS ONE 2013, 8, e79174. [Google Scholar] [CrossRef] [Green Version]
- Pyšek, P.; Jarošík, V.; Kučera, T. Patterns of invasion in temperate nature reserves. Biol. Conserv. 2002, 104, 13–24. [Google Scholar] [CrossRef]
- Celesti-Grapow, L.; Alessandrini, A.; Arrigoni, P.V.; Assini, S.; Banfi, E.; Bernardo, L.; Bovio, M.; Brundu, G.; Conti, F.; Galasso, G.; et al. The inventory of the non-native flora of Italy. Plant Biosyst. 2019, 143, 386–430. [Google Scholar] [CrossRef]
- Carboni, M.; Thuiller, W.; Izzi, F.; Acosta, A. Disentangling the relative effects of environmental versus human factors on the abundance of native and alien plant species in Mediterranean sandy shores. Divers. Distrib. 2010, 16, 537–546. [Google Scholar] [CrossRef]
- Lockwood, M. Global protected area framework. In Managing Protected Areas: A Global Guide; Lockwood, M., Worboys, G.L., Kothari, A., Eds.; Earthscan: London, UK, 2006; pp. 73–100. [Google Scholar]
- Gaston, K.J.; Jackson, S.F.; Nagy, A.; Cantú-Salazar, L.; Johnson, M. Protected areas in Europe: Principle and practice. Ann. N. Y. Acad. Sci. 2008, 1134, 97–119. [Google Scholar] [CrossRef]
- Hulme, P.E.; Pyšek, P.; Pergl, J.; Jarošík, V.; Schaffner, U.; Vilà, M. Greater focus needed on alien plant impacts in protected areas. Conserv. Lett. 2014, 7, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Perzanowska, J.; Korzeniak, J.; Chmura, D. Alien species as a potential threat for Natura 2000 habitats: A national survey. Peer J. 2019, 7, e8032. [Google Scholar] [CrossRef] [Green Version]
- Braun, M.; Schindler, S.; Essl, F. Distribution and management of invasive alien plant species in protected areas in Central Europe. J. Nat. Conserv. 2016, 33, 48–57. [Google Scholar] [CrossRef]
- Dimitrakopoulos, P.G.; Koukoulas, S.; Galanidis, A.; Delipetrou, P.; Gounaridis, D.; Touloumi, K.; Arianoutsou, M. Factors shaping alien plant species richness spatial patterns across Natura 2000 Special Areas of Conservation of Greece. Sci. Total Environ. 2017, 601, 461–468. [Google Scholar] [CrossRef]
- Landi, S.; Tordoni, E.; Amici, V.; Bacaro, G.; Carboni, M.; Filibeck, G.; Scoppola, A.; Bagella, S. Contrasting patterns of native and non-native plants in a network of protected areas across spatial scales. Biodivers. Conserv. 2020, 29, 2035–2053. [Google Scholar] [CrossRef]
- Bacchetta, G.; Berlanga, O.M.G.; Podda, L. Catálogo de la flora exótica de La Isla De Cerdeña (Italia). Flora Montiberica 2009, 41, 35–61. [Google Scholar]
- Pyšek, P.; Bacher, S.; Chytrý, M.; Jarošík, V.; Wild, J.; Celesti-Grapow, L.; Gassó, N.; Kenis, M.; Lambdon, Ρ.W.; Nentwig, W.; et al. Contrasting patterns in the invasions of European terrestrial and freshwater habitats by alien plants, insects and vertebrates. Glob. Ecol. Biogeogr. 2010, 19, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Randall, J.M. Protected areas. In Encyclopedia of Biological Invasions; Simberloff, D., Rejmánek, M., Eds.; University of California Press: Berkley, CA, USA, 2011; pp. 563–567. [Google Scholar]
- Nunes, A.N.; Tricarico, E.; Panov, V.E.; Cardoso, A.C.; Katsanevakis, S. Pathways and gateways of freshwater invasions in Europe. Aquat. Invasions 2015, 10, 359–370. [Google Scholar] [CrossRef] [Green Version]
- Pauchard, A.; Fuentes, N.; Jiménez, A.; Bustamante, R.; Marticorena, A. Alien plants homogenise protected areas: Evidence from the landscape and regional scales in south central Chile. In Plant Invasions in Protected Areas; Foxcroft, L.C., Pyšek, P., Richardson, D.M., Genovesi, P., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 191–208. [Google Scholar]
- Souza, L.; Bunn, W.A.; Simberloff, D.; Lawton, R.M.; Sanders, N.J. Biotic and abiotic influences on native and exotic richness relationship across spatial scales: Favourable environments for native species are highly invasible. Funct. Ecol. 2011, 25, 1106–1112. [Google Scholar] [CrossRef]
- Pauchard, A.; Alaback, P.B. Influence of elevation, land use, and landscape context on patterns of alien plant invasions along roadsides in protected areas of South-Central Chile. Conserv. Biol. 2004, 18, 238–248. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Bazos, I.; Kokkoris, I.; Zografidis, A.; Karadimou, E.; Kallimanis, A.; Raus, T.; Strid, A. Guide to Alien Plant. Species in Greece and the Natura 2000 Network of Protected Areas; The Natural Environment and Climate Change Agency (NECCA) and University of Patras: Athens, Greece, 2020; p. 112. (In Greek) [Google Scholar]
- Pyšek, P.; Richardson, D.M.; Rejmánek, M.; Webster, G.; Williamson, M.; Kirschner, J. Alien plants in checklists and floras: Towards better communication between taxonomists and ecologists. Taxon 2004, 53, 131–143. [Google Scholar] [CrossRef]
- Bazos, I.; Kokkoris, I.; Zikos, A.; Andriopoulos, P.; Dedipetrou, P.; Georghiou, K.; Yannitsaros, A.; Arianoutsou, M. The alien vascular flora of Greece: Floristic analysis and chorology. Bocconea 2009, 23, 281–284. [Google Scholar]
- Arianoutsou, M.; Bazos, I.; Delipetrou, P.; Kokkoris, Y. The alien flora of Greece: Taxonomy, life traits and habitat preferences. Biol. Invasions 2010a, 12, 3525–3549. [Google Scholar] [CrossRef]
- Krigas, N.; Tsiafouli, M.A.; Katsoulis, G.; Votsi, N.E.; van Kleunen, M. Investigating the Invasion Pattern of the Alien Plant Solanum elaeagnifolium Cav. (Silverleaf Nightshade): Environmental and Human-Induced Drivers. Plants 2021, 10, 805. [Google Scholar] [CrossRef]
- Hulme, P.H.; Brundu, G.; Camarda, I.; Dalias, P.; Lambdon, P.; Lloret, F.; Medail, F.; Moragues, E.; Suehs, C.; Traveset, A.; et al. Assessing the risks to Mediterranean islands ecosystems from alien plant invasions. In Plant Invasions: Human perception, Ecological Impacts and Management; Tokarska, B., Brock, G.J.H., Brundu, G., Child, L., Daehler, C.C., Pyšek, P., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2008; pp. 39–56. [Google Scholar]
- Vilà, M.; Bartomeus, I.; Dietzsch, A.C.; Petanidou, T.; Ingolf Steffan-Dewenter, I.; Jane, C.; Stout, J.C.; Tscheulin, T. Invasive plant integration into native plant–pollinator networks across Europe. Proc. R. Soc. B 2009, 276, 3887–3893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vimercati, G.; Kumschick, S.; Probert, A.F.; Volery, L.; Bacher, S. The importance of assessing positive and beneficial impacts of alien species. In Frameworks Used in Invasion Science; Wilson, J.R., Bacher, S., Daehler, C.C., Groom, Q.J., Kumschick, S., Lockwood, J.L., Robinson, T.B., Zengeya, T.A., Richardson, D.M., Eds.; NeoBiota: Sofia, Bulgaria, 2020; Volume 62, pp. 525–545. [Google Scholar] [CrossRef]
- Evans, D. Building the European Union’s Natura 2000 network. Nat. Conserv. 2012, 1, 11. [Google Scholar] [CrossRef]
- European Environment Agency. The Natura 2000 Protected Areas Network. Available online: https://www.eea.europa.eu/themes/biodiversity/natura-2000/the-natura-2000-protected-areas-network (accessed on 3 June 2021).
- Pyšek, P.; Sádlo, J.; Mandák, B. Catalogue of alien plants of the Czech Republic. Preslia 2002, 74, 97–186. [Google Scholar]
- Foxcroft, L.C.; Jarošík, V.; Pyšek, P.; Richardson, D.M.; Rouget, M. Protected-area boundaries as filters of plant invasions. Conserv. Biol. 2011, 25, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Lambdon, P.W.; Pyšek, P.; Basnou, C.; Hejda, M.; Arianoutsou, M.; Essl, F.; Jarošík, V.; Pergl, J.; Winter, M.; Anastasiu, P.; et al. Alien flora of Europe: Species diversity, temporal trends, geographical patterns and research needs. Preslia 2008, 80, 101–149. [Google Scholar]
- Arianoutsou, M.; Delipetrou, P.; Celesti-Grapow, L.; Basnou, C.; Bazos, I.; Kokkoris, Y.; Vilà, M. Comparing naturalized alien plants and recipient habitats across an east-west gradient in the Mediterranean Basin. J. Biogeogr. 2010b, 37, 1811–1823. [Google Scholar] [CrossRef]
- Pysek, P. Is there a taxonomic pattern to plant invasions? Oikos 1998, 82, 282–294. [Google Scholar] [CrossRef] [Green Version]
- Cronquist, A. An Integrated System of Classification of Flowering Plants; Columbia University Press: New York, NY, USA, 1981. [Google Scholar]
- Heywood, V.H. Patterns, extents and modes of invasions by terrestrial plants. In Biological Invasions: A Global Perspective; Drake, J.A., Mooney, H.A., Di Castri, F., Groves, R.H., Kruger, F.J., Rejmanek, M., Eds.; Wiley: Chichester, UK, 1989; pp. 31–60. [Google Scholar]
- Weber, E.; Sun, S.G.; Li, B. Invasive alien plants in China: Diversity and ecological highlights. Biol. Invasions 2008, 8, 1411–1429. [Google Scholar] [CrossRef] [Green Version]
- Georghiou, K.; Delipetrou, P. Patterns and traits of the endemic plants of Greece. Bot. J. Linn. Soc. 2010, 162, 130–422. [Google Scholar] [CrossRef] [Green Version]
- Crawley, M.J.; Harvey, P.H.; Purvis, A. Comparative ecology of the native and alien floras of the British Isles. Philos. Trans. R. Soc. B Biol. 1996, 351, 1251–1259. [Google Scholar]
- Arianoutsou, M.; Bazos, I.; Christopoulou, A.; Kokkoris, Y.; Zikos, A.; Zervou, S.; Delipetrou, P.; Cardoso, A.C.; Deriu, I.; Gervasini, E.; et al. Alien plants of Europe: Introduction pathways, gateways and time trends. Peer J. 2021, 9, e11270. [Google Scholar] [CrossRef] [PubMed]
- Vilà, Μ.; Basnou, C.; Pyšek, P.; Josefsson, M.; Genovesi, P.; Gollasch, S.; Nentwig, W.; Olenin, S.; Roques, A.A.; Roy, D.; et al. How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front. Ecol. Environ. 2010, 8, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Katsanevakis, S.; Deriu, I.; D’Amico, F.; Nunes, A.L.; Sanchez, S.P.; Crocetta, F.; Arianoutsou, M.; Bazos, I.; Christopoulou, A.; Curto, G.; et al. European Alien Species Information Network (EASIN): Supporting European policies and scientific research. Manag. Biol. Invasions 2015, 6, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Baquero, R.A.; Ayllón, D.; Nicola, G.G. Are the EU biosecurity legislative frameworks sufficiently effective to prevent biological invasions in the Natura 2000 network?—A case study in Mediterranean Europe. Environ. Sci. Policy 2021, 120, 21–28. [Google Scholar] [CrossRef]
- Traveset, A.; Brundu, G.; Carta, L.; Mprezetou, I.; Lambdon, P.; Manca, M.; Médail, F.; Moragues, E.; Rodríguez-Pérez, J.; Siamantziouras, A.S.; et al. Consistent performance of invasive plant species within and among islands of the Mediterranean basin. Biol. Invasions 2008, 10, 847–858. [Google Scholar] [CrossRef]
- Gimeno, I.; Vilà, M.; Hulme, P.E. Are islands more susceptible to plant invasion than continents? A test using Oxalis pes-caprae in the western Mediterranean. J. Biogeogr. 2006, 33, 1559–1565. [Google Scholar] [CrossRef]
- Vilà, M.; Gimeno, I. Does invasion by an alien plant species affect the soil seed bank? J. Veg. Sci. 2007, 18, 423–430. [Google Scholar] [CrossRef]
- Vilà, M.; Tessier, M.; Suehs, C.M.; Brundu, G.; Carta, L.; Galanidis, A.; Lambdon, P.; Manca, M.; Medail, F.; Moragues, E.; et al. Local and regional assessments of the impacts of plant invaders on vegetation structure and soil properties of Mediterranean islands. J. Biogeogr. 2006, 33, 853–861. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database (Vol. 12); Invasive Species Specialist Group: Auckland, New Zealand, 2000. [Google Scholar]
- Hardion, L.; Verlaque, R.; Saltonstall, K.; Leriche, A.; Vila, B. Origin of the invasive Arundo donax (Poaceae): A trans-Asian expedition in herbaria. Ann. Bot. Lond. 2014, 114, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular plants of Greece—an annotated checklist. Supplement. Willdenowia 2016, 46, 301–347. [Google Scholar] [CrossRef] [Green Version]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular Plants of Greece: An Annotated Checklist. Botanic Garden and Botanical Museum Berlin-Dahlem: Berlin, Germany; Hellenic Botanical Society: Athens, Greece. Englera 2013, 31, 1–370. [Google Scholar]
- Global Invasive Species Database (GISD). Available online: https://www.cabi.org/isc/abstract/20097200135 (accessed on 3 June 2021).
- Lucy, F.E.; Roy, H.; Simpson, A.; Carlton, J.T.; Hanson, J.M.; Magellan, K.; Campbell, M.L.; Costello, M.J.; Pagad, S.; Hewitt, C.L.; et al. INVASIVESNET towards an international association for open knowledge on invasive alien species. Manag. Biol. Invasions 2016, 7, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Quezel, P.; Barbero, M.; Bonin, G.; Loisel, R. Recent plant invasions in the Circum-Mediterranean region. In Biological Invasions in Europe and the Mediterranean Basin; Di Castri, F., Hansen, A.J., Debussche, M., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1990; pp. 51–60. [Google Scholar]
- Levine, J.M. Species diversity and biological invasions: Relating local process to community pattern. Science 2000, 288, 852–854. [Google Scholar] [CrossRef] [Green Version]
- Underwood, E.C.; Klinger, R.; Moore, P.E. Predicting patterns of non-native plant invasions in Yosemite National Park, California, USA. Divers. Distrib. 2004, 10, 447–459. [Google Scholar] [CrossRef]
- Foxcroft, L.C.; Rouget, M.; Richardson, D.M. Risk assessment of riparian plant invasions into protected areas. Conserv. Biol. 2007, 21, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Rejmánek, M.; Richardson, D.M.; Pyšek, P. Plant invasions and invasibility of plant communities. In Vegetation Ecology; van derMaarel, E., Ed.; Blackwell Science: Oxford, UK, 2005; pp. 332–355. [Google Scholar]
- McDougall, K.L.; Alexander, J.M.; Haider, S.; Pauchard, A.; Walsh, N.G.; Kueffer, C. Alien flora of mountains: Global comparisons for the development of local preventive measures against plant invasions. Divers. Distrib. 2011, 17, 103–111. [Google Scholar] [CrossRef]
- Bartomeus, I.; Sol, D.; Pino, J.; Vicente, P.; Font, X. Deconstructing the native–exotic richness relationship in plants. Glob. Ecol. Biogeogr. 2012, 21, 524–533. [Google Scholar] [CrossRef]
- Catford, J.A.; Jansson, R.; Nilsson, C. Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers. Distrib. 2009, 15, 22–40. [Google Scholar] [CrossRef] [Green Version]
- Melbourne, B.A.; Cornell, H.V.; Davies, K.F.; Dugaw, C.J.; Elmendorf, S.; Freestone, A.L.; Hall, R.J.; Harrison, S.; Hastings, A.; Holland, M.; et al. Invasion in a heterogeneous world: Resistance, coexistence or hostile takeover? Ecol. Lett. 2007, 10, 77–94. [Google Scholar] [CrossRef]
- Fyllas, N.M.; Christopoulou, A.; Galanidis, A.; Michelaki, C.Z.; Dimitrakopoulos, P.G.; Fulé, P.Z.; Arianoutsou, M. Tree growth-climate relationships in a forest-plot network on Mediterranean mountains. Sci. Total Environ. 2017, 598, 393–403. [Google Scholar] [CrossRef]
- Sarris, D.; Christodoulakis, D.; Körner, C. Impact of recent climatic change on growth of low elevation eastern Mediterranean forest trees. Clim. Chang. 2010, 106, 203–223. [Google Scholar] [CrossRef]
- Resasco, J.; Haddad, N.M.; Orrock, J.L.; Shoemaker, D.; Brudvig, L.A.; Damschen, E.I.; Tewksbury, J.J.; Levey, D.J. Landscape corridors can increase invasion by an exotic species and reduce diversity of native species. Ecology 2014, 95, 2033–2039. [Google Scholar] [CrossRef] [Green Version]
- Vokou, D.; Dimitrakopoulos, P.G.; Jones, N.; Damialis, A.; Monokrousos, N.; Pantis, J.D.; Mazaris, A.D.; The Natura 2000 Committee (2010–2013) members. Ten years of co-management in Greek protected areas: An evaluation. Biodivers. Conserv. 2014, 23, 2833–2855. [Google Scholar] [CrossRef]
- Natura 2000 Network Viewer. Available online: https://natura2000.eea.europa.eu/ (accessed on 3 June 2021).
- Van der Maarel, E. Transformation of cover-abundance values in phytosociology and its effects in community similarity. Vegetatio 1979, 39, 97–114. [Google Scholar]
- Dimopoulos, P.; Tsiripidis, I.; Xystrakis, F.; Kallimanis, A.; Panitsa, M. Methodology for Monitoring and Conservation Status Assessment of the Habitat Types in Greece; National Center of the Environment and Sustainable Development: Athens, Greece, 2018; p. 128. [Google Scholar]
- Richardson, D.M.; Pyšek, P.; Rejmánek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants: Concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- European Environmental Agency. Habitat Types Search. Available online: https://eunis.eea.europa.eu/habitats.jsp (accessed on 3 June 2021).
- WorldClim. Available online: http://worldclim.org/version2 (accessed on 3 June 2021).
- Seipel, T.; Kueffer, C.; Rew, L.J.; Daehler, C.C.; Pauchard, A.; Naylor, B.J.; Alexander, J.M.; Edwards, P.J.; Parks, C.G.; Arevalo, J.R.; et al. Processes at multiple scales affect richness and similarity of non-native plant species in mountains around the world. Glob. Ecol. Biogeogr. 2012, 21, 236–246. [Google Scholar] [CrossRef]
- Zenetos, A.; Arianoutsou, M.; Bazos, I.; Christopoulou, A.; Kokkoris, Y.; Zervou, S.; Zikos, A.; Jenna Wong, L.; Pagad, S. Global Register of Introduced and Invasive Species—Greece, Invasive Species Specialist Group ISSG; Checklist Dataset: 2020. Available online: https://doi.org/10.15468/td6afd(accessed on 1 October 2021). [CrossRef]
- Alien Plants in Greece: A Web-Based Platform. Available online: https://www.alienplants.gr (accessed on 3 May 2021).
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Wickham, H. Tidyverse: Easily Install and Load the ‘Tidyverse’. R Package Version 1.2.1. Available online: https://CRAN.R-project.org/package=tidyverse (accessed on 3 June 2021).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Zuur, A.F.; Leno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; p. 574. [Google Scholar]
- Säfken, B.; Rügamer, D.; Kneib, T.; Greven, S. Conditional model selection in mixed-effects models with cAIC4. J. Stat. Softw. 2021, 99, 1–30. [Google Scholar] [CrossRef]
- Richardson, D.M.; Holmes, P.M.; Esler, K.J.; Galatowitsch, S.M.; Stromberg, J.C.; Kirkman, S.P.; Pyšek, P.; Hobbs, R.J. Riparian vegetation: Degradation, alien plant invasions, and restoration prospects. Divers. Distrib. 2007, 13, 126–139. [Google Scholar] [CrossRef]
- Štajerová, K.; Šmilauer, P.; Brůna, J.; Pyšek, P. Distribution of invasive plants in urban environment is strongly spatially structured. Landsc. Ecol. 2017, 32, 681–692. [Google Scholar] [CrossRef]
- Mitrakos, K. A theory for Mediterranean plant life. Acta Oecol. Plant. 1980, 1, 1245–1252. [Google Scholar]
- European Commission. Towards an EU Strategy on Invasive Species; EC: Brussels, Belgium, 2008; p. 789.
- EU regulation on Invasive Alien Species 1143/2014. Available online: http://data.europa.eu/eli/reg/2014/1143/oj (accessed on 3 June 2021).
- Masters, G.; Norgrove, L.; Climate Change and Invasive Alien Species. CABI Working Paper 1; CABI: Wallingford, UK, 2010; p. 30.
- Flora of Greece Web: Vascular Plants of Greece—An Annotated Checklist. Available online: http://portal.cybertaxonomy.org/flora-greece/ (accessed on 11 June 2021).
| Taxa | Family | Percentage (%) of Natura 2000 Sites with the Presence of Each Taxon | Number of Plots | Number of Habitat Groups | ||
|---|---|---|---|---|---|---|
| Period A | Period B | Total | ||||
| Oxalis pes-caprae | Oxalidaceae | 20.8 | 13.8 | 25.8 | 174 | 6 |
| Arundo donax | Poaceae | 9.4 | 15.1 | 21.4 | 110 | 3 |
| Paspalum distichum | Poaceae | 5.0 | 6.3 | 9.4 | 50 | 2 |
| Erigeron canadensis | Asteraceae | 6.3 | 0 | 6.3 | 43 | 4 |
| Xanthium spinosum | Asteraceae | 4.4 | 1.3 | 5.0 | 63 | 3 |
| Ailanthus altissima | Simaroubaceae | 2.5 | 3.1 | 5.7 | 26 | 4 |
| Carpobrotus edulis | Aizoaceae | 0 | 5.7 | 5.7 | 12 | 2 |
| Medicago sativa subsp. microcarpa | Fabaceae | 5.0 | 0 | 5.7 | 10 | 4 |
| Robinia pseudoacacia | Fabaceae | 0.6 | 4.4 | 4.4 | 11 | 2 |
| Agave americana | Asparagaceae | 1.3 | 3.8 | 5.0 | 9 | 4 |
| 2000 Campaign | 2015 Campaign | |||||||
|---|---|---|---|---|---|---|---|---|
| Presence | All | Ratio | Presence | All | Ratio | X2 | p-Value | |
| Plot level | 367 | 9392 | 0.039 | 570 | 9028 | 0.063 | 54.70 | <0.001 |
| Habitat group Level | Presence | All | Ratio | Presence | All | Ratio | X2 | p-value |
| Forest | ||||||||
| All plots | 60 | 2688 | 0.022 | 109 | 2608 | 0.042 | 15.62 | <0.001 |
| 0–50 | 7 | 111 | 0.063 | 1 | 15 | 0.067 | 0.00 | 1.000 |
| 50–100 | 18 | 663 | 0.027 | 1 | 21 | 0.048 | 0.00 | 1.000 |
| 100–200 | 23 | 927 | 0.025 | 72 | 1487 | 0.048 | 7.89 | <0.001 |
| 200–300 | 5 | 486 | 0.010 | 1 | 28 | 0.036 | 0.10 | 0.754 |
| 300–400 | 5 | 281 | 0.018 | 34 | 1053 | 0.032 | 1.29 | 0.255 |
| 400+ | 2 | 220 | 0.009 | 0 | 4 | 0.000 | 0.00 | 1.000 |
| Shrubland | ||||||||
| All plots | 46 | 2543 | 0.018 | 81 | 1835 | 0.044 | 24.77 | <0.001 |
| 0–20 | 0 | 113 | 0.000 | 7 | 286 | 0.024 | 1.57 | 0.210 |
| 20–50 | 7 | 611 | 0.011 | 72 | 1491 | 0.048 | 15.25 | <0.001 |
| 50–100 | 35 | 1481 | 0.024 | 0 | 19 | 0.000 | 0.00 | 1.000 |
| 100+ | 4 | 338 | 0.012 | 2 | 39 | 0.051 | 1.41 | 0.235 |
| Grassland | ||||||||
| All plots | 37 | 690 | 0.054 | 28 | 791 | 0.035 | 2.50 | 0.114 |
| 0–5 | 1 | 69 | 0.015 | 10 | 134 | 0.075 | 2.15 | 0.143 |
| 5–10 | 3 | 36 | 0.083 | 0 | 3 | 0.000 | 0.00 | 1.000 |
| 10–20 | 3 | 142 | 0.021 | 15 | 591 | 0.025 | 0.00 | 1.000 |
| 20–30 | 2 | 178 | 0.011 | 0 | 39 | 0.000 | 0.00 | 1.000 |
| 30+ | 28 | 265 | 0.106 | 3 | 23 | 0.130 | 0.00 | 0.986 |
| Rock | ||||||||
| All plots | 6 | 674 | 0.008 | 50 | 714 | 0.070 | 31.90 | <0.001 |
| 0–30 | 1 | 281 | 0.004 | 42 | 589 | 0.071 | 17.17 | <0.001 |
| 30–50 | 0 | 155 | 0.000 | 8 | 122 | 0.066 | 8.26 | 0.004 |
| 50+ | 5 | 238 | 0.021 | 0 | 3 | 0.000 | 0.00 | 1.000 |
| Coastal | ||||||||
| All plots | 33 | 1704 | 0.019 | 105 | 1705 | 0.062 | 38.03 | <0.001 |
| 0–15 | 4 | 456 | 0.009 | 0 | 33 | 0.000 | 0.00 | 1.000 |
| 15–30 | 9 | 563 | 0.016 | 95 | 1466 | 0.065 | 18.97 | <0.001 |
| 30–50 | 15 | 355 | 0.042 | 9 | 187 | 0.048 | 0.01 | 0.923 |
| 50–100 | 3 | 283 | 0.011 | 1 | 6 | 0.167 | 2.17 | 0.141 |
| 100+ | 2 | 47 | 0.043 | 0 | 8 | 0.000 | <0.001 | 1.000 |
| Riparian—Wetland | ||||||||
| All plots | 185 | 1060 | 0.175 | 196 | 1351 | 0.145 | 3.65 | 0.056 |
| 0–5 | 10 | 119 | 0.084 | 29 | 240 | 0.121 | 0.77 | 0.382 |
| 5–10 | 10 | 45 | 0.222 | 1 | 6 | 0.167 | 0.00 | 1.000 |
| 10–20 | 22 | 80 | 0.275 | 84 | 416 | 0.201 | 1.72 | 0.190 |
| 20–50 | 53 | 322 | 0.165 | 10 | 89 | 0.112 | 1.09 | 0.296 |
| 50–100 | 52 | 237 | 0.219 | 2 | 6 | 0.333 | 0.03 | 0.868 |
| 100+ | 38 | 257 | 0.148 | 70 | 594 | 0.118 | 1.20 | 0.273 |
| Model No. | Model (beyond Optimal) | Random Parameters | df | LL | cAIC | ΔcAIC |
|---|---|---|---|---|---|---|
| 1 | only fixed effects | 0 | 11 | 224.25 | −426.49 | 62.06 |
| 2 | random intercept for site | 1 | 79.73 | 320.86 | −482.26 | 6.29 |
| 3 | random intercept for habitat group | 1 | 14.71 | 229.85 | −430.28 | 58.27 |
| 4 | random intercept for site and habitat group | 2 | 84.12 | 328.49 | −488.55 | 0 |
| Fixed effects of optimal model (no4) | Estimate | se | df | t | p | |
| intercept | 0.021 | 0.012 | 11.3 | 1.823 | 0.095 | |
| Lat | 0.059 | 0.020 | 123.6 | 2.918 | 0.004 | |
| TA | −0.117 | 0.037 | 132.8 | −3.189 | 0.002 | |
| Tmin | 0.086 | 0.039 | 126.5 | 2.196 | 0.030 | |
| PA | 0.025 | 0.010 | 140.4 | 2.537 | 0.012 | |
| Pdq | −0.092 | 0.039 | 123.4 | −2.387 | 0.019 |
| Forest | Shrubland | Rock | Coastal | |
|---|---|---|---|---|
| Intercept | 0.041 | 0.028 | 0.043 | 0.044 |
| Area | ||||
| Latitude (Lat) | 0.093 | 0.056 | 0.067 | |
| Average annual temperature (Ta) | –0.065 | –0.069 | ||
| Minimum annual temperature (Tmin) | 0.071 | –0.051 | ||
| Total annual precipitation (PA) | 0.027 | 0.035 | ||
| Precipitation during the dry quarter (Pdq) | –0.148 | –0.141 | –0.053 | |
| Hydrographic network density | –0.035 | |||
| Road network density | ||||
| Null deviance | 2.871 | 1.238 | 0.956 | 0.879 |
| Residual deviance | 2.618 | 1.080 | 0.848 | 0.843 |
| pseudo R2 | 0.088 | 0.128 | 0.113 | 0.041 |
| Variable Name | Abbreviation | Average | Range | Units or Scale |
|---|---|---|---|---|
| Area | Area | 96 | 0.32 to 606 | km2 |
| Latitude | Lat | - | - | degrees |
| Average annual temperature | TA | 13.91 | 5.92 to 19.03 | °C |
| Minimum annual temperature | Tmin | 0.35 | −8.8 to 8.78 | °C |
| Total annual precipitation | PA | 669 | 379 to 1734 | mm |
| Precipitation during the dry quarter | Pdq | 58.33 | 4 to 146 | mm |
| Hydrographic network density | Hydro | 15,910 | 0 to 379858 | m/m2 |
| Road network density | Road | 23,447 | 0 to 393675 | m/m2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christopoulou, A.; Christopoulou, A.; Fyllas, N.M.; Dimitrakopoulos, P.G.; Arianoutsou, M. How Effective Are the Protected Areas of the Natura 2000 Network in Halting Biological Invasions? A Case Study in Greece. Plants 2021, 10, 2113. https://doi.org/10.3390/plants10102113
Christopoulou A, Christopoulou A, Fyllas NM, Dimitrakopoulos PG, Arianoutsou M. How Effective Are the Protected Areas of the Natura 2000 Network in Halting Biological Invasions? A Case Study in Greece. Plants. 2021; 10(10):2113. https://doi.org/10.3390/plants10102113
Chicago/Turabian StyleChristopoulou, Aikaterini, Anastasia Christopoulou, Nikolaos M. Fyllas, Panayiotis G. Dimitrakopoulos, and Margarita Arianoutsou. 2021. "How Effective Are the Protected Areas of the Natura 2000 Network in Halting Biological Invasions? A Case Study in Greece" Plants 10, no. 10: 2113. https://doi.org/10.3390/plants10102113