Enhancing Salt Tolerance in Soybean by Exogenous Boron: Intrinsic Study of the Ascorbate-Glutathione and Glyoxalase Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growing Condition and Treatments
2.2. Growth Parameters
2.3. Measurement of Physiological Parameters
2.4. Estimation of Lipid Peroxidation and H2O2 Concentration
2.5. Ascorbate and Glutathione Estimation
2.6. Assays of Enzymes of the AsA-GSH Pathway and Glyoxalase System
2.7. Methylglyoxal Level
2.8. Statistical Analysis
3. Results
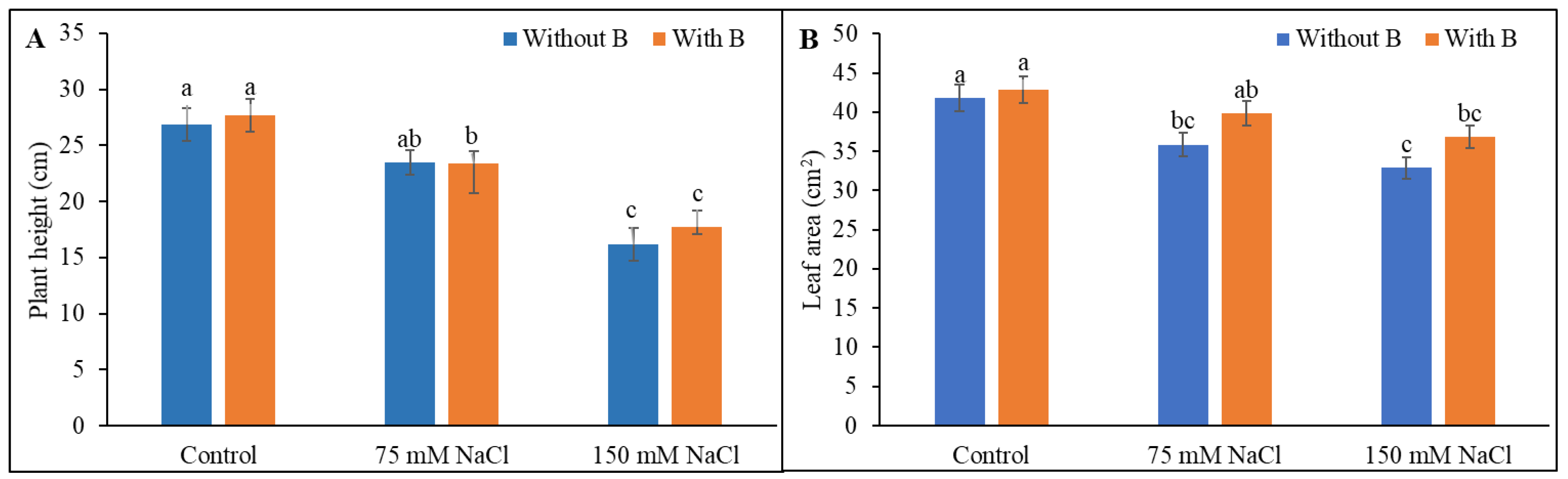
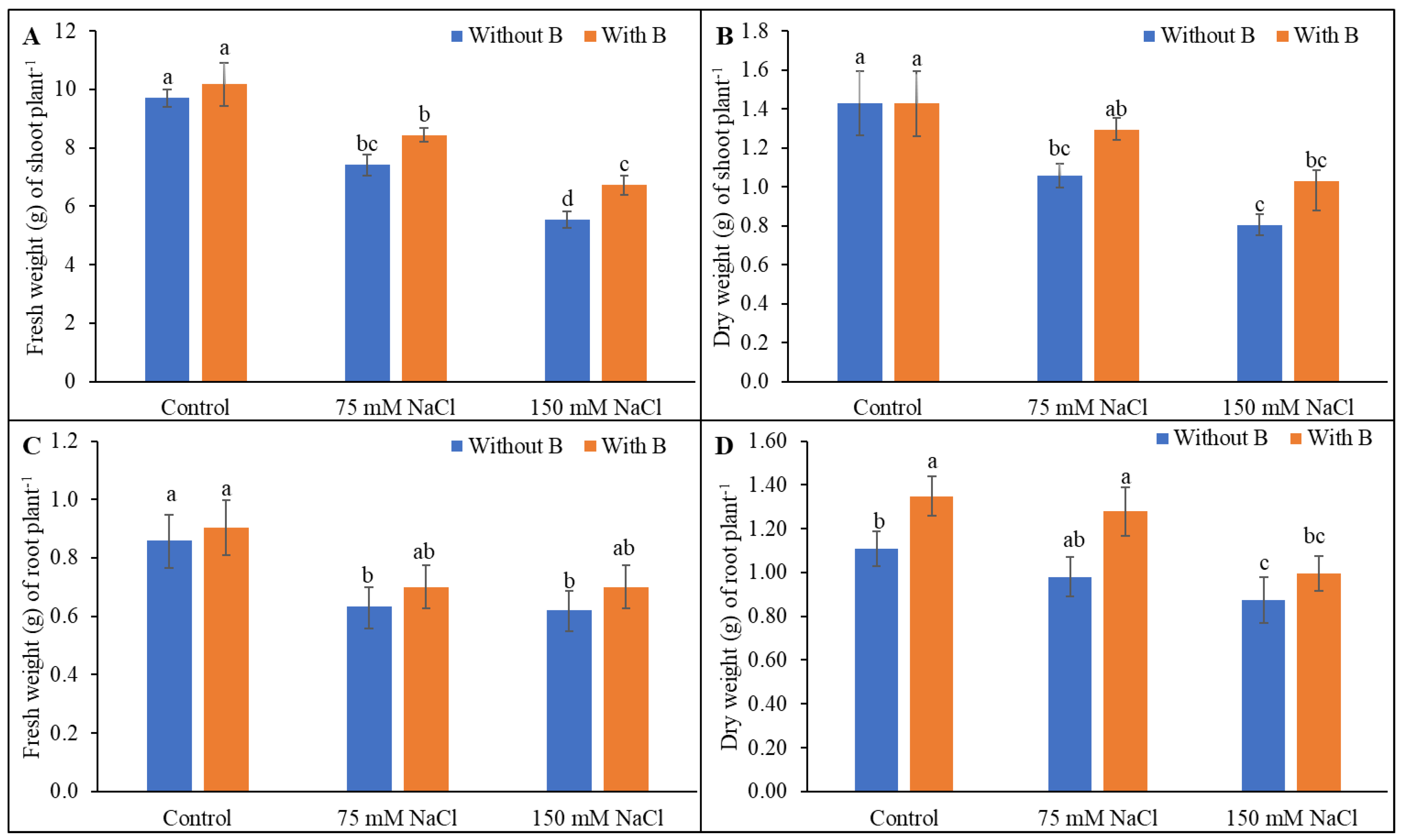
3.1. Growth Parameters
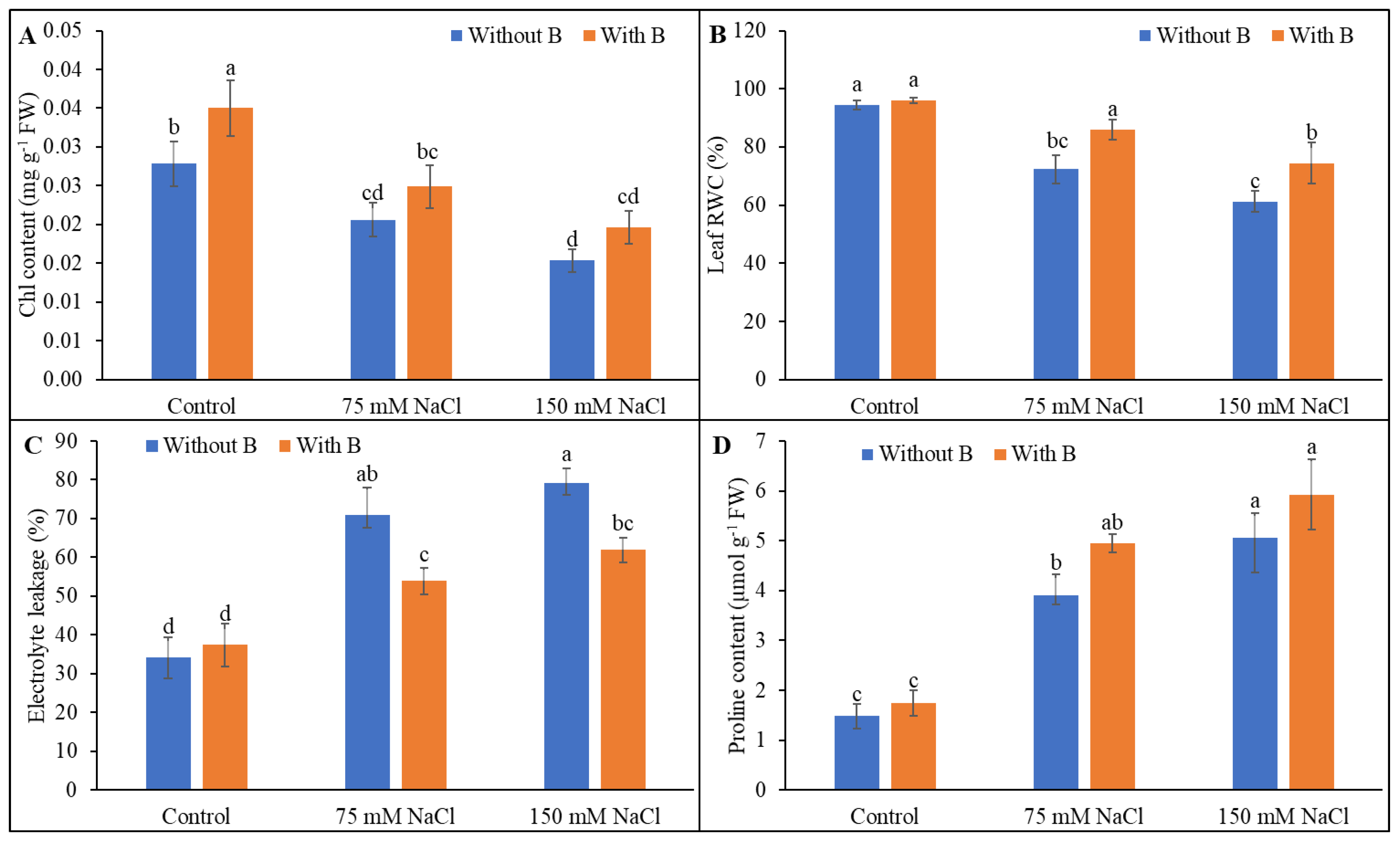
3.2. Physiological Parameters
3.3. Malondialdehyde and H2O2 Levels
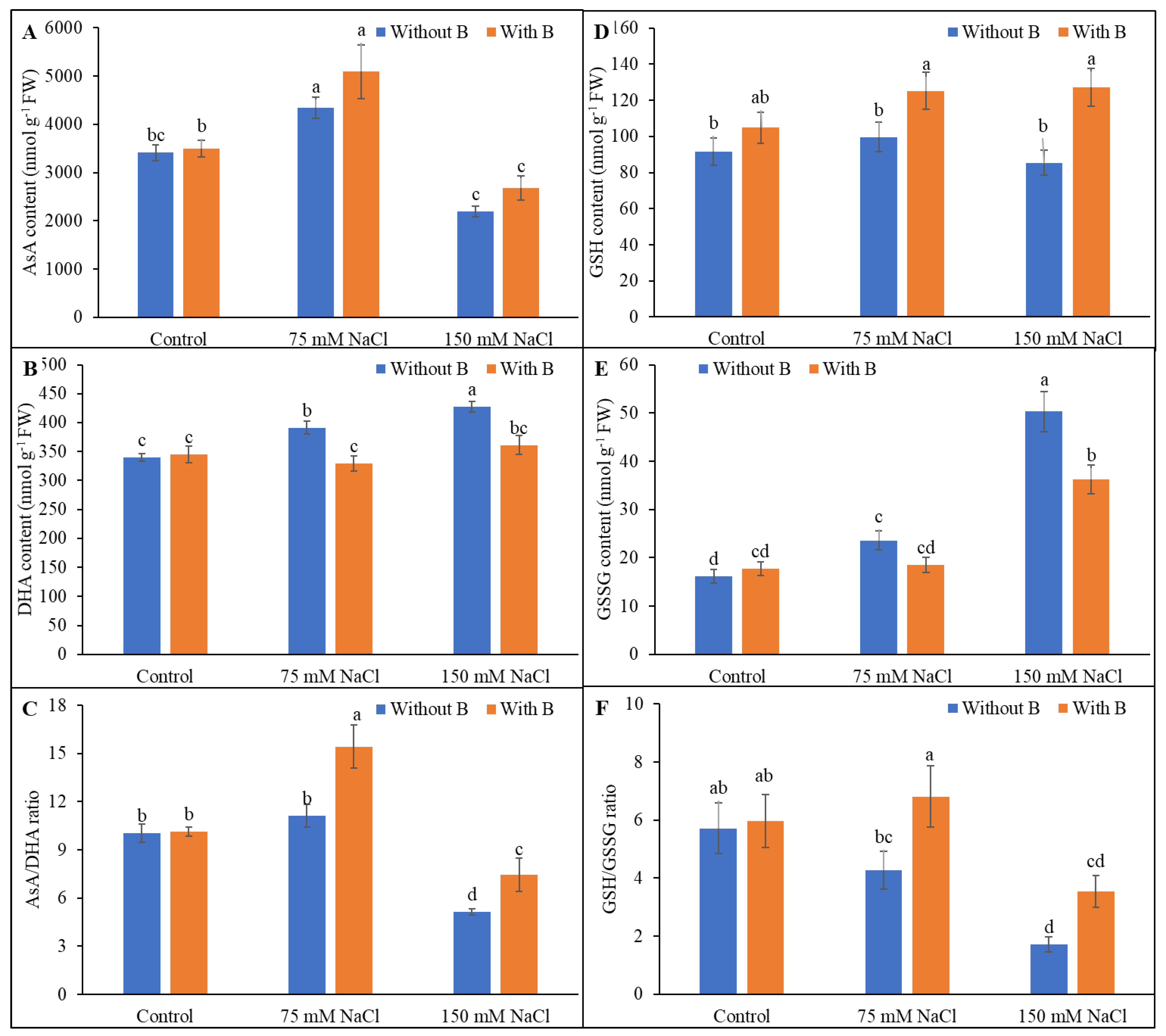
3.4. Ascorbate and Glutathione Pool
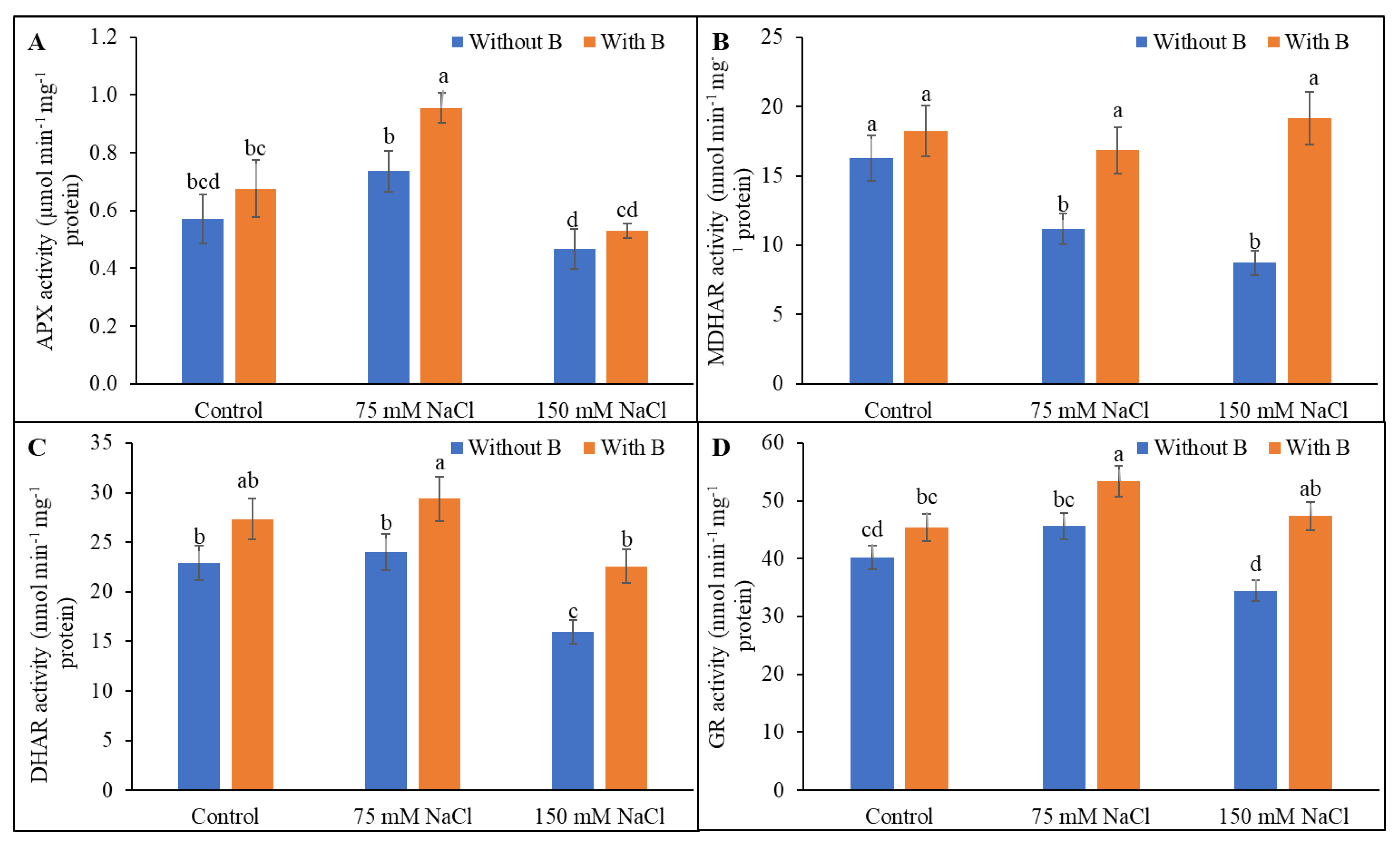
3.5. Activity of AsA-GSH Pathway Enzymes
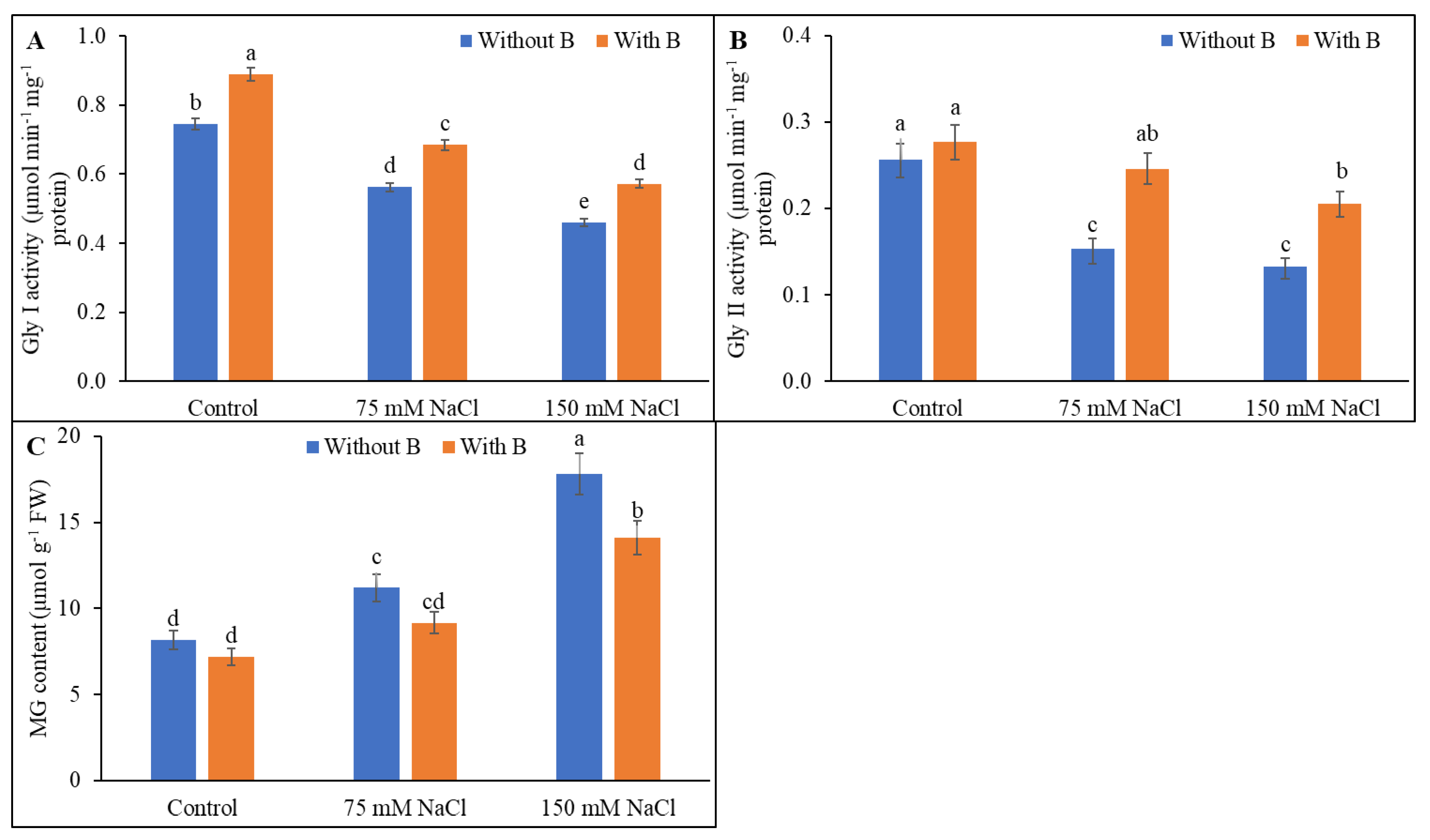
3.6. Methylglyoxal Detoxification System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Živanović, L.; Popović, V. Soybean (Glycine max) production in world and in Serbia. Zbornik Radova 2016, 21, 129–135. [Google Scholar]
- Gondek, M.; Weindorf, D.; Thiel, C.; Kleinheinz, G. Soluble salts in compost and their effects on soil and plants: A review. Compost Sci. Util. 2020, 28, 59–75. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Abdeldaym, E.A.; Abdelaziz, S.M.; El-Sawy, M.B.I.; Mottaleb, S.A. Synergetic effects of zinc, boron, silicon, and zeolite nanoparticles on confer tolerance in potato plants subjected to salinity. Agronomy 2020, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Polyamines confer salt tolerance in mung bean by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense and methylglyoxal detoxification systems. Front. Plant Sci. 2016, 7, 1104. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Liu, M.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Nie, C.; Yu, M.; Kuznetsov, V.V.; Allakhverdiev, S.I.; Shabala, S. Non-stomatal limitation of photosynthesis by soil salinity. Crit. Rev. Environ. Sci. Technol. 2021, 51, 791–825. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Tavallali, V.; Karimi, S.; Espargham, O. Boron enhances antioxidative defense in the leaves of salt-affected Pistacia vera seedlings. Hortic. J. 2018, 87, 55–62. [Google Scholar] [CrossRef]
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.B.; Mohsin, S.M.; Fujita, M. Exogenous vanillic acid enhances salt tolerance of tomato: Insight into plant antioxidant defense and glyoxalase systems. Plant Physiol. Biochem. 2020, 150, 109–120. [Google Scholar] [CrossRef]
- Yan, L.; Riaz, M.; Wu, X.; Du, C.; Liu, Y.; Lv, B.; Jiang, C. Boron inhibits aluminum-induced toxicity to citrus by stimulating antioxidant enzyme activity. J. Environ. Sci. Health Part C 2018, 36, 145–163. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.; Zulfiqar, F.; Alam, M.; Fujita, M. Regulation of ROS metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qin, C.; Begum, N.; Maodong, Q.; Dong, X.X.; El-Esawi, M.; El-Sheikh, M.A.; Alatar, A.A.; Zhang, L. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biol. 2019, 19, 479. [Google Scholar] [CrossRef] [PubMed]
- Blevins, D.G.; Lukaszewski, K.M. Boron in plant structure and function. Annu. Rev. Plant Phys. 1998, 49, 481–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saenz, J.L. Boron fertilization-A key for success. Vine Vin. View 2001, 17, 1–12. [Google Scholar]
- Saleem, M.; Khanif, Y.M.; Ishak, F.; Samsuri, A.W.; Hafeez, B. Importance of boron for agriculture productivity: A review. Int. Res. J. Agric. Sci. Soil Sci. 2011, 1, 293–300. [Google Scholar]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Wang, Y.; Imran, M.; Jiang, C. Boron alleviates the aluminum toxicity in trifoliate orange by regulating antioxidant defense system and reducing root cell injury. J. Environ. Manag. 2018, 15, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Dalir, N.; Rahnemaie, R.; Babaei, A. The alleviation of salinity-induced stress by using boron in soilless grown rose. J. Plant Nutr. 2019, 43, 1–12. [Google Scholar] [CrossRef]
- BARC. Fertilizer Recommendation Guide; Bangladesh Agricultural Research Council: Dhaka, Bangladesh, 2018. [Google Scholar]
- Ahmad, S.; Ali, H.; Rehman, A.; Khan, R.J.Z.; Ahmed, W.; Fatima, Z.; Abbas, G.; Irfan, M.; Ali, H.; Khan, M.A.; et al. Measuring leaf area of winter cereals by different techniques: A comparison. Pak. J. Life Soc. Sci. 2015, 13, 117–125. [Google Scholar]
- Zhu, J.; Tremblay, T.; Liang, Y. Comparing SPAD and at LEAF values for chlorophyll assessment in crop species. Can. J. Soil Sci. 2012, 92, 645–648. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teari, D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Yu, C.W.; Murphy, T.M.; Lin, C.H. Hydrogen peroxide-induces chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct. Plant Biol. 2003, 30, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradiso, A.; Berardino, R.; de Pinto, M.; di Toppi, L.S.; Storelli, F.T.; de Gara, L. Increase in ascorbate–glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant Cell Physiol. 2008, 49, 362–374. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- CoStat-Statistics Software Version 6.400; CoHort Software: Monterey, CA, USA, 2008.
- Naveed, M.; Sajid, H.; Mustafa, A.; Niamat, B.; Ahmad, Z.; Yaseen, M.; Kamran, M.; Rafique, M.; Ahmar, S.; Chen, J.T. Alleviation of salinity-induced oxidative stress, improvement in growth, physiology and mineral nutrition of canola (Brassica napus L.) through calcium-fortified composted animal manure. Sustainability 2020, 12, 846. [Google Scholar] [CrossRef] [Green Version]
- Salim, Q.T.; Joody, A.T. Effect of silicon calcium, boron on Proline and relative water contents in apple leaves. IOP Conf. Ser. Mat. Sci. Eng. 2018, 454, 012179. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Fujita, M. Roles of osmolytes in plants adaptation to drought and salinity. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R., Khan, N.A., Eds.; Springer: New York, NY, USA, 2016; pp. 37–68. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Inafuku, M.; Nahar, K.; Fujita, M.; Oku, H. Nitric oxide regulates plant growth, physiology, antioxidant defense, and ion homeostasis to confer salt tolerance in the mangrove species, Kandelia obovata. Antioxidants 2021, 10, 611. [Google Scholar] [CrossRef] [PubMed]
- Köse, C.; Güneş, A.; Kaya, Ö.; Kıtır, N.; Turan, M.; Sahin, F. Freeze injure and antioxidant enzyme activity of grapevine (Vitis vinifera) under bio-Boron fertilizer applications. Erwerbs-Obstbau 2018, 60, 3–10. [Google Scholar] [CrossRef]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Jiang, C. Boron supply maintains efficient antioxidant system, cell wall components and reduces aluminum concentration in roots of trifoliate orange. Plant Physiol. Biochem. 2019, 137, 93–101. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharby, H.F.; Nahar, K.; Al-Zahrani, H.S.; Hakeem, K.R.; Hasanuzzaman, M. Enhancing Salt Tolerance in Soybean by Exogenous Boron: Intrinsic Study of the Ascorbate-Glutathione and Glyoxalase Pathways. Plants 2021, 10, 2085. https://doi.org/10.3390/plants10102085
Alharby HF, Nahar K, Al-Zahrani HS, Hakeem KR, Hasanuzzaman M. Enhancing Salt Tolerance in Soybean by Exogenous Boron: Intrinsic Study of the Ascorbate-Glutathione and Glyoxalase Pathways. Plants. 2021; 10(10):2085. https://doi.org/10.3390/plants10102085
Chicago/Turabian StyleAlharby, Hesham F., Kamrun Nahar, Hassan S. Al-Zahrani, Khalid Rehman Hakeem, and Mirza Hasanuzzaman. 2021. "Enhancing Salt Tolerance in Soybean by Exogenous Boron: Intrinsic Study of the Ascorbate-Glutathione and Glyoxalase Pathways" Plants 10, no. 10: 2085. https://doi.org/10.3390/plants10102085
APA StyleAlharby, H. F., Nahar, K., Al-Zahrani, H. S., Hakeem, K. R., & Hasanuzzaman, M. (2021). Enhancing Salt Tolerance in Soybean by Exogenous Boron: Intrinsic Study of the Ascorbate-Glutathione and Glyoxalase Pathways. Plants, 10(10), 2085. https://doi.org/10.3390/plants10102085







