Multi-Walled Carbon Nanotubes Improved Development during In Vitro Multiplication of Sugarcane (Saccharum spp.) in a Semi-Automated Bioreactor
Abstract
:1. Introduction
2. Results
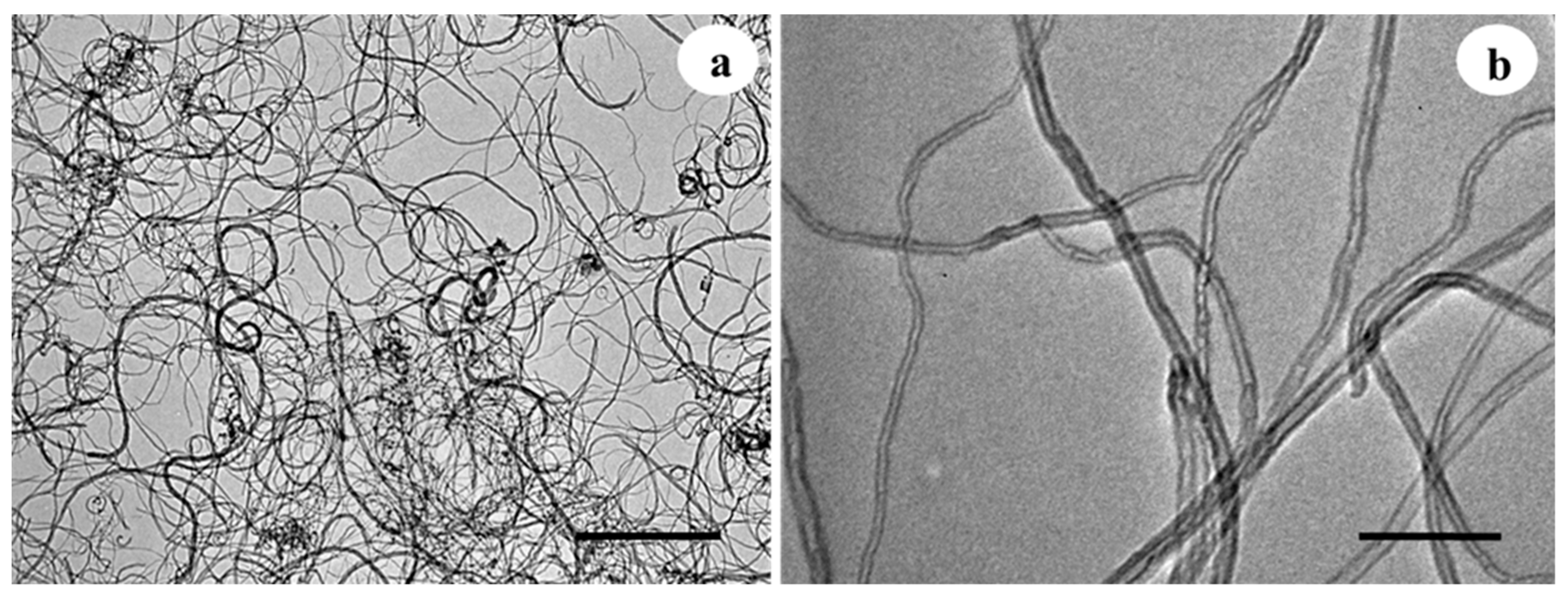
2.1. Characteristics of Carbon Nanotubes
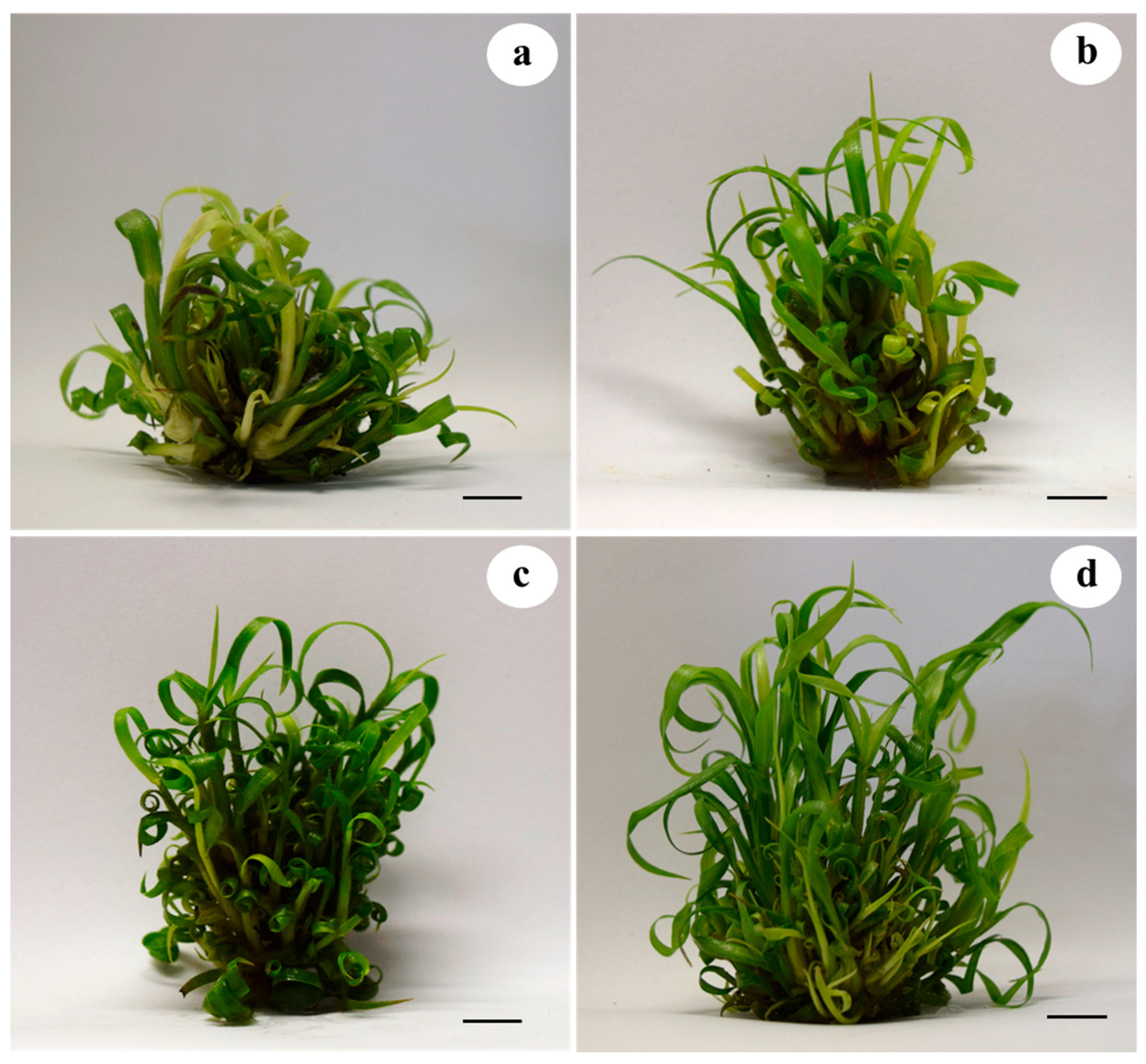
2.2. Effect of MWCNTs on Shoot Development, and Chlorophyll and Carbon Content
2.3. Effect of Carbon Nanotubes on Macro- and Micronutrient Content
3. Discussion
3.1. Plant Development and Carbon Content
3.2. Chlorophyll Content
3.3. Macro- and Micronutrient Content
4. Materials and Methods
4.1. Characterization of Multi-Walled Carbon Nanotubes
4.2. Establishment of In Vitro Sugarcane Explants
4.3. Application of Carbon Nanotubes in In Vitro Cultures
4.4. Evaluation of Macro- and Micronutrient Content
4.5. Quantification of Carbon Content
4.6. Chlorophyll Content
4.7. Experimental Design and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, A.H.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mali, S.C.; Raj, S.; Trivedi, R. Nanotechnology a novel approach to enhance crop productivity. Biochem. Biophys. Rep. 2020, 24, 100821. [Google Scholar]
- Verma, S.K.; Das, A.K.; Gantait, S.; Kumar, V.; Gurel, E. Applications of carbon nanomaterials in the plant system: A perspective view on the pros and cons. Sci. Total Environ. 2019, 667, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Zaidi, S.J. Recent developments in the application of nanomaterials in agroecoystems. Nanomaterials 2020, 10, 2411. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Silva, S.R.P. Low temperature growth of carbon nanotubes–A review. Carbon 2020, 158, 24–44. [Google Scholar] [CrossRef]
- Paramo, L.A.; Feregrino-Pérez, A.A.; Guevara, R.; Mendoza, S.; Esquivel, K. Nanoparticles in agroindustry: Applications, toxicity, challenges, and trends. Nanomaterials 2020, 10, 1654. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, R.; Khan, M.I. Carbon nanotubes: A review on properties, synthesis methods and applications in micro and nanotechnology. Microsyst. Technol. 2021, 1–10. [Google Scholar] [CrossRef]
- Samadi, S.; Lajayer, B.A.; Moghiseh, E.; Rodríguez-Couto, S. Effect of carbon nanomaterials on cell toxicity, biomass production, nutritional and active compound accumulation in plants. Environ. Technol. Innov. 2021, 21, 1–24. [Google Scholar] [CrossRef]
- Martinez-Ballesta, M.C.; Chelbi, N.; Lopez-Zaplana, A.; Carvajal, M. Discerning the mechanism of the multiwalled carbon nanotubes effect on root cell water and nutrient transport. Plant Physiol. Biochem. 2020, 146, 23–30. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.C.; Zapata, L.; Chalbi, N.; Carvajal, M. Multiwalled carbon nanotubes enter broccoli cells enhancing growth and water uptake of plants exposed to salinity. J. Nanobiotechnol. 2016, 14, 42. [Google Scholar] [CrossRef] [Green Version]
- Oloumi, H.; Mousavi, E.A.; Nejad, R.M. Multi-wall carbon nanotubes effects on plant seedlings growth and cadmium/lead uptake in vitro. Russ. J. Plant Physiol. 2018, 65, 260–268. [Google Scholar] [CrossRef]
- Pandey, K.; Lahiani, M.H.; Hicks, V.K.; Keith Hudson, M.; Green, M.J.; Khodakovskaya, M. Effects of carbon-based nanomaterials on seed germination, biomass accumulation and salt stress response of bioenergy crops. PLoS ONE 2018, 13, e0202274. [Google Scholar] [CrossRef] [Green Version]
- Samadi, S.; Saharkhiz, M.J.; Azizi, M.; Samiei, L.; Ghorbanpour, M. Multi-walled carbon nanotubes stimulate growth, redox reactions and biosynthesis of antioxidant metabolites in Thymus daenensis celak. in vitro. Chemosphere 2020, 249, 126069. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Deng, S.; Chu, Y.; Zhang, Y.; Zhao, H.; Chen, H.; Zhang, D. Single-wall carbon nanotubes improve cell survival rate and reduce oxidative injury in cryopreservation of Agapanthus praecox embryogenic callus. Plant Methods 2020, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.V.; De Silva, K.; Biris, A.S.; Dervishi, E.; Villagarcia, H. Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano 2012, 6, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Seddighinia, F.S.; Iranbakhsh, A.; Ardebili, Z.O.; Satari, T.N.; Soleimanpour, S. Seed priming with cold plasma and multi-walled carbon nanotubes modified growth, tissue differentiation, anatomy, and yield in bitter melon (Momordica charantia). J. Plant Growth Regul. 2020, 39, 87–98. [Google Scholar] [CrossRef]
- Yousefi, S.; Kartoolinejad, D.; Naghdi, R. Effects of priming with multi-walled carbon nanotubes on seed physiological characteristics of Hopbush (Dodonaea viscosa L.) under drought stress. Int. J. Environ. Sci. 2017, 74, 528–539. [Google Scholar]
- Sun, C.; Li, W.; Xu, Y.; Hu, N.; Ma, J.; Cao, W.; Huang, Q. Effects of carbon nanotubes on the toxicities of copper, cadmium and zinc toward the freshwater microalgae Scenedesmus obliquus. Aquat. Toxicol. 2020, 224, 105504. [Google Scholar] [CrossRef]
- López-Vargas, E.R.; González-García, Y.; Pérez-Álvarez, M.; Cadenas-Pliego, G.; González-Morales, S.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Seed Priming with Carbon Nanomaterials to Modify the Germination, Growth, and Antioxidant Status of Tomato Seedlings. Agronomy 2020, 10, 639. [Google Scholar] [CrossRef]
- Ghasempour, M.; Iranbakhsh, A.; Ebadi, M.; Ardebili, Z.O. Multi-walled carbon nanotubes improved growth, anatomy, physiology, secondary metabolism, and callus performance in Catharanthus roseus: An in vitro study. 3 Biotech 2019, 9, 404. [Google Scholar] [CrossRef]
- Rahmani, N.; Radjabian, T.; Soltani, B.M. Impacts of foliar exposure to multi-walled carbon nanotubes on physiological and molecular traits of Salvia verticillata L., as a medicinal plant. Plant Physiol. Biochem. 2020, 150, 27–38. [Google Scholar] [CrossRef]
- Hao, Y.; Yu, F.; Lv, R.; Ma, C.; Zhang, Z.; Rui, Y.; Xing, B. Carbon nanotubes filled with different ferromagnetic alloys affect the growth and development of rice seedlings by changing the C: N ratio and plant hormones concentrations. PLoS ONE 2016, 11, e0157264. [Google Scholar]
- Tiwari, D.K.; Dasgupta-Schubert, N.; Cendejas, L.V.; Villegas, J.; Montoya, L.C.; García, S.B. Interfacing carbon nanotubes (CNT) with plants: Enhancement of growth, water and ionic nutrient uptake in maize (Zea mays) and implications for nanoagriculture. Appl. Nanosci. 2014, 4, 577–591. [Google Scholar] [CrossRef] [Green Version]
- Taha, R.A.; Hassan, M.M.; Ibrahim, E.A.; Abou Baker, N.H.; Shaaban, E.A. Carbon nanotubes impact on date palm in vitro cultures. PCTOC 2016, 127, 525–534. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Dervishi, E.; Chen, J.; Nima, Z.; Gaume, A.; Biris, A.S.; Khodakovskaya, M.V. Impact of carbon nanotube exposure to seeds of valuable crops. ACS Appl. Mater. Interfaces 2013, 5, 7965–7973. [Google Scholar] [CrossRef]
- Li, G.; Santoni, V.; Maurel, C. Plant aquaporins: Roles in plant physiology. Biochim. Biophys. Acta 2014, 1840, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Verdoucq, L.; Maurel, C. Plant aquaporins. Adv. Bot. Res. 2018, 87, 25–56. [Google Scholar]
- Zhao, Q.; Ma, C.; White, J.C.; Dhankher, O.P.; Zhang, X.; Zhang, S.; Xing, B. Quantitative evaluation of multi-wall carbon nanotube uptake by terrestrial plants. Carbon 2017, 114, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Zhai, G.; Gutowski, S.M.; Walters, K.S.; Yan, B.; Schnoor, J.L. 2Charge, size, and cellular selectivity for multiwall carbon nanotubes by maize and soybean. Environ. Sci. Technol. 2015, 49, 7380–7390. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, A.; Gobikrishnan, S. Physicochemical Characterization of Native and Steam Explosion Pretreated Wild Sugarcane (Saccharum spontaneum). Int. J. Renew. Energy Dev. 2020, 9, 3. [Google Scholar] [CrossRef]
- González-García, Y.; López-Vargas, E.R.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; González-Morales, S.; Robledo-Olivo, A.; Juárez-Maldonado, A. Impact of carbon nanomaterials on the antioxidant system of tomato seedlings. Int. J. Mol. Sci. 2019, 20, 5858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 3, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Tiwari, D.K.; Tripathi, D. Interaction of carbon nanotubes with plant system: A review. Carbon Lett. 2020, 31, 1–10. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Escalona, M.; Lorenzo, J.C.; González, B.; Daquinta, M.; González, J.L.; Desjardins, Y.; Borroto, C.G. Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Rep. 1999, 18, 743–748. [Google Scholar] [CrossRef]
- Alcántar, G.G.M.; Sandoval, V. Manual de Análisis Químico de Tejido Vegetal; Publicación Especial 10; Sociedad Mexicana de la Ciencia del Suelo: Chapingo, Mexico, 1999. [Google Scholar]
- Bremner, J.T. Inorganic forms of nitrogen. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; John Wiley&Sons, Inc.: Hoboken, NJ, USA, 1965; Volume 9, pp. 1179–1237. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Harborne, J.B. Phenolic compounds. In Phytochemical Methods; Springer: Dordrecht, The Netherlands, 1973; pp. 33–88. [Google Scholar]

| MWCNT (mg L−1) | Macronutrients (mg kg−1) | ||||||
| N | P | K | Ca | Mg | S | ||
| 0 | 47,900 ± 1300 a | 3288.13 ± 63.92 a | 39,156.96 ± 3609.36 a | 1425.42 ± 25.07 b | 1006.97 ± 20.32 a | 3032.37 ± 72.02 b | |
| 50 | 45,800 ± 1400 a | 3172.63 ± 84.08 a | 39,263.50 ± 4270.20 a | 1561.81 ± 14.68 b | 1008.41 ± 16.54 a | 3267.52 ± 13.44 ab | |
| 100 | 42,400 ± 1500 ab | 3170.66 ± 126.87 a | 36,006.56 ± 3114.44 a | 1627.85 ± 60.79 ab | 1006.35 ± 8.31 a | 3549.03 ± 69.46 a | |
| 200 | 39,500 ± 1600 b | 3304.98 ± 108.87 a | 36,434.70 ± 4068.33 a | 1848.00 ± 70.56 a | 1051.42 ± 27.62 a | 3487.07 ± 122.15 a | |
| Micronutrients (mg kg−1) | |||||||
| Fe | Cu | Zn | Mn | B | Ni | Na | |
| 0 | 299.49 ± 7.04 ab | 6.41 ± 0.38 a | 69.73 ± 0.25 b | 62.04 ± 0.86 a | 20.75 ± 0.43 a | 1.42 ± 0.26 a | 508.29 ± 10.86 a |
| 50 | 318.00 ± 3.79 a | 3.32 ± 0.35 b | 74.45 ± 0.93 ab | 62.89 ± 0.64 a | 20.17 ± 0.64 a | 2.11 ± 0.50 a | 515.24 ± 9.16 a |
| 100 | 322.49 ± 2.45 a | 3.22 ± 0.17 b | 76.65 ± 0.44 a | 61.39 ± 0.42 a | 20.20 ± 0.29 a | 1.58 ± 0.90 a | 322.45 ± 1.50 b |
| 200 | 281.86 ± 10.04 b | 3.39 ± 0.02 b | 68.98 ± 2.61 b | 64.65 ± 2.61 a | 22.45 ± 0.93 a | 2.51 ± 0.79 a | 352.95 ± 16.30 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorcia-Morales, M.; Gómez-Merino, F.C.; Sánchez-Segura, L.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. Multi-Walled Carbon Nanotubes Improved Development during In Vitro Multiplication of Sugarcane (Saccharum spp.) in a Semi-Automated Bioreactor. Plants 2021, 10, 2015. https://doi.org/10.3390/plants10102015
Sorcia-Morales M, Gómez-Merino FC, Sánchez-Segura L, Spinoso-Castillo JL, Bello-Bello JJ. Multi-Walled Carbon Nanotubes Improved Development during In Vitro Multiplication of Sugarcane (Saccharum spp.) in a Semi-Automated Bioreactor. Plants. 2021; 10(10):2015. https://doi.org/10.3390/plants10102015
Chicago/Turabian StyleSorcia-Morales, Monserrat, Fernando Carlos Gómez-Merino, Lino Sánchez-Segura, José Luis Spinoso-Castillo, and Jericó Jabín Bello-Bello. 2021. "Multi-Walled Carbon Nanotubes Improved Development during In Vitro Multiplication of Sugarcane (Saccharum spp.) in a Semi-Automated Bioreactor" Plants 10, no. 10: 2015. https://doi.org/10.3390/plants10102015
APA StyleSorcia-Morales, M., Gómez-Merino, F. C., Sánchez-Segura, L., Spinoso-Castillo, J. L., & Bello-Bello, J. J. (2021). Multi-Walled Carbon Nanotubes Improved Development during In Vitro Multiplication of Sugarcane (Saccharum spp.) in a Semi-Automated Bioreactor. Plants, 10(10), 2015. https://doi.org/10.3390/plants10102015








