Metabolomic and Biochemical Analysis of Two Potato (Solanum tuberosum L.) Cultivars Exposed to In Vitro Osmotic and Salt Stresses
Abstract
1. Introduction
2. Results
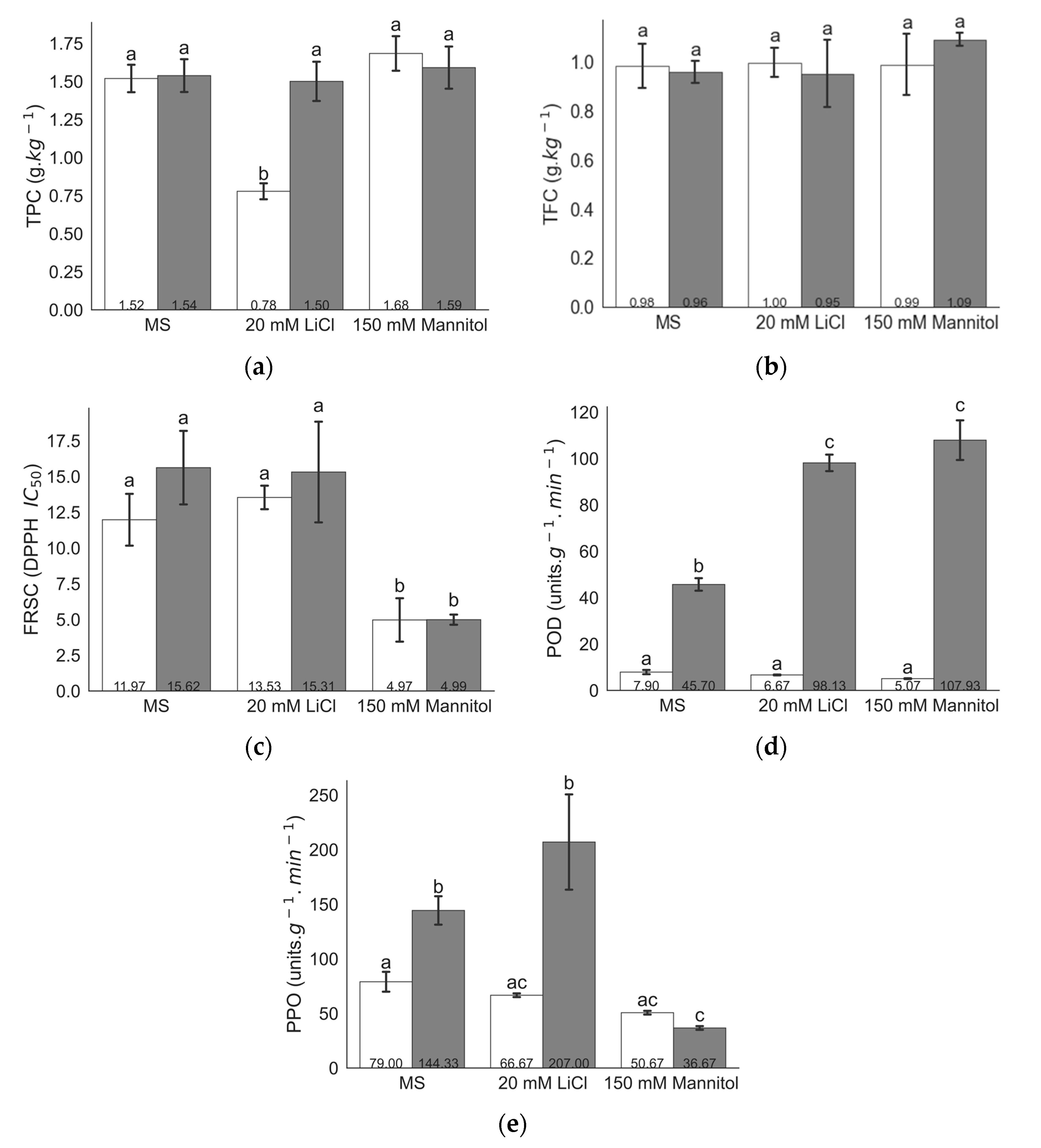
2.1. Biochemical Analysis under Different Levels of LiCl and Mannitol
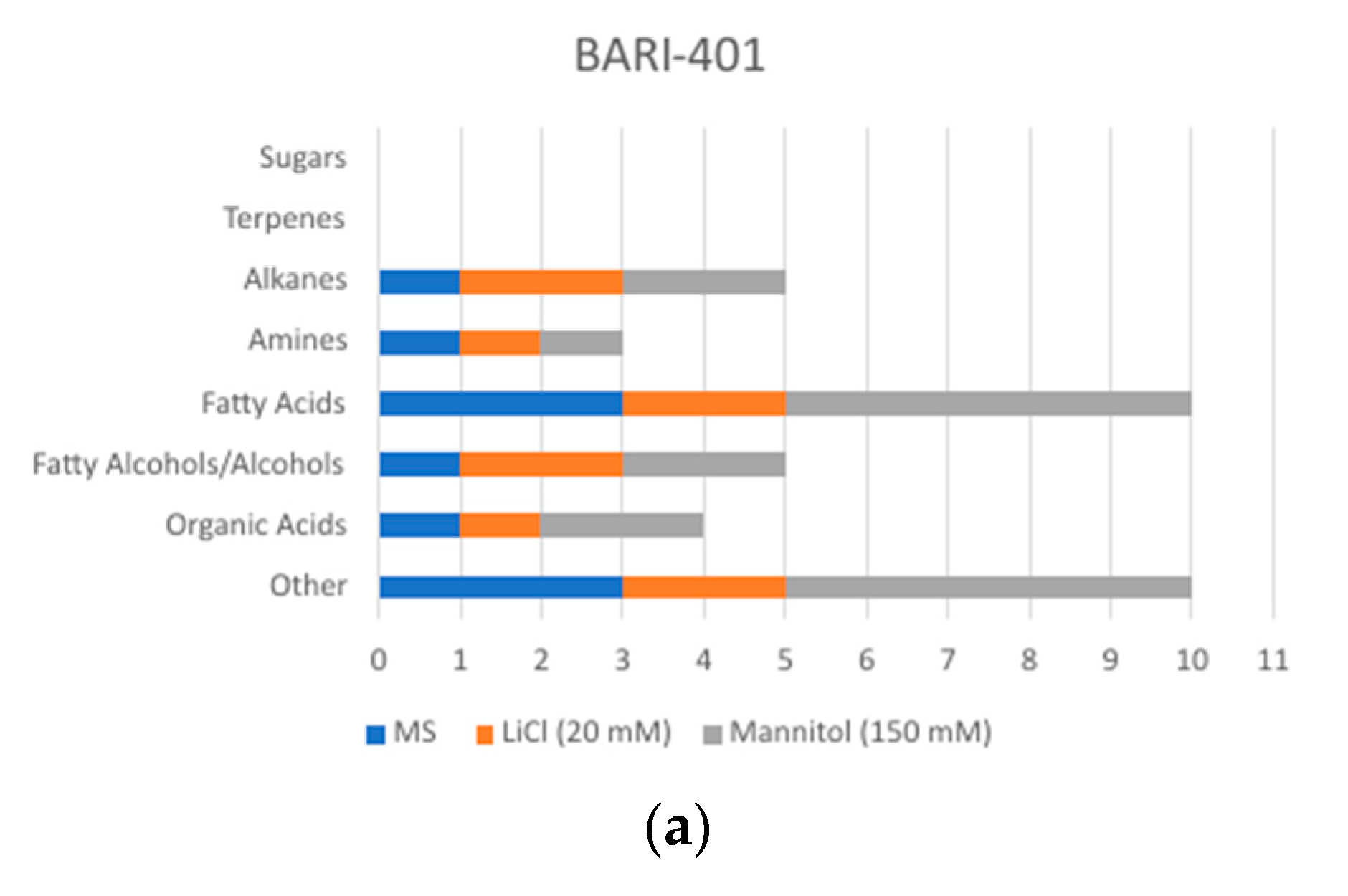
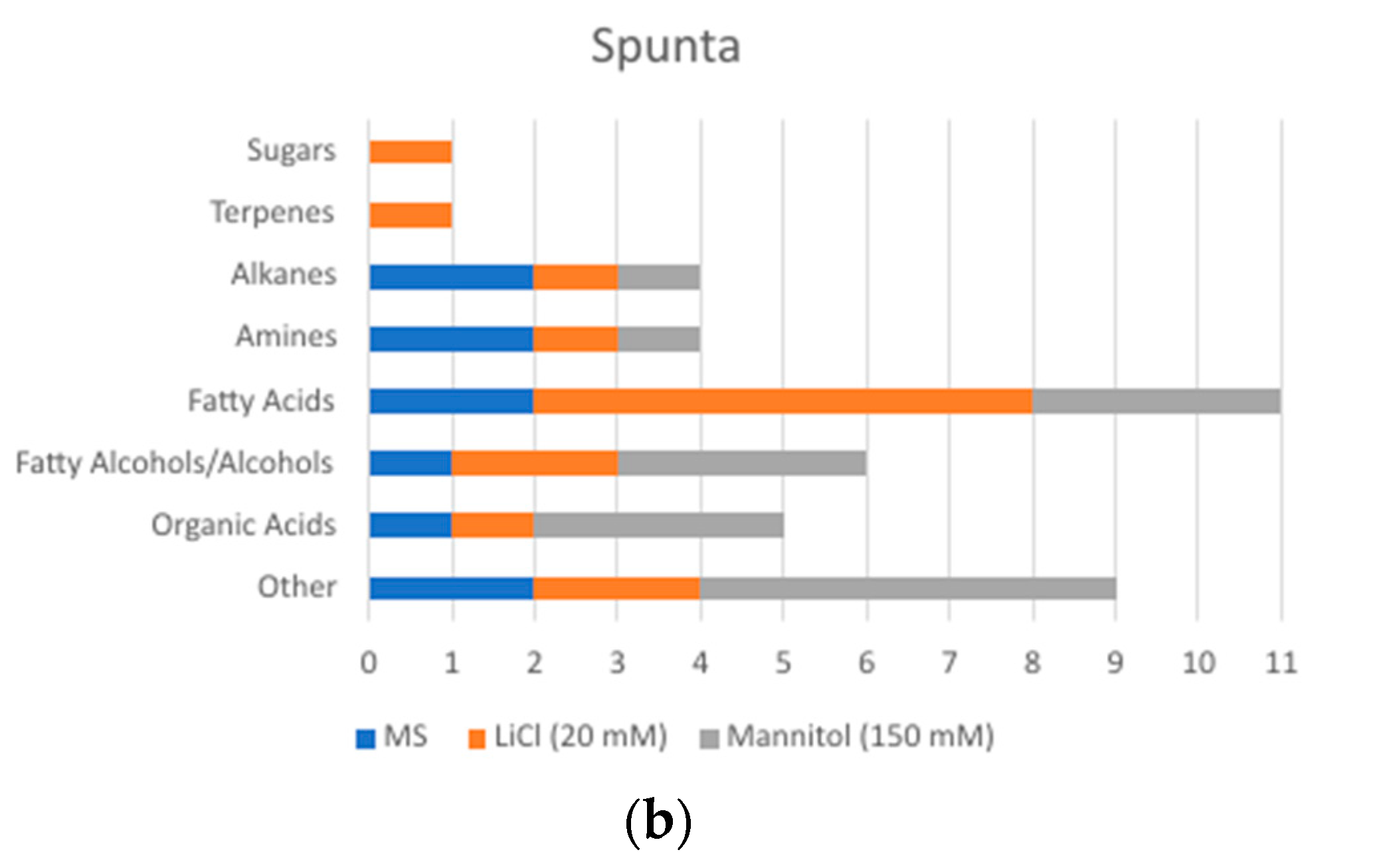
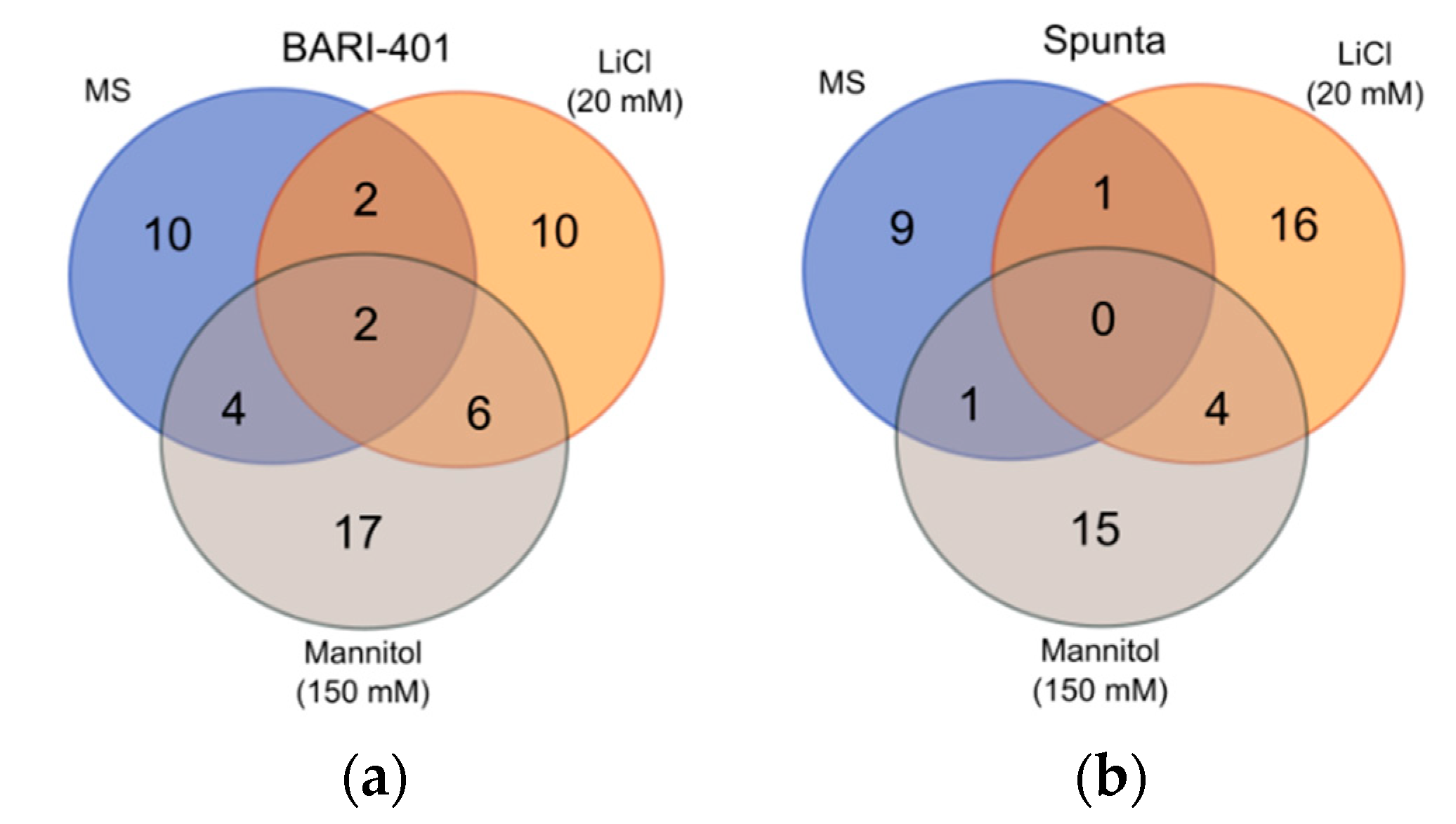
2.2. Changes in Metabolite Profile Induced by LiCl and Mannitol Exposure
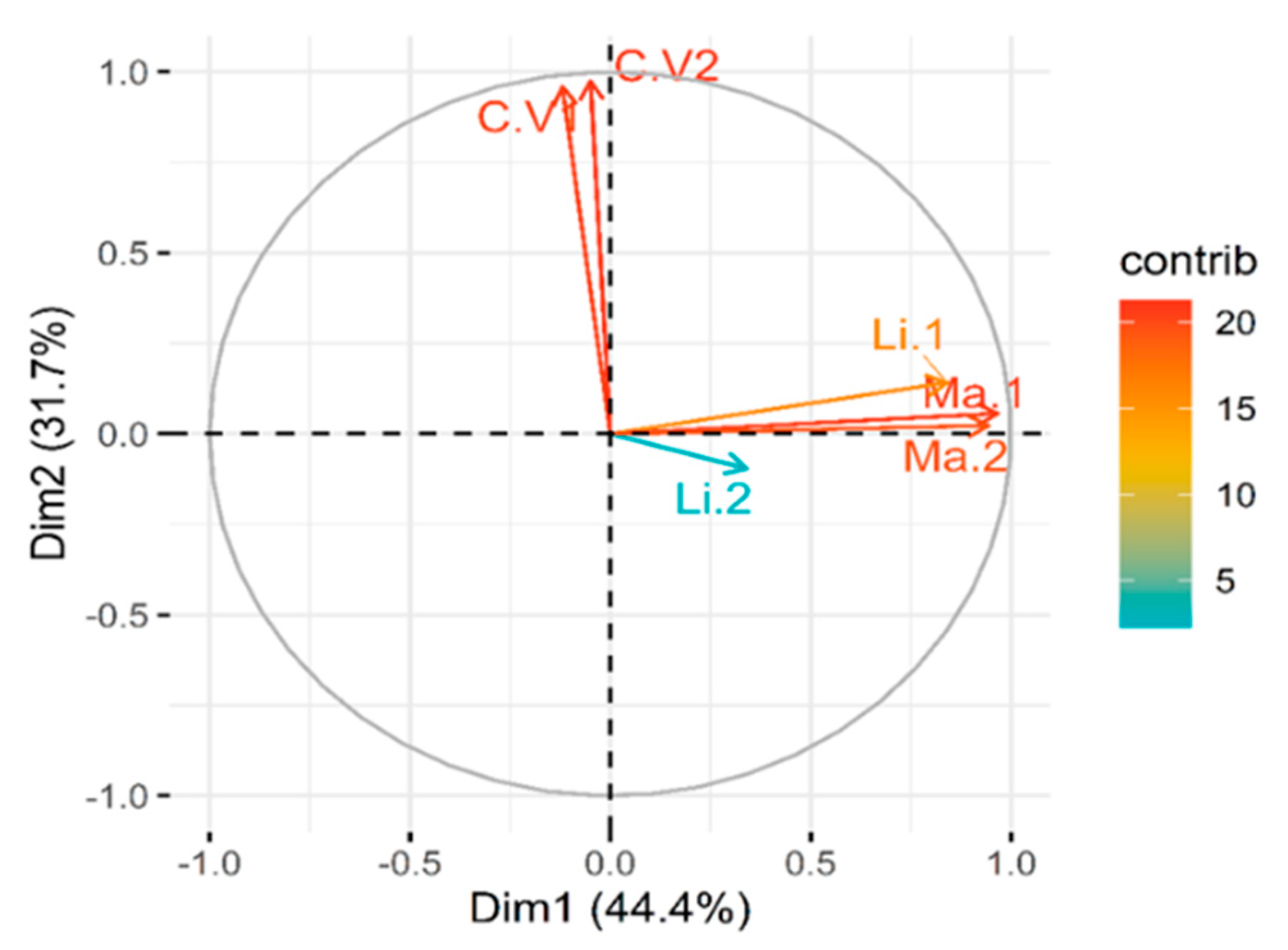
2.3. Principal Component Analysis (PCA) of Treatment Variables
3. Discussion
4. Materials and Methods
4.1. In Vitro Potato Plantlets Growth and Treatment with LiCl and Mannitol
4.2. Sample Preparation, Extraction, and Biochemical Analysis of Potato Plantlets
4.2.1. Extract Preparation for Total Phenols (TPC), Total Flavonoids (TFC), and Antioxidant Activity
4.2.2. Methanol Total Phenols Concentration (TPC) Estimation
4.2.3. Total Flavonoids Concentration (TFC) Estimation
4.2.4. Antioxidant Capacity by DPPH Free Radical Scavenging Capacity (FRSC) Assay
4.2.5. Enzymes Activity Evaluation
Peroxidase (POD) Assay
Polyphenol Oxidase (PPO) Assay
4.3. Potato Shoots Sample Preparation for GC-MS Metabolites Analysis
4.4. Experimental Design and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albiski, F.; Najla, S.; Sanoubar, R.; Alkabani, N.; Murshed, R. In Vitro Screening of Potato Lines for Drought Tolerance. Physiol. Mol. Biol. Plants 2012, 18, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Birch, P.R.; Bryan, G.; Fenton, B.; Gilroy, E.M.; Hein, I.; Jones, J.T.; Prashar, A.; Taylor, M.A.; Torrance, L.; Toth, I.K. Crops That Feed the World 8: Potato: Are the Trends of Increased Global Production Sustainable? Food Secur. 2012, 4, 477–508. [Google Scholar] [CrossRef]
- Vaitkevičienė, N.; Kulaitienė, J.; Jarienė, E.; Levickienė, D.; Danillčenko, H.; Średnicka-Tober, D.; Rembiałkowska, E.; Hallmann, E. Characterization of Bioactive Compounds in Colored Potato (Solanum Tuberosum L.) Cultivars Grown with Conventional, Organic, and Biodynamic Methods. Sustainability 2020, 12, 2701. [Google Scholar] [CrossRef]
- Brown, C.R. Antioxidants in Potato. Am. J. Potato Res. 2005, 82, 163–172. [Google Scholar] [CrossRef]
- Kazimierczak, R.; Średnicka-Tober, D.; Hallmann, E.; Kopczyńska, K.; Zarzyńska, K. The Impact of Organic vs. Conventional Agricultural Practices on Selected Quality Features of Eight Potato Cultivars. Agronomy 2019, 9, 799. [Google Scholar] [CrossRef]
- Monneveux, P.; Ramírez, D.A.; Pino, M.-T. Drought Tolerance in Potato (S. Tuberosum L.): Can We Learn from Drought Tolerance Research in Cereals? Plant Sci. 2013, 205, 76–86. [Google Scholar] [CrossRef]
- Demirel, U.; Morris, W.L.; Ducreux, L.J.; Yavuz, C.; Asim, A.; Tindas, I.; Campbell, R.; Morris, J.A.; Verrall, S.R.; Hedley, P.E. Physiological, Biochemical, and Transcriptional Responses to Single and Combined Abiotic Stress in Stress-Tolerant and Stress-Sensitive Potato Genotypes. Front. Plant Sci. 2020, 11, 169. [Google Scholar] [CrossRef]
- Katerji, N.; Van Hoorn, J.W.; Hamdy, A.; Mastrorilli, M. Salt Tolerance Classification of Crops According to Soil Salinity and to Water Stress Day Index. Agric. Water Manag. 2000, 43, 99–109. [Google Scholar] [CrossRef]
- Teixeira, J.; Pereira, S. High Salinity and Drought Act on an Organ-Dependent Manner on Potato Glutamine Synthetase Expression and Accumulation. Environ. Exp. Bot. 2007, 60, 121–126. [Google Scholar] [CrossRef]
- Boyer, J.S. Plant Productivity and Environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef]
- Bray, E.A. Plant Responses to Water Deficit. Trends Plant Sci. 1997, 2, 48–54. [Google Scholar] [CrossRef]
- Pérez-Clemente, R.M.; Gómez-Cadenas, A. In Vitro Tissue Culture, a Tool for the Study and Breeding of Plants Subjected to Abiotic Stress Conditions. Recent Adv. Plant In Vitro Cult. 2012, 91–108. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and Salt Tolerance in Plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS Regulation during Abiotic Stress Responses in Crop Plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and Glutathione: Keeping Active Oxygen under Control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Hancock, J.T. Cell Signalling; Oxford University Press: Oxford, UK, 2017; ISBN 0-19-965848-X. [Google Scholar]
- Jamil, M.; Rha, E.S. NaCl Stress-Induced Reduction in Grwoth, Photosynthesis and Protein in Mustard. J. Agric. Sci. 2013, 5, 114. [Google Scholar] [CrossRef]
- Abdel-Farid, I.B.; Marghany, M.R.; Rowezek, M.M.; Sheded, M.G. Effect of Salinity Stress on Growth and Metabolomic Profiling of Cucumis Sativus and Solanum Lycopersicum. Plants 2020, 9, 1626. [Google Scholar] [CrossRef]
- Rai, M.K.; Kalia, R.K.; Singh, R.; Gangola, M.P.; Dhawan, A.K. Developing Stress Tolerant Plants through in Vitro Selection—An Overview of the Recent Progress. Environ. Exp. Bot. 2011, 71, 89–98. [Google Scholar] [CrossRef]
- Zhu, J.-K. Salt and Drought Stress Signal Transduction in Plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Nakayama, H.; Horie, T.; Yonamine, I.; Shinmyo, A.; Yoshida, K. Improving Salt Tolerance in Plant Cells. Plant Biotechnol. 2005, 22, 477–487. [Google Scholar] [CrossRef]
- Shulaev, V.; Cortes, D.; Miller, G.; Mittler, R. Metabolomics for Plant Stress Response. Physiol. Plant. 2008, 132, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cela, E.; Kiaitsi, E.; Medina, A.; Sulyok, M.; Krska, R.; Magan, N. Interacting Environmental Stress Factors Affects Targeted Metabolomic Profiles in Stored Natural Wheat and That Inoculated with F. Graminearum. Toxins 2018, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Che-Othman, M.H.; Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Wheat Mitochondrial Respiration Shifts from the Tricarboxylic Acid Cycle to the GABA Shunt under Salt Stress. New Phytol. 2020, 225, 1166–1180. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Fuentes, D.; Threthowan, R.; Mohammad, F.; Ahmad, M. Comparative Metabolite Profiling of Two Wheat Genotypes as Affected by Nitrogen Stress at Seedling Stage. J. Anim. Plant Sci. 2019, 29, 260–268. [Google Scholar]
- Yang, L.; Fountain, J.C.; Ji, P.; Ni, X.; Chen, S.; Lee, R.D.; Kemerait, R.C.; Guo, B. Deciphering Drought-induced Metabolic Responses and Regulation in Developing Maize Kernels. Plant Biotechnol. J. 2018, 16, 1616–1628. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Fragasso, M.; Nigro, F.; Platani, C.; Papa, R.; Beleggia, R.; Trono, D. Analysis of Metabolic and Mineral Changes in Response to Salt Stress in Durum Wheat (Triticum Turgidum ssp. Durum) Genotypes, Which Differ in Salinity Tolerance. Plant Physiol. Biochem. 2018, 133, 57–70. [Google Scholar] [CrossRef]
- Rouphael, Y.; Raimondi, G.; Lucini, L.; Carillo, P.; Kyriacou, M.C.; Colla, G.; Cirillo, V.; Pannico, A.; El-Nakhel, C.; De Pascale, S. Physiological and Metabolic Responses Triggered by Omeprazole Improve Tomato Plant Tolerance to NaCl Stress. Front. Plant Sci. 2018, 9, 249. [Google Scholar] [CrossRef]
- Zörb, C.; Geilfus, C.-M.; Mühling, K.H.; Ludwig-Müller, J. The Influence of Salt Stress on ABA and Auxin Concentrations in Two Maize Cultivars Differing in Salt Resistance. J. Plant Physiol. 2013, 170, 220–224. [Google Scholar] [CrossRef]
- Degenkolbe, T.; Do, P.T.; Kopka, J.; Zuther, E.; Hincha, D.K.; Köhl, K.I. Identification of Drought Tolerance Markers in a Diverse Population of Rice Cultivars by Expression and Metabolite Profiling. PLoS ONE 2013, 8, e63637. [Google Scholar] [CrossRef]
- Hein, J.A.; Sherrard, M.E.; Manfredi, K.P.; Abebe, T. The Fifth Leaf and Spike Organs of Barley (Hordeum Vulgare L.) Display Different Physiological and Metabolic Responses to Drought Stress. BMC Plant Biol. 2016, 16, 248. [Google Scholar] [CrossRef]
- Kang, Z.; Babar, M.A.; Khan, N.; Guo, J.; Khan, J.; Islam, S.; Shrestha, S.; Shahi, D. Comparative Metabolomic Profiling in the Roots and Leaves in Contrasting Genotypes Reveals Complex Mechanisms Involved in Post-Anthesis Drought Tolerance in Wheat. PLoS ONE 2019, 14, e0213502. [Google Scholar] [CrossRef] [PubMed]
- Silvente, S.; Sobolev, A.P.; Lara, M. Metabolite Adjustments in Drought Tolerant and Sensitive Soybean Genotypes in Response to Water Stress. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Witt, S.; Galicia, L.; Lisec, J.; Cairns, J.; Tiessen, A.; Araus, J.L.; Palacios-Rojas, N.; Fernie, A.R. Metabolic and Phenotypic Responses of Greenhouse-Grown Maize Hybrids to Experimentally Controlled Drought Stress. Mol. Plant 2012, 5, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Paszkowski, J. Identification of Genes Preventing Transgenerational Transmission of Stress-Induced Epigenetic States. Proc. Natl. Acad. Sci. USA 2014, 111, 8547–8552. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gopal, J.; Iwama, K. In Vitro Screening of Potato against Water-Stress Mediated through Sorbitol and Polyethylene Glycol. Plant Cell Rep. 2007, 26, 693–700. [Google Scholar] [CrossRef]
- Shah, A.H.; Shah, S.H.; Ahmad, H.; Baig, A.; Swati, Z.A.; Aiman, U.; Din, I.; Khalid, Q. Co Adaptation of LiCl Tolerant Solanum Tuberosum L. Callus Cultures to NaCl Stress. Afr. J. Biotechnol. 2011, 10, 13444–13452. [Google Scholar]
- Daneshmand, F.; Arvin, M.J.; Kalantari, K.M. Physiological Responses to NaCl Stress in Three Wild Species of Potato in Vitro. Acta Physiol. Plant. 2010, 32, 91. [Google Scholar] [CrossRef]
- Purohit, M.; Srivastava, S.; Srivastava, P.S. Stress Tolerant Plants through Tissue Culture. In Plant Tissue Culture and Molecular Biology: Application and Prospects; Srivastava, P.S., Ed.; Narosa Publishing House: New Delhi, India, 1998; pp. 554–578. [Google Scholar]
- Solecka, D. Role of Phenylpropanoid Compounds in Plant Responses to Different Stress Factors. Acta Physiol. Plant. 1997, 19, 257–268. [Google Scholar] [CrossRef]
- Boo, H.-O.; Hwang, S.-J.; Bae, C.-S.; Park, S.-H.; Heo, B.-G.; Gorinstein, S. Extraction and Characterization of Some Natural Plant Pigments. Ind. Crops Prod. 2012, 40, 129–135. [Google Scholar] [CrossRef]
- Korayem, A.M.; El-Bassiouny, H.M.S.; El-Monem, A.A.A.; Mohamed, M.M.M. Physiological and Biochemical Changes in Different Sugar Beet Genotypes Infected with Root-Knot Nematode. Acta Physiol. Plant. 2012, 34, 1847–1861. [Google Scholar] [CrossRef]
- Navarro, J.M.; Flores, P.; Garrido, C.; Martinez, V. Changes in the Contents of Antioxidant Compounds in Pepper Fruits at Different Ripening Stages, as Affected by Salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- Roussos, P.A. Growth and Biochemical Responses of Jojoba (Simmondsia Chinensis (Link) Schneid) Explants Cultured under Mannitol-Simulated Drought Stress in vitro. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2013, 147, 272–284. [Google Scholar] [CrossRef]
- Vaziri, A.; Motamed, N.; Naghavi, M.R.; Niknam, V.N. Physiological and Biochemical Responses of Aeluropus Lagopoides and Aeluropus Littoralis to Drought Stress. Open Access J. Med. Aromat. Plants 2011, 2, 5. [Google Scholar]
- Sen, A.; Alikamanoglu, S. Antioxidant Enzyme Activities, Malondialdehyde, and Total Phenolic Content of PEG-Induced Hyperhydric Leaves in Sugar Beet Tissue Culture. Vitro Cell. Dev. Biol. Plant 2013, 49, 396–404. [Google Scholar] [CrossRef]
- Weidner, S.; Karamać, M.; Amarowicz, R.; Szypulska, E.; Gołgowska, A. Changes in Composition of Phenolic Compounds and Antioxidant Properties of Vitis Amurensis Seeds Germinated under Osmotic Stress. Acta Physiol. Plant. 2007, 29, 283–290. [Google Scholar] [CrossRef]
- Weidner, S.; Karolak, M.; Karamac, M.; Kosinska, A.; Amarowicz, R. Phenolic Compounds and Properties of Antioxidants in Grapevine Roots [Vitis Vinifera L.] under Drought Stress Followed by Recovery. Acta Soc. Bot. Pol. 2009, 78, 97–103. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Awad, M.A.; Al-Qurashi, A.D.; Mohamed, S.A.; El-Shishtawy, R.M.; Ali, M.A. Postharvest Chitosan, Gallic Acid and Chitosan Gallate Treatments Effects on Shelf Life Quality, Antioxidant Compounds, Free Radical Scavenging Capacity and Enzymes Activities of ‘Sukkari’Bananas. J. Food Sci. Technol. 2017, 54, 447–457. [Google Scholar] [CrossRef]
- Bowler, C.; van Montagu, M.; Inze, D. Superoxide Dismutase and Stress Tolerance. Annu. Rev. Plant Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Gossett, D.R.; Millhollon, E.P.; Lucas, M. Antioxidant Response to NaCl Stress in Salt-Tolerant and Salt-Sensitive Cultivars of Cotton. Crop Sci. 1994, 34, 706–714. [Google Scholar] [CrossRef]
- Gossett, D.R.; Banks, S.W.; Millhollon, E.P.; Lucas, M.C. Antioxidant Response to NaCl Stress in a Control and an NaCl-Tolerant Cotton Cell Line Grown in the Presence of Paraquat, Buthionine Sulfoximine, and Exogenous Glutathione. Plant Physiol. 1996, 112, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and Activity of Superoxide Dismutase, Peroxidase and Glutathione Reductase in Cotton under Salt Stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Rahnama, H.; Ebrahimzadeh, H. The Effect of NaCl on Antioxidant Enzyme Activities in Potato Seedlings. Biol. Plant. 2005, 49, 93–97. [Google Scholar] [CrossRef]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.-K. Methods and Concepts in Quantifying Resistance to Drought, Salt and Freezing, Abiotic Stresses That Affect Plant Water Status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef]
- Visser, E.J.; Voesenek, L.A. Acclimation to soil flooding—sensing and signal-transduction. In Root Physiology: From Gene to Function; Springer: Dordrech, The Netherlands, 2005; pp. 197–214. [Google Scholar]
- Bohnert, H.J.; Jensen, R.G. Strategies for Engineering Water-Stress Tolerance in Plants. Trends Biotechnol. 1996, 14, 89–97. [Google Scholar] [CrossRef]
- Roessner, U.; Wagner, C.; Kopka, J.; Trethewey, R.N.; Willmitzer, L. Simultaneous Analysis of Metabolites in Potato Tuber by Gas Chromatography–Mass Spectrometry. Plant J. 2000, 23, 131–142. [Google Scholar] [CrossRef]
- Vogel, G.; Fiehn, O.; Jean-Richard-dit-Bressel, L.; Boller, T.; Wiemken, A.; Aeschbacher, R.A.; Wingler, A. Trehalose Metabolism in Arabidopsis: Occurrence of Trehalose and Molecular Cloning and Characterization of Trehalose-6-phosphate Synthase Homologues. J. Exp. Bot. 2001, 52, 1817–1826. [Google Scholar] [CrossRef]
- Xu, G.; Liu, D.; Chen, J.; Ye, X.; Shi, J. Composition of Major Flavanone Glycosides and Antioxidant Capacity of Three Citrus Varieties. J. Food Biochem. 2009, 33, 453–469. [Google Scholar] [CrossRef]
- El-Bashiti, T.; Hamamcı, H.; Öktem, H.A.; Yücel, M. Biochemical Analysis of Trehalose and Its Metabolizing Enzymes in Wheat under Abiotic Stress Conditions. Plant Sci. 2005, 169, 47–54. [Google Scholar] [CrossRef]
- Henry, C.; Bledsoe, S.W.; Griffiths, C.A.; Kollman, A.; Paul, M.J.; Sakr, S.; Lagrimini, L.M. Differential Role for Trehalose Metabolism in Salt-Stressed Maize. Plant Physiol. 2015, 169, 1072–1089. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.B.; Engler, J.D.A.; Iyer, S.; Gerats, T.; Van Montagu, M.; Caplan, A.B. Effects of Osmoprotectants upon NaCl Stress in Rice. Plant Physiol. 1997, 115, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-E.; Park, S.-R.; Kwon, H.-B.; Yi, B.-Y.; Lee, G.-B.; Byun, M.-O. Genetic Engineering of Drought-Resistant Tobacco Plants by Introducing the Trehalose Phosphorylase (TP) Gene from Pleurotus Sajor-Caju. Plant Cell Tissue Organ Cult. 2005, 82, 151–158. [Google Scholar] [CrossRef]
- Karim, S.; Aronsson, H.; Ericson, H.; Pirhonen, M.; Leyman, B.; Welin, B.; Mäntylä, E.; Palva, E.T.; Van Dijck, P.; Holmström, K.-O. Improved Drought Tolerance without Undesired Side Effects in Transgenic Plants Producing Trehalose. Plant Mol. Biol. 2007, 64, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B.; Kwon, H.-B.; Kwon, S.-J.; Park, S.-C.; Jeong, M.-J.; Han, S.-E.; Byun, M.-O.; Daniell, H. Accumulation of Trehalose within Transgenic Chloroplasts Confers Drought Tolerance. Mol. Breed. 2003, 11, 1–13. [Google Scholar] [CrossRef]
- Garg, A.K.; Kim, J.-K.; Owens, T.G.; Ranwala, A.P.; Do Choi, Y.; Kochian, L.V.; Wu, R.J. Trehalose Accumulation in Rice Plants Confers High Tolerance Levels to Different Abiotic Stresses. Proc. Natl. Acad. Sci. USA 2002, 99, 15898–15903. [Google Scholar] [CrossRef]
- Jang, I.C.; JS, O.S.S.; Choi, W.B.; Song, S.I.; Kim, C.H.; Kim, Y.S.; Seo, H.S.; Choi, Y.D.; Nahm, B.H.; Kim, J.K. Expression of a Bifunctional Fusion of the E. Coli Genes for Trehalose-6-Phosphate Phosphatase in Transgenic Rice Plants Increase Trehalose Accumulation and Abiotic Stress Tolerance without Stunting Growth. Plant Physiol. 2003, 131, 516–524. [Google Scholar] [CrossRef]
- Cortina, C.; Culiáñez-Macià, F.A. Tomato Abiotic Stress Enhanced Tolerance by Trehalose Biosynthesis. Plant Sci. 2005, 169, 75–82. [Google Scholar] [CrossRef]
- Goddijn, O.J.; Verwoerd, T.C.; Voogd, E.; Krutwagen, R.W.; De Graff, P.; Poels, J.; van Dun, K.; Ponstein, A.S.; Damm, B.; Pen, J. Inhibition of Trehalase Activity Enhances Trehalose Accumulation in Transgenic Plants. Plant Physiol. 1997, 113, 181–190. [Google Scholar] [CrossRef]
- Govind, S.R.; Jogaiah, S.; Abdelrahman, M.; Shetty, H.S.; Tran, L.-S.P. Exogenous Trehalose Treatment Enhances the Activities of Defense-Related Enzymes and Triggers Resistance against Downy Mildew Disease of Pearl Millet. Front. Plant Sci. 2016, 7, 1593. [Google Scholar] [CrossRef] [PubMed]
- Zeid, I.M. Trehalose as Osmoprotectant for Maize under Salinity-Induced Stress. Res. J. Agric. Biol. Sci. 2009, 5, 613–622. [Google Scholar]
- Akram, N.A.; Waseem, M.; Ameen, R.; Ashraf, M. Trehalose Pretreatment Induces Drought Tolerance in Radish (Raphanus Sativus L.) Plants: Some Key Physio-Biochemical Traits. Acta Physiol. Plant. 2016, 38, 3. [Google Scholar]
- Kaplan, F.; Guy, C.L. β-Amylase Induction and the Protective Role of Maltose during Temperature Shock. Plant Physiol. 2004, 135, 1674–1684. [Google Scholar] [CrossRef]
- Szabados, L.; Savoure, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Lunn, J.E.; Feil, R.; Hendriks, J.H.; Gibon, Y.; Morcuende, R.; Osuna, D.; Scheible, W.-R.; Carillo, P.; Hajirezaei, M.-R.; Stitt, M. Sugar-Induced Increases in Trehalose 6-Phosphate Are Correlated with Redox Activation of ADPglucose Pyrophosphorylase and Higher Rates of Starch Synthesis in Arabidopsis Thaliana. Biochem. J. 2006, 397, 139–148. [Google Scholar] [CrossRef]
- Wingler, A.; Fritzius, T.; Wiemken, A.; Boller, T.; Aeschbacher, R.A. Trehalose Induces the ADP-Glucose Pyrophosphorylase Gene, ApL3, and Starch Synthesis in Arabidopsis. Plant Physiol. 2000, 124, 105–114. [Google Scholar] [CrossRef]
- Kolbe, A.; Tiessen, A.; Schluepmann, H.; Paul, M.; Ulrich, S.; Geigenberger, P. Trehalose 6-Phosphate Regulates Starch Synthesis via Posttranslational Redox Activation of ADP-Glucose Pyrophosphorylase. Proc. Natl. Acad. Sci. USA 2005, 102, 11118–11123. [Google Scholar] [CrossRef]
- Baena-González, E.; Rolland, F.; Thevelein, J.M.; Sheen, J. A Central Integrator of Transcription Networks in Plant Stress and Energy Signalling. Nature 2007, 448, 938–942. [Google Scholar] [CrossRef]
- Avonce, N.; Leyman, B.; Mascorro-Gallardo, J.O.; Van Dijck, P.; Thevelein, J.M.; Iturriaga, G. The Arabidopsis Trehalose-6-P Synthase AtTPS1 Gene Is a Regulator of Glucose, Abscisic Acid, and Stress Signaling. Plant Physiol. 2004, 136, 3649–3659. [Google Scholar] [CrossRef]
- Iba, K. Acclimative Response to Temperature Stress in Higher Plants: Approaches of Gene Engineering for Temperature Tolerance. Annu. Rev. Plant Biol. 2002, 53, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Khalid, Q.; Shah, S.H.; Zaeem, F.; Shah, S.H. Assessment of Adaptation, Regeneration Capability and Fatty Acid Profiles of LiCl Adapted and Unadapted Cell Lines of Oryza Sativa L. Cv. Swat-1. Sarhad J. Agric. 2017, 33, 549–555. [Google Scholar]
- Sui, N.; Li, M.; Li, K.; Song, J.; Wang, B.-S. Increase in Unsaturated Fatty Acids in Membrane Lipids of Suaeda Salsa L. Enhances Protection of Photosystem II under High Salinity. Photosynthetica 2010, 48, 623–629. [Google Scholar] [CrossRef]
- Harrathi, J.; Hosni, K.; Karray-Bouraoui, N.; Attia, H.; Marzouk, B.; Magné, C.; Lachaâl, M. Effect of Salt Stress on Growth, Fatty Acids and Essential Oils in Safflower (Carthamus Tinctorius L.). Acta Physiol. Plant. 2012, 34, 129–137. [Google Scholar] [CrossRef]
- López-Pérez, L.; del Carmen Martínez-Ballesta, M.; Maurel, C.; Carvajal, M. Changes in Plasma Membrane Lipids, Aquaporins and Proton Pump of Broccoli Roots, as an Adaptation Mechanism to Salinity. Phytochemistry 2009, 70, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Gigon, A.; Matos, A.-R.; Laffray, D.; Zuily-Fodil, Y.; Pham-Thi, A.-T. Effect of Drought Stress on Lipid Metabolism in the Leaves of Arabidopsis Thaliana (Ecotype Columbia). Ann. Bot. 2004, 94, 345–351. [Google Scholar] [CrossRef]
- Zhang, M.; Barg, R.; Yin, M.; Gueta-Dahan, Y.; Leikin-Frenkel, A.; Salts, Y.; Shabtai, S.; Ben-Hayyim, G. Modulated Fatty Acid Desaturation via Overexpression of Two Distinct Ω-3 Desaturases Differentially Alters Tolerance to Various Abiotic Stresses in Transgenic Tobacco Cells and Plants. Plant J. 2005, 44, 361–371. [Google Scholar] [CrossRef]
- Beisson, F.; Li, Y.; Bonaventure, G.; Pollard, M.; Ohlrogge, J.B. The Acyltransferase GPAT5 Is Required for the Synthesis of Suberin in Seed Coat and Root of Arabidopsis. Plant Cell 2007, 19, 351–368. [Google Scholar] [CrossRef]
- Mikami, K.; Murata, N. Membrane Fluidity and the Perception of Environmental Signals in Cyanobacteria and Plants. Prog. Lipid Res. 2003, 42, 527–543. [Google Scholar] [CrossRef]
- Upchurch, R.G. Fatty Acid Unsaturation, Mobilization, and Regulation in the Response of Plants to Stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Kosma, D.K.; Bourdenx, B.; Bernard, A.; Parsons, E.P.; Lü, S.; Joubès, J.; Jenks, M.A. The Impact of Water Deficiency on Leaf Cuticle Lipids of Arabidopsis. Plant Physiol. 2009, 151, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Bourdenx, B.; Bernard, A.; Domergue, F.; Pascal, S.; Léger, A.; Roby, D.; Pervent, M.; Vile, D.; Haslam, R.P.; Napier, J.A. Overexpression of Arabidopsis ECERIFERUM1 Promotes Wax Very-Long-Chain Alkane Biosynthesis and Influences Plant Response to Biotic and Abiotic Stresses. Plant Physiol. 2011, 156, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, S.; Tal, M. Development of Callus and Suspension Cultures of Potato Resistant to NaCl and Mannitol and Their Response to Stress. Plant Cell Tissue Organ Cult. 1990, 21, 119–128. [Google Scholar] [CrossRef]
- Kumlay, A.M.; Ercisli, S. Callus Induction, Shoot Proliferation and Root Regeneration of Potato (Solanum Tuberosum L.) Stem Node and Leaf Explants under Long-Day Conditions. Biotechnol. Biotechnol. Equip. 2015, 29, 1075–1084. [Google Scholar] [CrossRef]
- Hoff, J.E.; Singleton, K.I. A Method for Determination of Tannins in Foods by Means of Immobilized Protein. J. Food Sci. 1977, 42, 1566–1569. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Mar’ia, V.M.; Cascone, O. Horseradish Peroxidase Extraction and Purification by Aqueous Two-Phase Partition. Appl. Biochem. Biotechnol. 1995, 53, 147–154. [Google Scholar]
- Jiang, Y.; Zhang, Z.; Joyce, D.C.; Ketsa, S. Postharvest Biology and Handling of Longan Fruit (Dimocarpus Longan Lour.). Postharvest Biol. Technol. 2002, 26, 241–252. [Google Scholar] [CrossRef]
- Gelmesa, D.; Dechassa, N.; Mohammed, W.; Gebre, E.; Monneveux, P.; Bündig, C.; Winkelmann, T. In vitro Screening of Potato Genotypes for Osmotic Stress Tolerance. Open Agric. 2017, 2, 308–316. [Google Scholar] [CrossRef]
- Jain, S.M. Tissue Culture-Derived Variation in Crop Improvement. Euphytica 2001, 118, 153–166. [Google Scholar] [CrossRef]
- Iturriaga, G.; Suárez, R.; Nova-Franco, B. Trehalose Metabolism: From Osmoprotection to Signaling. Int. J. Mol. Sci. 2009, 10, 3793–3810. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamooh, B.T.; Sattar, F.A.; Wellman, G.; Mousa, M.A.A. Metabolomic and Biochemical Analysis of Two Potato (Solanum tuberosum L.) Cultivars Exposed to In Vitro Osmotic and Salt Stresses. Plants 2021, 10, 98. https://doi.org/10.3390/plants10010098
Hamooh BT, Sattar FA, Wellman G, Mousa MAA. Metabolomic and Biochemical Analysis of Two Potato (Solanum tuberosum L.) Cultivars Exposed to In Vitro Osmotic and Salt Stresses. Plants. 2021; 10(1):98. https://doi.org/10.3390/plants10010098
Chicago/Turabian StyleHamooh, Bahget Talat, Farooq Abdul Sattar, Gordon Wellman, and Magdi Ali Ahmed Mousa. 2021. "Metabolomic and Biochemical Analysis of Two Potato (Solanum tuberosum L.) Cultivars Exposed to In Vitro Osmotic and Salt Stresses" Plants 10, no. 1: 98. https://doi.org/10.3390/plants10010098
APA StyleHamooh, B. T., Sattar, F. A., Wellman, G., & Mousa, M. A. A. (2021). Metabolomic and Biochemical Analysis of Two Potato (Solanum tuberosum L.) Cultivars Exposed to In Vitro Osmotic and Salt Stresses. Plants, 10(1), 98. https://doi.org/10.3390/plants10010098





