Plant Sources Responsible for the Chemical Composition and Main Bioactive Properties of Poplar-Type Propolis
Abstract
1. Introduction
2. Plant Sources for Poplar-Type Propolis
3. Chemical Composition and Principal Properties of Plant Extracts
4. Poplar-Type Propolis
4.1. Chemical Composition and Analysis Methods
4.2. Main Bioactive Properties of Propolis
5. Propolis and New Trends in Medicine: Antiviral Activity
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DeGrandi-Hoffman, G.; Eckholm, B.J.; Huang, M. Methods for comparing nutrients in beebread made by Africanized and European honey bees and the effects on hemolymph protein titers. J. Vis. Exp. 2015, 97, e52448. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Islam, B.; Akram, M.; Shakil, S.; Ahmad, A.A.; Ali, S.M.; Siddiqui, M.; Khan, A.U. Antimicrobial Activity of Five Herbal Extracts Against Multi Drug Resistant (MDR) Strains of Bacteria and Fungus of Clinical Origin. Molecules 2009, 14, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Popravko, S.A. Chemical Composition of Propolis, its Origin and Standardization. In A Remarcable Hive Product: PROPOLIS; Harnaj, V., Ed.; Apimondia Publishing House: Bucharest, Romania, 1978; pp. 15–18. [Google Scholar]
- De Marco, S.; Piccioni, M.; Pagiotti, R.; Pietrella, D. Antibiofilm and Antioxidant Activity of Propolis and Bud Poplar Resins versusPseudomonas aeruginosa. Evid. Based Complement. Altern. Med. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vardar-Ünlü, G.; Silici, S.; Ünlü, M. Composition and in vitro antimicrobial activity of Populus buds and poplar-type propolis. World J. Microbiol. Biotechnol. 2008, 24, 1011–1017. [Google Scholar] [CrossRef]
- Zaccaria, V.; Garzarella, E.U.; Di Giovanni, C.; Galeotti, F.; Gisone, L.; Campoccia, D.; Volpi, N.; Arciola, C.R.; Daglia, M. Multi Dynamic Extraction: An Innovative Method to Obtain a Standardized Chemically and Biologically Reproducible Polyphenol Extract from Poplar-Type Propolis to Be Used for Its Anti-Infective Properties. Materials 2019, 12, 3746. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.D.; Spivak, M. Propolis and bee health: The natural history and significance of resin use by honey bees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.D.; Evans, J.D.; Spivak, M. Resin collection and social immunity in honey bees. Evolution 2009, 63, 3016–3022. [Google Scholar] [CrossRef]
- Crane, E. Beekeeping: Science, Practice and World Resources; Heinemann: London, UK, 1988. [Google Scholar]
- Bankova, V.; De Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Popravko, S.A.; Kononenko, G.P.; Tikhomirova, V.I.; Vulfson, N.S. Secondary metabolites of birch. IV. Identification of group of flavonoid aglycones in silver birch buds (Betula verucosa). Bioorg. Chem. 1979, 5, 1662–1667. [Google Scholar]
- Tămaş, M.; Marinescu, I.; Ionescu, F. Flavonoidele din muguri de plop. Stud. Cercet. Biochim. 1979, 22, 207–213. [Google Scholar]
- Nagy, E.; Papay, V.; Litkei, G.; Dinya, Z. Investigation of the chemical constituents, particularly the flavonoid components, of propolis and Populi gemma by GC/MS method. Stud. Org. Chem. 1986, 23, 223–232. [Google Scholar]
- Greenaway, W.; Scaysbrook, T.; Whatley, F.R. The analysis of bud exudates of Populus x euramericana and of propolis, by gas chromatography-mass spectrometry. Proc. R. Soc. Lond. 1987, 232, 249–272. [Google Scholar]
- Bankova, V.; Dyulgerov, A.; Popov, S.; Evstatieva, L.; Kuleva, L.; Pureb, O.; Zamjansan, Z. Propolis produced in Bulgaria and Mongolia: Phenolic composition and plant origin. Apidologie 1992, 23, 79–85. [Google Scholar] [CrossRef]
- Garciaviguera, C.; Ferreres, F.; Tomasbarberan, F.A. Study of Canadian Propolis by GC-MS and HPLC. Z. Nat. C 1993, 48, 731–735. [Google Scholar] [CrossRef]
- Markham, K.R.; Mitchell, K.A.; Wilkins, A.L.; Daldy, J.A.; Lu, Y. HPLC and GC-MS identification of the major organic constituents in New Zeland propolis. Phytochemistry 1996, 42, 205–211. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.; Wang, K.; Li, G.Q.; Hu, F.-L. Recent Advances in the Chemical Composition of Propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Greenaway, W.; Scaysbrook, T.; Whatley, F.R. Composition of Propolis in Oxfordshire, U.K. and its Relation to Poplar Bud Exudate. Z. Nat. C 1988, 43, 301–304. [Google Scholar] [CrossRef]
- Taber, S. Bees and Beekeeping. Bull. Èntomol. Soc. Am. 1976, 22, 30. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. Plant Sources of Propolis: An Update from a Chemist’s Point of View. Nat. Prod. Commun. 2006, 1, 1023–1028. [Google Scholar] [CrossRef]
- Popravko, S.A.; Sokolov, I.V.; Torgov, I.V. New natural phenolic triglycerides. Chem. Nat. Compd. 1982, 18, 153–157. [Google Scholar] [CrossRef]
- Popravko, S.A.; Tikhomirova, V.I.; Vulfson, N.S. Comparative Study of the Chemical Composition and Biological Activity of Propolis and Its Sources. In Propolis; Apimondia Pub. Housem: Bucharest, Romania, 1985; pp. 35–37. [Google Scholar]
- Gawroński, S.W.; Greger, M.; Gawrońska, H. Plant Taxonomy and Metal Phytoremediation. Soil Biol. 2011, 30, 91–109. [Google Scholar] [CrossRef]
- Fischer, A.; Lindner, M.; Abs, C.; Lasch, P. Vegetation dynamics in central european forest ecosystems (near-natural as well as managed) after storm events. Folia Geobot. Phytotaxon. 2002, 37, 17–32. [Google Scholar] [CrossRef]
- Hynynen, J.; Niemisto, P.; Vihera-Aarnio, A.; Brunner, A.; Hein, S.; Velling, P. Silviculture of birch (Betula pendula Roth and Betula pubescens Ehrh.) in northern Europe. Forestry 2010, 83, 103–119. [Google Scholar] [CrossRef]
- Smart, L.B.; Cameron, K.D. Genetic Improvement of Willow (Salix spp.) as a Dedicated Bioenergy Crop. In Genetic Improvement of Bioenergy Crops; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Rasul, A.; Millimouno, F.M.; Eltayb, W.A.; Ali, M.; Li, J.; Li, X. Pinocembrin: A Novel Natural Compound with Versatile Pharmacological and Biological Activities. BioMed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.K.; Jaiswal, G.; Bhutani, K.K. A review on biological sources, chemistry and pharmacological activities of pinostrobin. Nat. Prod. Res. 2016, 30, 2017–2027. [Google Scholar] [CrossRef]
- Pobłocka-Olech, L.; Inkielewicz-Stepniak, I.; Krauze-Baranowska, M. Anti-inflammatory and antioxidative effects of the buds from different species of Populus in human gingival fibroblast cells: Role of bioflavanones. Phytomedicine 2019, 56, 1–9. [Google Scholar] [CrossRef]
- Daugsch, A.; Moraes, C.S.; Fort, P.; Park, Y.K. Brazilian Red Propolis—Chemical Composition and Botanical Origin. Evid. Based Complement. Altern. Med. 2008, 5, 435–441. [Google Scholar] [CrossRef]
- Farooqui, T. Beneficial effects of propolis on human health and neurological diseases. Front. Biosci. 2012, E4, 779. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Bogdanov, S.; Sabatini, A.-G. Chemical composition of European propolis: Expected and unexpected results. Z. Nat. C 2002, 57, 530–533. [Google Scholar] [CrossRef]
- Popova, M.; Trusheva, B.; Khismatullin, R.; Gavrilova, N.; Legotkina, G.; Lyapunov, J.; Bankova, V. The Triple Botanical Origin of Russian Propolis from the Perm Region, Its Phenolic Content and Antimicrobial Activity. Nat. Prod. Commun. 2013, 8, 617–621. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Szczepaniak, L.; Bakier, S. Rapid GC/MS determination of botanical precursors of Eurasian propolis. Food Chem. 2014, 142, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.A.; Bagan, R.; Szczepaniak, L.; Swiecicka, I. Chemical profile and antimicrobial activity of extractable compounds of Betula litwinowii (Betulaceae) buds. Open Chem. 2015, 13, 125–137. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Blüthgen, N. A Sticky Affair: Resin Collection by Bornean Stingless Bees. Biotropica 2009, 41, 730–736. [Google Scholar] [CrossRef]
- Wilson, M.B.; Spivak, M.; Hegeman, A.D.; Rendahl, A.; Cohen, J.D. Metabolomics Reveals the Origins of Antimicrobial Plant Resins Collected by Honey Bees. PLoS ONE 2013, 8, e77512. [Google Scholar] [CrossRef]
- Wilson, M.B.; Brinkman, D.; Spivak, M.; Gardner, G.; Cohen, J.D. Regional variation in composition and antimicrobial activity of US propolis against Paenibacillus larvae and Ascosphaera apis. J. Invertebr. Pathol. 2015, 124, 44–50. [Google Scholar] [CrossRef]
- Dudonné, S.; Poupard, P.; Coutière, P.; Woillez, M.; Richard, T.; Mérillon, J.-M.; Vitrac, X. Phenolic Composition and Antioxidant Properties of Poplar Bud (Populus nigra) Extract: Individual Antioxidant Contribution of Phenolics and Transcriptional Effect on Skin Aging. J. Agric. Food Chem. 2011, 59, 4527–4536. [Google Scholar] [CrossRef]
- Tree Names. Available online: https://www.treenames.net/ti/populus/populus_names_index.html (accessed on 7 April 2018).
- Marcucci, M.C. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Vinogorova, V.T. GC-MS Analysis of Compounds Extracted from Buds of Populus balsamifera and Populus nigra. Z. Nat. C 2003, 58, 355–360. [Google Scholar] [CrossRef]
- Pobłocka-Olech, L.; Migas, P.; Krauze-Baranowska, M. TLC determination of some flavanones in the buds of different genus Populus species and hybrids. Acta Pharm. 2018, 68, 199–210. [Google Scholar] [CrossRef]
- Xu, L.; Liu, H.; Ma, Y.; Wu, C.; Li, R.; Chao, Z. Comparative study of volatile components from male and female flower buds of Populus x tomentosa by HS-SPME-GC-MS. Nat. Prod. Res. 2019, 33, 2105–2108. [Google Scholar] [CrossRef]
- Ellis, G. Special Issue 40th ISEO: Toxicological challenges for essential oils in REACH. Flavour Fragr. J. 2010, 25, 138–144. [Google Scholar] [CrossRef]
- Park, Y.K.; Alencar, S.M.; Aguiar, C.L. Botanical Origin and Chemical Composition of Brazilian Propolis. J. Agric. Food Chem. 2002, 50, 2502–2506. [Google Scholar] [CrossRef] [PubMed]
- Adelmann, J.; Passos, M.; Breyer, D.H.; Dos Santos, M.H.R.; Lenz, C.; Leite, N.F.; Lancas, F.M.; Fontana, J.D. Exotic flora dependence of an unusual Brazilian propolis: The pinocembrin biomarker by capillary techniques. J. Pharm. Biomed. Anal. 2007, 43, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Isaeva, E.V.; Lozhkina, G.A.; Ryazanova, T.V. A study of the alcohol extract from balsam poplar buds. Russ. J. Bioorg. Chem. 2010, 36, 929–933. [Google Scholar] [CrossRef]
- Bertrams, J.; Müller, M.B.; Kunz, N.; Kammerer, D.R.; Stintzing, F.C. Phenolic compounds as marker compounds for botanical origin determination of German propolis samples based on TLC and TLC-MS. J. Appl. Bot. Food Qual. 2013, 86, 143–153. [Google Scholar] [CrossRef]
- Rubiolo, P.; Casetta, C.; Cagliero, C.; Brevard, H.; Sgorbini, B.; Bicchi, C. Populus nigra L. bud absolute: A case study for a strategy of analysis of natural complex substances. Anal. Bioanal. Chem. 2012, 405, 1223–1235. [Google Scholar] [CrossRef]
- Al Mărghitaş, L.; Dezmirean, D.S.; Bobiş, O. Important Developments in Romanian Propolis Research. Evid. Based Complement. Altern. Med. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Miguel, M.G. Chemical and biological properties of propolis from the western countries of the Mediteraneean basin and Portugal. Int. J. Pharm. Pharmaceut. Sci. 2013, 5, 403–409. [Google Scholar]
- Dimkić, I.; Ristivojević, P.; Janakiev, T.; Beric, T.; Trifković, J.; Milojković-Opsenica, D.; Stanković, S. Phenolic profiles and antimicrobial activity of various plant resins as potential botanical sources of Serbian propolis. Ind. Crop. Prod. 2016, 94, 856–871. [Google Scholar] [CrossRef]
- Gardini, S.; Bertelli, D.; Marchetti, L.; Graziosi, R.; Pinetti, D.; Plessi, M.; Marcazzan, G.L. Chemical composition of Italian propolis of different ecoregional origin. J. Apic. Res. 2018, 57, 639–647. [Google Scholar] [CrossRef]
- Maciejewicz, W.; Daniewski, M.; Dzio, T.B.; Bal, K. GC-MS and HPLC analysis of phenolic acids extracted from propolis and from Populus nigra bud exudates. Chem. Anal. 2002, 47, 21–30. [Google Scholar]
- Jerković, I.; Mastelić, J. Volatile compounds from leaf-buds of Populus nigra L. (Salicaceae). Phytochemistry 2003, 63, 109–113. [Google Scholar] [CrossRef]
- Greenaway, W. Compositions of Bud and Leaf Exudates of Some Populus Species Compared. Z. Nat. C 1992, 47, 329–334. [Google Scholar] [CrossRef]
- Debbache-Benaida, N.; Atmani, D.; Atmani, D. Chemical analysis and biological activities of Populus nigra, flower buds extracts as source of propolis in Algeria. Ind. Crop. Prod. 2014, 53, 85–92. [Google Scholar] [CrossRef]
- Heinrich, M.; Barnes, J.; Gibbons, S.; Williamson, E.M. Fundamentals of Pharmacognosy and Phytotherapy; Churchill Livingstone: Edinburgh, UK, 2018. [Google Scholar]
- McCune, L.M.; Johns, T. Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the Indigenous Peoples of the North American boreal forest. J. Ethnopharmacol. 2002, 82, 197–205. [Google Scholar] [CrossRef]
- Stanciu, G.; Chililă, E.; Dobrinaş, S.; Negreanu-Pîrjol, T. Studies regarding the determination of antioxidant properties of new plant extracts for cosmetic purposes. Rev. Chim. 2010, 61, 41–44. [Google Scholar]
- Wang, K.; Zhang, J.; Ping, S.; Ma, Q.; Chen, X.; Xuan, H.; Shi, J.; Zhang, C.; Hu, F. Anti-inflammatory effects of ethanol extracts of Chinese propolis and buds from poplar (Populus x canadensis). J. Ethnopharmacol. 2014, 155, 300–311. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, X.; Xueping, C.; Wang, K.; Shi, J.; Zhang, C.; Zheng, H.; Huoqing, Z. Comparisons of Ethanol Extracts of Chinese Propolis (Poplar Type) and Poplar Gums Based on the Antioxidant Activities and Molecular Mechanism. Evid. Based Complement. Altern. Med. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Falcão, S.I.; Freire, C.; Figueiredo, A.C.; Vilas-Boas, M. The volatile composition of Portuguese propolis towards its origin discrimination. Rec. Nat. Prod. 2016, 10, 176–188. [Google Scholar]
- Ramallo, I.A.; Zacchino, S.A.; Furlan, R.L. A rapid TLC autographic method for the detection of xanthine oxidase inhibitors and superoxide scavengers. Phytochem. Anal. 2005, 17, 15–19. [Google Scholar] [CrossRef]
- Medina, C.; Inkielewicz-Stępniak, I.; Santos-Martínez, M.J.O.; Radomski, M.W. Pharmacological and toxicological effects of co-exposure of human gingival fibroblasts to silver nanoparticles and sodium fluoride. Int. J. Nanomed. 2014, 9, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, W.; Jin, L. Caffeic acid alleviates inflammatory response in rheumatoid arthritis fibroblast-like synoviocytes by inhibiting phosphorylation of IκB kinase α/β and IκBα. Int. Immunopharmacol. 2017, 48, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ristivojević, P.; Trifković, J.; Andrić, F.; Milojković-Opsenica, D. Poplar-type Propolis: Chemical Composition, Botanical Origin and Biological Activity. Nat. Prod. Commun. 2015, 10, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.C.; Popova, M.P.; Bankova, V.S. An Update on the Constituents of Poplar-Type Propolis; Acdegroot Publishing: Wapserveen, The Netherlands, 2014; ISBN 978-90-813233-0-7. [Google Scholar]
- Fernández-Calderón, M.C.; Navarro-Pérez, M.L.; Blanco-Roca, M.T.; Gómez-Navia, C.; Pérez-Giraldo, C.; Vadillo-Rodríguez, V. Chemical Profile and Antibacterial Activity of a Novel Spanish Propolis with New Polyphenols also Found in Olive Oil and High Amounts of Flavonoids. Molecules 2020, 25, 3318. [Google Scholar] [CrossRef] [PubMed]
- Aliboni, A.; D’Andrea, A.; Massanisso, P. Propolis Specimens from Different Locations of Central Italy: Chemical Profiling and Gas Chromatography–Mass Spectrometry (GC−MS) Quantitative Analysis of the Allergenic Esters Benzyl Cinnamate and Benzyl Salicylate. J. Agric. Food Chem. 2011, 59, 282–288. [Google Scholar] [CrossRef]
- Papotti, G.; Bertelli, D.; Bortolotti, L.; Plessi, M. Chemical and Functional Characterization of Italian Propolis Obtained by Different Harvesting Methods. J. Agric. Food Chem. 2012, 60, 2852–2862. [Google Scholar] [CrossRef]
- Hegazi, A.G.; El-Hady, F.K.A.; Allah, F.A.M.A. Chemical Composition and Antimicrobial Activity of European Propolis. Z. Nat. C 2000, 55, 70–75. [Google Scholar] [CrossRef]
- Boisard, S.; Shahali, Y.; Aumond, M.; Derbré, S.; Blanchard, P.; Dadar, M.; Le Ray, A.; Richomme, P. Anti-AGE activity of poplar-type propolis: Mechanism of action of main phenolic compounds. Int. J. Food Sci. Technol. 2019, 55, 453–460. [Google Scholar] [CrossRef]
- Volpi, N.; Bergonzini, G. Analysis of flavonoids from propolis by on-line HPLC–electrospray mass spectrometry. J. Pharm. Biomed. Anal. 2006, 42, 354–361. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Lyoussi, B.; Miguel, M.G. Insight on Propolis from Mediterranean Countries: Chemical Composition, Biological Activities and Application Fields. Chem. Biodivers. 2019, 16, e1900094. [Google Scholar] [CrossRef]
- Falcão, S.I.; Vilas-Boas, M.; Estevinho, L.M.; Barros, C.; Domingues, M.D.R.; Cardoso, S.M. Phenolic characterization of Northeast Portuguese propolis: Usual and unusual compounds. Anal. Bioanal. Chem. 2009, 396, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Falcão, S.I.; Vale, N.; Gomes, P.; Domingues, M.R.M.; Freire, C.; Cardoso, S.M.; Vilas-Boas, M. Phenolic Profiling of Portuguese Propolis by LC-MS Spectrometry: Uncommon Propolis Rich in Flavonoid Glycosides. Phytochem. Anal. 2013, 24, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Christov, R.; Trusheva, B.; Popova, M.; Bankova, V.; Bertrand, M. Chemical composition of propolis from Canada, its antiradical activity and plant origin. Nat. Prod. Res. 2006, 20, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.P.; Bankova, V.; Bogdanov, S.; Tsvetkova, I.; Naydenski, C.; Marcazzan, G.L.; Sabatini, A.-G. Chemical characteristics of poplar type propolis of different geographic origin. Apidologie 2007, 38, 306. [Google Scholar] [CrossRef]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent Progress of Propolis for Its Biological and Chemical Compositions and Its Botanical Origin. Evid. Based Complement. Altern. Med. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- De Groot, A.C. Propolis. Dermatitis 2013, 24, 263–282. [Google Scholar] [CrossRef]
- Saleh, K.; Zhang, T.; Fearnley, J.; Watson, D.G. A Comparison of the Constituents of Propolis from Different Regions of the United Kingdom by Liquid Chromatography-High Resolution Mass Spectrometry using a Metabolomics Approach. Curr. Metab. 2015, 3, 42–53. [Google Scholar] [CrossRef]
- Miguel, M.G.; Nunes, S.; Cruz, C.; Duarte, J.; Antunes, M.D.C.; Cavaco, A.M.; Mendes, M.D.D.S.; Lima, A.S.; Pedro, L.G.; Barroso, J.G.; et al. Propolis volatiles characterisation from acaricide-treated and -untreated beehives maintained at Algarve (Portugal). Nat. Prod. Res. 2013, 27, 743–749. [Google Scholar] [CrossRef]
- Trusheva, B.; Popova, M.; Bankova, V.; Tsvetkova, I.; Naydensky, H.; Sabatini, A.G. A new type of European propolis, containing bioactive labdanes. Riv. Ital. 2003, 36, 3–7. [Google Scholar]
- García-Viguera, C.; Greenaway, W.; Whatley, F.R. Composition of Propolis from Two Different Spanish Regions. Z. Nat. C 1992, 47, 634–637. [Google Scholar] [CrossRef]
- Chasset, T.; Häbe, T.T.; Ristivojevic, P.; Morlock, G.E. Profiling and classification of French propolis by combined multivariate data analysis of planar chromatograms and scanning direct analysis in real time mass spectra. J. Chromatogr. A 2016, 1465, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.S.; Popov, S.S.; Marekov, N.L. A Study on Flavonoids of Propolis. J. Nat. Prod. 1983, 46, 471–474. [Google Scholar] [CrossRef]
- Bankova, V.; Dyulgerov, A.; Popov, S.; Marekov, N. A GC/MS Study of the Propolis Phenolic Constituents. Z. Nat. C 1987, 42, 147–151. [Google Scholar] [CrossRef]
- Peycheva, S.; Apostolova, E.; Gardjeva, P.; Peychev, Z.; Kokova, V.; Angelov, A.; Slavov, A.; Murdjeva, M. Effect of Bulgarian propolis on the oral microflora in adolescents with plaque-induced gingivitis. Rev. Bras. Farm. 2019, 29, 271–277. [Google Scholar] [CrossRef]
- Keskin, N.; Hazir, S.; Can Baser, K.H.; Kürkçüoglu, M. Antibacterial activity and chemical composition of Turkish propolis. Z. Nat. 2001, 56, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Silici, S.; Kaftanoglu, O.; Bankova, V. Antibacterial activity of Turkish propolis and its qualitative and quantitative chemical composition. Phytomedicine 2005, 12, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Guzelmeric, E.; Ristivojevic, P.; Trifkovic, J.; Dastan, T.; Yilmaz, O.; Cengiz, O.; Yeşilada, E. Authentication of Turkish propolis through HPTLC fingerprints combined with multivariate analysis and palynological data and their comparative antioxidant activity. LWT 2018, 87, 23–32. [Google Scholar] [CrossRef]
- Ristivojevic, P.; Dimkić, I.; Guzelmeric, E.; Trifkovic, J.; Knežević, M.; Beric, T.; Yeşilada, E.; Milojković-Opsenica, D.; Stanković, S. Profiling of Turkish propolis subtypes: Comparative evaluation of their phytochemical compositions, antioxidant and antimicrobial activities. LWT 2018, 95, 367–379. [Google Scholar] [CrossRef]
- Coneac, G.; Gafiţeanu, E.; Hadărugă, D.I.; Hădărugă, N.G.; Pînzaru, I.A.; Bandur, G.; Urşica, L.; Păunescu, V.; Gruia, A. Flavonoid contents of propolis from the West Side of Romania and correlation with the antioxidant activity. Chem. Bull. Timişoara Univ. 2008, 53, 56–60. [Google Scholar]
- Duca, A.; Sturza, D.M.A.; Moacă, E.-A.; Negrea, M.; Lalescu, V.-D.; Lungeanu, D.; Dehelean, C.; Muntean, D.M.; Alexa, E. Identification of Resveratrol as Bioactive Compound of Propolis from Western Romania and Characterization of Phenolic Profile and Antioxidant Activity of Ethanolic Extracts. Molecules 2019, 24, 3368. [Google Scholar] [CrossRef]
- Svečnjak, L.; Marijanović, Z.; Okińczyc, P.; Kuś, P.M.; Jerković, I. Mediterranean Propolis from the Adriatic Sea Islands as a Source of Natural Antioxidants: Comprehensive Chemical Biodiversity Determined by GC-MS, FTIR-ATR, UHPLC-DAD-QqTOF-MS, DPPH and FRAP Assay. Antioxidants 2020, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Graikou, K.; Popova, M.; Gortzi, O.; Bankova, V.; Chinou, I. Characterization and biological evaluation of selected Mediterranean propolis samples. Is it a new type? LWT 2016, 65, 261–267. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Anastasiadou, P.; Papadopoulos, A.; Machera, K. Revisiting Greek Propolis: Chromatographic Analysis and Antioxidant Activity Study. PLoS ONE 2017, 12, e0170077. [Google Scholar] [CrossRef] [PubMed]
- Melliou, E.; Stratis, E.; Chinou, I. Volatile constituents of propolis from various regions of Greece—Antimicrobial activity. Food Chem. 2007, 103, 375–380. [Google Scholar] [CrossRef]
- Szliszka, E.; Sokół-Łętowska, A.; Kucharska, A.Z.; Jaworska, D.; Czuba, Z.P.; Król, W. Ethanolic Extract of Polish Propolis: Chemical Composition and TRAIL-R2 Death Receptor Targeting Apoptotic Activity against Prostate Cancer Cells. Evid. Based Complement. Altern. Med. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Popova, M.; Giannopoulou, E.; Skalicka-Woźniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.P.; Sivolapenko, G.B.; Gaweł-Bęben, K.; Antosiewicz, B.; et al. Characterization and Biological Evaluation of Propolis from Poland. Molecules 2017, 22, 1159. [Google Scholar] [CrossRef] [PubMed]
- Wezgowiec, J.; Wieczynska, A.; Wieckiewicz, W.; Kulbacka, J.; Saczko, J.; Pachura, N.; Wieckiewicz, M.; Gancarz, R.; Wilk, K.A. Polish Propolis—Chemical Composition and Biological Effects in Tongue Cancer Cells and Macrophages. Molecules 2020, 25, 2426. [Google Scholar] [CrossRef]
- Morlock, G.E.; Ristivojevic, P.; Chernetsova, E.S. Combined multivariate data analysis of high-performance thin-layer chromatography fingerprints and direct analysis in real time mass spectra for profiling of natural products like propolis. J. Chromatogr. A 2014, 1328, 104–112. [Google Scholar] [CrossRef]
- Mouse, H.A.; Tilaoui, M.; Jaafari, A.; M’Barek, L.A.; Aboufatima, R.; Chait, A.; Zyad, A. Evaluation of the in vitro and in vivo anticancer properties of Moroccan propolis extracts. Rev. Bras. Farm. 2012, 22, 558–567. [Google Scholar] [CrossRef][Green Version]
- Segueni, N.; Khadraoui, F.; Moussaoui, F.; Zellagui, A.; Gherraf, N.; Lahouel, M.; Rhouati, S. Volatile constituents of Algerian propolis. Ann. Biol. Res. 2010, 1, 103–107. [Google Scholar]
- Lahouel, M.; Boutabet, K.; Kebsa, W.; Alyane, M. Polyphenolic fraction of Algerian propolis protects rat kidney against acute oxidative stress induced by doxorubicin. Indian J. Nephrol. 2011, 21, 101–106. [Google Scholar] [CrossRef]
- Segueni, N.; Magid, A.A.; Decarme, M.; Rhouati, S.; Lahouel, M.; Antonicelli, F.; Lavaud, C.; Hornebeck, W. Inhibition of Stromelysin-1 by Caffeic Acid Derivatives from a Propolis Sample from Algeria. Planta Med. 2011, 77, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Segueni, N.; Zellagui, A.; Moussaoui, F.; Lahouel, M.; Rhouati, S. Flavonoids from Algerian propolis. Arab. J. Chem. 2016, 9, S425–S428. [Google Scholar] [CrossRef]
- Martos, I.; Cossentini, M.; Ferreres, F.; Tomás-Barberán, F.A. Flavonoid Composition of Tunisian Honeys and Propolis. J. Agric. Food Chem. 1997, 45, 2824–2829. [Google Scholar] [CrossRef]
- Cottica, S.M.; Sabik, H.; Antoine, C.; Fortin, J.; Graveline, N.; Visentainer, J.V.; Britten, M. Characterization of Canadian propolis fractions obtained from two-step sequential extraction. LWT 2015, 60, 609–614. [Google Scholar] [CrossRef]
- Islam, K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Development and validation of an HPTLC–DPPH assay and its application to the analysis of honey. J. Planar Chromatogr. Mod. TLC 2020, 33, 301–311. [Google Scholar] [CrossRef]
- Russo, A.; Cardile, V.; Sánchez, F.; Troncoso, N.; Vanella, A.; Garbarino, J. Chilean propolis: Antioxidant activity and antiproliferative action in human tumor cell lines. Life Sci. 2004, 76, 545–558. [Google Scholar] [CrossRef]
- Miguel, M.G.; Nunes, S.; Dandlen, S.A.; Cavaco, A.M.; Antunes, M.D. Phenols and antioxidant activity of hydro-alcoholic extracts of propolis from Algarve, South of Portugal. Food Chem. Toxicol. 2010, 48, 3418–3423. [Google Scholar] [CrossRef]
- Torres, A.; Sandjo, L.; Friedemann, M.; Tomazzoli, M.; Maraschin, M.; Mello, C.; Dos Santos, A.R.S. Chemical characterization, antioxidant and antimicrobial activity of propolis obtained from Melipona quadrifasciata quadrifasciata and Tetragonisca angustula stingless bees. Braz. J. Med Biol. Res. 2018, 51, e7118. [Google Scholar] [CrossRef]
- Seibert, J.B.; Bautista-Silva, J.P.; Amparo, T.R.; Petit, A.; Pervier, P.; Almeida, J.C.D.S.; Azevedo, M.C.; Silveira, B.M.; Brandão, G.C.; De Souza, G.H.B.; et al. Development of propolis nanoemulsion with antioxidant and antimicrobial activity for use as a potential natural preservative. Food Chem. 2019, 287, 61–67. [Google Scholar] [CrossRef]
- Araujo, M.A.R.; Libério, S.A.; Guerra, R.N.M.; Ribeiro, M.N.S.; Nascimento, F.R.F. Mechanisms of action underlying the anti-inflammatory and immunomodulatory effects of propolis: A brief review. Rev. Bras. Farm. 2012, 22, 208–219. [Google Scholar] [CrossRef]
- Boyanova, L.; Kolarov, R.; Gergova, G.; Mitov, I. In vitro activity of Bulgarian propolis against 94 clinical isolates of anaerobic bacteria. Anaerobe 2006, 12, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Koru, O.; Toksoy, F.; Acikel, C.H.; Tunca, Y.M.; Baysallar, M.; Guclu, A.U.; Akca, E.; Tuylu, A.O.; Sorkun, K.; Tanyuksel, M.; et al. In vitro antimicrobial activity of propolis samples from different geographical origins against certain oral pathogens. Anaerobe 2007, 13, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Kharsany, K.; Viljoen, A.; Leonard, C.; Van Vuuren, S. The new buzz: Investigating the antimicrobial interactions between bioactive compounds found in South African propolis. J. Ethnopharmacol. 2019, 238, 111867. [Google Scholar] [CrossRef]
- Veloz, J.J.; Alvear, M.; Salazar, L.A. Antimicrobial and Antibiofilm Activity against Streptococcus mutans of Individual and Mixtures of the Main Polyphenolic Compounds Found in Chilean Propolis. BioMed Res. Int. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Christov, R.; Popov, S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef]
- Banskota, A.; Tezuka, Y.; Adnyana, I.K.; Ishii, E.; Midorikawa, K.; Matsushige, K.; Kadota, S. Hepatoprotective and anti-Helicobacter pylori activities of constituents from Brazilian propolis. Phytomedicine 2001, 8, 16–23. [Google Scholar] [CrossRef]
- Ota, C.; Unterkircher, C.; Fantinato, V.; Shimizu, M. Antifungal activity of propolis on different species of Candida. Mycoses 2001, 44, 375–378. [Google Scholar] [CrossRef]
- Akao, Y.; Maruyama, H.; Matsumoto, K.; Ohguchi, K.; Nishizawa, K.; Sakamoto, T.; Araki, Y.; Mishima, S.; Nozawa, Y. Cell Growth Inhibitory Effect of Cinnamic Acid Derivatives from Propolis on Human Tumor Cell Lines. Biol. Pharm. Bull. 2003, 26, 1057–1059. [Google Scholar] [CrossRef]
- Valente, M.J.; Baltazar, A.F.; Henrique, R.; Estevinho, L.; Carvalho, M.; Estevinho, L.M. Biological activities of Portuguese propolis: Protection against free radical-induced erythrocyte damage and inhibition of human renal cancer cell growth in vitro. Food Chem. Toxicol. 2011, 49, 86–92. [Google Scholar] [CrossRef]
- Da Cunha, M.G.; Franchin, M.; Galvão, L.; Ruiz, A.L.; Carvalho, J.E.; Ikegaki, M.; De Alencar, S.M.; Koo, H.; Rosalen, P. Antimicrobial and antiproliferative activities of stingless bee Melipona scutellaris geopropolis. BMC Complement. Altern. Med. 2013, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.L.; Queiroz, R.F.; Sawaya, A.C.H.F.; Lopez, B.G.-C.; Soares, M.B.; Bezerra, D.P.; Rodrigues, A.C.B.; De Paula, V.F.; Waldschmidt, A.M. Melipona mondury produces a geopropolis with antioxidant, antibacterial and antiproliferative activities. An. da Acad. Bras. de Ciências 2017, 89, 2247–2259. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, P.; Neuner, A.; Nolkemper, S.; Zundel, C.; Nowack, H.; Sensch, K.H.; Reichling, J. Antiviral Activity and Mode of Action of Propolis Extracts and Selected Compounds. Phytother. Res. 2010, 24 (Suppl. 1), S20–S28. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Guo, Z.; Shen, Z.; Wang, J.; Hu, Y.; Wang, D. The immune enhancement of propolis adjuvant on inactivated porcine parvovirus vaccine in guinea pig. Cell. Immunol. 2011, 270, 13–18. [Google Scholar] [CrossRef]
- Shimizu, T.; Takeshita, Y.; Takamori, Y.; Kai, H.; Sawamura, R.; Yoshida, H.; Watanabe, W.; Tsutsumi, A.; Park, Y.K.; Yasukawa, K.; et al. Efficacy of Brazilian Propolis against Herpes Simplex Virus Type 1 Infection in Mice and Their Modes of Antiherpetic Efficacies. Evid. Based Complement. Altern. Med. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Fernandes, M.H.V.; Ferreira, L.D.N.; Vargas, G.D.; Fischer, G.; Hübner, S.D.O. Efeito do extrato aquoso de própolis marrom sobre a produção de ifn-γ após imunização contra parvovírus canino (cpv) e coronavírus canino (ccov). Ciên. Anim. Bras. 2015, 16, 235–242. [Google Scholar] [CrossRef]
- Hazem, A.; Pitică-Aldea, I.M.; Popescu, C.; Matei, L.; Dragu, D.; Economescu, M.; Alexiu, I.; Crişan, I.; Diaconu, C.C.; Bleotu, C.; et al. The antiviral/virucidal effects of alcoholic and aqueous extracts with propolis. Farmacia 2017, 65, 868–876. [Google Scholar]
- Braakhuis, A.J. Evidence on the Health Benefits of Supplemental Propolis. Nutrients 2019, 11, 2705. [Google Scholar] [CrossRef]
- Mihai, C.M.; Mărghitaş, L.A. Antioxidant capacity of Transylvanian propolis. Bull. Anim. Sci. Biotechnol. 2010, 67, 266–270. [Google Scholar]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Different Forms and in Different Solvents Useful for Finished Products. Foods 2018, 7, 41. [Google Scholar] [CrossRef]
- Cottica, S.M.; Sawaya, A.C.H.F.; Eberlin, M.N.; Franco, S.L.; Zeoula, L.M.; Visentainer, J.V. Antioxidant activity and composition of propolis obtained by different methods of extraction. J. Braz. Chem. Soc. 2011, 22, 929–935. [Google Scholar] [CrossRef]
- Wagh, V.D. Propolis: A Wonder Bees Product and Its Pharmacological Potentials. Adv. Pharmacol. Sci. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Propolis: A new frontier for wound healing? Burn. Trauma 2015, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dezmirean, D.S.; Mărghitaş, L.A.; Chirila, F.; Copaciu, F.; Simonca, V.; Bobiş, O.; Erler, S. Influence of geographic origin, plant source and polyphenolic substances on antimicrobial properties of propolis against human and honey bee pathogens. J. Apic. Res. 2017, 56, 588–597. [Google Scholar] [CrossRef]
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Mihai, C.M.; Mărghitaş, L.A.; Dezmirean, D.S.; Chirilă, F.; Moritz, R.F.; Schlüns, H. Interactions among flavonoids of propolis affect antibacterial activity against the honeybee pathogen Paenibacillus larvae. J. Invertebr. Pathol. 2012, 110, 68–72. [Google Scholar] [CrossRef]
- Parolia, A.; Thomas, M.S.; Kundabala, M.; Mohan, M. Propolis and its potential used in oral health. Int. J. Med. Med. Sci. 2010, 2, 210–215. [Google Scholar]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef]
- Machado, B.; Pulcino, T.; Silva, A.; Melo, D.; Silva, R.; Mendonca, I. Propolis as an alternative in prevention and control of dental cavity. J. Apither. 2016, 1. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial properties of propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed]
- Orsolic, N.; Basic, I. Immunomodulatory by water-soluble derivative of propolis: A factor of antitumor reactivity. J. Ethnopharm. 2003, 84, 265–273. [Google Scholar] [CrossRef]
- Seven, P.T.; Seven, I.; Karakus, S.; Mutlu, S.I.; Arkali, G.; Sahin, Y.M.; Kilislioglu, A. Turkish Propolis and Its Nano Form Can Ameliorate the Side Effects of Cisplatin, Which Is a Widely Used Drug in the Treatment of Cancer. Plants 2020, 9, 1075. [Google Scholar] [CrossRef]
- Magro-Filho, O.; De Carvalho, A.C.P. Topical Effect of Propolis in the Repair of Sulcoplasties by the Modified Kazanjian Technique. Cytological and Clinical Evaluation. J. Nihon Univ. Sch. Dent. 1994, 36, 102–111. [Google Scholar] [CrossRef][Green Version]
- Martin, M.P.; Pileggi, R. A quantitative analysis of Propolis: A promising new storage media following avulsion. Dent. Traumatol. 2004, 20, 85–89. [Google Scholar] [CrossRef]
- Özan, F.; Sümer, Z.; Polat, Z.A.; Er, K.; Özan, U.; Değer, O. Effect of mouth rinse containing propolis on oral microorganisms and human gingival fibroblast. Eur. J. Dent. 2007, 11, 195–200. [Google Scholar]
- Sabir, A.; Tabbu, C.R.; Agustiono, P.; Sosroseno, W. Histological analysis of rat dental pulp tissue capped with propolis. J. Oral Sci. 2005, 47, 135–138. [Google Scholar] [CrossRef]
- Al-Qathami, H.; Al-Madi, E. Comparison of sodium hypochlorite, propolis and salineas root canal irrigants: A pilot study. Saudi Dent. J. 2003, 5, 100–102. [Google Scholar]
- Koo, H.; Cury, J.A.; Rosalen, P.L.; Ambrosano, G.M.; Ikegaki, M.; Park, Y.K. Effect of a Mouthrinse Containing Selected Propolis on 3-Day Dental Plaque Accumulation and Polysaccharide Formation. Caries Res. 2002, 36, 445–448. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Almas, K.; Dahlan, A.A. The effect of propolis on dentinal hypersensitivity and level of satisfaction among patients from a university hospital Riyadh, Saudi Arabia. Indian J. Dent. Res. 2000, 10, 130–137. [Google Scholar]
- Almas, K.; Mahmoud, A.; Dahlan, A. A comparative study of propolis and saline application on human dentin. A SEM study. Indian J. Dent. Res. 2001, 12, 21–27. [Google Scholar] [PubMed]
- Toker, H.; Ozan, F.; Ozer, H.; Ozdemir, H.; Eren, K.; Yeler, H. A Morphometric and Histopathologic Evaluation of the Effects of Propolis on Alveolar Bone Loss in Experimental Periodontitis in Rats. J. Periodontol. 2008, 79, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.R.; Gomes, R.T.; De Mesquita, R.A.; De Moura, M.D.G.; França, E.C.; De Aguiar, E.G.; Naves, M.D.; Abreu, J.A.S.; Abreu, S.R.L. Efficacy of Brazilian propolis gel for the management of denture stomatitis: A pilot study. Phytother. Res. 2008, 22, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Oncag, O.; Cogulu, D.; Uzel, A.; Sorkun, K. Efficacy of propolis as an intracanal medicament against Enterococcus faecalis. Gen. Dent. 2006, 54, 319–322. [Google Scholar] [PubMed]
- Samet, N.; Laurent, C.; Susarla, S.M.; Samet-Rubinsteen, N. The effect of bee propolis on recurrent aphthous stomatitis: A pilot study. Clin. Oral Investig. 2007, 11, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.S.; Péreira, E.S.J.; Lima, S.M.; Senna, M.I.B.; Mesquita, R.A.; Santos, V.R. Effect of commercial ethanol propolis extract on the in vitro growth of Candida albicans collected from HIV-seropositive and HIV-seronegative Brazilian patients with oral candidiasis. J. Oral Sci. 2002, 44, 41–48. [Google Scholar] [CrossRef][Green Version]
- Bachevski, D.; Damevska, K.; Simeonovski, V.; Dimova, M. Back to the basics: Propolis and COVID-19. Dermatol. Ther. 2020, 33, e13780. [Google Scholar] [CrossRef]
- Berretta, A.A.; Silveira, M.A.D.; Capcha, J.M.C.; De Jong, D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease. Biomed. Pharmacother. 2020, 131, 110622. [Google Scholar] [CrossRef]
- Oliver, C.M.; Campbell, M.; Dulan, O.; Hamilton, N.; Birchall, M. Appearance and management of COVID-19 laryngo-tracheitis: Two case reports. F1000Research 2020, 9, 310. [Google Scholar] [CrossRef]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef]
- Amoros, M.; Simõs, C.M.O.; Girre, L.; Sauvager, F.; Cormier, M. Synergistic Effect of Flavones and Flavonols Against Herpes Simplex Virus Type 1 in Cell Culture. Comparison with the Antiviral Activity of Propolis. J. Nat. Prod. 1992, 55, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Serkedjieva, J.; Manolova, N.; Bankova, V. Anti-influenza Virus Effect of Some Propolis Constituents and Their Analogues (Esters of Substituted Cinnamic Acids). J. Nat. Prod. 1992, 55, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Harish, Z.; Rubinstein, A.; Golodner, M.; Elmaliah, M.; Mizrachi, Y. Suppresion of HIV-1 replication by propolis and its immunoregulatory effect. Drugs Exp. Clin. Res. 1997, 23, 89–96. [Google Scholar] [PubMed]
- Ito, J.; Chang, F.-R.; Wang, H.-K.; Park, Y.K.; Ikegaki, M.; Kilgore, N.; Lee, K.-H. Anti-AIDS Agents. 48.1Anti-HIV Activity of Moronic Acid Derivatives and the New Melliferone-Related Triterpenoid Isolated from Brazilian Propolis. J. Nat. Prod. 2001, 64, 1278–1281. [Google Scholar] [CrossRef]
- Fischer, G.; Cleff, M.B.; Dummer, L.A.; Paulino, N.; Paulino, A.S.; Vilela, C.D.O.; Campos, F.S.; Storch, T.; Vargas, G.D.; Hübner, S.D.O.; et al. Adjuvant effect of green propolis on humoral immune response of bovines immunized with bovine herpesvirus type 5. Vet. Immunol. Immunopathol. 2007, 116, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Hino, A.; Tsutsumi, A.; Park, Y.K.; Watanabe, W.; Kurokawa, M. Anti-Influenza Virus Activity of Propolisin Vitroand its Efficacy against Influenza Infection in Mice. Antivir. Chem. Chemother. 2008, 19, 7–13. [Google Scholar] [CrossRef]
- Bufalo, M.; Figueiredo, A.S.; De Sousa, J.; Candeias, J.M.G.; Bastos, J.; Sforcin, J.M. Anti-poliovirus activity of Baccharis dracunculifolia and propolis by cell viability determination and real-time PCR. J. Appl. Microbiol. 2009, 107, 1669–1680. [Google Scholar] [CrossRef]
- Nolkemper, S.; Reichling, J.; Sensch, K.H.; Schnitzler, P. Mechanism of herpes simplex virus type 2 suppression by propolis extracts. Phytomedicine 2010, 17, 132–138. [Google Scholar] [CrossRef]
- Fesen, M.R.; Pommier, Y.; Leteurtre, F.; Hiroguchi, S.; Yung, J.; Kohn, K.W. Inhibition of HIV-1 integrase by flavones, caffeic acid phenethyl ester (CAPE) and related compounds. Biochem. Pharmacol. 1994, 48, 595–608. [Google Scholar] [CrossRef]
- Wang, G.-F.; Shi, L.-P.; Ren, Y.-D.; Liu, Q.-F.; Liu, H.-F.; Zhang, R.-J.; Li, Z.; Zhu, F.-H.; He, P.-L.; Tang, W.; et al. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antivir. Res. 2009, 83, 186–190. [Google Scholar] [CrossRef]
- Shvarzbeyn, J.; Huleihel, M. Effect of propolis and caffeic acid phenethyl ester (CAPE) on NFκB activation by HTLV-1 Tax. Antivir. Res. 2011, 90, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Wang, Q.; Nanua, S.; Hershenson, M.B.; Sajjan, U.S. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antivir. Res. 2012, 94, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Ichinose, M.; Ikeda, K.; Uozaki, M.; Morishita, J.; Kuwahara, T.; Koyama, A.H.; Yamasaki, H. Inhibition by caffeic acid of the influenza A virus multiplication in vitro. Int. J. Mol. Med. 2014, 34, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Sun, G.; Guo, W.; Huang, Y.; Sun, W.; Zhao, F.; Hu, K. Inhibition of hepatitis B virus replication by quercetin in human hepatoma cell lines. Virol. Sin. 2015, 30, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rojas, Á.; Del Campo, J.A.; Clement, S.; Lemasson, M.; García-Valdecasas, M.; Gil-Gómez, A.; Ranchal, I.; Bartosch, B.; Bautista, J.D.; Rosenberg, A.R.; et al. Effect of Quercetin on Hepatitis C Virus Life Cycle: From Viral to Host Targets. Sci. Rep. 2016, 6, 31777. [Google Scholar] [CrossRef]
- Kwon, M.J.; Shin, H.M.; Perumalsamy, H.; Wang, X.; Ahn, Y.-J. Antiviral effects and possible mechanisms of action of constituents from Brazilian propolis and related compounds. J. Apic. Res. 2019, 59, 413–425. [Google Scholar] [CrossRef]
- Babaei, S.; Torshizi, M.A.K.; Tahmasebi, G.; Miran, S.N.K. Effects of propolis, royal jelly, honey and bee pollen on growth performance and immune system of Japanese quails. Vet. Res. Forum Int. Q. J. 2016, 7, 13–20. [Google Scholar]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Fang, L.; Karakiulakis, G.; Rotha, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776. [Google Scholar] [CrossRef] [PubMed]
- Sforcin, J.M. Propolis and the immune system: A review. J. Ethnopharmacol. 2007, 113, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; Brito, J.C.M.; Nizer, W.S.D.C. Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID-19 (SARS-CoV -2). Phytother. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Scorza, C.A.; Gonçalves, V.C.; Scorza, F.A.; Fiorini, A.C.; De Almeida, A.-C.G.; Fonseca, M.C.; Finsterer, J. Propolis and coronavirus disease 2019 (COVID-19): Lessons from nature. Complement. Ther. Clin. Pract. 2020, 41, 101227. [Google Scholar] [CrossRef] [PubMed]
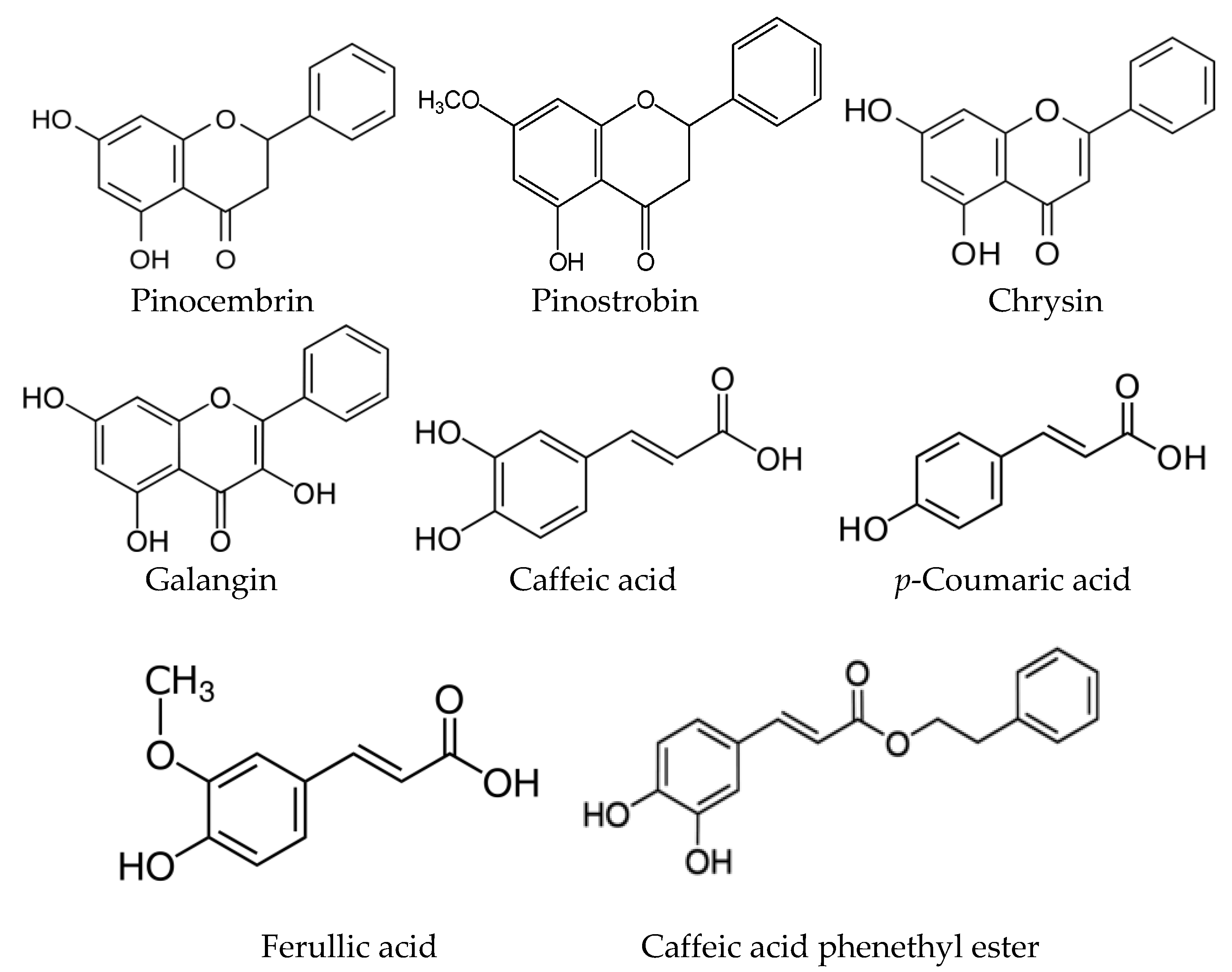
| Plant Material | Separated Compounds | Adsorbent/Mobile Phases | Reference |
|---|---|---|---|
| Brazilian poplar buds | Flavonoid profiles | HPTLC RP-18 F254Merck/Ethanol-water (55:54, v/v) | [47] |
| Brazilian poplar buds | Gallic, ferulic, caffeic p-coumaric acids, quercetin, kaempferol, chrysin, pinocembrin, pinostrobin | TLC Silica gel 60 Merck/Hexane-ethyl acetate (3:2, v/v) | [48] |
| Populus balsamifera | Neutral substances (acylglycerides and sterols) | TLC Silica gel L40/100/petroleum ether-diethyl ether-acetic acid (80:20:1, v/v/v or 70:30:1, v/v/v); heptanes-benzene (9:1, v/v) | [49] |
| Populus nigra, P. nigra “Italica”. P.x can.”Robusta”, P.x canescens, P. berolinensis, P. maximowiczii, P. balsamifera, P. tremula | Apigenin, quercetin, kaempferol, chrysin, naringenin, caffeic acid phenethylester (CAPE), galangin, pinocembrin, caffeic acid | TLC Silica gel 60 Merck/hexane-ethylacetate-glacial acetic acid (5:3:1, v/v/v) | [50] |
| Populus alba, P. tremula, P. nigra “Italica”, P. x canadensis “Robusta”, P. canadensis “Marilandica”, P. balsamifera, P. candicans, P. simonii | Apigenin, luteolin, genkwanin, chrysin, tectochrysin, galangin, isorhamnetin, kaempferol, quercetin, myricetin, eriodictyol, naringenin, pinocembrin, pinostrobin, pinobanksin, chrysin 5, 7-dimethylether, pinocembrin 5, 7-dimethylether | TLC Silica gel 60 Merck/hexane-ethyl acetate-formic acid (60:40:1.3, v/v/v) | [44] |
| Origin | Separated Compounds | Analytical Method | Reference |
|---|---|---|---|
| Portugal | Caffeic acid, ellagic acid, p-coumaric acid, ferulic and isoferulic acid, quercetin, luteolic, apigenin, kaempherol, rhamnetin, chrysin, galangin, acacetin, kaempferide, kaempferol dimethyl ether, other flavonoid glycosides, sesquiterpene, monoterpene, aliphatic and aromatic alcohols, fatty acids, carbonyl compounds, hydroxarbons | LC-DAD ESI-MS; GC-MS | [65,78,79,85] |
| Italy | Communic acid, isocupressic acid, acetylisocupressic acid, caffeic acid, p-coumaric acid, ferulic acid, quercetin, apigenin, kaempferol, chrysin, caffeic acid phenethyl ester, pinocembrin, galangin, benzyl salycilate, benzyl cinnamate, caffedic acid cinnamyl ester, pinobanksin-3-O-acetate | GC-MS, HPLC-MS/MS | [32,72,73,86] |
| Spain | Naringenin, genistein, kaempferol, apigenin, pinocembrin, galangin, acacetin, chrysin, benzoic acid, guaiol, pinostrobin, pinobanksin, galangin-7-methyl ether, pinobanksin-3-acetate, glyceryl trans-caffeate, henicosane, tricosane, pentacosane, hexacosane, heptacosane, nonacosane, tetracosanoic acid | GC-MS, HPLC-ESI-MS | [71,76,87] |
| France | Benzyl caffeate, pinocembrin, trans-p-coumaric acid, caffeic acid, p-coumaric acid, chrysin, pinobanksin, pinobanksin-3-acetate, galangin, kaempferol, tectochrysin | GC-MS, RP-HPTLC-FLD, RP-HPTLC-DART-MS | [74,88] |
| Bulgaria | Dihydrocaffeic acid, dihydroferulic acid, pinostrobin, dimethyl kaempferil, benzyl alcohol pinobanksin, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid, quercetin, myricetin, kaempferol, rutin, catechin, quercetin-3-β-glucside, alcohols, aromatic acids, organic acids, terpenoids | HPLC, GC-MS | [89,90,91] |
| Turkey | Apigenin, pinocembrin, pinobanksin, chrysin, galangin, quercetin, rutin, kaempferol, p-coumaric acid, ferulic acid, caffeic acid and their esters, p-hidroxybenzoic acid, vanillic acid, protocatechuic acid, cinnamyl cinamate, abietic acid, isopimaric acid, dihydroabietic acid, hydroxy fatty acids, phenolic glicerides | TLC; HPTLC, GC-MS; UHPLC-LTQ/Orbitrap/MS/MS | [92,93,94,95] |
| Romania | Gallic acid, protocatechuic acid, syringic acid, caffeic acid, vanillin, p-coumaric acid, ferulic acid, t-cinnamic acid, rosmarinic acid, pinocembrin, chrysin, galangin, pinostrobin, caffeic acid phenethyl ester, rutin, quercetin, apigenin, resveratrol | HPLC-DAD, LC-MS | [52,96,97] |
| Croatia (islands in Mediterranean Sea) | 108 Volatiles determined by HS-SPME/GC-MS 118 Compounds determined by UHPLC-DAD-QqTOF-MS (terpenes, phenolic acids, flavonoids and their derivatives) | GC-MS, FT-MIR, UHPLC-DAD-QqTOF-MS | [98] |
| Greece | 59 Phenolic compounds determined by HPLC-PDA-ESI/MS | HPLC-PDA-ESI/MS, GC-MS | [99,100,101] |
| Poland | Aromatic acids, fatty acids, esters, flavonoids, and chalcones (85 constituents) 37 Phenolic compounds identified by LC-MS | GC-MS, HPLC-DAD, LC-MS, UPLC-Q-TOF-MS | [102,103,104] |
| Germany | Chrysin, pinocembrin, naringenin, pinobanksin, kaempferol, luteolin, pinobanksin-5-methylether, coumaric acid, galangin, apigenin, pinostrobin, benzyl caffeate | HPTLC | [105] |
| Morocco | Wogonoside, quercetin-arabinoseglucoside, apigenin dihexoside, rhamnetin hexoside, baicalin, rhamnetin, isorhamnetin, saphnin, daphnitin, afzelechin-catechin dimmer | HPLC-ESI/MS | [106] |
| Algeria | Pinostrobin chalcone, galangin, naringenic, tectochrysin, methoxychrisin, prenitated coumarin, pectolinaringenin, pilosin, ladanein, chicoric acid, caftaric acid, 2-hexanal, myristic acid, linoleic acid, spathulenol, isooctgane, hexadecane, p-cymene, palnitic acid, 4-terpineol, charvacol, α-cedrol | Two-dimensional paper chromatography, identification by 1H NMR and 13C NMR, HPLC-DAD, GC-MS | [107,108,109,110] |
| Tunisia | Chrysin, galangin, tectochrysin, pinocembrin, pinobanksin, dimerthyallil caffeate, phenylethyl caffeate, myricetin 3, 7, 4′, 5′-tetramethyl ether, quercetin 3, 7, 3′-trimethyl ether | HPLC | [111] |
| Canada | Chrysin, pinocembrin, ellagic acid, pinostrobin, benzyl caffeate, palmitic acid, naringenin, pinobanksin, isopentenyl caffeate, acacetin, caffeic acid, acacetin, caffeic acid phenethyl ester, other aromatic acids, fatty acids, esters, dihydrochalcones | HPLC-ESI/MS, GC-MS | [16,80,112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dezmirean, D.S.; Paşca, C.; Moise, A.R.; Bobiş, O. Plant Sources Responsible for the Chemical Composition and Main Bioactive Properties of Poplar-Type Propolis. Plants 2021, 10, 22. https://doi.org/10.3390/plants10010022
Dezmirean DS, Paşca C, Moise AR, Bobiş O. Plant Sources Responsible for the Chemical Composition and Main Bioactive Properties of Poplar-Type Propolis. Plants. 2021; 10(1):22. https://doi.org/10.3390/plants10010022
Chicago/Turabian StyleDezmirean, Daniel Severus, Claudia Paşca, Adela Ramona Moise, and Otilia Bobiş. 2021. "Plant Sources Responsible for the Chemical Composition and Main Bioactive Properties of Poplar-Type Propolis" Plants 10, no. 1: 22. https://doi.org/10.3390/plants10010022
APA StyleDezmirean, D. S., Paşca, C., Moise, A. R., & Bobiş, O. (2021). Plant Sources Responsible for the Chemical Composition and Main Bioactive Properties of Poplar-Type Propolis. Plants, 10(1), 22. https://doi.org/10.3390/plants10010022








