The Use of Essential Oils from Thyme, Sage and Peppermint against Colletotrichum acutatum
Abstract
1. Introduction
2. Results
2.1. Essential Oils Chemical Composition
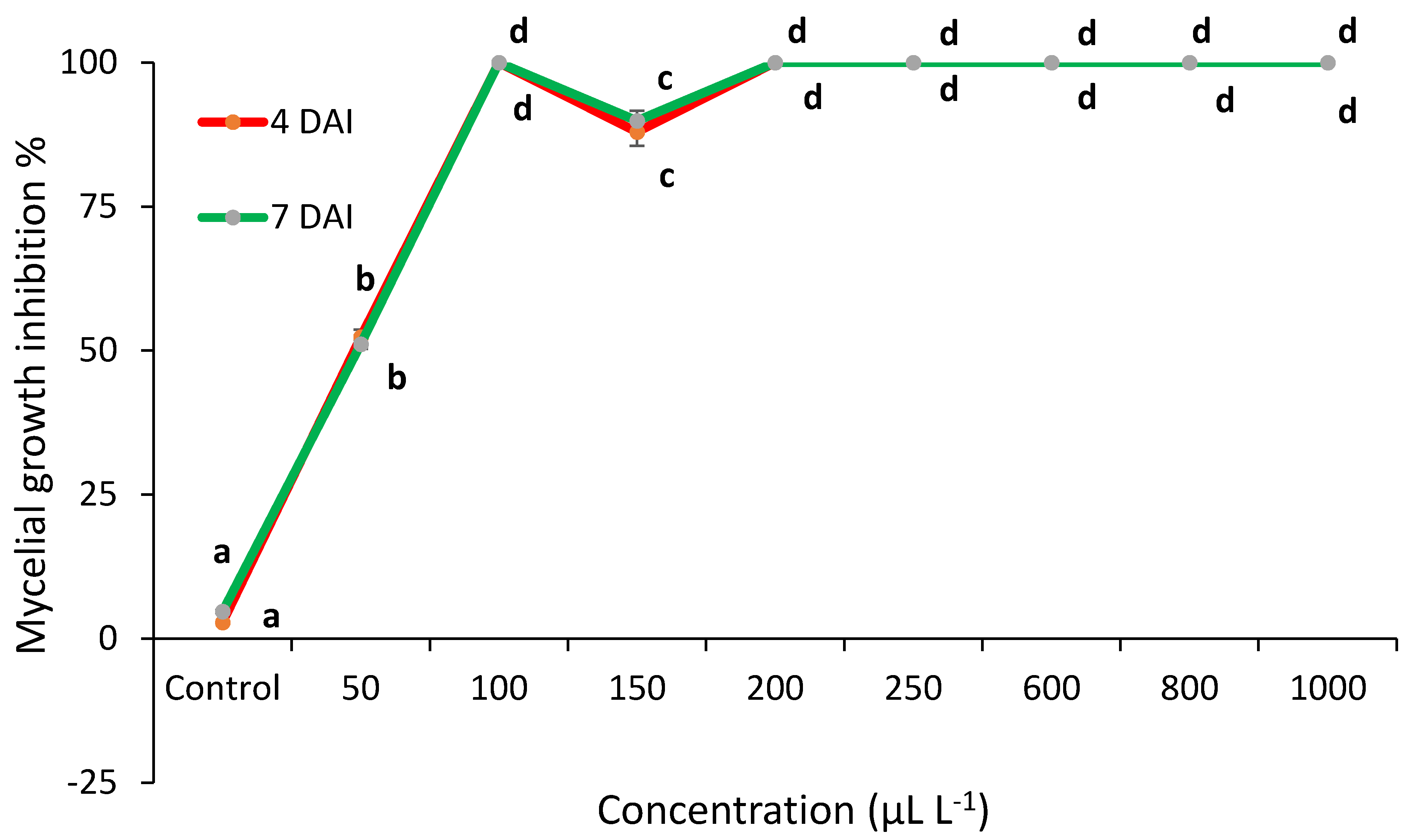
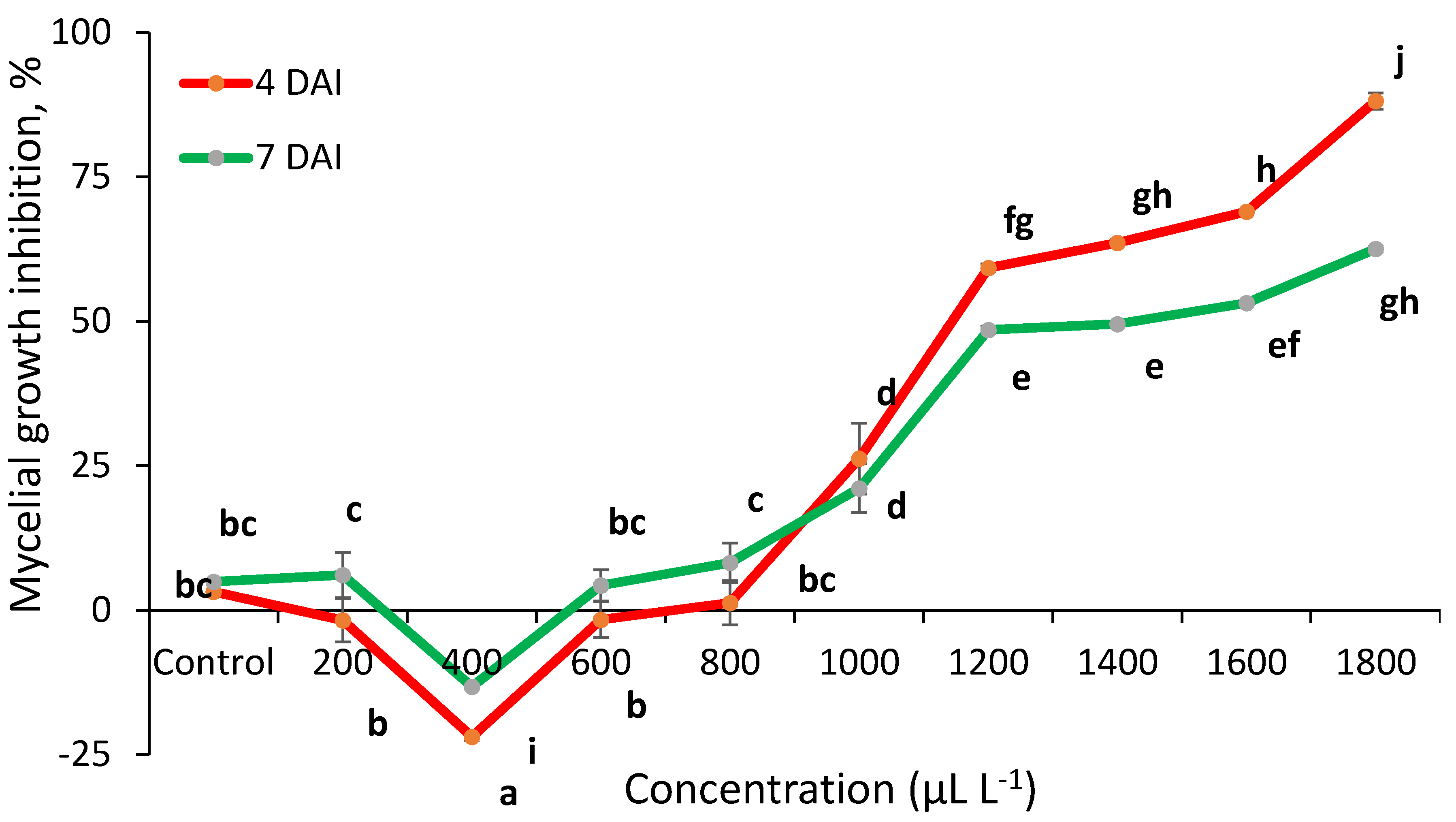
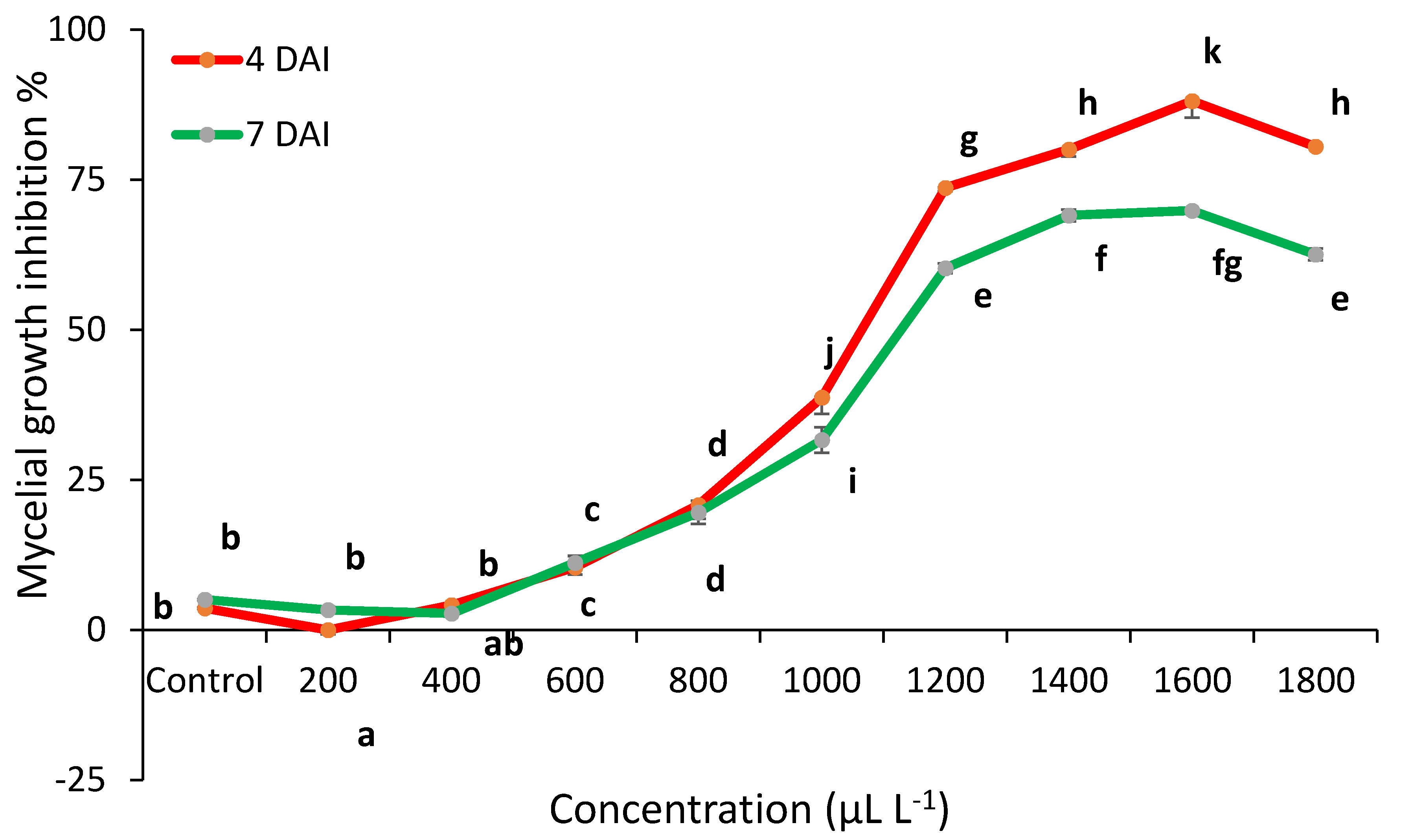
2.2. Essential Oils Antifungal Activity In Vitro
2.3. Antifungal Activity on Detached Strawberry Leaves
3. Discussion
4. Materials and Methods
4.1. Essential Oil Extraction
4.2. Identification of the Essential Oils Chemical Composition
4.3. C. acutatum Isolates
4.4. Essential Oils Antifungal Activity In Vitro
4.5. Essential Oils Antifungal Activity on Detached Strawberry Leaves
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum—Current status and future directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef] [PubMed]
- Udayanga, D.; Manamgoda, D.S.; Liu, X.; Chukeatirote, E.; Hyde, K.D. What are the common anthracnose pathogens of tropical fruits? Fungal Divers. 2013, 61, 165–179. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Huang, J.K.; Jin, B.C.; Yan, J.Y.; Li, X.H.; Hyde, K.D.; Bahkali, A.H.; Yin, S.L.; Zhang, G.Z. An account of Colletotrichum species associated with strawberry anthracnose in china based on morphology and molecular data. Mycosphere 2016, 7, 1147–1163. [Google Scholar] [CrossRef]
- He, L.; Li, X.; Gao, Y.; Li, B.; Mu, W.; Liu, F. Characterisation and fungicide sensitivity of Colletotrichum spp. from different hosts in Shandong, China. Plant Dis. 2019, 103, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Forcelini, B.B.; Seijo, T.E.; Amiri, A.; Peres, N.A. Resistance in strawberry isolates of Colletotrichum acutatum from Florida to quinone-outside inhibitor fungicides. Plant Dis. 2016, 100, 2050–2056. [Google Scholar] [CrossRef]
- Hong, J.K.; Yang, H.I.; Jung, H.; Yoon, D.J.; Sang, M.K.; Jeun, Y.C. Application of volatile antifungal plant essential oils for controlling pepper fruit anthracnose by Colletotrichum gloeosporioides. Plant Pathol. 2015, 31, 269–277. [Google Scholar] [CrossRef]
- Rasiukevičiūtė, N.; Uselis, N.; Valiuškaitė, A. The use of forecasting model IMETOS® for strawberry grey mould management. Zemdirb. Agric. 2019, 106, 143–150. [Google Scholar] [CrossRef]
- Zhang, L.; Song, L.; Xu, X.; Zou, X.; Duan, K.; Gao, Q. Characterisation and fungicide sensitivity of Colletotrichum species causing strawberry anthracnose in Eastern China. Plant Dis. 2020, 7, 104. [Google Scholar]
- Aguado, A.; Pastrana, A.M.; Santos, B.; Romero, F.; Sánchez, M.C.; Capote, N. The efficiency of natural products for the control of Colletotrichum acutatum monitored by real-time PCR. Acta Hortic. 2014, 1049, 329–334. [Google Scholar] [CrossRef]
- Mahilrajan, S.; Nandakumar, J.; Kailayalingam, R.; Manoharan, N.A.; Srivijeindran, S. Screening the antifungal activity of essential oils against decay fungi from palmyrah leaf handicrafts. Biol. Res. 2014, 47, 35. [Google Scholar] [CrossRef]
- Šernaitė, L.; Rasiukevičiūtė, N.; Valiuškaitė, A. The extracts of cinnamon and clove as potential biofungicides against strawberry grey mould. Plants 2020, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Šernaitė, L.; Rasiukevičiūtė, N.; Valiuškaitė, A. Application of plant extracts to control postharvest gray mold and susceptibility of apple fruits to B. cinerea from different plant hosts. Foods 2020, 9, 1430. [Google Scholar] [CrossRef] [PubMed]
- Lopez, N.L.; Gutiérrez-Grijalva, E.P.; Vazquez-Oliva, G.; Heredia, J.B. Essential oils of Oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Naeini, A.; Ziglari, T.; Shokri, H.; Khosravi, A.R. Assessment of growth inhibiting effect of some plant essential oils on different Fusarium isolates. J. Mycol. Med. 2010, 20, 174–178. [Google Scholar] [CrossRef]
- Foltinová, D.; Tančinová, D.; Císarová, M. Influence of essential oils on the growth of Aspergillus flavus. Potravinarstvo 2017, 11, 322–331. [Google Scholar] [CrossRef]
- Oliveira, R.C.; Moreno, M.C.; Correa, B.; Callejas, F.J. Cellular, physiological and molecular approaches to investigate the antifungal and anti-aflatoxigenic effects of thyme essential oil on Aspergillus flavus. Food Chem. 2020, 315, 126096. [Google Scholar] [CrossRef]
- Palfi, M.; Konjevoda, P.; Vrandečić, K. Antifungal activity of essential oils on mycelial growth of Fusarium oxysporum and Botrytis cinerea. Emir. J. Food Agric. 2019, 31, 544–554. [Google Scholar] [CrossRef]
- Sil, A.; Pramanik, K.; Samantaray, P.; Firoz, M.; Yadav, V. Essential oils: A boon towards eco-friendly management of phytopathogenic fungi. J. Entomol. Zool. Stud. 2020, 8, 1884–1891. [Google Scholar]
- Kim, J.E.; Lee, J.E.; Huh, M.J.; Lee, S.C.; Seo, S.M.; Kwon, J.H.; Park, I.K. Fumigant Antifungal Activity via Reactive Oxygen Species of Thymus vulgaris and Satureja hortensis Essential Oils and Constituents against Raffaelea quercus-mongolicae and Rhizoctonia solani. Biomolecules 2019, 9, 561. [Google Scholar] [CrossRef]
- Moumni, S.; Elaissi, A.; Trabelsi, A.; Merghni, A.; Chraief, I.; Jelassi, B.; Chemli, R.; Ferchichi, S. Correlation between chemical composition and antibacterial activity of some Lamiaceae species essential oils from Tunisia. BMC Complement. Med. Ther. 2020, 20, 103. [Google Scholar] [CrossRef]
- Kumar, A.; Kudachikar, V.B. Antifungal properties of essential oils against anthracnose disease: A critical appraisal. J. Plant Dis. Prot. 2018, 125, 133–144. [Google Scholar] [CrossRef]
- Starović, M.; Ristic, D.; Pavlović, S.; Ristic, M.; Stevanović, M.; AlJuhaimi, F.; Svetlana, N.; Özcan, M.M. Antifungal activities of different essential oils against anise seeds mycopopulations. J. Food Saf. Food Qual. 2016, 67, 72–78. [Google Scholar]
- Micucci, M.; Protti, M.; Aldini, R.; Frosini, M.; Corazza, I.; Marzetti, C.; Mattioli, L.B.; Tocci, G.; Chiarini, A.; Mercolini, L.; et al. Thymus vulgaris L. Essential Oil Solid Formulation: Chemical Profile and Spasmolytic and Antimicrobial Effects. Biomolecules 2020, 10, 860. [Google Scholar] [CrossRef] [PubMed]
- Desam, N.R.; Al-Rajab, A.J.; Sharma, M.; Mylabathula, M.M.; Gowkanapalli, R.R.; Albratty, M. Chemical constituents, in vitro antibacterial and antifungal activity of Mentha × piperita L. (peppermint) essential oils. J. King Saud. Univ. Sci. 2017, 31, 528–533. [Google Scholar] [CrossRef]
- Santoro, K.; Maghenzani, M.; Chiabrando, V.; Bosio, P.; Gullino, M.L.; Spadaro, D.; Giacalone, G. Thyme and savory essential oil vapor treatments control brown rot and improve the storage quality of peaches and nectarines, but could favor gray mold. Foods 2018, 7, 7. [Google Scholar] [CrossRef]
- Taghavi, T.; Kim, C.; Rahemi, A. Role of Natural Volatiles and Essential Oils in Extending Shelf Life and Controlling Postharvest Microorganisms of Small Fruits. Microorganisms 2018, 6, 104. [Google Scholar] [CrossRef]
- Çetin, B.; Çakmakçi, S.; Çakmakçi, R. The investigation of antimicrobial activity of thyme and oregano essential oils. Turk. J. Agric. For. 2011, 35, 145–154. [Google Scholar]
- Park, J.Y.; Kim, S.H.; Kim, N.H.; Lee, S.W.; Jeun, Y.C.; Hong, J.K. Differential Inhibitory Activities of Four Plant Essential Oils on In Vitro Growth of Fusarium oxysporum f. sp. fragariae Causing Fusarium Wilt in Strawberry Plants. Plant Pathol. J. 2017, 33, 582–588. [Google Scholar]
- Tančinová, D.; Mašková, Z.; Foltinová, D.; Štefániková, J.; Árvay, J. Effect of essential oils of Lamiaceae plants on the Rhizopus spp. Potravinarstvo 2018, 12, 491–498. [Google Scholar] [CrossRef]
- Wan, J.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Physical properties, antifungal and mycotoxin inhibitory activities of five essential oil nanoemulsions: Impact of oil compositions and processing parameters. Food Chem. 2019, 291, 199–206. [Google Scholar] [CrossRef]
- Duduk, N.; Markovic, T.; Vasic, M.; Duduk, B.; Vico, I.; Obradovic, A. Antifungal activity of three essential oils against Colletotrichum acutatum, the casual agent of strawberry anthracnose. Essent. Oil-Bear. Plants 2015, 18, 529–537. [Google Scholar] [CrossRef]
- Oliveira, K.Á.R.; Berger, L.R.R.; Araújo, S.A.; Câmara, M.P.S.; Souza, E.L. Synergistic mixtures of chitosan and Mentha piperita L. essential oil to inhibit Colletotrichum species and anthracnose development in mango cultivar Tommy Atkins. Food Microbiol. 2017, 66, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Tančinová, D.; Medo, J.; Mašková, Z.; Foltinová, D.; Árvay, J. Effect of essential oils of Lamiaceae plants on the Penicillium commune. J. Microbiol. Biotech. Food Sci. 2019, 8, 1111–1117. [Google Scholar] [CrossRef]
- Yilmaz, A.; Ermis, E.; Boyraz, N. Investigation of in vitro and in vivo antifungal activities of different plant essential oils against postharvest apple rot diseases—Colletotrichum gloeosporioides, Botrytis cinerea and Penicillium expansum. J. Food Saf. Food Qual. 2016, 67, 113–148. [Google Scholar]
- Idris, F.M.; Ibrahim, A.M.; Forsido, S.V. Essential oils to control Colletotrichum musae in vitro and in vivo on banana fruits. AEJAES 2015, 15, 291–302. [Google Scholar]
- Živković, S.; Stojanović, S.; Ivanović, Ž.; Trkulja, N.; Dolovac, N.; Aleksić, G.; Balaž, J. Morphological and Molecular Identification of Colletotrichum acutatum from Tomato Fruit. Pestic. Phytomed. 2010, 25, 231–239. [Google Scholar] [CrossRef]
- Moreira, R.R.; Silva, G.A.; Mio, L.L.M. Colletotrichum acutatum complex causing anthracnose on peach 457 in Brazil. Australas. Plant Pathol. 2020, 49, 179–189. [Google Scholar] [CrossRef]
- Šernaitė, L.; Rasiukevičiūtė, N.; Dambrauskienė, E.; Viškelis, P.; Valiuškaitė, A. Efficacy of plant extracts and essential oils for biocontrol of strawberry pathogen Botrytis cinerea. Zemdirb. Agric. 2020, 107, 147–152. [Google Scholar] [CrossRef]
- Oliveira, J.; Gloria, E.M.; Parisi, M.C.M.; Baggio, J.S.; Silva, P.P.M.; Ambrosio, C.M.S.; Spoto, M.H.F. Antifungal activity of essential oils associated with carboxymethylcellulose against Colletotrichum acutatum in strawberries. Sci. Hortic. 2019, 243, 261–267. [Google Scholar] [CrossRef]
- EPPO. Efficacy Evaluation of Fungicides: Leafspots of Vegetables; European and Mediterranean Plant Protection Organization: Paris, France, 1996. [Google Scholar]
- Bajpai, S.; Shukla, P.S.; Asiedu, S.; Pruski, K.; Prithiviraj, B. A biostimulant preparation of brown seaweed Ascophyllum nodosum suppresses powdery mildew of strawberry. Plant Pathol. J. 2019, 35, 406–416. [Google Scholar]
- Kone, N.; Asare-Bediako, E.; Silue, S.; Kone, D.; Koita, O.; Menzel, W.; Winter, S. Influence of planting date on incidence and severity of viral disease on cucurbits under field condition. Ann. Agric. Sci. 2017, 62, 99–104. [Google Scholar] [CrossRef]
- Hosseini, S.; Amini, J.; Saba, M.K.; Karimi, K.; Pertot, I. Preharvest and postharvest application of garlic and rosemary essential oils for controlling anthracnose and quality assessment of strawberry fruit during cold storage. Front. Microbiol. 2020, 11, 1855. [Google Scholar] [CrossRef] [PubMed]


| Essential Oils | Thymus vulgaris | Salvia officinalis | Mentha piperita | |||
|---|---|---|---|---|---|---|
| Component | PA 1 (%) | RT 2 | PA (%) | RT | PA (%) | RT |
| Tricyclene | 0.16 | 6.388 | ||||
| α-thujene | 1.06 | 6.488 | 0.17 | 6.503 | ||
| α-pinene | 1.09 | 6.667 | 3.23 | 6.689 | 0.73 | 6.682 |
| Camphene | 0.38 | 7.079 | 5.5 | 7.079 | ||
| Sabinene | 0.12 | 7.702 | 0.55 | 7.701 | ||
| β-pinene | 0.43 | 7.779 | 2.65 | 7.798 | 0.87 | 7.795 |
| 1-octen-3-ol | 0.94 | 7.888 | ||||
| Myrcene | 2.42 | 8.139 | 1.18 | 8.147 | 0.48 | 8.147 |
| 3-octanol | 0.21 | 8.344 | ||||
| α-phellandrene | 0.32 | 8.517 | ||||
| δ-3-carene | 0.15 | 8.673 | ||||
| α-terpinene | 2.52 | 8.863 | 0.15 | 8.871 | 0.47 | 8.869 |
| p-cymene | 16.95 | 9.157 | 0.18 | 9.107 | 0.47 | 9.1 |
| Limonene | 0.81 | 9.228 | 1.21 | 9.237 | 0.92 | 9.219 |
| Eucalyptol | 1.88 | 9.285 | 10.33 | 9.3 | 3.35 | 9.283 |
| cis-β-ocimene | 0.11 | 9.432 | 0.23 | 9.444 | 0.16 | 9.442 |
| γ-terpinene | 10.81 | 10.092 | 0.32 | 10.053 | 0.73 | 10.053 |
| 4-pentenyl butyrate | 0.15 | 10.214 | ||||
| cis-sabinene hydrate | 1.04 | 10.328 | 0.16 | 10.351 | 2.51 | 10.352 |
| Terpinolene | 0.17 | 10.876 | 0.32 | 10.894 | 0.2 | 10.895 |
| trans-sabinene hydrate | 0.21 | 11.257 | ||||
| Linalool | 3.47 | 11.255 | 0.47 | 11.339 | 0.33 | 11.291 |
| α-thujone | 0.6 | 11.39 | 25.8 | 11.507 | ||
| β-thujone | 0.21 | 11.708 | 8.65 | 11.775 | ||
| Isothujol | 0.17 | 12.371 | ||||
| cis-p-menth-2-en-1-ol | 0.19 | 11.939 | ||||
| trans-p-menth-2-en--1ol + trans-Sabinol | 0.22 | 12.548 | ||||
| Menthone | 44.56 | 12.964 | ||||
| Isomenthone | 12.81 | 13.2 | ||||
| Camphor | 0.74 | 12.619 | 20.46 | 12.616 | ||
| trans-pinocamphone | 0.14 | 12.98 | ||||
| Borneol | 0.75 | 13.208 | 4.37 | 13.223 | ||
| δ-terpineol + borneol | 0.24 | 13.24 | ||||
| cis-pinocamphone | 0.2 | 13.385 | ||||
| Menthol | 7.95 | 13.473 | ||||
| Terpinen-4-ol | 1.05 | 13.483 | 0.28 | 13.494 | 2.58 | 13.549 |
| α-terpineol | 0.25 | 13.999 | 0.22 | 13.879 | 0.32 | 13.916 |
| Thymol methyl ether | 0.61 | 15.01 | ||||
| Carvacrol methyl ether | 0.63 | 15.272 | ||||
| Carvone Z, dihydro | 0.31 | 14.039 | ||||
| Myrtenol | 0.28 | 14.056 | ||||
| cis-3-hexenyl-isovalerate | 0.11 | 15.072 | ||||
| Pulegone | 10.74 | 15.282 | ||||
| Bornyl acetate | 1.39 | 16.431 | ||||
| Thymol | 41.35 | 16.984 | ||||
| Carvone | 0.45 | 15.346 | ||||
| Carvacrol | 2.57 | 17.123 | ||||
| trans-sabinyl acetate + thujyl acetate | 0.1 | 16.597 | ||||
| Piperitone | 1.65 | 15.636 | ||||
| Caryophyllene E | 1.86 | 20.018 | 2.84 | 20.013 | 0.75 | 20.004 |
| Menthyl acetate | 1.41 | 16.639 | ||||
| α-humulene | 0.17 | 20.859 | 3.25 | 20.872 | ||
| Geranyl propanoate | 0.12 | 21.212 | ||||
| γ-cadinene | 0.14 | 22.324 | ||||
| δ-cadinene | 0.22 | 22.517 | ||||
| Piperitenone | 0.36 | 17.97 | ||||
| β-elemene | 0.34 | 19.258 | ||||
| Germacrene D | 0.79 | 21.536 | ||||
| Bicyclogermacren | 0.2 | 21.908 | ||||
| Caryophyllene oxide | 0.31 | 24.066 | 0.19 | 24.059 | ||
| Viridiflorol | 0.11 | 24.337 | 3.03 | 24.363 | ||
| Humulene epoxide II | 0.26 | 24.796 | ||||
| Manool | 0.74 | 32.227 | ||||
| α-muurolol | ||||||
| Unknown | 0.49 | |||||
| Squalene | 1.19 | 36.912 | ||||
| Di-n-octyl phthalate | 0.13 | 37.496 | 0.2 | 37.486 | ||
| Other 3 | 1.64 | 1.41 | 1.68 | |||
| Total Identified | 99.33 | 99.94 | 99.94 | |||
| Treatments | Disease Severity (%) | Disease Reduction (%) |
|---|---|---|
| Inoculated control | 79.2 ± 0.2 | n.a.* |
| Thymus vulgaris 800 μL/L | 75 ± 0.3 | 5.3 |
| Salvia officinalis 1000 μL/L | 80.6 ± 0.2 | 0 |
| Mentha piperita 1000 μL/L | 66.7 ± 0.2 | 15.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morkeliūnė, A.; Rasiukevičiūtė, N.; Šernaitė, L.; Valiuškaitė, A. The Use of Essential Oils from Thyme, Sage and Peppermint against Colletotrichum acutatum. Plants 2021, 10, 114. https://doi.org/10.3390/plants10010114
Morkeliūnė A, Rasiukevičiūtė N, Šernaitė L, Valiuškaitė A. The Use of Essential Oils from Thyme, Sage and Peppermint against Colletotrichum acutatum. Plants. 2021; 10(1):114. https://doi.org/10.3390/plants10010114
Chicago/Turabian StyleMorkeliūnė, Armina, Neringa Rasiukevičiūtė, Lina Šernaitė, and Alma Valiuškaitė. 2021. "The Use of Essential Oils from Thyme, Sage and Peppermint against Colletotrichum acutatum" Plants 10, no. 1: 114. https://doi.org/10.3390/plants10010114
APA StyleMorkeliūnė, A., Rasiukevičiūtė, N., Šernaitė, L., & Valiuškaitė, A. (2021). The Use of Essential Oils from Thyme, Sage and Peppermint against Colletotrichum acutatum. Plants, 10(1), 114. https://doi.org/10.3390/plants10010114






