The Developmental Phases of Zebrafish Myogenesis
Abstract
1. Introduction
2. Primary Myotome Formation in Zebrafish
2.1. Molecular Signalling and Fibre Fate of Adaxial Cells
2.2. Posterior Paraxial Mesoderm Differentiation
2.3. Primary Myotome Variation in Teleosts
2.4. Development of the External Cell Layer
3. Secondary Myogenesis and the Role of the External Cell Layer
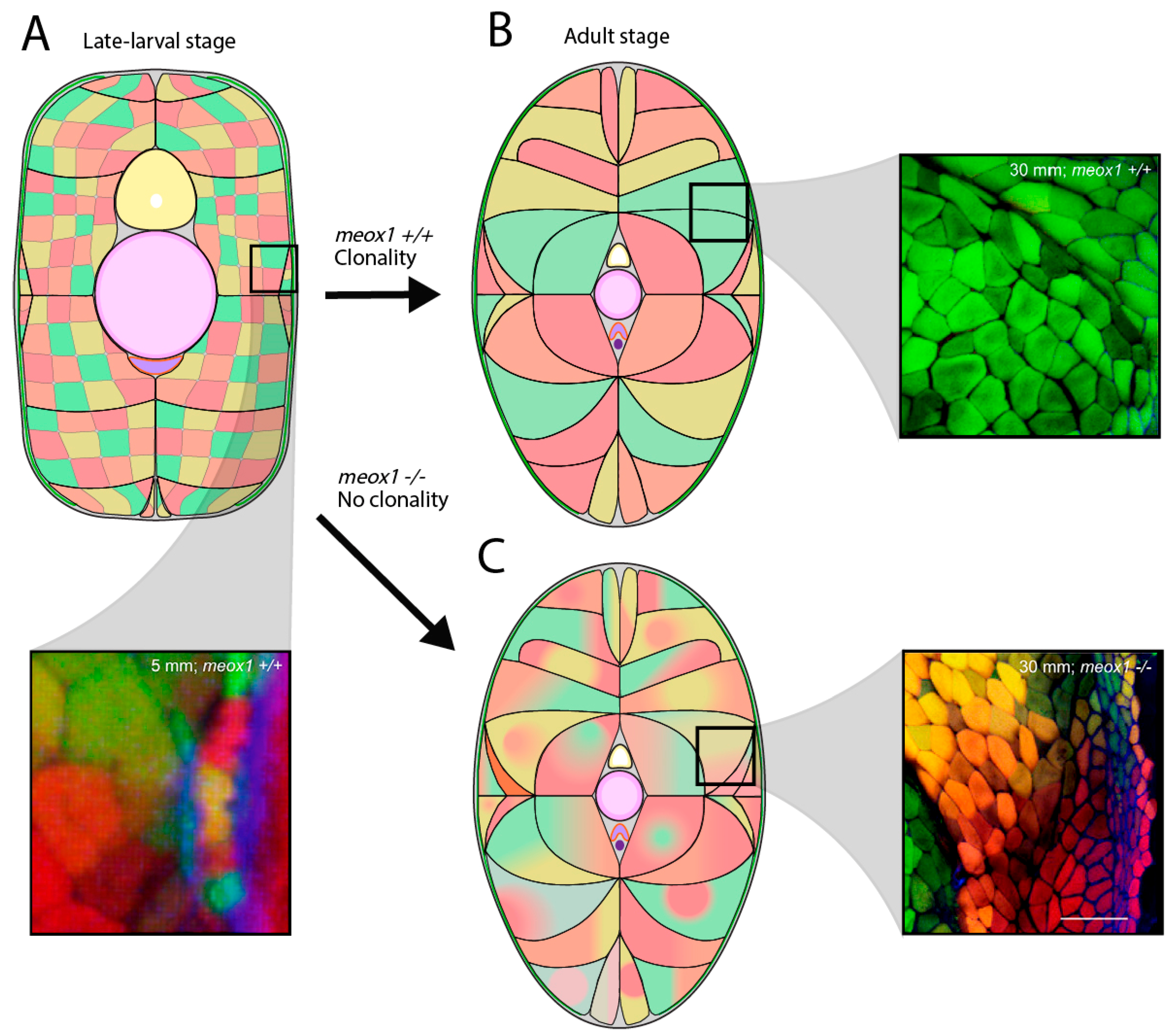
3.1. Mechanisms of Muscle Growth
3.2. External Cell Layer Dynamics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blagden, C.S.; Currie, P.D.; Ingham, P.W.; Hughes, S.M. Notochord induction of zebrafish slow muscle mediated by Sonic hedgehog. Genes Dev. 1997, 11, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Du, S.J.; Devoto, S.H.; Westerfield, M.; Moon, R.T. Positive and negative regulation of muscle cell identity by members of the hedgehog and TGF-β gene families. J. Cell Biol. 1997, 139, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, D.; Siegel, A.; Currie, P.D. Skeletal Myogenesis in the Zebrafish and Its Implications for Muscle Disease Modelling; Brand-Saberi, B., Ed.; Results and Problems in Cell Differentiation; Springer: Berlin, Germany, 2015; Volume 56, ISBN 978-3-662-44607-2. [Google Scholar]
- Devoto, S.H.; Stoiber, W.; Hammond, C.L.; Steinbacher, P.; Haslett, J.R.; Barresi, M.J.F.; Patterson, S.E.; Adiarte, E.G.; Hughes, S.M. Generality of vertebrate developmental patterns: Evidence for a dermomyotome in fish. Evol. Dev. 2006, 8, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Hollway, G.E.; Bryson-Richardson, R.J.; Berger, S.; Cole, N.J.; Hall, T.E.; Currie, P.D. Whole-somite rotation generates muscle progenitor cell compartments in the developing zebrafish embryo. Dev. Cell 2007, 12, 207–219. [Google Scholar] [CrossRef]
- Stellabotte, F.; Dobbs-McAuliffe, B.; Fernandez, D.A.; Feng, X.; Devoto, S.H. Dynamic somite cell rearrangements lead to distinct waves of myotome growth. Development 2007, 134, 1253–1257. [Google Scholar] [CrossRef]
- Stellabotte, F.; Devoto, S.H. The teleost dermomyotome. Dev. Dyn. 2007, 236, 2432–2443. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Hollway, G.E.; Sonntag, C.; Miles, L.B.; Hall, T.E.; Berger, S.; Fernandez, K.J.; Gurevich, D.B.; Cole, N.J.; Alaei, S.; et al. Haematopoietic stem cell induction by somite-derived endothelial cells controlled by meox1. Nature 2014, 512, 314–318. [Google Scholar] [CrossRef]
- Stickney, H.L.; Barresi, M.J.F.; Devoto, S.H. Somite Development in Zebrafish. Dev. Dyn. 2000, 219, 287–303. [Google Scholar] [CrossRef]
- Ma, R.C.; Jacobs, C.T.; Sharma, P.; Kocha, K.M.; Huang, P. Stereotypic generation of axial tenocytes from bipartite sclerotome domains in zebrafish. PLoS Genet. 2018, 14, e1007775. [Google Scholar] [CrossRef]
- Tahmisian, T.N.; Wright, B.J.; Hall, B.R. Biological and Medical Research Division Semiannual Report, July-December 1962; Argonne National Laboratory: Lemont, IL, USA, 1963. [Google Scholar]
- Gordon, A.M.; Huxley, A.F.; Julian, F.J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. 1966, 184, 170–192. [Google Scholar] [CrossRef]
- van Raamsdonk, W.; van’t Veer, L.; Veeken, K.; Heyting, C.; Pool, C.W. Differentiation of Muscle Fiber Types in the Teleost Brachydanio rerio, the Zebrafish. Anat. Embryol. 1982, 164, 51–62. [Google Scholar] [CrossRef]
- Devoto, S.H.; Melançon, E.; Eisen, J.S.; Westerfield, M. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development 1996, 122, 3371–3380. [Google Scholar]
- Talbot, J.; Maves, L. Skeletal muscle fiber type: Using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 518–534. [Google Scholar] [CrossRef]
- Hatch, K.; Pabon, A.; DiMario, J.X. EMX2 activates slow myosin heavy chain 2 gene expression in embryonic muscle fibers. Mech. Dev. 2017, 147, 8–16. [Google Scholar] [CrossRef]
- Sefton, E.M.; Kardon, G. Connecting muscle development, birth defects, and evolution: An essential role for muscle connective tissue. Curr. Top. Dev. Biol. 2019, 132, 137–176. [Google Scholar]
- Rowland, L.A.; Bal, N.C.; Periasamy, M. The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy. Biol. Rev. 2015, 90, 1279–1297. [Google Scholar] [CrossRef]
- Glickman, N.S. Shaping the zebrafish notochord. Development 2003, 130, 873–887. [Google Scholar] [CrossRef]
- Weinberg, E.S.; Allende, M.L.; Kelly, C.S.; Abdelhamid, A.; Murakami, T.; Andermann, P.; Doerre, O.G.; Grunwald, D.J.; Riggleman, B. Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development 1996, 122, 271–280. [Google Scholar]
- Delalande, J.M.; Rescan, P.Y. Differential expression of two nonallelic MyoD genes in developing and adult myotomal musculature of the trout (Oncorhynchus mykiss). Dev. Genes Evol. 1999, 209, 432–437. [Google Scholar] [CrossRef]
- Coutelle, O.; Blagden, C.S.; Hampson, R.; Halai, C.; Rigby, P.W.J.; Hughes, S.M. Hedgehog signalling is required for maintenance of myf5 and myoD expression and timely terminal differentiation in zebrafish adaxial myogenesis. Dev. Biol. 2001, 236, 136–150. [Google Scholar] [CrossRef]
- Bryson-Richardson, R.J.; Daggett, D.F.; Cortes, F.; Neyt, C.; Keenan, D.G.; Currie, P.D. Myosin heavy chain expression in zebrafish and slow muscle composition. Dev. Dyn. 2005, 233, 1018–1022. [Google Scholar] [CrossRef]
- Jackson, H.E.; Ingham, P.W. Control of muscle fibre-type diversity during embryonic development: The zebrafish paradigm. Mech. Dev. 2013, 130, 447–457. [Google Scholar] [CrossRef]
- Ingham, P.W.; Nakano, Y.; Seger, C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat. Rev. Genet. 2011, 12, 393–406. [Google Scholar] [CrossRef]
- Baxendale, S.; Davison, C.; Muxworthy, C.; Wolff, C.; Ingham, P.W.; Roy, S. The B-cell maturation factor Blimp-1 specifies vertebrate slow-twitch muscle fiber identity in response to Hedgehog signaling. Nat. Genet. 2004, 36, 88–93. [Google Scholar] [CrossRef]
- Maurya, A.K.; Tan, H.; Souren, M.; Wang, X.; Wittbrodt, J.; Ingham, P.W. Integration of Hedgehog and BMP signalling by the engrailed2a gene in the zebrafish myotome. Development 2012, 139, 1885. [Google Scholar] [CrossRef][Green Version]
- Nguyen-Chi, M.E.; Bryson-Richardson, R.; Sonntag, C.; Hall, T.E.; Gibson, A.; Sztal, T.; Chua, W.; Schilling, T.F.; Currie, P.D. Morphogenesis and cell fate determination within the adaxial cell equivalence group of the zebrafish myotome. PLoS Genet. 2012, 8, e1003014. [Google Scholar] [CrossRef]
- Lewis, K.; Currie, P.; Roy, S.; Schauerte, H.; Haffter, P.; Ingham, P. Control of muscle cell-type specification in the zebrafish embryo by hedgehog signalling. Dev. Biol. 1999, 216, 469–480. [Google Scholar] [CrossRef]
- Barresi, M.J.; Stickney, H.L.; Devoto, S.H. The zebrafish slow-muscle-omitted gene product is required for Hedgehog signal transduction and the development of slow muscle identity. Development 2000, 127, 2189–2199. [Google Scholar]
- Wolff, C.; Roy, S.; Ingham, P.W. Multiple muscle cell identities induced by distinct levels and timing of hedgehog activity in the zebrafish embryo. Curr. Biol. 2003, 13, 1169–1181. [Google Scholar] [CrossRef]
- Ingham, P.W.; Kim, H.R. Hedgehog signalling and the specification of muscle cell identity in the zebrafish embryo. Exp. Cell Res. 2005, 306, 336–342. [Google Scholar] [CrossRef]
- Cortés, F.; Daggett, D.; Bryson-Richardson, R.J.; Neyt, C.; Maule, J.; Gautier, P.; Hollway, G.E.; Keenan, D.; Currie, P.D. Cadherin-mediated differential cell adhesion controls slow muscle cell migration in the developing zebrafish myotome. Dev. Cell 2003, 5, 865–876. [Google Scholar] [CrossRef]
- Ono, Y.; Yu, W.; Jackson, H.E.; Parkin, C.A.; Ingham, P.W. Adaxial cell migration in the zebrafish embryo is an active cell autonomous property that requires the Prdm1a transcription factor. Differentiation 2015, 89, 77–86. [Google Scholar] [CrossRef]
- Yin, J.; Lee, R.; Ono, Y.; Ingham, P.W.; Saunders, T.E. Spatiotemporal coordination of FGF and Shh signaling underlies the specification of myoblasts in the zebrafish embryo. Dev. Cell 2018, 46, 735–750.e4. [Google Scholar] [CrossRef]
- Felsenfeld, A.L.; Curry, M.; Kimmel, C.B. The fub-1 mutation blocks initial myofibril formation in zebrafish muscle pioneer cells. Dev. Biol. 1991, 148, 23–30. [Google Scholar] [CrossRef]
- Hatta, K.; Bremiller, R.; Westerfield, M.; Kimmel, C.B. Diversity of expression of engrailed-like antigens in zebrafish. Development 1991, 112, 821–832. [Google Scholar]
- Currie, P.D.; Ingham, P.W. Induction of a specific muscle cell type by a hedgehog-like protein in zebrafish. Nature 1996, 382, 452–455. [Google Scholar] [CrossRef]
- Gibert, J.M. The evolution of engrailed genes after duplication and speciation events. Dev. Genes Evol. 2002, 212, 307–318. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Maurya, A.K.; Cheng, L.; Jorge, E.C.; Schubert, F.R.; Maire, P.; Basson, M.A.; Ingham, P.W.; Dietrich, S. Engrailed controls epaxial-hypaxial muscle innervation and the establishment of vertebrate three-dimensional mobility. Dev. Biol. 2017, 430, 90–104. [Google Scholar] [CrossRef]
- Melançon, E.; Liu, D.W.C.; Westerfield, M.; Eisen, J.S. Pathfinding by Identified Zebrafish Motoneurons in the Absence of Muscle Pioneers. J. Neurosci. 1997, 17, 7796–7804. [Google Scholar] [CrossRef]
- Lewis, K.E.; Eisen, J.S. From cells to circuits: Development of the zebrafish spinal cord. Prog. Neurobiol. 2003, 69, 419–449. [Google Scholar] [CrossRef]
- Pagnon-Minot, A.; Malbouyres, M.; Haftek-Terreau, Z.; Kim, H.R.; Sasaki, T.; Thisse, C.; Thisse, B.; Ingham, P.W.; Ruggiero, F.; Le Guellec, D. Collagen XV, a novel factor in zebrafish notochord differentiation and muscle development. Dev. Biol. 2008, 316, 21–35. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z.; Muller, J.; Iyu, A.; Khng, A.J.; Guccione, E.; Ruan, Y.; Ingham, P.W. Targeted inactivation and identification of targets of the Gli2a transcription factor in the zebrafish. Biol. Open 2013, 2, 1203–1213. [Google Scholar] [CrossRef]
- Daggett, D.F.; Domingo, C.R.; Currie, P.D.; Amacher, S.L. Control of morphogenetic cell movements in the early zebrafish myotome. Dev. Biol. 2007, 309, 169–179. [Google Scholar] [CrossRef]
- Rost, F.; Eugster, C.; Schroter, C.; Oates, A.C.; Brusch, L. Chevron formation of the zebrafish muscle segments. J. Exp. Biol. 2014, 217, 3870–3882. [Google Scholar] [CrossRef]
- Temple, G.K.; Cole, N.J.; Johnston, I.A. Embryonic temperature and the relative timing of muscle-specific genes during development in herring (Clupea harengus L.). J. Exp. Biol. 2001, 204, 3629–3637. [Google Scholar]
- Steinbacher, P.; Haslett, J.R.; Six, M.; Gollmann, H.P.; Sänger, A.M.; Stoiber, W. Phases of myogenic cell activation and possible role of dermomyotome cells in teleost muscle formation. Dev. Dyn. 2006, 235, 3132–3143. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, X.; Zhang, P.-J.; Xu, Y. Characterization of muscle-regulatory gene, myod, from flounder (paralichthys olivaceus) and analysis of its expression patterns during embryogenesis. Mar. Biotechnol. 2006, 8, 139–148. [Google Scholar] [CrossRef]
- Cole, N.J.; Hall, T.E.; Martin, C.I.; Chapman, M.A.; Kobiyama, A.; Nihei, Y.; Watabe, S.; Johnston, I.A. Temperature and the expression of myogenic regulatory factors (MRFs) and myosin heavy chain isoforms during embryogenesis in the common carp Cyprinus carpio L. J. Exp. Biol. 2004, 207, 4239–4248. [Google Scholar] [CrossRef]
- Steinbacher, P.; Haslett, J.R.; Sänger, A.M.; Stoiber, W. Evolution of myogenesis in fish: A sturgeon view of the mechanisms of muscle development. Anat. Embryol. (Berl) 2006, 211, 311–322. [Google Scholar] [CrossRef]
- Ono, Y.; Kinoshita, S.; Ikeda, D.; Watabe, S. Early development of medaka Oryzias latipes muscles as revealed by transgenic approaches using embryonic and larval types of myosin heavy chain genes. Dev. Dyn. 2010, 239, 1807–1817. [Google Scholar] [CrossRef]
- Atit, R.; Sgaier, S.K.; Mohamed, O.A.; Taketo, M.M.; Dufort, D.; Joyner, A.L.; Niswander, L.; Conlon, R.A. β-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev. Biol. 2006, 296, 164–176. [Google Scholar] [CrossRef]
- Moustakas, J.E. Development of the carapacial ridge: Implications for the evolution of genetic networks in turtle shell development. Evol. Dev. 2008, 10, 29–36. [Google Scholar] [CrossRef]
- Tanaka, M.; Münsterberg, A.; Anderson, W.G.; Prescott, A.R.; Hazon, N.; Tickle, C. Fin development in a cartilaginous fish and the origin of vertebrate limbs. Nature 2002, 416, 527–531. [Google Scholar] [CrossRef]
- Flynt, A.S.; Li, N.; Thatcher, E.J.; Solnica-Krezel, L.; Patton, J.G. Zebrafish miR-214 modulates Hedgehog signaling to specify muscle cell fate. Nat. Genet. 2007, 39, 259–263. [Google Scholar] [CrossRef]
- Wang, X.; Ono, Y.; Tan, S.C.; Chai, R.J.; Parkin, C.; Ingham, P.W. Prdm1a and miR-499 act sequentially to restrict Sox6 activity to the fast-twitch muscle lineage in the zebrafish embryo. Development 2011, 138, 4399–4404. [Google Scholar] [CrossRef]
- Dolez, M.; Nicolas, J.-F.; Hirsinger, E. Laminins, via heparan sulfate proteoglycans, participate in zebrafish myotome morphogenesis by modulating the pattern of Bmp responsiveness. Development 2011, 138, 97–106. [Google Scholar] [CrossRef]
- Lee, Y.; Manegold, J.E.; Kim, A.D.; Pouget, C.; Stachura, D.L.; Clements, W.K.; Traver, D. FGF signalling specifies haematopoietic stem cells through its regulation of somitic Notch signalling. Nat. Commun. 2014, 5, 5583. [Google Scholar] [CrossRef]
- Groves, J.A. Fgf8 drives myogenic progression of a novel lateral fast muscle fibre population in zebrafish. Development 2005, 132, 4211–4222. [Google Scholar] [CrossRef]
- Hamade, A.; Deries, M.; Begemann, G.; Bally-Cuif, L.; Genêt, C.; Sabatier, F.; Bonnieu, A.; Cousin, X. Retinoic acid activates myogenesis in vivo through Fgf8 signalling. Dev. Biol. 2006, 289, 127–140. [Google Scholar] [CrossRef]
- Janesick, A.; Tang, W.; Nguyen, T.T.L.; Blumberg, B. RARβ2 is required for vertebrate somitogenesis. Development 2017, 144, 1997–2008. [Google Scholar] [CrossRef]
- Henry, C.A.; Amacher, S.L. Zebrafish slow muscle cell migration induces a wave of fast muscle morphogenesis. Dev. Cell 2004, 7, 917–923. [Google Scholar] [CrossRef]
- Reifers, F.; Böhli, H.; Walsh, E.C.; Crossley, P.H.; Stainier, D.Y.R.; Brand, M. Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development 1998, 125, 2381–2395. [Google Scholar]
- Rowlerson, A.; Veggetti, A. Cellular mechanisms of post-embryonic muscle growth in aquaculture species. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2001; pp. 103–140. [Google Scholar]
- Sanger, A.M.; Stoiber, W. Muscle fiber diversity and plasticity. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2001; pp. 187–250. [Google Scholar]
- Reyes, N.L.; Banks, G.B.; Tsang, M.; Margineantu, D.; Gu, H.; Djukovic, D.; Chan, J.; Torres, M.; Liggitt, H.D.; Hirenallur-S, D.K.; et al. Fnip1 regulates skeletal muscle fiber type specification, fatigue resistance, and susceptibility to muscular dystrophy. Proc. Natl. Acad. Sci. USA 2015, 112, 424–429. [Google Scholar] [CrossRef]
- Kivelä, R.; Salmela, I.; Nguyen, Y.H.; Petrova, T.V.; Koistinen, H.A.; Wiener, Z.; Alitalo, K. The transcription factor Prox1 is essential for satellite cell differentiation and muscle fibre-type regulation. Nat. Commun. 2016, 7, 13124. [Google Scholar] [CrossRef]
- te Kronnie, G.; Tatarczuch, L.; Raamsdonk, W.; Kilarski, W. Muscle fibre types in the myotome of stickleback, Gasterosteus aculeatus L.; a histochemical, immunohistochemical and ultrastructural study. J. Fish Biol. 1983, 22, 303–316. [Google Scholar] [CrossRef]
- Nelson, J.S. Fishes of the World; John Wiley and Sons: New York, NY, USA, 2006. [Google Scholar]
- Boddeke, R.; Slijper, E.J.; Van der Stelt, A. Histological characteristics of the body musculature of fishes in connection with their mode of life. Proc. K. Ned. Akad. Wet. Ser. 1959, 62, 576–588. [Google Scholar]
- Graham, J.B.; Dickson, K.A. The evolution of thunniform locomotion and heat conservation in scombrid fishes: New insights based on the morphology of Allothunnus fallai. Zool. J. Linn. Soc. 2000, 129, 419–466. [Google Scholar] [CrossRef]
- Davenport, J.; Phillips, N.D.; Cotter, E.; Eagling, L.E.; Houghton, J.D.R. The locomotor system of the ocean sunfish Mola mola (L.): Role of gelatinous exoskeleton, horizontal septum, muscles and tendons. J. Anat. 2018, 233, 347–357. [Google Scholar] [CrossRef]
- Feng, X.; Adiarte, E.G.; Devoto, S.H. Hedgehog acts directly on the zebrafish dermomyotome to promote myogenic differentiation. Dev. Biol. 2006, 300, 736–746. [Google Scholar] [CrossRef]
- Hammond, C.L.; Hinits, Y.; Osborn, D.P.S.; Minchin, J.E.N.; Tettamanti, G.; Hughes, S.M. Signals and myogenic regulatory factors restrict pax3 and pax7 expression to dermomyotome-like tissue in zebrafish. Dev. Biol. 2007, 302, 504–521. [Google Scholar] [CrossRef]
- Windner, S.E.; Doris, R.A.; Ferguson, C.M.; Nelson, A.C.; Valentin, G.; Tan, H.; Oates, A.C.; Wardle, F.C.; Devoto, S.H. Tbx6, Mesp-b and Ripply1 regulate the onset of skeletal myogenesis in zebrafish. Development 2015, 142, 1159–1168. [Google Scholar] [CrossRef]
- Waterman, R.E. Development of the lateral musculature in the teleost, Brachydanio rerio: A fine structural study. Am. J. Anat. 1969, 125, 457–493. [Google Scholar] [CrossRef]
- Veggetti, A.; Mascarello, F.; Scapolo, P.A.; Rowlerson, A. Hyperplastic and hypertrophic growth of lateral muscle in Dicentrarchus labrax (L.) An ultrastructural and morphometric study. Anat. Embryol. 1990, 182, 1–10. [Google Scholar] [CrossRef]
- Johnston, I.A. Marine Biology of organogenesis in herring (Clupea harengus) larvae. Mar. Biol. 1993, 116, 363–379. [Google Scholar] [CrossRef]
- Ramirez-Zarzosa, G.; Gil, F.; Latorre, R.; Ortega, A.; Garcia-Alcaraz, A.; Abellan, E.; Vazquez, J.M.; Lopez-Albors, O.; Arencibia, A.; Moreno, F. The larval development of lateral musculature in gilthead sea bream Sparus aurata and sea bass Dicentrarchus labrax. Cell Tissue Res. 1995, 280, 217–224. [Google Scholar] [CrossRef]
- Patruno, M.; Radaelli, G.; Mascarello, F.; Candia Carnevali, M.D. Muscle growth in response to changing demands of functions in the teleost Sparus aurata (L.) during development from hatching to juvenile. Anat. Embryol. 1998, 198, 487–504. [Google Scholar] [CrossRef]
- Stoiber, W.; Sanger, A.M. An electron microscopic investigation into the possible source of new muscle fibres in teleost fish. Anat. Embryol. (Berl) 1996, 194, 569–579. [Google Scholar] [CrossRef]
- Stoiber, W.; Haslett, J.R.; Goldschmid, A.; Sänger, A.M. Patterns of superficial fibre formation in the European pearlfish (Rutilus frisii meidingeri) provide a general template for slow muscle development in teleost fish. Anat. Embryol. 1998, 197, 485–496. [Google Scholar] [CrossRef]
- Kahane, N.; Ribes, V.; Kicheva, A.; Briscoe, J.; Kalcheim, C. The transition from differentiation to growth during dermomyotome-derived myogenesis depends on temporally restricted hedgehog signaling. Development 2013, 140, 1740–1750. [Google Scholar] [CrossRef]
- Applebaum, M.; Kalcheim, C. Vertebrate Myogenesis; Brand-Saberi, B., Ed.; Results and Problems in Cell Differentiation; Springer: Berlin, Germany, 2015; Volume 56, ISBN 978-3-662-44607-2. [Google Scholar]
- Buckingham, M.; Mayeuf, A. Skeletal muscle development. In Muscle; Academic Press: Cambridge, MA, USA, 2012; pp. 749–762. [Google Scholar]
- Mayeuf-Louchart, A.; Montarras, D.; Bodin, C.; Kume, T.; Vincent, S.D.; Buckingham, M. Endothelial cell specification in the somite is compromised in Pax3-positive progenitors of Foxc1/2 conditional mutants, with loss of forelimb myogenesis. Development 2016, 143, 872–879. [Google Scholar] [CrossRef]
- Davidson, A.J.; Zon, L.I. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene 2004, 23, 7233–7246. [Google Scholar] [CrossRef]
- Bertrand, J.Y.; Chi, N.C.; Santoso, B.; Teng, S.; Stainier, D.Y.R.; Traver, D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 2010, 464, 108–111. [Google Scholar] [CrossRef]
- Kissa, K.; Herbomel, P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 2010, 464, 112–115. [Google Scholar] [CrossRef]
- Clements, W.K.; Traver, D. Signalling pathways that control vertebrate haematopoietic stem cell specification. Nat. Rev. Immunol. 2013, 13, 336–348. [Google Scholar] [CrossRef]
- Bone, Q. Locomotor muscle. In Fish Physiology; Academic Press: Cambridge, MA, USA, 1978; pp. 361–424. [Google Scholar]
- Lovell, T. The nutrients. In Nutrition and Feeding of Fish; Van Nostrand Reinhold: New York, NY, USA, 1989; pp. 11–71. [Google Scholar]
- Watanabe, T. Nutrition and growth. In Intensive Fish Farming; Blackwell Scientific Publications: Oxford, UK, 1992; pp. 154–197. [Google Scholar]
- Houlihan, D.F.; Mathers, E.M.; Foster, A. Biochemical correlates of growth rate in fish. In Fish Ecophysiology; Chapman & Hall: London, UK, 1993; pp. 45–71. [Google Scholar]
- Stickland, N.C.; White, R.N.; Mescall, P.E.; Crook, A.R.; Thorpe, J.E. The effect of temperature on myogenesis in embryonic development of the Atlantic salmon (Salmo salar L.). Anat. Embryol. 1988, 178, 253–257. [Google Scholar] [CrossRef]
- Nathanailides, C.; Lopez-Albors, O.; Stickland, N.C. Influence of prehatch temperature on the development of muscle cellularity in posthatch Atlantic salmon (Salmo salar). Can. J. Fish Aquat. Sci. 1995, 52, 675–680. [Google Scholar] [CrossRef]
- Johnston, I.A.; Fleming, J.D.; Crockford, T. Thermal acclimation and muscle contractile properties in cyprinid fish. Am. J. Physiol. 1990, 259, R231–R236. [Google Scholar] [CrossRef]
- Langfeld, K.S.; Crockford, T.; Johnston, I.A. Temperature acclimation in the common carp: Force-velocity characteristics and myosin subunit composition of slow muscle fibres. J. Exp. Biol. 1991, 155, 291–304. [Google Scholar]
- Matschak, T.W.; Stickland, N.C.; Mason, P.S.; Crook, A.R. Oxygen availability and temperature affect embryonic muscle development in Atlantic salmon (Salmo salar L.). Differentiation 1997, 61, 229–235. [Google Scholar] [CrossRef]
- Matschak, T.W.; Hopcroft, T.; Mason, P.S.; Crook, A.R.; Stickland, N.C. Temperature and oxygen tension influence the development of muscle cellularity in embryonic rainbow trout. J. Fish Biol. 1998, 53, 581–590. [Google Scholar] [CrossRef]
- Davison, W.; Goldspink, G. The effect of training on the swimming muscles of the goldfish (Carassius auratus). J. Exp. Biol. 1978, 74, 115–122. [Google Scholar]
- Christiansen, J.S.; Martinez, I.; Jobling, M.; Amin, A.B. Rapid somatic growth and muscle damage in a salmonid fish. Basic Appl. Myol. 1992, 2, 235–239. [Google Scholar]
- Johnston, I.A. Genetic and environmental determinants of muscle growth patterns. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2001; pp. 141–186. [Google Scholar]
- Bryson-Richardson, R.J.; Currie, P.D. The genetics of vertebrate myogenesis. Nat. Rev. Genet. 2008, 9, 632–646. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Gurevich, D.B.; Sonntag, C.; Hersey, L.; Alaei, S.; Nim, H.T.; Siegel, A.; Hall, T.E.; Rossello, F.J.; Boyd, S.E.; et al. Muscle stem cells undergo extensive clonal drift during tissue growth via meox1-mediated induction of G2 cell-cycle arrest. Cell Stem Cell 2017, 21, 107–119.e6. [Google Scholar] [CrossRef]
- Gurevich, D.B.; Nguyen, P.D.; Siegel, A.L.; Ehrlich, O.V.; Sonntag, C.; Phan, J.M.N.; Berger, S.; Ratnayake, D.; Hersey, L.; Berger, J.; et al. Asymmetric division of clonal muscle stem cells coordinates muscle regeneration in vivo. Science 2016, 353, aad9969. [Google Scholar] [CrossRef]
- Johnston, I.A.; Bower, N.I.; Macqueen, D.J. Growth and the regulation of myotomal muscle mass in teleost fish. J. Exp. Biol. 2011, 214, 1617–1628. [Google Scholar] [CrossRef]
- Greer-Walker, M.G.; Bird, A.C.; Pull, G.A. The total number of white skeletal muscle fibres in cross section as a character for stock separation in North Sea herring (Clupea harengus). J. Cons. Int. Explor. Mer 1972, 34, 238–243. [Google Scholar] [CrossRef]
- Fine, M.L.; Bernard, B.; Harris, T.M. Functional morphology of toadfish sonic muscle fibers: Relationship to possible fiber division. Can. J. Zool. 1993, 71, 2262–2274. [Google Scholar] [CrossRef]
- Parichy, D.M.; Elizondo, M.R.; Mills, M.G.; Gordon, T.N.; Engeszer, R.E. Normal table of postembryonic zebrafish development: Staging by externally visible anatomy of the living fish. Dev. Dyn. 2009, 238, 2975–3015. [Google Scholar] [CrossRef]
- Seger, C.; Hargrave, M.; Wang, X.; Chai, R.J.; Elworthy, S.; Ingham, P.W. Analysis of Pax7 expressing myogenic cells in zebrafish muscle development, injury, and models of disease. Dev. Dyn. 2011, 240, 2440–2451. [Google Scholar] [CrossRef]
- Ross, J.J.; Duxson, M.J.; Harris, A.J. Formation of primary and secondary myotubes in rat lumbrical muscles. Development 1987, 100, 383–394. [Google Scholar]
- Gros, J.; Manceau, M.; Thomé, V.; Marcelle, C. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature 2005, 435, 954–958. [Google Scholar] [CrossRef]
- Lepper, C.; Conway, S.J.; Fan, C.-M. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 2009, 460, 627–631. [Google Scholar] [CrossRef]
- Ontell, M.; Feng, K.C.; Klueber, K.; Dunn, R.F.; Taylor, F. Myosatellite cells, growth, and regeneration in murine dystrophic muscle: A quantitative study. Anat. Rec. 1984, 208, 159–174. [Google Scholar] [CrossRef]
- Du, M.; Tong, J.; Zhao, J.; Underwood, K.R.; Zhu, M.; Ford, S.P.; Nathanielsz, P.W. Fetal programming of skeletal muscle development in ruminant animals1. J. Anim. Sci. 2010, 88, E51–E60. [Google Scholar] [CrossRef]
- Steinbacher, P.; Haslett, J.R.; Obermayer, A.; Marschallinger, J.; Bauer, H.C.; Sänger, A.M.; Stoiber, W. MyoD andMyogenin expression during myogenic phases in brown trout: A precocious onset of mosaic hyperplasia is a prerequisite for fast somatic growth. Dev. Dyn. 2007, 236, 1106–1114. [Google Scholar] [CrossRef]
- Alexander, M.S.; Kawahara, G.; Kho, A.T.; Howell, M.H.; Pusack, T.J.; Myers, J.A.; Montanaro, F.; Zon, L.I.; Guyon, J.R.; Kunkel, L.M. Isolation and transcriptome analysis of adult zebrafish cells enriched for skeletal muscle progenitors. Muscle Nerve 2011, 43, 741–750. [Google Scholar] [CrossRef]
- Steinbacher, P.; Stadlmayr, V.; Marschallinger, J.; Sänger, A.M.; Stoiber, W. Lateral fast muscle fibers originate from the posterior lip of the teleost dermomyotome. Dev. Dyn. 2008, 237, 3233–3239. [Google Scholar] [CrossRef]
- Fung, T.K.; Ma, H.T.; Poon, R.Y.C. Specialized Roles of the Two Mitotic Cyclins in Somatic Cells: Cyclin A as an Activator of M Phase–promoting Factor. Mol. Biol. Cell 2007, 18, 1861–1873. [Google Scholar] [CrossRef]
- Gong, D.; Ferrell, J.E. The Roles of Cyclin A2, B1, and B2 in Early and Late Mitotic Events. Mol. Biol. Cell 2010, 21, 3149–3161. [Google Scholar] [CrossRef]
- Sutcu, H.H.; Ricchetti, M. Loss of heterogeneity, quiescence, and differentiation in muscle stem cells. Stem Cell Investig. 2018, 5, 9. [Google Scholar] [CrossRef]
- Delfini, M.-C.; De La Celle, M.; Gros, J.; Serralbo, O.; Marics, I.; Seux, M.; Scaal, M.; Marcelle, C. The timing of emergence of muscle progenitors is controlled by an FGF/ERK/SNAIL1 pathway. Dev. Biol. 2009, 333, 229–237. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keenan, S.R.; Currie, P.D. The Developmental Phases of Zebrafish Myogenesis. J. Dev. Biol. 2019, 7, 12. https://doi.org/10.3390/jdb7020012
Keenan SR, Currie PD. The Developmental Phases of Zebrafish Myogenesis. Journal of Developmental Biology. 2019; 7(2):12. https://doi.org/10.3390/jdb7020012
Chicago/Turabian StyleKeenan, Samuel R., and Peter D. Currie. 2019. "The Developmental Phases of Zebrafish Myogenesis" Journal of Developmental Biology 7, no. 2: 12. https://doi.org/10.3390/jdb7020012
APA StyleKeenan, S. R., & Currie, P. D. (2019). The Developmental Phases of Zebrafish Myogenesis. Journal of Developmental Biology, 7(2), 12. https://doi.org/10.3390/jdb7020012



