Regulation of Actin Dynamics in the C. elegans Somatic Gonad
Abstract
:1. Introduction
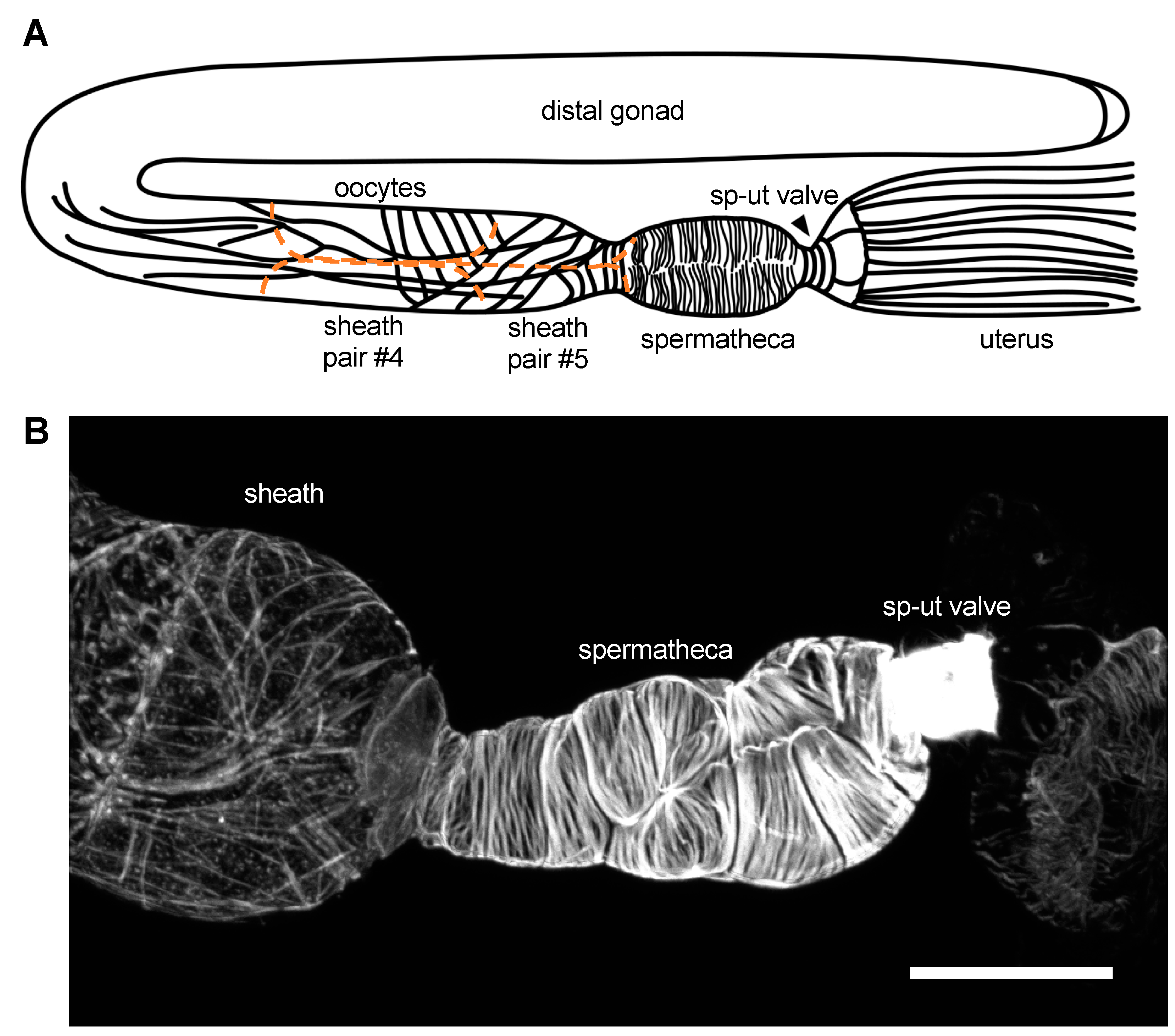
2. The Gonadal Sheath Cells
3. The Spermatheca
4. The SP–UT Valve
5. Shared Features, Unanswered Questions, and Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Pollard, T.D. Actin and Actin-Binding Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a018226. [Google Scholar] [CrossRef] [PubMed]
- Plastino, J.; Blanchoin, L. Dynamic stability of the actin ecosystem. J. Cell Sci. 2018, 132, jcs219832. [Google Scholar] [CrossRef] [PubMed]
- Velarde, N.; Gunsalus, K.C.; Piano, F. Diverse roles of actin in C. elegans early embryogenesis. BMC Dev. Biol. 2007, 7, 142. [Google Scholar] [CrossRef]
- Reymann, A.-C.; Staniscia, F.; Erzberger, A.; Salbreux, G.; Grill, S.W. Cortical flow aligns actin filaments to form a furrow. Elife 2016, 5, e17807. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L. The contractile ring. Curr. Biol. 2011, 21, R976–R978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, J.; Szep, G.; Nemethova, M.; de Vries, I.; Lieber, A.D.; Winkler, C.; Kruse, K.; Small, J.V.; Schmeiser, C.; Keren, K.; et al. Load Adaptation of Lamellipodial Actin Networks. Cell 2017, 171, 188–200.e16. [Google Scholar] [CrossRef]
- Williams-Masson, E.M.; Malik, A.N.; Hardin, J. An actin-mediated two-step mechanism is required for ventral enclosure of the C. elegans hypodermis. Development 1997, 124, 2889–2901. [Google Scholar]
- Burridge, K.; Wittchen, E.S. The tension mounts: Stress fibers as force-generating mechanotransducers. J. Cell Biol. 2013, 200, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Tojkander, S.; Gateva, G.; Lappalainen, P. Actin stress fibers—Assembly, dynamics and biological roles. J. Cell Sci. 2012, 125, 1855–1864. [Google Scholar] [CrossRef]
- Blanchoin, L.; Boujemaa-Paterski, R.; Sykes, C.; Plastino, J. Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 2014, 94, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, N.F.; Hsu, S. Mechanotransduction in endothelial cell migration. J. Cell. Biochem. 2005, 96, 1110–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wozniak, M.A.; Chen, C.S. Mechanotransduction in development: A growing role for contractility. Nat. Rev. Mol. Cell Biol. 2009, 10, 34–43. [Google Scholar] [CrossRef]
- Nelson, C.M.; Gleghorn, J.P. Sculpting Organs: Mechanical Regulation of Tissue Development. Annu. Rev. Biomed. Eng. 2012, 14, 129–154. [Google Scholar] [CrossRef] [PubMed]
- Jodoin, J.N.; Coravos, J.S.; Perkins, L.A.; Perrimon, N.; Correspondence, A.C.M.; Chanet, S.; Vasquez, C.G.; Tworoger, M.; Kingston, E.R.; Martin, A.C. Stable Force Balance between Epithelial Cells Arises from F-Actin Turnover Article. Dev. Cell 2015, 35, 685–697. [Google Scholar] [CrossRef]
- Brugues, A.; Anon, E.; Conte, V.; Veldhuis, J.H.; Gupta, M.; Colombelli, J.; Munoz, J.J.; Brodland, G.W.; Ladoux, B.; Trepat, X. Forces driving epithelial wound healing. Nat. Phys. 2014, 10, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, M.J.; Wu, X.; Nurkiewicz, T.R.; Kawasaki, J.; Davis, G.E.; Hill, M.A.; Meininger, G.A. Integrins and mechanotransduction of the vascular myogenic response. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1427–H1433. [Google Scholar] [CrossRef]
- Boudou, T.; Legant, W.R.; Mu, A.; Borochin, M.A.; Thavandiran, N.; Radisic, M.; Zandstra, P.W.; Epstein, J.A.; Margulies, K.B.; Chen, C.S. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng. Part A 2012, 18, 910–919. [Google Scholar] [CrossRef]
- Skwarek-Maruszewska, A.; Hotulainen, P.; Mattila, P.K.; Lappalainen, P. Contractility-dependent actin dynamics in cardiomyocyte sarcomeres. J. Cell Sci. 2009, 122, 2119–2126. [Google Scholar] [CrossRef] [Green Version]
- Greiner, A.M.; Chen, H.; Spatz, J.P.; Kemkemer, R. Cyclic Tensile Strain Controls Cell Shape and Directs Actin Stress Fiber Formation and Focal Adhesion Alignment in Spreading Cells. PLoS ONE 2013, 8, e77328. [Google Scholar] [CrossRef]
- Hannezo, E.; Dong, B.; Recho, P.; Joanny, J.-F.; Hayashi, S. Cortical instability drives periodic supracellular actin pattern formation in epithelial tubes. Proc. Natl. Acad. Sci. USA 2015, 112, 8620–8625. [Google Scholar] [CrossRef] [Green Version]
- Mason, F.M.; Tworoger, M.; Martin, A.C. Apical domain polarization localizes actin-myosin activity to drive ratchet-like apical constriction. Nat. Cell Biol. 2013, 15, 926–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosono, C.; Matsuda, R.; Adryan, B.; Samakovlis, C. Transient junction anisotropies orient annular cell polarization in the Drosophila airway tubes. Nat. Cell Biol. 2015, 17, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Pellegrin, S.; Mellor, H. Actin stress fibres. J. Cell Sci. 2007, 120, 3491–3499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naumanen, P.; Lappalainen, P.; Hotulainen, P. Mechanisms of actin stress fibre assembly. J. Microsc. 2008, 231, 446–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollard, T.D.; Blanchoin, L.; Mullins, R.D. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 545–576. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Cooper, J.A. Actin, a Central Player in Cell Shape and Movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cetera, M.; Ramirez-San Juan, G.R.; Oakes, P.W.; Lewellyn, L.; Fairchild, M.J.; Tanentzapf, G.; Gardel, M.L.; Horne-Badovinac, S. Epithelial rotation promotes the global alignment of contractile actin bundles during Drosophila egg chamber elongation. Nat. Commun. 2014, 5, 5511. [Google Scholar] [CrossRef] [PubMed]
- Kasza, K.E.; Farrell, D.L.; Zallen, J.A. Spatiotemporal control of epithelial remodeling by regulated myosin phosphorylation. Proc. Natl. Acad. Sci. USA 2014, 111, 11732–11737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashi, T.; Arnold, T.R.; Stephenson, R.E.; Dinshaw, K.M.; Miller, A.L. Maintenance of the Epithelial Barrier and Remodeling of Cell-Cell Junctions during Cytokinesis. Curr. Biol. 2016, 26, 1829–1842. [Google Scholar] [CrossRef]
- Stephenson, R.E.; Higashi, T.; Erofeev, I.S.; Arnold, T.R.; Leda, M.; Goryachev, A.B.; Miller, A.L. Rho Flares Repair Local Tight Junction Leaks. Dev. Cell 2019, 48, 445–459.e5. [Google Scholar] [CrossRef] [PubMed]
- Mandato, C.A.; Bement, W.M. Contraction and polymerization cooperate to assemble and close actomyosin rings around Xenopus oocyte wounds. J. Cell Biol. 2001, 154, 785–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merriam, R.W.; Christensen, K. A contractile ring-like mechanism in wound healing and soluble factors affecting structural stability in the cortex of Xenopus eggs and oocytes. J. Embryol. Exp. Morphol. 1983, 75, 11–20. [Google Scholar] [PubMed]
- Sonnemann, K.J.; Bement, W.M. Wound Repair: Toward Understanding and Integration of Single-Cell and Multicellular Wound Responses. Annu. Rev. Cell Dev. Biol. 2011, 27, 237–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherwood, D.R.; Plastino, J. Invading, leading and navigating cells in caenorhabditis elegans: Insights into cell movement in vivo. Genetics 2018, 208, 53–78. [Google Scholar] [CrossRef]
- Tang, N.H.; Jin, Y. Shaping neurodevelopment: Distinct contributions of cytoskeletal proteins. Curr. Opin. Neurobiol. 2018, 51, 111–118. [Google Scholar] [CrossRef]
- Ono, S. Regulation of structure and function of sarcomeric actin filaments in striated muscle of the nematode Caenorhabditis elegans. Anat. Rec. 2014, 297, 1548–1559. [Google Scholar] [CrossRef]
- Hubbard, E.J.A.; Greenstein, D. The Caenorhabditis elegans Gonad: A Test Tube for Cell and Developmental Biology. Dev. Dyn. 2000, 218, 2–22. [Google Scholar] [CrossRef]
- Whitten, S.J.; Miller, M.A. The role of gap junctions in Caenorhabditis elegans oocyte maturation and fertilization. Dev. Biol. 2006, 301, 432–446. [Google Scholar] [CrossRef]
- Altun, Z.F.; Chen, B.; Wang, Z.-W.; Hall, D.H. High resolution map of Caenorhabditis elegans gap junction proteins. Dev. Dyn. 2009, 238, 1936–1950. [Google Scholar] [CrossRef]
- Kimble, J.; Hirsh, D. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev. Biol. 1979, 70, 396–417. [Google Scholar] [CrossRef]
- Lints, R.; Hall, D.H. Reproductive system, somatic gonad. WormAtlas 2009. [Google Scholar] [CrossRef]
- McCarter, J.; Bartlett, B.; Dang, T.; Schedl, T. Soma–Germ Cell Interactions in Caenorhabditis elegans: Multiple Events of Hermaphrodite Germline Development Require the Somatic Sheath and Spermathecal Lineages. Dev. Biol. 1997, 181, 121–143. [Google Scholar] [CrossRef]
- McCarter, J.; Bartlett, B.; Dang, T.; Schedl, T. On the Control of Oocyte Meiotic Maturation and Ovulation in Caenorhabditis elegans. Dev. Biol. 1999, 205, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.; Hirsh, D. Receptor-mediated Endocytosis in the Caenorhabditis elegans Oocyte. Mol. Biol. Cell 1999, 10, 4311–4326. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.H.; Winfrey, V.P.; Blaeuer, G.; Hoffman, L.H.; Furuta, T.; Rose, K.L.; Hobert, O.; Greenstein, D. Ultrastructural Features of the Adult Hermaphrodite Gonad of Caenorhabditis elegans: Relations between the Germ Line and Soma. Dev. Biol. 1999, 212, 101–123. [Google Scholar] [CrossRef]
- Strome, S. Fluorescence Visualization of the Distribution of Microfilaments in Gonads and Early Embryos of the Nematode Caenorhabditis elegans. J. Cell Biol. 1986, 103, 2241–2252. [Google Scholar] [CrossRef]
- Yin, X.Y.; Gower, N.J.D.; Baylis, H.A.; Strange, K. Inositol 1,4,5-Trisphosphate Signaling Regulates Rhythmic Contractile Activity of Myoepithelial Sheath Cells in Caenorhabditis elegans. Mol. Biol. Cell 2004, 15, 3938–3949. [Google Scholar] [CrossRef] [PubMed]
- Clandinin, T.R.; DeModena, J.A.; Sternberg, P.W. Inositol trisphosphate mediates a RAS-independent response to LET-23 receptor tyrosine kinase activation in C. elegans. Cell 1998, 92, 523–533. [Google Scholar] [CrossRef]
- Norman, K.R.; Fazzio, R.T.; Mellem, J.E.; Espelt, M.V.; Strange, K.; Beckerle, M.C.; Maricq, A.V. The Rho/Rac-Family Guanine Nucleotide Exchange Factor VAV-1 Regulates Rhythmic Behaviors in C. elegans. Cell 2005, 123, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Ardizzi, J.P.; Epstein, H.F. Immunochemical Localization of Myosin Heavy Chain Isoforms and Paramyosin in Developmentally and Structurally Diverse Muscle Cell Types of the Nematode Caenorhabditis elegans. J. Cell Biol. 1987, 105, 2763–2770. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Yu, R.; Ono, S. Structural components of the nonstriated contractile apparatuses in the Caenorhabditis elegans gonadal myoepithelial sheath and their essential roles for ovulation. Dev. Dyn. 2007, 236, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Ono, S. Two distinct myosin II populations coordinate ovulatory contraction of the myoepithelial sheath in the Caenorhabditis elegans somatic gonad. Mol. Biol. Cell 2016, 27, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Obinata, T.; Ono, K.; Ono, S. Troponin I controls ovulatory contraction of non-striated actomyosin networks in the C. elegans somatic gonad. J. Cell Sci. 2010, 123, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Ono, S. Tropomyosin and Troponin Are Required for Ovarian Contraction in the Caenorhabditis elegans Reproductive System. Mol. Biol. Cell 2004, 15, 2782–2793. [Google Scholar] [CrossRef]
- Myers, C.D.; Goh, P.Y.; Allen, T.S.; Bucher, E.A.; Bogaert, T. Developmental genetic analysis of troponin T mutations in striated and nonstriated muscle cells of Caenorhabditis elegans. J. Cell Biol. 1996, 132, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Ciobanasu, C.; Faivre, B.; Le Clainche, C. Actin Dynamics Associated with Focal Adhesions. Int. J. Cell Biol. 2012, 2012, 941292. [Google Scholar] [CrossRef]
- Xu, X.; Rongali, S.C.; Miles, J.P.; Lee, K.D.; Lee, M. pat-4/ILK and unc-112/Mig-2 are required for gonad function in Caenorhabditis elegans. Exp. Cell Res. 2006, 312, 1475–1483. [Google Scholar] [CrossRef]
- Cram, E.J.; Clark, S.G.; Schwarzbauer, J.E. Talin loss-of-function uncovers roles in cell contractility and migration in C. elegans. J. Cell Sci. 2003, 116, 3871–3878. [Google Scholar] [CrossRef]
- Wioland, H.; Guichard, B.; Senju, Y.; Myram, S.; Lappalainen, P.; Jégou, A.; Romet-Lemonne, G. ADF/Cofilin Accerlerates Actin Dynamics by Severing Filaments and Promoting Their Depolymerization at Both Ends. Curr. Biol. 2017, 27, 1956–1967. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Baillie, D.L.; Benian, G.M. UNC-60B, an ADF/Cofilin Family Protein, Is Required for Proper Assembly of Actin into Myofibrils in Caenorhabditis elegans Body Wall Muscle. J. Cell Biol. 1999, 145, 491–502. [Google Scholar] [CrossRef]
- Ono, K.; Yamashiro, S.; Ono, S. Essential role of ADF/cofilin for assembly of contractile actin networks in the C. elegans somatic gonad. J. Cell Sci. 2008, 121, 2662–2670. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Hayakawa, K.; Tatsumi, H.; Ono, S. Actin-interacting Protein 1 Promotes Disassembly of Actin-depolymerizing Factor/Cofilin-bound Actin Filaments in a pH-dependent Manner. J. Biol. Chem. 2016, 291, 5146–5156. [Google Scholar] [CrossRef]
- Ono, K.; Ono, S. Two actin-interacting protein 1 isoforms function redundantly in the somatic gonad and are essential for reproduction in Caenorhabditis elegans. Cytoskeleton 2014, 71, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Rodal, A.A.; Tetreault, J.W.; Lappalainen, P.; Drubin, D.G.; Amberg, D.C. Aip1p Interacts with Cofilin to Disassemble Actin Filaments. J. Cell Biol. 1999, 145, 1251–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, S. Functions of actin-interacting protein 1 (AIP1)/WD repeat protein 1 (WDR1) in actin filament dynamics and cytoskeletal regulation. Biochem. Biophys. Res. Commun. 2018, 506, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Zaidel-Bar, R.; Joyce, M.J.; Lynch, A.M.; Witte, K.; Audhya, A.; Hardin, J. The F-BAR domain of SRGP-1 facilitates cell-cell adhesion during C. elegans morphogenesis. J. Cell Biol. 2010, 191, 761–769. [Google Scholar] [CrossRef]
- Hong, F.; Haldeman, B.D.; Jackson, D.; Carter, M.; Baker, J.E.; Cremo, C.R. Biochemistry of Smooth Muscle Myosin Light Chain Kinase. Arch. Biochem. Biophys. 2011, 510, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Kovacevic, I.; Orozco, J.M.; Cram, E.J. Filamin and phospholipase C-ε are required for calcium signaling in the Caenorhabditis elegans spermatheca. PLoS Genet. 2013, 9, e1003510. [Google Scholar] [CrossRef]
- Kelley, C.A.; Wirshing, A.C.E.; Zaidel-Bar, R.; Cram, E.J. The myosin light-chain kinase MLCK-1 relocalizes during Caenorhabditis elegans ovulation to promote actomyosin bundle assembly and drive contraction. Mol. Biol. Cell 2018, 29, 1975–1991. [Google Scholar] [CrossRef]
- Wissmann, A.; Ingles, J.; McGhee, J.D.; Mains, P.E. Caenorhabditis elegans LET-502 is related to Rho-binding kinases and human myotonic dystrophy kinase and interacts genetically with a homolog of the regulatory subunit of smooth muscle myosin phosphatase to affect cell shape. Genes Dev. 1997, 11, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Wissmann, A.; Ingles, J.; Mains, P.E. The Caenorhabditis elegans mel-11 Myosin Phosphatase Regulatory Subunit Affects Tissue Contraction in the Somatic Gonad and the Embryonic Epidermis and Genetically Interacts with the Rac Signaling Pathway. Dev. Biol. 1999, 209, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.Y.; Zaidel-Bar, R. Transient membrane localization of SPV-1 drives cyclical actomyosin contractions in the C. elegans spermatheca. Curr. Biol. 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Ito, M.; Amano, M.; Chihara, K.; Fukata, Y.; Nakafuku, M.; Yamamori, B.; Feng, J.; Nakano, T.; Okawa, K.; et al. Regulation of Myosin Phosphatase by Rho and Rho-Associated Kinase (Rho-Kinase). Science 1996, 273, 245–248. [Google Scholar] [CrossRef]
- Wirshing, A.C.E.; Cram, E.J. Myosin activity drives actomyosin bundle formation and organization in contractile cells of the Caenorhabditis elegans spermatheca. Mol. Biol. Cell 2017, 28, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, I.; Cram, E.J. FLN-1/Filamin is required for maintenance of actin and exit of fertilized oocytes from the spermatheca in C. elegans. Dev. Biol. 2010, 347, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Xia, D.; Fang, B.; Zhang, H. The flightless I homolog, fli-1, regulates anterior/posterior polarity, asymmetric cell division and ovulation during Caenorhabditis elegans development. Genetics 2007, 177, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Hegsted, A.; Wright, F.A.; Votra, S.; Pruyne, D. INF2- and FHOD-related formins promote ovulation in the somatic gonad of C. elegans. Cytoskeleton 2016, 73, 712–728. [Google Scholar] [CrossRef]
- Bouffard, J.; Cecchetelli, A.D.; Clifford, C.; Sethi, K.; Zaidel-Bar, R.; Cram, E.J. The RhoGAP SPV-1 regulates calcium signaling to control the contractility of the C. elegans spermatheca during embryo transits. Mol. Biol. Cell 2019. [Google Scholar] [CrossRef]
- Aono, S.; Legouis, R.; Hoose, W.A.; Kemphues, K.J. PAR-3 is required for epithelial cell polarity in the distal spermatheca of C. elegans. Development 2004, 131, 2865–2874. [Google Scholar] [CrossRef]
- Kim, H.; McCulloch, C.A. Filamin A mediates interactions between cytoskeletal proteins that control cell adhesion. FEBS Lett. 2011, 585, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Djinovic-Carugo, K.; Carugo, O. Structural portrait of filamin interaction mechanisms. Curr. Protein Peptide Sci. 2010, 11, 639–650. [Google Scholar] [CrossRef]
- McKeown, C.; Praitis, V.; Austin, J. sma-1 encodes a betaH-spectrin homolog required for Caenorhabditis elegans morphogenesis. Development 1998, 125, 2087–2098. [Google Scholar] [PubMed]
- Buechner, M.; Hall, D.H.; Bhatt, H.; Hedgecock, E.M. Cystic canal mutants in Caenorhabditis elegans are defective in the apical membrane domain of the renal (excretory) cell. Dev. Biol. 1999, 214, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.R.; Moerman, D.G. α spectrin is essential for morphogenesis and body wall muscle formation in Caenorhabditis elegans. J. Cell Biol. 2002, 157, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, A.; Charron, A.; Sadozai, Y.; Switaj, L.; Szutenbach, A.; Smith, P.A. Multiple phenotypes resulting from a mutagenesis screen for pharynx muscle mutations in Caenorhabditis elegans. PLoS ONE 2011, 6, e26594. [Google Scholar] [CrossRef] [PubMed]
- Wirshing, A.C.E.; Cram, E.J. Spectrin regulates cell contractility through production and maintenance of actin bundles in the Caenorhabditis elegans spermatheca. Mol. Biol. Cell 2018, 29, 2433–2449. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Fischman, D.A.; Steck, T.L. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J. Supramol. Struct. 1973, 1, 233–248. [Google Scholar] [CrossRef]
- Greenquist, A.C.; Shohet, S.B.; Bernstein, S.E. Marked reduction of spectrinin hereditary spherocytosis in the common house mouse. Blood 1978, 51, 1149–1155. [Google Scholar]
- Tse, W.T.; Lecomte, M.C.; Costa, F.F.; Garbarz, M.; Feo, C.; Boivin, P.; Dhermy, D.; Forget, B.G. Point mutation in the beta-spectrin gene associated with alpha I/74 hereditary elliptocytosis. Implications for the mechanism of spectrin dimer self-association. J. Clin. Investig. 1990, 86, 909–916. [Google Scholar] [CrossRef]
- Hammarlund, M.; Jorgensen, E.M.; Bastiani, M.J. Axons break in animals lacking β-spectrin. J. Cell Biol. 2007, 176, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruyama, R.; Velarde, N.V.; Klancer, R.; Gordon, S.; Kadandale, P.; Parry, J.M.; Hang, J.S.; Rubin, J.; Stewart-Michaelis, A.; Schweinsberg, P.; et al. EGG-3 Regulates Cell-Surface and Cortex Rearrangements during Egg Activation in Caenorhabditis elegans. Curr. Biol. 2007, 17, 1555–1560. [Google Scholar] [CrossRef]
- Rattan, S.; De Godoy, M.A.F.; Patel, C.A. Rho Kinase as a Novel Molecular Therapeutic Target for Hypertensive Internal Anal Sphincter. Gastroenterology 2006, 131, 108–116. [Google Scholar] [CrossRef] [Green Version]
- DeMaso, C.R.; Kovacevic, I.; Uzun, A.; Cram, E.J. Structural and functional evaluation of C. elegans filamins FLN-1 and FLN-2. PLoS ONE 2011, 6, e22428. [Google Scholar] [CrossRef]
- Sawa, M.; Suetsugu, S.; Sugimoto, A.; Miki, H.; Yamamoto, M.; Takenawa, T. Essential role of the C. elegans Arp2/3 complex in cell migration during ventral enclosure. J. Cell Sci. 2003, 116, 1505–1518. [Google Scholar] [CrossRef]
- Vanneste, C.A.; Pruyne, D.; Mains, P.E. The role of the formin gene fhod-1 in C. elegans embryonic morphogenesis. Worm 2013, 2, e25040. [Google Scholar] [CrossRef] [PubMed]
- Gettner, S.N.; Kenyon, C.; Reichardt, L.E. Characterization of/3pat-3 Heterodimers, a Family of Essential Integrin Receptors in C. elegans. J. Cell Biol. 1995, 129, 1127–1141. [Google Scholar] [CrossRef]
- Lynch, A.M.; Hardin, J. The assembly and maintenance of epithelial junctions in C. elegans. Front. Biosci. 2009, 1, 1414–1432. [Google Scholar] [CrossRef]
- Nance, J.; Munro, E.M.; Priess, J.R. C. elegans PAR-3 and PAR-6 are required for apicobasal asymmetries associated with cell adhesion and gastrulation. Development 2003, 130, 5339–5350. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Raich, W.; Agbunag, C.; Leung, B.; Hardin, J.; Priess, J.R. A Putative Catenin-Cadherin System Mediates Morphogenesis of the Caenorhabditis elegans Embryo. J. Cell Biol. 1998, 141, 297–308. [Google Scholar] [CrossRef]
- Pilipiuk, J.; Lefebvre, C.; Wiesenfahrt, T.; Legouis, R.; Bossinger, O. Increased IP3/Ca2+ signaling compensates depletion of LET-413/DLG-1 in C. elegans epithelial junction assembly. Dev. Biol. 2009, 327, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ward, J.D.; Cheng, Z.; Dernburg, A.F. The auxin-inducible degradation (AID) system enables versatile conditional protein depletion in C. elegans. Development 2015, 142, 4374–4384. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Furuse, M. Molecular organization and function of invertebrate occluding junctions. Semin. Cell Dev. Biol. 2014, 36, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Babatz, F.; Naffin, E.; Klämbt, C. The Drosophila Blood-Brain Barrier Adapts to Cell Growth by Unfolding of Pre-existing Septate Junctions. Dev. Cell 2018, 47, 697–710.e3. [Google Scholar] [CrossRef]
- Hatan, M.; Shinder, V.; Schnorrer, F.; Volk, T. The Drosophila blood brain barrier is maintained by GPCR-dependent dynamic actin structures. J. Cell Biol. 2011, 192, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Simske, J.S. Claudins reign: The claudin/EMP/PMP22/γ channel protein family in C. elegans. Tissue Barriers 2013, 1, e25502. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Fehon, R.G. Analysis of protein dynamics within the septate junction reveals a highly stable core protein complex that does not include basolateral polarity protein Discs large. J. Cell Sci. 2011, 124, 2861–2871. [Google Scholar] [CrossRef] [PubMed]
- Wiener, J.; Spiro, D.; Loewenstein, W.R. Studies on an epithelial (gland) cell junction. II. Surfact Structure. J. Cell Biol. 1964, 22, 587–598. [Google Scholar] [CrossRef]
- Noirot-Timothee, C.; Smith, D.S.; Cayer, M.L.; Noirot, C. Septate Junctions in Insects: Comparison between Intercellular and Intramembranous Structures. Tissue Cell 1978, 10, 125–136. [Google Scholar] [CrossRef]
- Genova, J.L.; Fehon, R.G. Neuroglian, Gliotactin, and the Na/K ATPase are essential for septate junction function in Drosophila. J. Cell Biol. 2003, 161, 979–989. [Google Scholar] [CrossRef]
- Haklai-Topper, L.; Soutschek, J.; Sabanay, H.; Scheel, J.; Hobert, O.; Peles, E. The neurexin superfamily of Caenorhabditis elegans. Gene Expr. Patterns 2011, 11, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Draper, B.W.; Priess, J.R. The Role of Actin Filaments in Patterning the Caenorhabditis elegans Cuticle. Dev. Biol. 1997, 184, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Priess, J.R.; Hirsh, D.I. Caenorhabditis elegans morphogenesis: The role of the cytoskeleton in elongation of the embryo. Dev. Biol. 1986, 117, 156–173. [Google Scholar] [CrossRef]
- Tamiello, C.; Buskermolen, A.B.C.; Baaijens, F.P.T.; Broers, J.L.V.; Bouten, C.V.C. Heading in the Right Direction: Understanding Cellular Orientation Responses to Complex Biophysical Environments. Cell. Mol. Bioeng. 2015, 9, 12–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plastino, J.; Blanchoin, L. Adaptive Actin Networks. Dev. Cell 2017, 42, 565–566. [Google Scholar] [CrossRef] [PubMed]
- Kaunas, R.; Nguyen, P.; Usami, S.; Chien, S. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc. Natl. Acad. Sci. USA 2005, 102, 15895–15900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaCroix, A.S.; Rothenberg, K.E.; Berginski, M.E.; Urs, A.N.; Hoffman, B.D. Construction, imaging, and analysis of FRET-based tension sensors in living cells. Methods Cell Biol. 2015, 125, 161–186. [Google Scholar] [PubMed] [Green Version]
- Kobb, A.B.; Zulueta-Coarasa, T.; Fernandez-Gonzalez, R. Tension regulates myosin dynamics during Drosophila embryonic wound repair. J. Cell Sci. 2017, 130, 689–696. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelley, C.A.; Cram, E.J. Regulation of Actin Dynamics in the C. elegans Somatic Gonad. J. Dev. Biol. 2019, 7, 6. https://doi.org/10.3390/jdb7010006
Kelley CA, Cram EJ. Regulation of Actin Dynamics in the C. elegans Somatic Gonad. Journal of Developmental Biology. 2019; 7(1):6. https://doi.org/10.3390/jdb7010006
Chicago/Turabian StyleKelley, Charlotte A., and Erin J Cram. 2019. "Regulation of Actin Dynamics in the C. elegans Somatic Gonad" Journal of Developmental Biology 7, no. 1: 6. https://doi.org/10.3390/jdb7010006



