Molecular and Cellular Characterization of Avian Reticulate Scales Implies the Evo–Devo Novelty of Skin Appendages in Foot Sole
Abstract
1. Introduction
2. Method
2.1. Juvenile Alligators and Adult Chickens
2.2. Pulse BrdU Labeling and Identification of Label Retaining Cells
2.3. Immunostaining and Whole-Mount/Section In Situ Hybridization
2.4. RNAscope
2.5. Transgenic Quail Eggs and Confocal Imaging
2.6. Reticulate Scale Wound Healing and Regeneration
3. Results
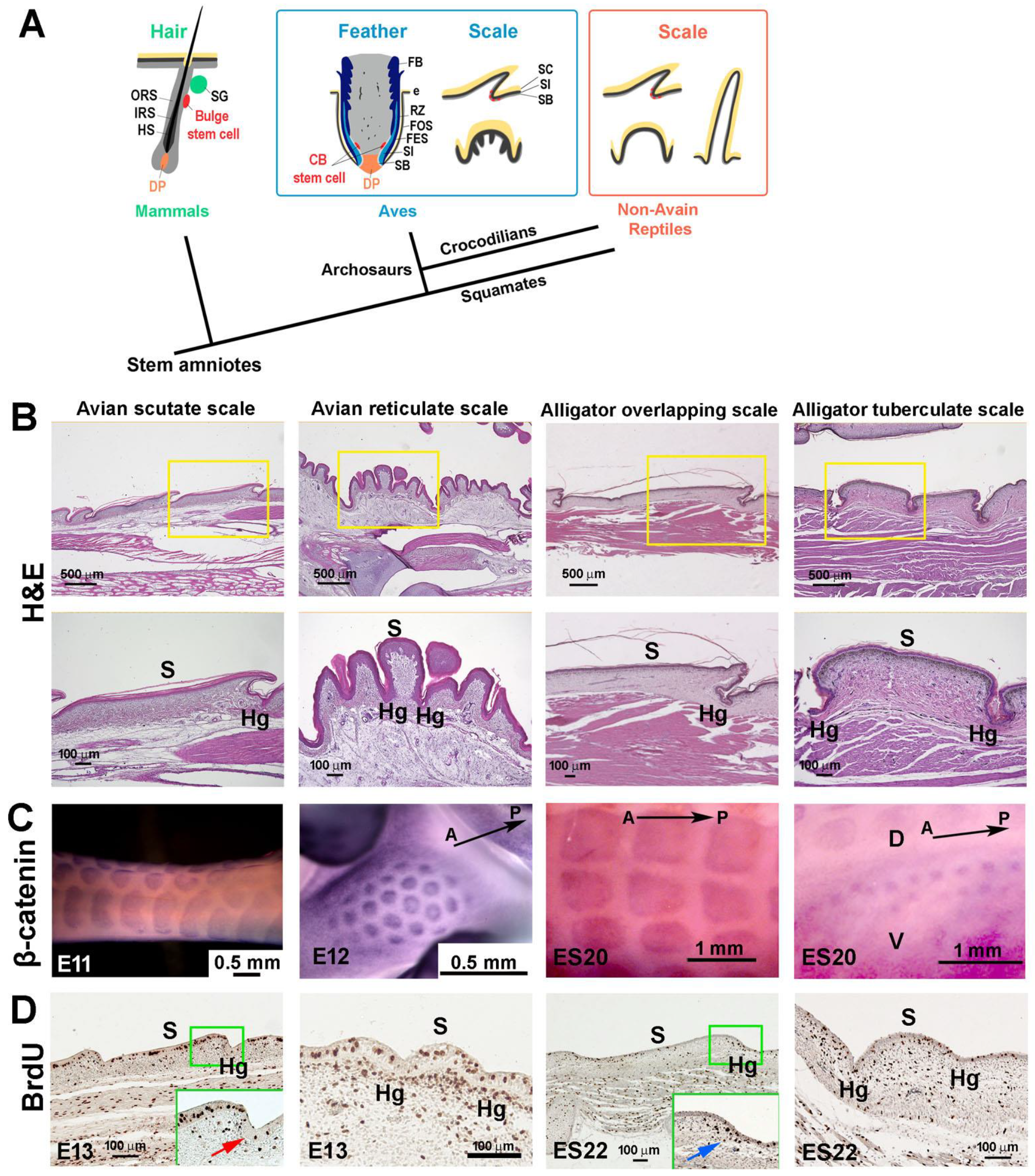
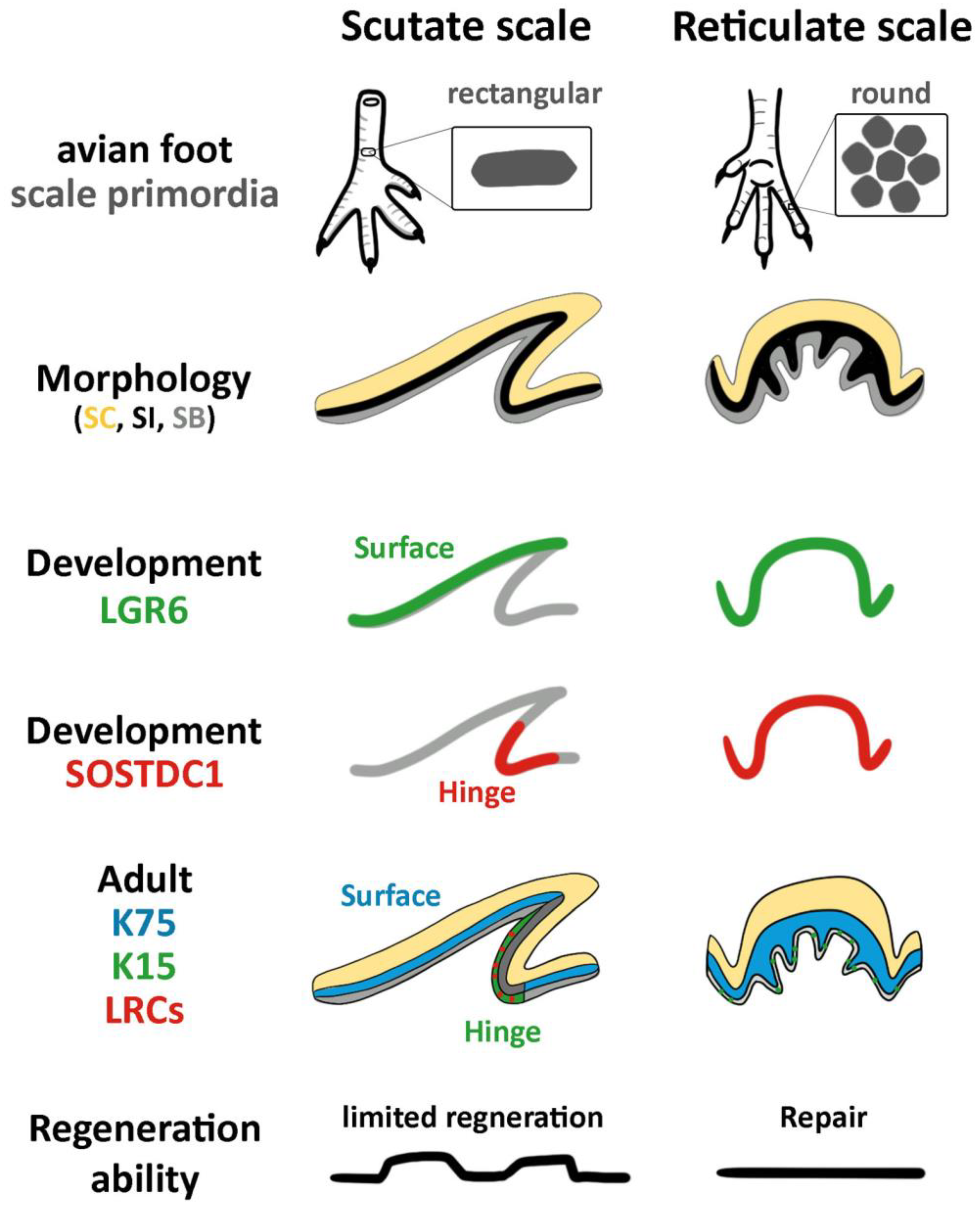
3.1. The Structure of Reticulate Scales and Comparison with Other Scales
3.2. Difference in Morphogen Expression between Avian Scutate Scales and Reticulate Scales
3.3. Difference in Epidermal Cell Arrangements in Developing Scutate and Reticulate Scales Using Transgenic Quail Embryos
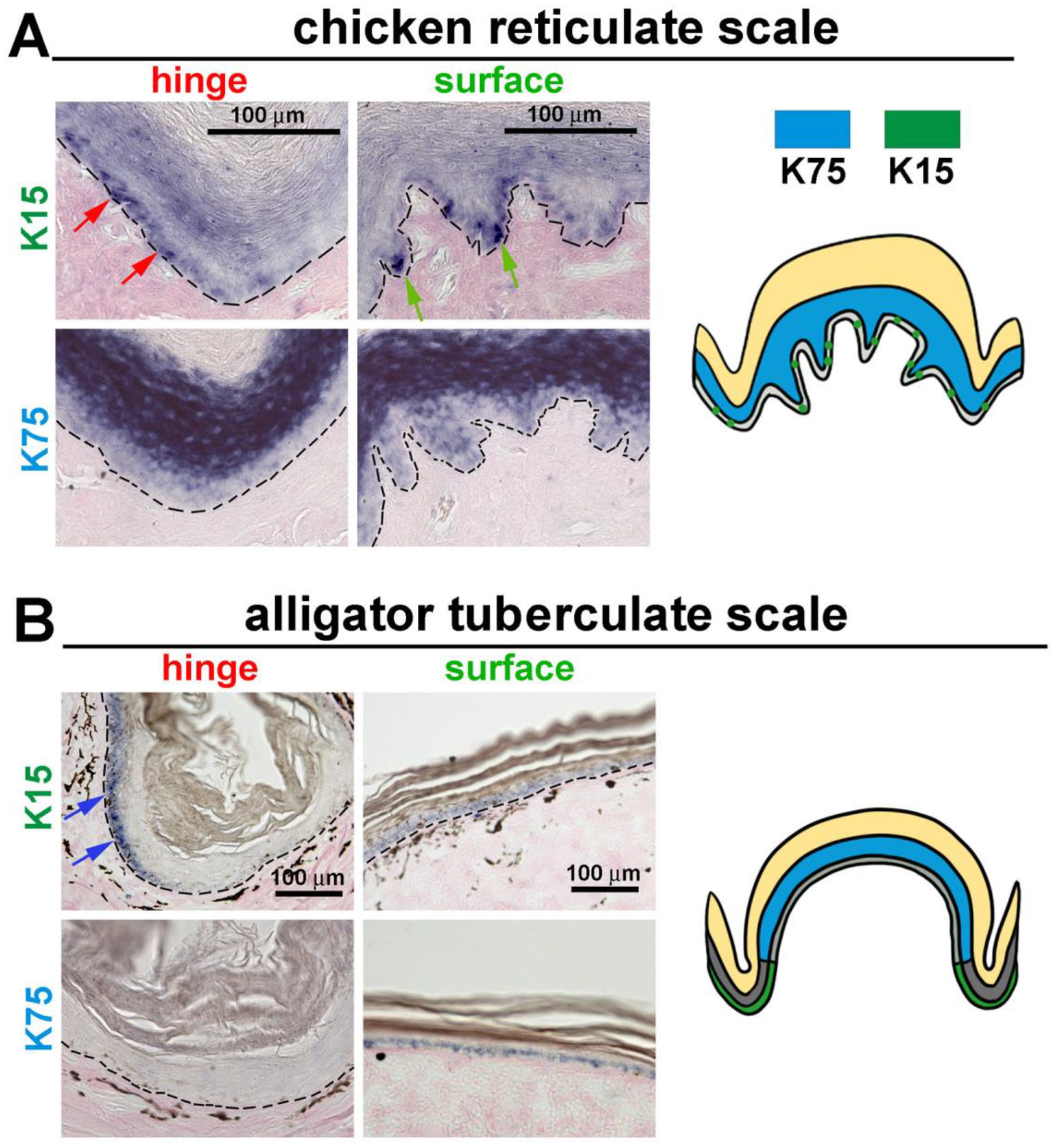
3.4. The Expression of K15 Is Sporadic and Not Restricted in the Reticulate Scale Hinge like in the Scutate Scale
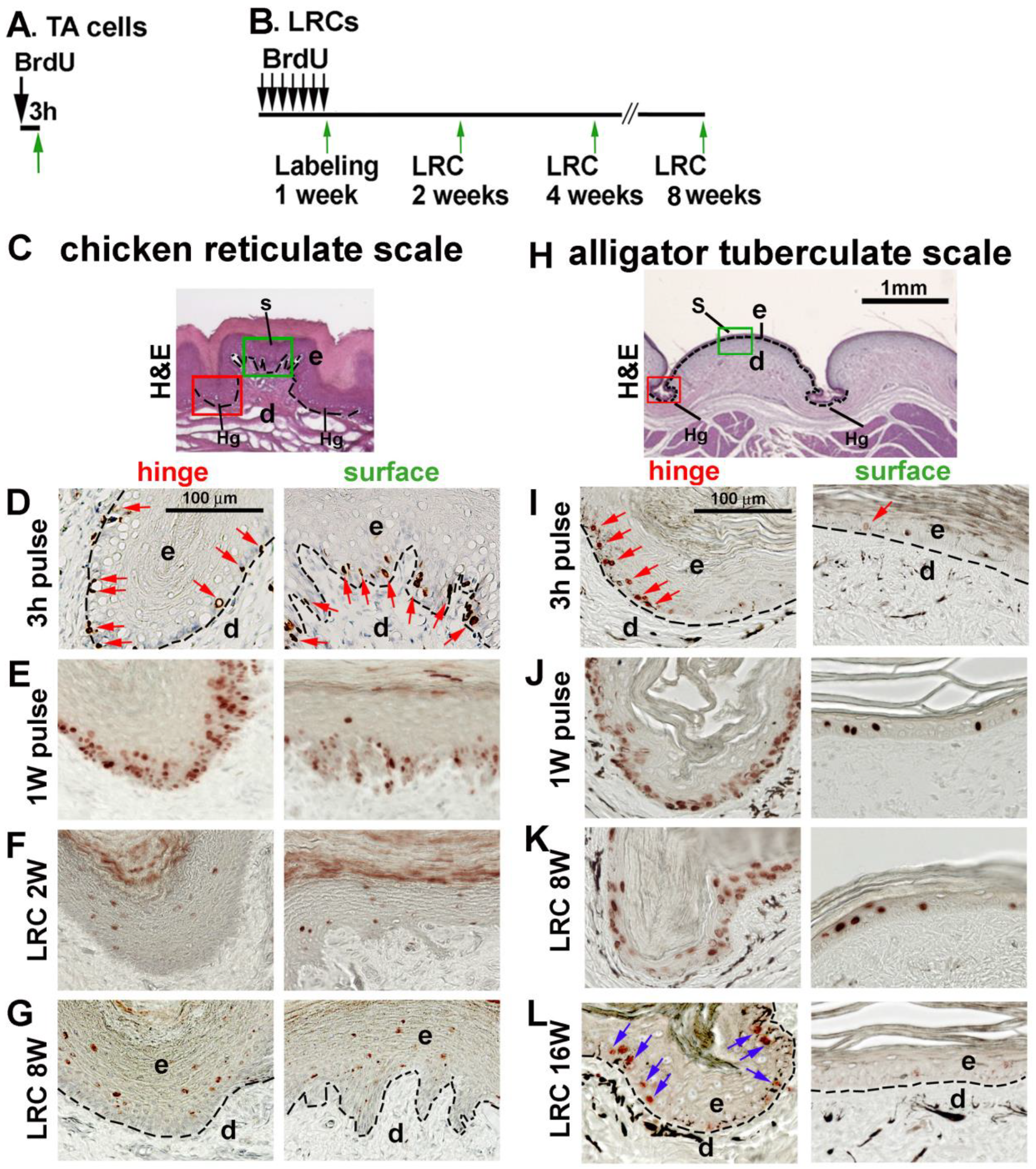
3.5. LRCs in Reticulate Scales Are Not Distributed in Clusters like in Avian Scutate Scales and Alligator Overlapping Scales
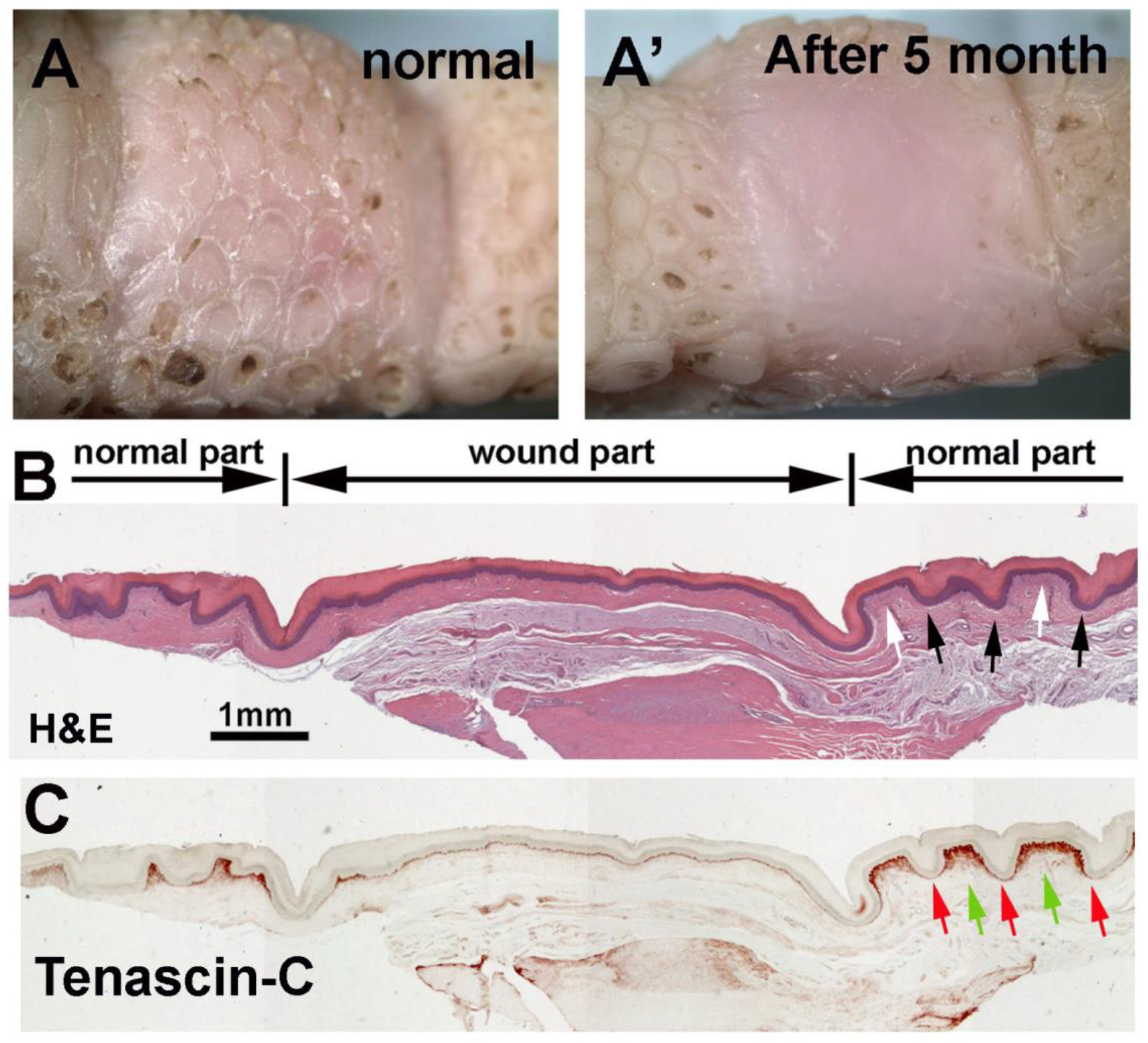
3.6. Wound Healing Response of Avian Foot Sole Skin and Reticulate Scales
4. Discussion
4.1. The Molecular Expression in Embryonic Stages Specifies Different Adult Properties in Avian Scales
4.2. The Stem Cell Niche Configuration Determines the Mode of Physiological Regeneration and Response to Wounding
4.3. Hinge versus Surface in Different Scale Architectures
4.4. The Unique Feature of Reticulate Scales on Avian Foot Sole
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, P.; Hou, L.; Plikus, M.; Hughes, M.; Scehnet, J.; Suksaweang, S.; Widelitz, R.; Jiang, T.-X.; Chuong, C.-M. Evo-Devo of amniote integuments and appendages. Int. J. Dev. Biol. 2004, 48, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Wu, P.; Baker, R.E.; Maini, P.K.; Alibardi, L.; Chuong, C.-M. Reptile scale paradigm: Evo-Devo, pattern formation and regeneration. Int. J. Dev. Biol. 2009, 53, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Chuong, C.; Wu, P.; Plikus, M.; Jiang, T.; Widelitz, R.B. Engineering stem cells into organs: Topobiological transformations demonstrated by beak, feather, and other ectodermal organ morphogenesis. Curr. Top. Dev. Biol. 2006, 72, 237–274. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Jiang, T.-X.; Lei, M.; Chen, C.-K.; Li, S.-M.H.; Widelitz, R.B.; Chuong, C.-M. Cyclic growth of dermal papilla and regeneration of follicular mesenchymal components during feather cycling. Development 2021, 148, dev.198671. [Google Scholar] [CrossRef] [PubMed]
- Blanpain, C.; Lowry, W.E.; Geoghegan, A.; Polak, L.; Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 2004, 118, 635–648. [Google Scholar] [CrossRef]
- Yue, Z.; Jiang, T.-X.; Widelitz, R.B.; Chuong, C.-M. Mapping stem cell activities in the feather follicle. Nature 2005, 438, 1026–1029. [Google Scholar] [CrossRef]
- Sun, T.-T.; Costsarelis, G.; Lavker, R.M. Hair follicular stem cells: The bulge-activation hypothesis. J. Investig. Dermatol. 1991, 96, 77S–78S. [Google Scholar] [CrossRef]
- Wu, P.; Lai, Y.-C.; Widelitz, R.; Chuong, C.-M. Comprehensive molecular and cellular studies suggest avian scutate scales are secondarily derived from feathers, and more distant from reptilian scales. Sci. Rep. 2018, 8, 16766-y. [Google Scholar] [CrossRef]
- Sawyer, R.H.; Knapp, L.W.; O’Guin, W.M. Biology of the Integument; Springer: Berlin/Heidelberg, Germany, 1986; pp. 194–238. [Google Scholar]
- Cooper, R.L.; Lloyd, V.J.; Di-Poï, N.; Fletcher, A.G.; Barrett, P.M.; Fraser, G.J. Conserved gene signalling and a derived patterning mechanism underlie the development of avian footpad scales. Evodevo 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Wu, P.; Ng, C.S.; Yan, J.; Lai, Y.C.; Chen, C.K.; Lai, Y.T.; Wu, S.M.; Chen, J.J.; Luo, W.; Widelitz, R.B.; et al. Topographical mapping of α- and β-keratins on developing chicken skin integuments. Proc. Natl. Acad. Sci. USA 2015, 112, E6770–E6779. [Google Scholar] [CrossRef]
- Dhouailly, D.; Hardy, M.H.; Sengel, P. Formation of feathers on chick foot scales: A stage-dependent morphogenetic response to retinoic acid. J. Embryol. Exp. Morphol. 1980, 58, 63–78. [Google Scholar] [CrossRef]
- Widelitz, R.B.; Jiang, T.X.; Lu, J.; Chuong, C.M. Beta-Catenin in Epithelial Morphogenesis: Conversion of Part of Avian Foot Scales into Feather Buds with a Mutated Beta-Catenin. Dev. Biol. 2000, 219, 98–114. [Google Scholar] [CrossRef]
- Crowe, R.; Niswander, L. Disruption of scale development by Delta-1 misexpression. Dev. Biol. 1998, 195, 70–74. [Google Scholar] [CrossRef]
- Zou, H.; Niswander, L. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science 1996, 272, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.L.; Milinkovitch, M.C. Transient agonism of the sonic hedgehog pathway triggers a permanent transition of skin appendage fate in the chicken embryo. Sci. Adv. 2023, 9, eadg9619. [Google Scholar] [CrossRef]
- Wu, P.; Yan, J.; Lai, Y.-C.; Ng, C.S.; Li, A.; Jiang, X.; Elsey, R.M.; Widelitz, R.; Bajpai, R.; Li, W.-H.; et al. Multiple Regulatory Modules Are Required for Scale-to-Feather Conversion. Mol. Biol. Evol. 2018, 35, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Jiang, T.-X.; Suksaweang, S.; Widelitz, R.B.; Chuong, C.-M. Molecular Shaping of the Beak. Science 2004, 305, 1465–1466. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wu, X.; Jiang, T.-X.; Elsey, R.M.; Temple, B.L.; Divers, S.J.; Glenn, T.C.; Yuan, K.; Chen, M.-H.; Widelitz, R.B.; et al. Specialized stem cell niche enables repetitive renewal of alligator teeth. Proc. Natl. Acad. Sci. USA 2013, 110, E2009–E2018. [Google Scholar] [CrossRef]
- Jiang, T.; Stott, N.; Widelitz, R.; Chuong, C. Molecular Basis of Epithelial Appendage Morphogenesis; Chuong, C., Ed.; Landes Bioscience: Austin, TX, USA, 1998; pp. 395–408. [Google Scholar]
- Moreau, C.; Caldarelli, P.; Rocancourt, D.; Roussel, J.; Denans, N.; Pourquie, O.; Gros, J. Timed Collinear Activation of Hox Genes during Gastrulation Controls the Avian Forelimb Position. Curr. Biol. 2019, 29, 35–50.e4. [Google Scholar] [CrossRef]
- Huss, D.; Benazeraf, B.; Wallingford, A.; Filla, M.; Yang, J.; Fraser, S.E.; Lansford, R. Data from: A transgenic quail model that enables dynamic imaging of amniote embryogenesis. Development 2015, 142, 2850–2859. [Google Scholar] [CrossRef]
- Barker, N.; Tan, S.; Clevers, H. Lgr proteins in epithelial stem cell biology. Development 2013, 140, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wu, P.; Widelitz, R.B.; Chuong, C.-M. The morphogenesis of feathers. Nature 2002, 420, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Ting-Berreth, S.A.; Chuong, C.M. Local delivery of TGF beta2 can substitute for placode epithelium to induce mesenchymal condensation during skin appendage morphogenesis. Dev. Biol. 1996, 179, 347–359. [Google Scholar] [CrossRef]
- Närhi, K.; Tummers, M.; Ahtiainen, L.; Itoh, N.; Thesleff, I.; Mikkola, M.L. Sostdc1 defines the size and number of skin appendage placodes. Dev. Biol. 2012, 364, 149–161. [Google Scholar] [CrossRef]
- Cotsarelis, G. Epithelial stem cells: A folliculocentric view. J. Investig. Dermatol. 2006, 126, 1459–1468. [Google Scholar] [CrossRef]
- Lyle, S.; Christofidou-Solomidou, M.; Liu, Y.; Elder, D.E.; Albelda, S.; Cotsarelis, G. The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J. Cell. Sci. 1998, 111 Pt 21, 3179–3188. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Mayer, J.A.; de la Cruz, D.; Baker, R.E.; Maini, P.K.; Maxson, R.; Chuong, C.-M. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 2008, 451, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Alibardi, L.; Chuong, C. Regeneration of reptilian scales after wounding: Neogenesis, regional difference, and molecular modules. Regeneration 2014, 1, 15–26. [Google Scholar] [CrossRef]
- Alibardi, L.; Toni, M. Cytochemical, biochemical and molecular aspects of the process of keratinization in the epidermis of reptilian scales. Prog. Histochem. Cytochem. 2006, 40, 73–134. [Google Scholar] [CrossRef]
- Alibardi, L.; Toni, M.; Valle, L.D. Hard cornification in reptilian epidermis in comparison to cornification in mammalian epidermis. Exp. Dermatol. 2007, 16, 961–976. [Google Scholar] [CrossRef]
- Vela-Romera, A.; Carriel, V.; Martín-Piedra, M.A.; Aneiros-Fernández, J.; Campos, F.; Chato-Astrain, J.; Prados-Olleta, N.; Campos, A.; Alaminos, M.; Garzón, I. Characterization of the human ridged and non-ridged skin: A comprehensive histological, histochemical and immunohistochemical analysis. Histochem. Cell Biol. 2019, 151, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Wai, V.; Roberts, L.; Michaud, J.; Bent, L.R.; Clark, A.L. The Anatomical Distribution of Mechanoreceptors in Mouse Hind Paw Skin and the Influence of Integrin alpha1beta1 on Meissner-Like Corpuscle Density in the Footpads. Front. Neuroanat. 2021, 15, 628711. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.-Y.; Hughes, M.W.; Wang, H.-V.; Yang, W.-C.; Chuong, C.-M.; Wu, P. Molecular and Cellular Characterization of Avian Reticulate Scales Implies the Evo–Devo Novelty of Skin Appendages in Foot Sole. J. Dev. Biol. 2023, 11, 30. https://doi.org/10.3390/jdb11030030
Liu T-Y, Hughes MW, Wang H-V, Yang W-C, Chuong C-M, Wu P. Molecular and Cellular Characterization of Avian Reticulate Scales Implies the Evo–Devo Novelty of Skin Appendages in Foot Sole. Journal of Developmental Biology. 2023; 11(3):30. https://doi.org/10.3390/jdb11030030
Chicago/Turabian StyleLiu, Tzu-Yu, Michael W. Hughes, Hao-Ven Wang, Wei-Cheng Yang, Cheng-Ming Chuong, and Ping Wu. 2023. "Molecular and Cellular Characterization of Avian Reticulate Scales Implies the Evo–Devo Novelty of Skin Appendages in Foot Sole" Journal of Developmental Biology 11, no. 3: 30. https://doi.org/10.3390/jdb11030030
APA StyleLiu, T.-Y., Hughes, M. W., Wang, H.-V., Yang, W.-C., Chuong, C.-M., & Wu, P. (2023). Molecular and Cellular Characterization of Avian Reticulate Scales Implies the Evo–Devo Novelty of Skin Appendages in Foot Sole. Journal of Developmental Biology, 11(3), 30. https://doi.org/10.3390/jdb11030030






