Evo Devo of the Vertebrates Integument
Abstract
1. Introduction
2. Specification of Special Area of the Integument Largely Precedes the Formation of Clade Typical Appendages
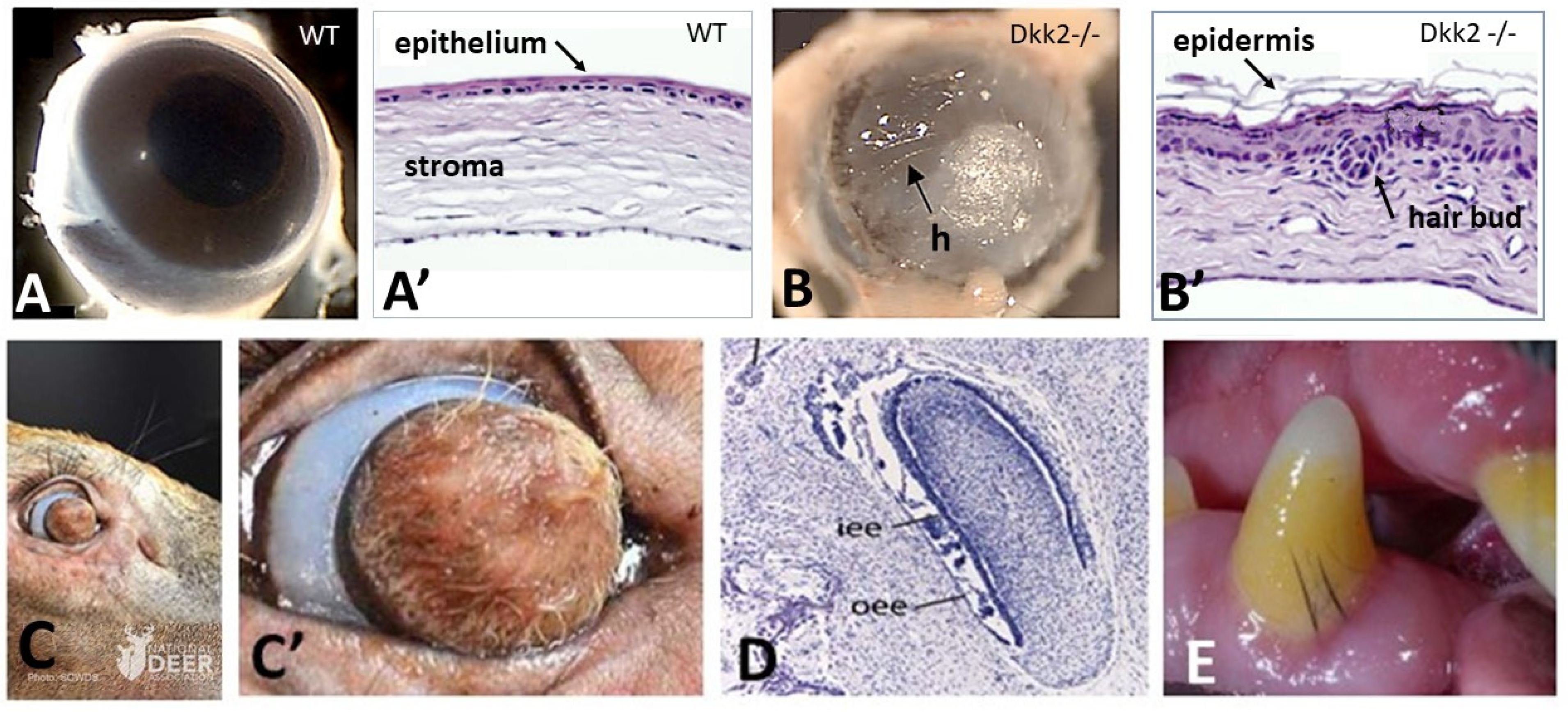
2.1. Corneal Ectoderm
2.2. Oral Ectoderm
2.3. Avian Beak
2.4. Mammary Ectoderm
2.5. Palmar/Plantar Epidermis
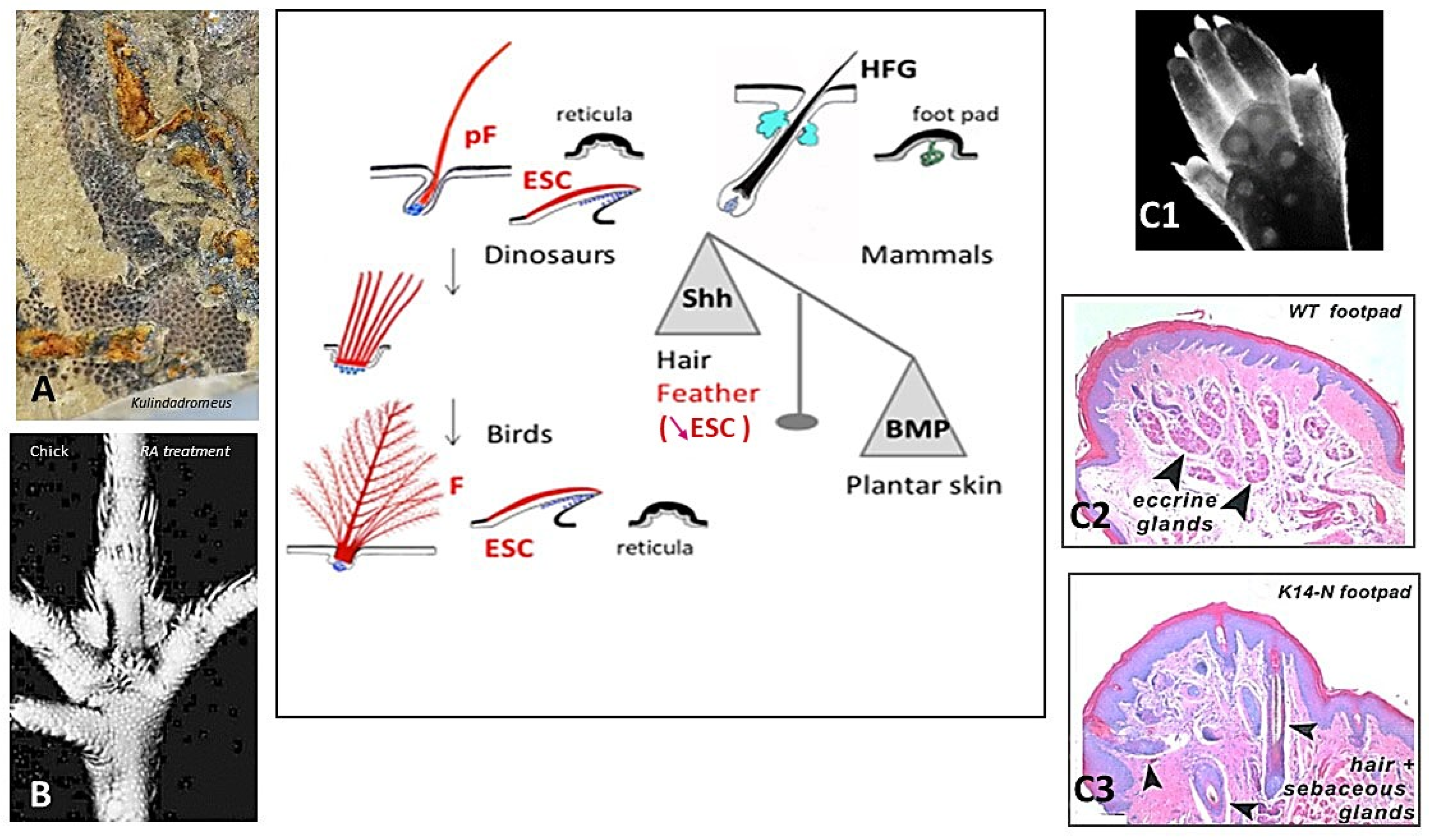
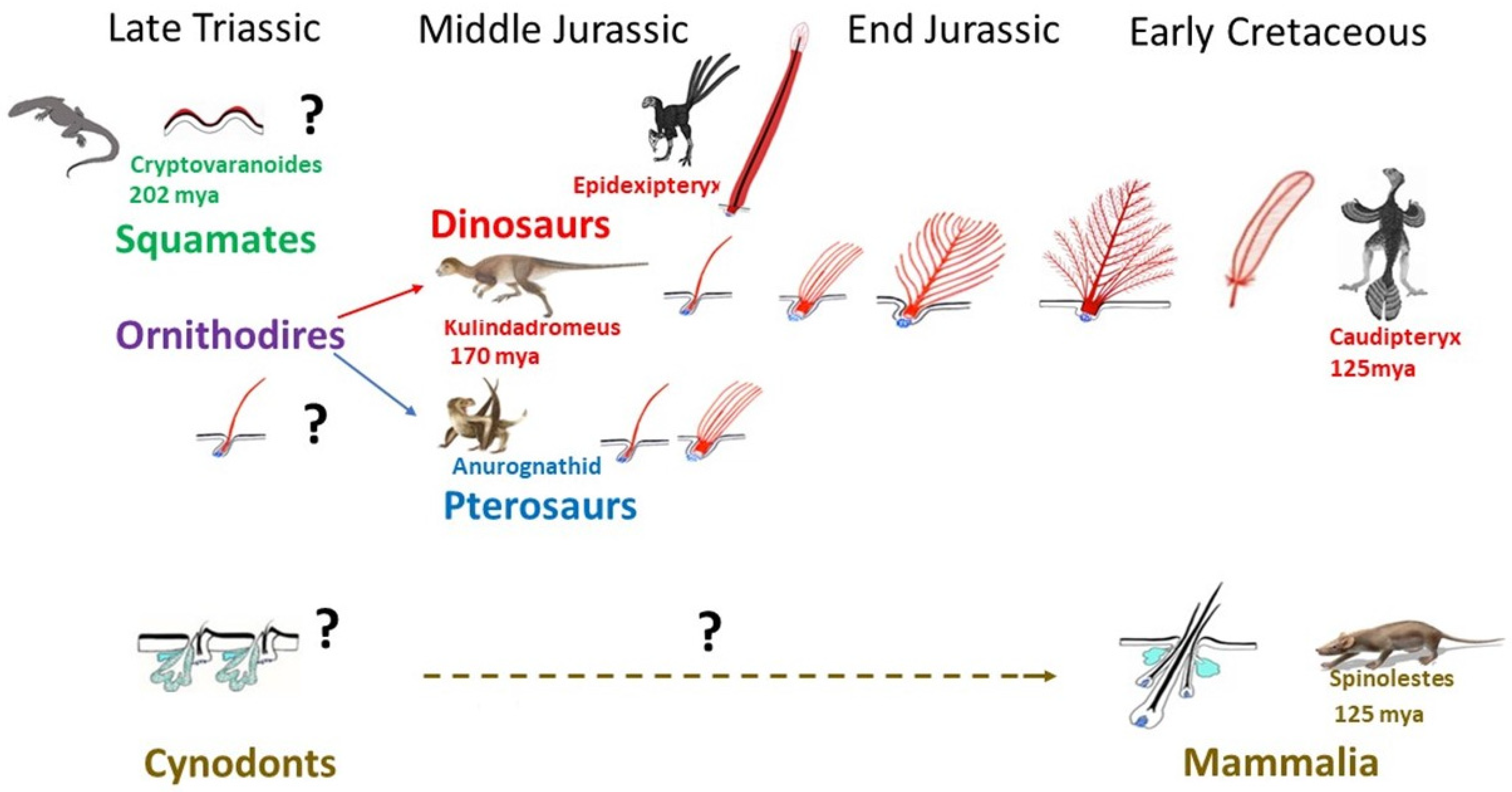
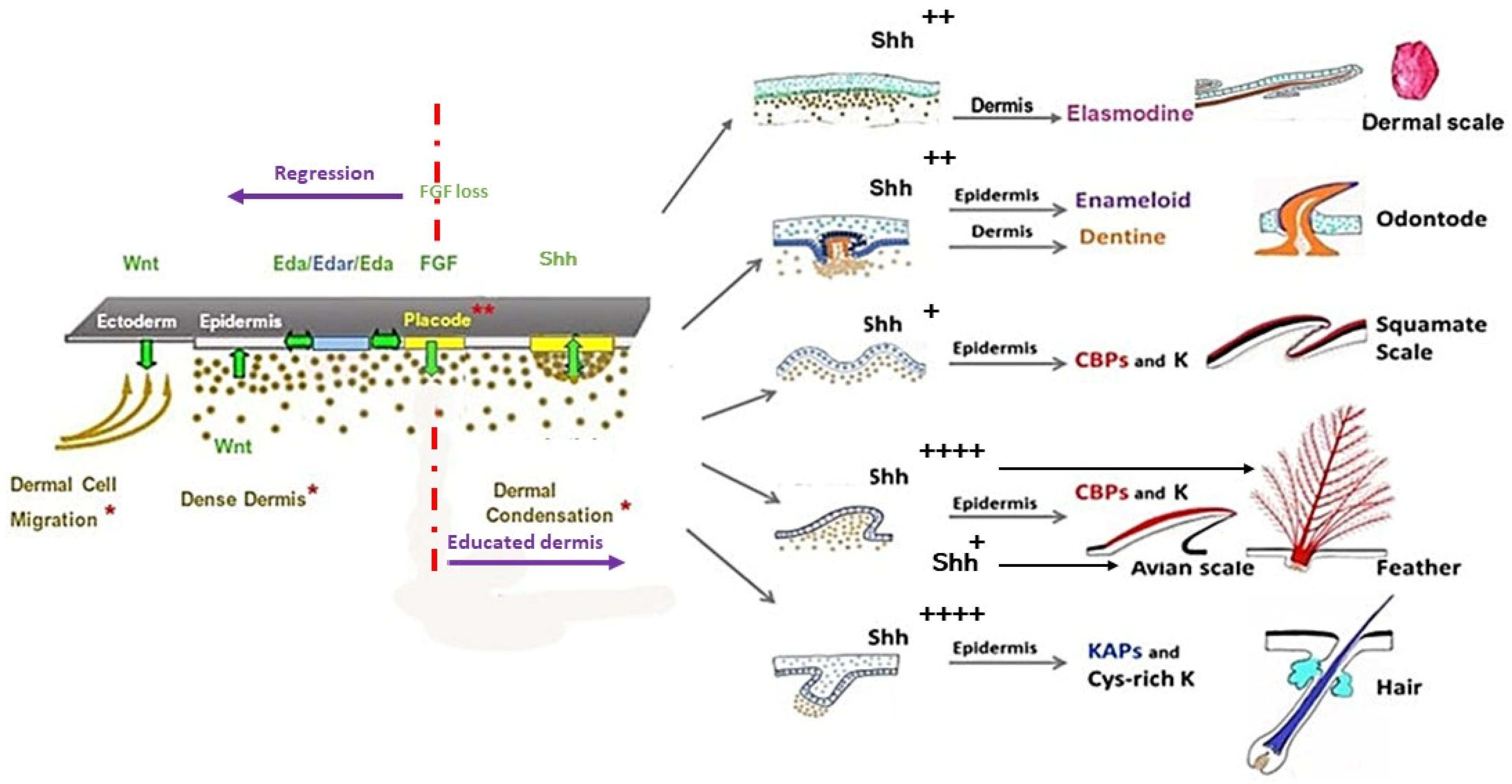
3. Skin Appendages: Different Scales, Feathers, and Hair
3.1. Fish Skin Appendages: Odontodes and Elasmoid Scales
3.2. Amniotes Scales, Feathers, and Hair
4. Squamate, Avian, and Mammalian Ectoderms Are Genetically Programmed to Build Scales, Feathers, or Hairs, Respectively, and Their Early Morphogenesis Pathways Were Conserved during Evolution
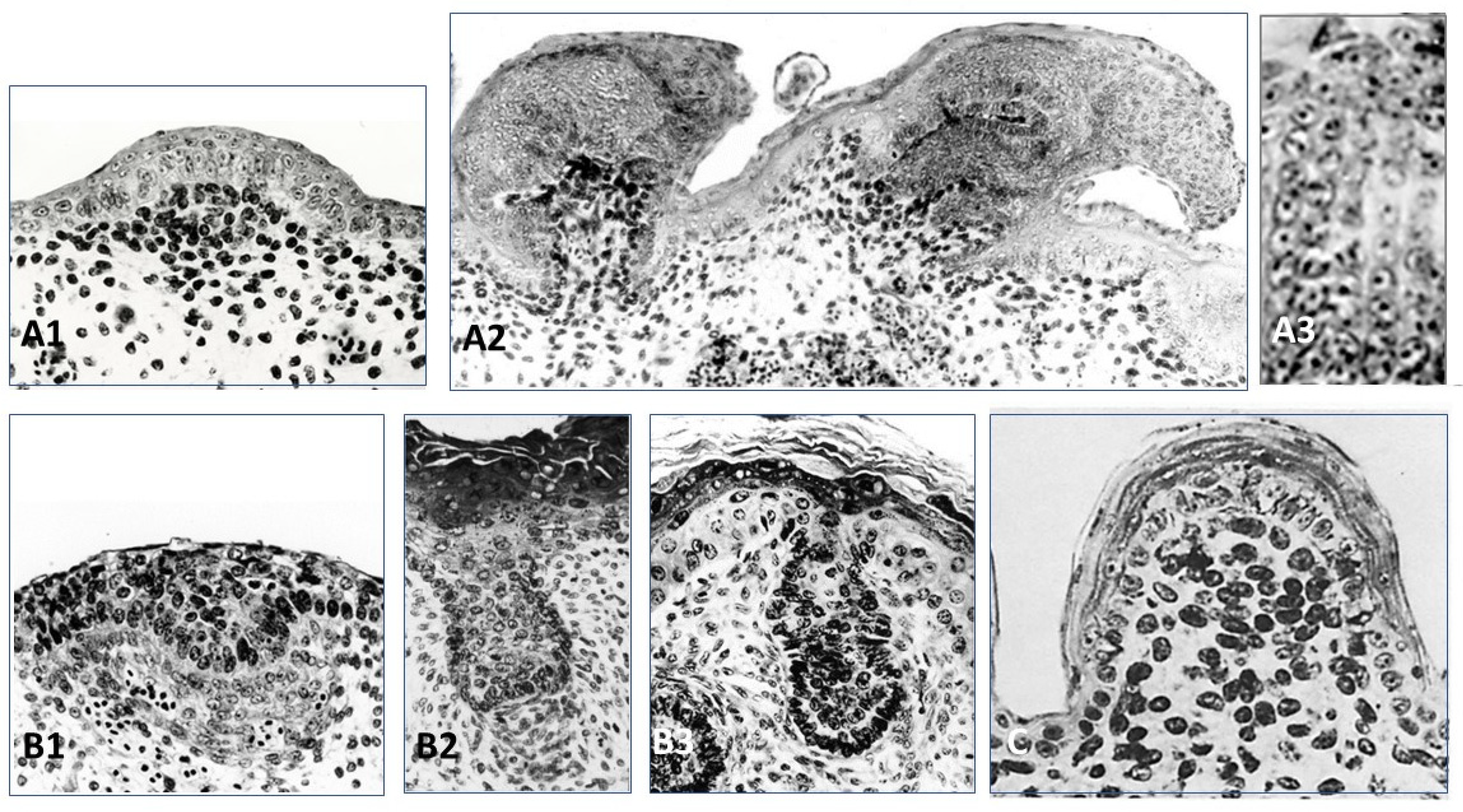
5. Placode Formation, a Dynamic Process Common to All Vertebrate Cutaneous Appendages
6. Conclusions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Chammas, S.M.; Carneiro, S.M.; Ferro, R.S.; Antoniazzi, M.M.; Jared, C. Development of integument and cutaneous glands in larval, juvenile and adult toads (Rhinella granulosa): A morphological and morphometric study. Acta Zool. 2015, 96, 460–477. [Google Scholar] [CrossRef]
- Çömden, E.A.; Yenmiş, M.; Çakır, B. The Complex Bridge between Aquatic and Terrestrial Life: Skin Changes during Development of Amphibians. J. Dev. Biol. 2023, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, T.; Nagashima, H.; Kuratani, S. The endoskeletal origin of the turtle carapace. Nat. Commun. 2013, 4, 2107. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.H.; Yang, W.; Meyers, M.A. Alligator osteoderms: Mechanical behavior and hierarchical structure. Mater. Sci. Eng. C 2014, 35, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.M.; Stettenheim, P.R. Avian Anatomy Integument; US Department of Agriculture: Washington, DC, USA, 1972. [Google Scholar]
- Chernova, O.F. Evolutionary aspects of hair polymorphism. Biol. Bull. 2006, 33, 43–52. [Google Scholar] [CrossRef]
- Hieronymus, T.L.; Witmer, L.M.; Ridgely, R.C. Structure of white rhinoceros (Ceratotherium simum) horn investigated by X-ray computed tomography and histology with implications for growth and external form. J. Morphol. 2006, 267, 1172–1176. [Google Scholar] [CrossRef]
- Alibardi, L.; Rogers, G. Observations on fur development in echidna (Monotremata, Mammalia) indicate that spines precede hairs in ontogeny. Anat. Rec. 2014, 298, 761–770. [Google Scholar] [CrossRef]
- Wang, B.; Yang, W.; Sherman, V.R.; Meyers, M.A. Pangolin armor: Overlapping, structure, and mechanical properties of the keratinous scales. Acta Biomater. 2016, 41, 60–74. [Google Scholar] [CrossRef]
- Martin, T.; Marugán-Lobón, J.; Vullo, R.; Martín-Abad, H.; Luo, Z.X.; Buscalioni, A.D. A Cretaceous eutriconodont and integument evolution in early mammals. Nature 2015, 526, 380–384. [Google Scholar] [CrossRef]
- Alibardi, L.; Segalla, A. The process of cornification in the horny teeth of the lamprey involves proteins in the keratin range and other keratin-associated proteins. Zool. Stud. 2011, 50, 416–425. [Google Scholar]
- Alibardi, L. The Process of Cornification Evolved from the Initial Keratinization in the Epidermis and Epidermal Derivatives of Vertebrates: A New Synthesis and the Case of Sauropsids. Int. Rev. Cell Mol. Biol. 2016, 327, 263–319. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Keratinization and Cornification are not equivalent processes but keratinization in fish and amphibians evolved into cornification in terrestrial vertebrates. Exp. Dermatol. 2022, 31, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.E. Hair follicle differentiation and regulation. Int. J. Dev. Biol. 2004, 48, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L.; Sawyer, R.H. Immunocytochemical analysis of beta keratins in the epidermis of chelonians, lepidosaurians, and archosaurians. J. Exp. Zool. 2002, 293, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Fraser, R.D.; Parry, D.A. The molecular structure of reptilian keratin. Int. J. Biol. Macromol. 1996, 19, 207–211. [Google Scholar] [CrossRef]
- Miyazaki, M.; Man, W.C.; Ntambi, J.M. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J. Nutr. 2001, 131, 2260–2268. [Google Scholar] [CrossRef]
- Chen, H.C.; Smith, S.J.; Tow, B.; Elias, P.M.; Farese, R.V., Jr. Leptin modulates the effects of acyl CoA:diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J. Clin. Investig. 2002, 109, 175–181. [Google Scholar] [CrossRef]
- Sire, J.Y.; Akimenko, M.A. Scale development in fish: A review, with description of sonic hedgehog (shh) expression in the zebrafish (Danio rerio). Int. J. Dev. Biol. 2004, 48, 233–247. [Google Scholar] [CrossRef]
- Parichy, D.M.; Elizondo, M.R.; Mills, M.G.; Gordon, T.N.; Engeszer, R.E. Normal table of postembryonic zebrafish development: Staging by externally visible anatomy of the living fish. Dev. Dyn. 2009, 238, 2975–3015. [Google Scholar] [CrossRef]
- Aman, A.J.; Fulbright, A.N.; Parichy, D.M. Wnt/β-catenin regulates an ancient signaling network during zebrafish scale development. eLife 2018, 7, e37001. [Google Scholar] [CrossRef]
- Gibert, Y.; Samarut, E.; Ellis, M.K.; Jackman, W.R.; Laudet, V. The first formed tooth serves as a signalling centre to induce the formation of the dental row in zebrafish. Proc. Biol. Sci. 2019, 286, 20190401. [Google Scholar] [CrossRef] [PubMed]
- Dhouailly, D.; Saxod, R. Les stades du développement de Lacerta muralis Laur, entre la ponte et l’éclosion. Bull. Soc. Zool. France 1974, 99, 489–494. [Google Scholar]
- Hu, D.; Young, N.M.; Xu, Q.; Jamniczky, H.; Green, R.M.; Mio, W.; Marcucio, R.S.; Hallgrimsson, B. Signals from the brain induce variation in avian facial shape. Dev. Dyn. 2015, 244, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Gehring, W.J.; Ikeo, K. Pax 6: Mastering eye morphogenesis and eye evolution. Trends Genet. 1999, 15, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Chaloin-Dufau, C.; Pavitt, I.; Delorme, P.; Dhouailly, D. Identification of keratins 3 and 12 in corneal epithelium of vertebrates. Epithelial. Cell Biol. 1993, 2, 120–125. [Google Scholar]
- Dhouailly, D.; Pearton, D.J.; Michon, F. The vertebrate corneal epithelium: From early specification to constant renewal. Dev. Dyn. 2014, 243, 1226–1241. [Google Scholar] [CrossRef]
- Streit, A. The preplacodal region: An ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int. J. Dev. Biol. 2007, 51, 447–461. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Bailey, A.P.; Bronner-Fraser, M.; Streit, A. Segregation of lens and olfactory precursors from a common territory: Cell sorting and reciprocity of Dlx5 and Pax6 expression. Dev. Biol. 2004, 271, 403–414. [Google Scholar] [CrossRef]
- Collomb, E.; Yang, Y.; Foriel, S.; Cadau, S.; Pearton, D.J.; Dhouailly, D. The corneal epithelium and lens develop independently from a common pool of precursors. Dev. Dyn. 2013, 242, 401–413. [Google Scholar] [CrossRef]
- Shalom-Feuerstein, R.; Serror, L.; De La Forest Divonne, S.; Petit, I.; Aberdam, E.; Camargo, L.; Damour, O.; Vigouroux, C.; Solomon, A.; Gaggioli, C.; et al. Pluripotent stem cell model reveals essential roles for miR-450b-5p and miR-184 in embryonic corneal lineage specification. Stem. Cells. 2012, 30, 898–909. [Google Scholar] [CrossRef]
- Ferraris, C.; Chevalier, G.; Favier, B.; Jahoda, C.A.; Dhouailly, D. Adult corneal epithelium basal cells possess the capacity to activate epidermal, pilosebaceous and sweat gland genetic programs in response to embryonic dermal stimuli. Development 2000, 127, 5487–5495. [Google Scholar] [CrossRef] [PubMed]
- Pearton, D.J.; Yang, Y.; Dhouailly, D. Transdifferentiation of corneal epithelium into epidermis occurs by means of a multistep process triggered by dermal developmental signals. Proc. Natl. Acad. Sci. USA 2005, 102, 3714–3719. [Google Scholar] [CrossRef] [PubMed]
- Majo, F.; Rochat, A.; Nicolas, M.; Jaoudé, G.A.; Barrandon, Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature 2008, 456, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, M.; Gorivodsky, M.; Shtrom, S.; Grinberg, A.; Niehrs, C.; Morasso, M.I.; Westphal, H. Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development 2006, 133, 2149–2154, Erratum in Development 2006, 133, 2595. Erratum in Development 2006, 133, 2447. [Google Scholar] [CrossRef] [PubMed]
- Thewissen, J.G.; Hieronymus, T.L.; George, J.C.; Suydam, R.; Stimmelmayr, R.; McBurney, D. Evolutionary aspects of the development of teeth and baleen in the bowhead whale. J. Anat. 2017, 230, 549–566. [Google Scholar] [CrossRef]
- Blais, S.A.; MacKenzie, L.A.; Wilson, M.V.H. Tooth-like scales in Early Devonian eugnathostomes and the ‘outside-in’ hypothesis for the origins of teeth in vertebrates. J. Vertebr. Paleontol. 2011, 31, 1189–1199. [Google Scholar] [CrossRef]
- Donoghue, P.C.; Rücklin, M. The ins and outs of the evolutionary origin of teeth. Evol. Dev. 2016, 18, 19–30. [Google Scholar] [CrossRef]
- Fraser, G.J.; Cerny, R.; Soukup, V.; Bronner-Fraser, M.; Streelman, J.T. The odontode explosion: The origin of tooth-like structures in vertebrates. Bioessays 2010, 32, 808–817. [Google Scholar] [CrossRef]
- Thesleff, I. The Developmental Anatomy of Teeth. Kaufman’s Atlas of Mouse Development; Baldock, R., Bard, J., Davidson, D.R., Morriss-Kay, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 231–238. [Google Scholar] [CrossRef]
- Jussila, M.; Thesleff, I. Signaling networks regulating tooth organogenesis and regeneration, and the specification of dental mesenchymal and epithelial cell lineages. Cold Spring Harb. Perspect Biol. 2012, 4, a008425. [Google Scholar] [CrossRef]
- Biggs, L.C.; Mikkola, M.L. Early inductive events in ectodermal appendage morphogenesis. Semin. Cell Dev. Biol. 2014, 25–26, 11–21. [Google Scholar] [CrossRef]
- Szewciw, L.J.; de Kerckhove, D.G.; Grime, G.W.; Fudge, D.S. Calcification provides mechanical reinforcement to whale baleen alpha-keratin. Proc. Biol. Sci. 2010, 277, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Amasaki, H.; Dohguchi, H.; Furuya, A.; Suzuki, K. Immunohistological distributions of fibronectin, tenascin, type I, III and IV collagens, and laminin during tooth development and degeneration in fetuses of minke whale, Balaenoptera acutorostrata. J. Vet. Med. Sci. 1999, 61, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Zhurakivska, K.; Toni, G.; Laino, G.; Franco, R.; Troiano, G.; Laino, L.; Ronchi, A. An unusual case of recurrent gingival hirsutism. Oral Surg. Oral Med. Oral Pathol Oral Radiol. 2020, 129, e200–e203. [Google Scholar] [CrossRef]
- Agha-Hosseini, F.; Etesam, F.; Rohani, B. A boy with oral hair: Case report. Med. Oral Patol. Oral Cir. Bucal. 2007, 12, E357–E359. [Google Scholar]
- Bhullar, B.-A.S.; Morris, Z.S.; Sefton, E.M.; Tok, A.; Tokita, M.; Namkoong, B.; Camacho, J.; Burnham, D.A.; Abzhanov, A. A molecular mechanism for the origin of a key evolutionary innovation, the bird beak and palate, revealed by an integrative approach to major transitions in vertebrate history. Evolution 2015, 69, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Mitsiadis, T.A.; Chéraud, Y.; Sharpe, P.; Fontaine-Pérus, J. Development of teeth in chick embryos after mouse neural crest transplantations. Proc. Natl. Acad. Sci. USA 2003, 100, 6541–6545. [Google Scholar] [CrossRef]
- Veltmaat, J.M.; Relaix, F.; Le, L.T.; Kratochwil, K.; Sala, F.G.; van Veelen, W.; Rice, R.; Spencer-Dene, B.; Mailleux, A.A.; Rice, D.P.; et al. Gli3-mediated somitic Fgf10 expression gradients are required for the induction and patterning of mammary epithelium along the embryonic axes. Development 2006, 133, 2325–2335. [Google Scholar] [CrossRef]
- Mayer, J.A.; Foley, J.; De La Cruz, D.; Chuong, C.M.; Widelitz, R. Conversion of the nipple to hair-bearing epithelia by lowering bone morphogenetic protein pathway activity at the dermal-epidermal interface. Am. J. Pathol. 2008, 173, 1339–1348. [Google Scholar] [CrossRef]
- Godefroit, P.; Sinitsa, S.M.; Dhouailly, D.; Bolotsky, Y.L.; Sizov, A.V.; McNamara, M.E.; Benton, M.J.; Spagna, P. Dinosaur evolution. A Jurassic ornithischian dinosaur from Siberia with both feathers and scales. Science 2014, 345, 451–455. [Google Scholar] [CrossRef]
- Sawyer, R.H.; Glenn, T.; French, J.O.; Mays, B.; Shames, R.B.; Barnes, G.L.; Rhodes, W.; Ishikawa, Y. The expression of beta (β) keratins in the epidermal appendages of reptiles and birds. Am. Zool. 2000, 40, 530–539. [Google Scholar] [CrossRef]
- Dhouailly, D.; Hardy, M.H.; Sengel, P. Formation of feathers on chick foot scales: A stage-dependent morphogenetic response to retinoic acid. J. Embryol. Exp. Morphol. 1980, 58, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Prin, F.; Logan, C.; D’Souza, D.; Ensini, M.; Dhouailly, D. Dorsal versus ventral scales and the dorsoventral patterning of chick foot epidermis. Dev. Dyn. 2004, 229, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Prin, F.; Dhouailly, D. How and when the regional competence of chick epidermis is established: Feathers vs. scutate and reticulate scales, a problem en route to a solution. Int. J. Dev. Biol. 2004, 48, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Dhouailly, D. A new scenario for the evolutionary origin of hair, feather, and avian scales. J. Anat. 2009, 214, 587–606. [Google Scholar] [CrossRef]
- Plikus, M.; Wang, W.P.; Liu, J.; Wang, X.; Jiang, T.X.; Chuong, C.M. Morpho-regulation of ectodermal organs: Integument pathology and phenotypic variations in K14-Noggin engineered mice through modulation of bone morphogenic protein pathway. Am. J. Pathol. 2004, 164, 1099–1114. [Google Scholar] [CrossRef]
- Loomis, C.A.; Harris, E.; Michaud, J.; Wurst, W.; Hanks, M.; Joyner, A.L. The mouse Engrailed-1 gene and ventral limb patterning. Nature 1996, 382, 360–363. [Google Scholar] [CrossRef]
- Lu, C.P.; Polak, L.; Keyes, B.E.; Fuchs, E. Spatiotemporal antagonism in mesenchymal-epithelial signaling in sweat versus hair fate decision. Science 2016, 354, aah6102. [Google Scholar] [CrossRef]
- Alibardi, L. The Periodic Replacement of Adhesive Setae in Pad Lamellae of Climbing Lizards Is Driven by Patterns of Corneous Layer Growth. J. Dev. Biol. 2023, 11, 3. [Google Scholar] [CrossRef]
- Cooper, R.L.; Nicklin, E.F.; Rasch, L.J.; Fraser, G.J. Teeth outside the mouth: The evolution and development of shark denticles. Evol. Dev. 2023, 25, 54–72. [Google Scholar] [CrossRef]
- Chen, D.; Blom, H.; Sanchez, S.; Tafforeau, P.; Märss, T.; Ahlberg, P.E. The developmental relationship between teeth and dermal odontodes in the most primitive bony fish Lophosteus. eLife 2020, 9, e60985. [Google Scholar] [CrossRef]
- Sire, J.Y.; Huysseune, A. Formation of dermal skeletal and dental tissues in fish: A comparative and evolutionary approach. Biol. Rev. Camb. Philos. Soc. 2003, 78, 219–249. [Google Scholar] [CrossRef]
- Harris, M.P.; Rohner, N.; Schwarz, H.; Perathoner, S.; Konstantinidis, P.; Nüsslein-Volhard, C. Zebrafish eda and edar mutants reveal conserved and ancestral roles of ectodysplasin signaling in vertebrates. PLoS Genet. 2008, 4, e1000206. [Google Scholar] [CrossRef] [PubMed]
- Maderson, P.F.A. When? Why? and How? Some speculations on the evolution of the vertebrate integument. Am. Zool. 1972, 12, 159–171. Available online: http://www.jstor.org/stable/3881739 (accessed on 5 May 2022). [CrossRef]
- Jones, T.D.; Ruben, J.A.; Martin, L.D.; Kurochkin, E.N.; Feduccia, A.; Maderson, P.F.; Hillenius, W.J.; Geist, N.R.; Alifanov, V. Nonavian feathers in a late Triassic archosaur. Science 2000, 288, 2202–2205. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.P.; Fallon, J.F.; Prum, R.O. Shh-Bmp2 signaling module and the evolutionary origin and diversification of feathers. J. Exp. Zool. 2002, 294, 160–176. [Google Scholar] [CrossRef]
- Wu, P.; Hou, L.; Plikus, M.; Hughes, M.; Scehnet, J.; Suksaweang, S.; Widelitz, R.; Jiang, T.X.; Chuong, C.M. Evo-Devo of amniote integuments and appendages. Int. J. Dev. Biol. 2004, 48, 249–270. [Google Scholar] [CrossRef]
- Chang, C.; Wu, P.; Baker, R.E.; Maini, P.K.; Alibardi, L.; Chuong, C.M. Reptile scale paradigm: Evo-Devo, pattern formation and regeneration. Int. J. Dev. Biol. 2009, 53, 813–826. [Google Scholar] [CrossRef]
- Whiteside, D.I.; Chambi-Trowell, S.A.V.; Benton, M.J. A Triassic crown squamate. Sci. Adv. 2022, 8, eabq8274. [Google Scholar] [CrossRef]
- Lindgren, J.; Everhart, M.J.; Caldwell, M.W. Three-dimensionally preserved integument reveals hydrodynamic adaptations in the extinct marine lizard Ectenosaurus (Reptilia, Mosasauridae). PLoS ONE 2011, 6, e27343. [Google Scholar] [CrossRef]
- Conrad, J.L.; Head, J.J.; Carrano, M.T. Unusual soft-tissue preservation of a crocodile lizard (Squamata, Shinisauria) from the green river formation (Eocene) and shinisaur relationships. Anat. Rec. 2014, 297, 545–559. [Google Scholar] [CrossRef]
- Kellner, A.W.; Wang, X.; Tischlinger, H.; de Almeida Campos, D.; Hone, D.W.; Meng, X. The soft tissue of Jeholopterus (Pterosauria, Anurognathidae, Batrachognathinae) and the structure of the pterosaur wing membrane. Proc. Biol. Sci. 2010, 277, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Bakhurina, N.N.; Unwin, D.M. Sixth Symposium on Mesozoic Terrestrial Ecosystems and Biota; Sun, A., Wang, Y., Eds.; China Ocean Press: Beijing, China, 1995; pp. 79–82. [Google Scholar]
- Yang, Z.; Jiang, B.; McNamara, M.E.; Kearns, S.L.; Pittman, M.; Kaye, T.G.; Orr, P.J.; Xu, X.; Benton, M.J. Pterosaur integumentary structures with complex feather-like branching. Nat. Ecol. Evol. 2019, 3, 24–30. [Google Scholar] [CrossRef]
- Czerkas, S. The History and Interpretation of Sauropod Skin Impressions. GAIA 1994, 10, 173–182. Available online: http://www.arca.museus.ul.pt/ArcaSite/obj/gaia/MNHNL-0000273-MG-DOC-web.PDF (accessed on 5 May 2022).
- Ayer, J. The Howe Ranch Dinosaurs: 10 Years of Dinosaur Digging in Wyoming; Sauriermuseum Aathal: Aathal, Switzerland, 1999. [Google Scholar]
- Barrett, P.M.; Evans, D.C.; Campione, N.E. Evolution of dinosaur epidermal structures. Biol. Lett. 2015, 11, 20150229. [Google Scholar] [CrossRef]
- Xu, X.; Norell, M.A.; Kuang, X.; Wang, X.; Zhao, Q.; Jia, C. Basal tyrannosauroids from China and evidence for protofeathers in tyrannosauroids. Nature 2004, 431, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Benton, M.J.; Dhouailly, D.; Jiang, B.; McNamara, M. The Early Origin of Feathers. Trends Ecol. Evol. 2019, 34, 856–869. [Google Scholar] [CrossRef]
- Xu, X.; Guo, Y. The origin and early evolution of feathers: Insights from recent paleontological and neontological data. Vertebr. PalAsiatica 2009, 47, 311–329. [Google Scholar]
- Prum, R.O.; Brush, A.H. The evolutionary origin and diversification of feathers. Q. Rev. Biol. 2002, 77, 261–295. [Google Scholar] [CrossRef]
- Prum, R.O. Evolution of the morphological innovations of feathers. J. Exp. Zool. B Mol. Dev. Evol. 2005, 304, 570–579. [Google Scholar] [CrossRef]
- Chuong, C.M.; Homberger, D.G. Development and evolution of the amniote integument: Current landscape and future horizon. J. Exp. Zool. B Mol. Dev. Evol. 2003, 298, 1–11. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, Z.; Xu, X.; Wang, X.; Sullivan, C. A bizarre Jurassic maniraptoran from China with elongate ribbon-like feathers. Nature 2008, 455, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, X.; You, H. Exceptional dinosaur fossils show ontogenetic development of early feathers. Nature 2010, 464, 1338–1341. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, Z.; Wang, X.; Zhang, F.; Zhang, X.; Wang, Y.; Wei, G.; Wang, S.; Xu, X. Hind wings in Basal birds and the evolution of leg feathers. Science 2013, 339, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Lai, Y.C.; Widelitz, R.; Chuong, C.M. Comprehensive molecular and cellular studies suggest avian scutate scales are secondarily derived from feathers, and more distant from reptilian scales. Sci. Rep. 2018, 8, 16766. [Google Scholar] [CrossRef]
- Widelitz, R.B.; Jiang, T.X.; Lu, J.; Chuong, C.M. Beta-catenin in epithelial morphogenesis: Conversion of part of avian foot scales into feather buds with a mutated beta-catenin. Dev. Biol. 2000, 219, 98–114. [Google Scholar] [CrossRef]
- Crowe, R.; Henrique, D.; Ish-Horowicz, D.; Niswander, L. A new role for Notch and Delta in cell fate decisions: Patterning the feather array. Development 1998, 125, 767–775. [Google Scholar] [CrossRef]
- Zou, H.; Niswander, L. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science 1996, 272, 738–741. [Google Scholar] [CrossRef]
- Kanzler, B.; Prin, F.; Thelu, J.; Dhouailly, D. CHOXC-8 and CHOXD-13 expression in embryonic chick skin and cutaneous appendage specification. Dev. Dyn. 1997, 210, 274–287. [Google Scholar] [CrossRef]
- Wu, P.; Yan, J.; Lai, Y.C.; Ng, C.S.; Li, A.; Jiang, X.; Elsey, R.M.; Widelitz, R.; Bajpai, R.; Li, W.H.; et al. Multiple Regulatory Modules Are Required for Scale-to-Feather Conversion. Mol. Biol. Evol. 2018, 35, 417–430. [Google Scholar] [CrossRef]
- Eckhart, L.; Dalla Valle, L.; Jaeger, K.; Ballaun, C.; Szabo, S.; Nardi, A.; Buchberger, M.; Hermann, M.; Alibardi, L.; Tschachler, E. Identification of reptilian genes encoding hair keratin-like proteins suggests a new scenario for the evolutionary origin of hair. Proc. Natl. Acad. Sci. USA 2008, 105, 18419–18423. [Google Scholar] [CrossRef]
- Elias, H.; Bortner, S. On the phylogeny of hair. Am. Mus. Nat. Hist. Novitates. 1957, 1820, 1–15. Available online: http://hdl.handle.net/2246/4703 (accessed on 10 May 2022).
- Stenn, K.S.; Zheng, Y.; Parimoo, S. Phylogeny of the hair follicle: The sebogenic hypothesis. J. Investig. Dermatol. 2008, 128, 1576–1578. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.A.; Polak, L.; Pasolli, H.A.; Fuchs, E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem. Cell 2008, 3, 33–43. [Google Scholar] [CrossRef]
- Schneider, M.R. Fifty years of the asebia mouse: Origins, insights and contemporary developments. Exp. Dermatol. 2015, 24, 340–341. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Luo, Z.X.; Yuan, C.X.; Tabrum, A.R. A swimming mammaliaform from the Middle Jurassic and ecomorphological diversification of early mammals. Science 2006, 311, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Oftedal, O.T.; Dhouailly, D. Evo-devo of the mammary gland. J. Mammary Gland Biol. Neoplasia 2013, 18, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Oftedal, O.T. The mammary gland and its origin during synapsid evolution. J. Mammary Gland Biol. Neoplasia 2002, 7, 225–252. [Google Scholar] [CrossRef]
- Dhouailly, D. Dermo-epidermal interactions between birds and mammals: Differentiation of cutaneous appendages. J. Embryol. Exp. Morphol. 1973, 30, 587–603. [Google Scholar] [CrossRef]
- Dhouailly, D. Formation of cutaneous appendages in dermo-epidermal recombinations between reptiles, birds and mammals. Wilehm. Roux. Arch. Dev. Biol. 1975, 177, 323–340. [Google Scholar] [CrossRef]
- Dhouailly, D. Dermo-epidermal interactions during morphogenesis of cutaneous appendages in amniotes. Front. Matrix. Biol. 1977, 4, 86–121. [Google Scholar]
- Atit, R.; Sgaier, S.K.; Mohamed, O.A.; Taketo, M.M.; Dufort, D.; Joyner, A.L.; Niswander, L.; Conlon, R.A. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev. Biol. 2006, 296, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Dhouailly, D. Analyse des facteurs de la différenciation spécifique de la plume néoptile chez le Canard et le Poulet [Analysis of the factors in the specific differenciation of the neoptile feathers in the duck and chicken]. J. Embryol. Exp. Morphol. 1967, 18, 389–400. (In French) [Google Scholar] [PubMed]
- Dhouailly, D. Déterminisme de la différenciation spécifique des plumes néoptiles et téléptiles chez le poulet et le canard [The determination of specific differentiation of neoptile and teleoptile feathers in the chick and the duck]. J. Embryol. Exp. Morphol. 1970, 24, 73–94. (In French) [Google Scholar]
- Yue, Z.; Jiang, T.X.; Widelitz, R.B.; Chuong, C.M. Wnt3a gradient converts radial to bilateral feather symmetry via topological arrangement of epithelia. Proc. Natl. Acad. Sci. USA 2006, 103, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.P.; Williamson, S.; Fallon, J.F.; Meinhardt, H.; Prum, R.O. Molecular evidence for an activator-inhibitor mechanism in development of embryonic feather branching. Proc. Natl. Acad. Sci. USA 2005, 102, 11734–11739. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Juan, W.T.; Liang, Y.C.; Wu, P.; Chuong, C.M. Making region-specific integumentary organs in birds: Evolution and modifications. Curr. Opin. Genet. Dev. 2021, 69, 103–111. [Google Scholar] [CrossRef]
- Fliniaux, I.; Viallet, J.P.; Dhouailly, D. Signaling dynamics of feather tract formation from the chick somatopleure. Development 2004, 131, 3955–3966. [Google Scholar] [CrossRef]
- Jahoda, C.A.; Horne, K.A.; Oliver, R.F. Induction of hair growth by implantation of cultured dermal papilla cells. Nature 1984, 311, 560–562. [Google Scholar] [CrossRef]
- Rompolas, P.; Deschene, E.R.; Zito, G.; Gonzalez, D.G.; Saotome, I.; Haberman, A.M.; Greco, V. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature 2012, 487, 496–499. [Google Scholar] [CrossRef]
- Mesler, A.L.; Veniaminova, N.A.; Lull, M.V.; Wong, S.Y. Hair Follicle Terminal Differentiation Is Orchestrated by Distinct Early and Late Matrix Progenitors. Cell Rep. 2017, 19, 809–821. [Google Scholar] [CrossRef]
- Sequeira, I.; Nicolas, J.F. Redefining the structure of the hair follicle by 3D clonal analysis. Development 2012, 139, 3741–3751. [Google Scholar] [CrossRef] [PubMed]
- Kobielak, K.; Pasolli, H.A.; Alonso, L.; Polak, L.; Fuchs, E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J. Cell Biol. 2003, 163, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Mehrani, T.; Millar, S.E.; Morasso, M.I. Dlx3 is a crucial regulator of hair follicle differentiation and cycling. Development 2008, 135, 3149–3159. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Lee, J.; Kopan, R.; Ma, L. Genetic interplays between Msx2 and Foxn1 are required for Notch1 expression and hair shaft differentiation. Dev. Biol. 2009, 326, 420–430. [Google Scholar] [CrossRef]
- Hu, X.M.; Li, Z.X.; Zhang, D.Y.; Yang, Y.C.; Fu, S.A.; Zhang, Z.Q.; Yang, R.H.; Xiong, K. A systematic summary of survival and death signalling during the life of hair follicle stem cells. Stem. Cell Res. Ther. 2021, 12, 453. [Google Scholar] [CrossRef]
- Di-Poï, N.; Milinkovitch, M.C. The anatomical placode in reptile scale morphogenesis indicates shared ancestry among skin appendages in amniotes. Sci. Adv. 2016, 2, e1600708. [Google Scholar] [CrossRef]
- Sawyer, R.H.; Borg, T.K. Avian scale development. VI. Ultrastructure of the keratinizing cells of reticulate scales. J. Morphol. 1979, 161, 111–121. [Google Scholar] [CrossRef]
- Glover, J.D.; Sudderick, Z.R.; Shih, B.B.; Batho-Samblas, C.; Charlton, L.; Krause, A.L.; Anderson, C.; Riddell, J.; Balic, A.; Li, J.; et al. The developmental basis of fingerprint pattern formation and variation. Cell 2023, 186, 940–956.e20. [Google Scholar] [CrossRef]
- Olivera-Martinez, I.; Coltey, M.; Dhouailly, D.; Pourquié, O. Mediolateral somitic origin of ribs and dermis determined by quail-chick chimeras. Development 2000, 127, 4611–4617. [Google Scholar] [CrossRef]
- Houzelstein, D.; Chéraud, Y.; Auda-Boucher, G.; Fontaine-Pérus, J.; Robert, B. The expression of the homeobox gene Msx1 reveals two populations of dermal progenitor cells originating from the somites. Development 2000, 127, 2155–2164. [Google Scholar] [CrossRef]
- Thesleff, I.; Sharpe, P. Signalling networks regulating dental development. Mech. Dev. 1997, 67, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.L.; Bohnsack, B.L. The Ocular Neural Crest: Specification, Migration, and Then What? Front. Cell Dev. Biol. 2020, 8, 595896. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Martinez, I.; Missier, S.; Fraboulet, S.; Thélu, J.; Dhouailly, D. Differential regulation of the chick dorsal thoracic dermal progenitors from the medial dermomyotome. Development 2002, 129, 4763–4772. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cserjesi, P.; Olson, E.N. Dermo-1: A novel twist-related bHLH protein expressed in the developing dermis. Dev. Biol. 1995, 172, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Scaal, M.; Füchtbauer, E.M.; Brand-Saberi, B. cDermo-1 expression indicates a role in avian skin development. Anat. Embryol. 2001, 203, 1–7. [Google Scholar] [CrossRef]
- Jacob, T.; Chakravarty, A.; Panchal, A.; Patil, M.; Ghodadra, G.; Sudhakaran, J.; Nuesslein-Volhard, C. Zebrafish twist2/dermo1 regulates scale shape and scale organization during skin development and regeneration. Cells Dev. 2021, 166, 203684. [Google Scholar] [CrossRef]
- Olivera-Martinez, I.; Viallet, J.P.; Michon, F.; Pearton, D.J.; Dhouailly, D. The different steps of skin formation in vertebrates. Int. J. Dev. Biol. 2004, 48, 107–115. [Google Scholar] [CrossRef]
- Dhouailly, D.; Olivera-Martinez, I.; Fliniaux, I.; Missier, S.; Viallet, J.P.; Thélu, J. Skin field formation: Morphogenetic events. Int. J. Dev. Biol. 2004, 48, 85–91. [Google Scholar] [CrossRef]
- Viallet, J.P.; Prin, F.; Olivera-Martinez, I.; Hirsinger, E.; Pourquié, O.; Dhouailly, D. Chick Delta-1 gene expression and the formation of the feather primordia. Mech. Dev. 1998, 72, 159–168. [Google Scholar] [CrossRef]
- Houghton, L.; Lindon, C.; Morgan, B.A. The ectodysplasin pathway in feather tract development. Development 2005, 132, 863–872. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, X.; Liu, Y.; Tao, Y.; Feng, G.; He, L.; Guo, X.; Ma, G. Wls Is Expressed in the Epidermis and Regulates Embryonic Hair Follicle Induction in Mice. PLoS ONE 2012, 7, e45904. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, R.H. Avian scale development. I. Histogenesis and morphogenesis of the epidermis and dermis during formation of the scale ridge. J. Exp. Zool. 1972, 181, 365–384. [Google Scholar] [CrossRef]
- Cooper, R.L.; Lloyd, V.J.; Di-Poï, N.; Fletcher, A.G.; Barrett, P.M.; Fraser, G.J. Conserved gene signalling and a derived patterning mechanism underlie the development of avian footpad scales. Evodevo 2019, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Sengel, P. Morphogenesis of Skin. In Developmental and Cell Biology Series; Abercrombie, M., Newth, D.R., Torrey, J.G., Eds.; Cambridge University Press: Cambridge, UK, 1976; pp. 1–277. [Google Scholar]
- Millar, S.E. Molecular mechanisms regulating hair follicle development. J. Investig. Dermatol. 2002, 118, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.L.; Martin, K.J.; Rasch, L.J.; Fraser, G.J. Developing an ancient epithelial appendage: FGF signalling regulates early tail denticle formation in sharks. Evodevo 2017, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Veltmaat, J.M.; Van Veelen, W.; Thiery, J.P.; Bellusci, S. Identification of the mammary line in mouse by Wnt10b expression. Dev. Dyn. 2004, 229, 349–356. [Google Scholar] [CrossRef]
- Ferguson, C.A.; Tucker, A.S.; Christensen, L.; Lau, A.L.; Matzuk, M.M.; Sharpe, P.T. Activin is an essential early mesenchymal signal in tooth development that is required for patterning of the murine dentition. Genes Dev. 1998, 12, 2636–2649. [Google Scholar] [CrossRef]
- Nakamura, M.; Matzuk, M.M.; Gerstmayer, B.; Bosio, A.; Lauster, R.; Miyachi, Y.; Werner, S.; Paus, R. Control of pelage hair follicle development and cycling by complex interactions between follistatin and activin. FASEB J. 2003, 17, 497–499. [Google Scholar] [CrossRef]
- Dhouailly, D. Specification of Feather and Scale Patterns. In Pattern Formation; Malacinski, G.M., Bryant, S.V., Eds.; MacMillan Pub.: New York, NY, USA; London, UK, 1984; pp. 581–601. [Google Scholar]
- Ho, W.K.W.; Freem, L.; Zhao, D.; Painter, K.J.; Woolley, T.E.; Gaffney, E.A.; McGrew, M.J.; Tzika, A.; Milinkovitch, M.C.; Schneider, P.; et al. Feather arrays are patterned by interacting signalling and cell density waves. PLoS Biol. 2019, 17, e3000132. [Google Scholar] [CrossRef]
- Ahtiainen, L.; Lefebvre, S.; Lindfors, P.H.; Renvoisé, E.; Shirokova, V.; Vartiainen, M.K.; Thesleff, I.; Mikkola, M.L. Directional cell migration, but not proliferation, drives hair placode morphogenesis. Dev. Cell 2014, 28, 588–602. [Google Scholar] [CrossRef]
- Wessells, N.K. Morphology and proliferation during early feather development. Dev. Biol. 1965, 12, 131–153. [Google Scholar] [CrossRef]
- Wessells, N.K.; Roessner, K.D. Nonproliferation in dermal condensations of mouse vibrissae and pelage hairs. Dev. Biol. 1965, 12, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Biggs, L.C.; Mäkelä, O.J.; Myllymäki, S.M.; Das Roy, R.; Närhi, K.; Pispa, J.; Mustonen, T.; Mikkola, M.L. Hair follicle dermal condensation forms via Fgf20 primed cell cycle exit, cell motility, and aggregation. eLife 2018, 7, e36468. [Google Scholar] [CrossRef] [PubMed]
- Noramly, S.; Freeman, A.; Morgan, B.A. Beta-catenin signaling can initiate feather bud development. Development 1999, 126, 3509–3521. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Jiang, T.X.; Lin, C.M.; Burrus, L.W.; Chuong, C.M.; Widelitz, R. Distinct Wnt members regulate the hierarchical morphogenesis of skin regions (spinal tract) and individual feathers. Mech. Dev. 2004, 121, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Närhi, K.; Järvinen, E.; Birchmeier, W.; Taketo, M.M.; Mikkola, M.L.; Thesleff, I. Sustained epithelial beta-catenin activity induces precocious hair development but disrupts hair follicle down-growth and hair shaft formation. Development 2008, 135, 1019–1028. [Google Scholar] [CrossRef]
- Chen, D.; Jarrell, A.; Guo, C.; Lang, R.; Atit, R. Dermal β-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development 2012, 139, 1522–1533. [Google Scholar] [CrossRef]
- Glinka, A.; Wu, W.; Delius, H.; Monaghan, A.P.; Blumenstock, C.; Niehrs, C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 1998, 391, 357–362. [Google Scholar] [CrossRef]
- Chu, Q.; Cai, L.; Fu, Y.; Chen, X.; Yan, Z.; Lin, X.; Zhou, G.; Han, H.; Widelitz, R.B.; Chuong, C.M.; et al. Dkk2/Frzb in the dermal papillae regulates feather regeneration. Dev. Biol. 2014, 387, 167–178. [Google Scholar] [CrossRef]
- Gat, U.; DasGupta, R.; Degenstein, L.; Fuchs, E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 1998, 95, 605–614. [Google Scholar] [CrossRef]
- Sadier, A.; Viriot, L.; Pantalacci, S.; Laudet, V. The ectodysplasin pathway: From diseases to adaptations. Trends Genet. 2014, 30, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Goetinck, P.F.; Abbott, U.K. Tissue interaction in the scaleless mutant and the use of scaleless as an ectodermal marker in studies of normal limb differentiation. J. Exp. Zool. 1963, 154, 7–19. [Google Scholar] [CrossRef]
- Sengel, P.; Abbott, U.K. In vitro studies with the scaleless mutant: Interaction during feather and scale differentiation. J. Hered. 1963, 54, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Wells, K.L.; Hadad, Y.; Ben-Avraham, D.; Hillel, J.; Cahaner, A.; Headon, D.J. Genome-wide SNP scan of pooled DNA reveals nonsense mutation in FGF20 in the scaleless line of featherless chickens. BMC Genom. 2012, 13, 257. [Google Scholar] [CrossRef] [PubMed]
- Houghton, L.; Lindon, C.M.; Freeman, A.; Morgan, B.A. Abortive placode formation in the feather tract of the scaleless chicken embryo. Dev. Dyn. 2007, 236, 3020–3030. [Google Scholar] [CrossRef] [PubMed]
- Michon, F.; Charveron, M.; Dhouailly, D. Dermal condensation formation in the chick embryo: Requirement for integrin engagement and subsequent stabilization by a possible notch/integrin interaction. Dev. Dyn. 2007, 236, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Francis-West, P.H.; Widelitz, R.B.; Jiang, T.X.; Ting-Berreth, S.; Tickle, C.; Wolpert, L.; Chuong, C.M. Local inhibitory action of BMPs and their relationships with activators in feather formation: Implications for periodic patterning. Dev. Biol. 1998, 196, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Song, H.K.; Lee, S.H.; Goetinck, P.F. FGF-2 signaling is sufficient to induce dermal condensations during feather development. Dev. Dyn. 2004, 231, 741–749. [Google Scholar] [CrossRef]
- Botchkarev, V.A.; Paus, R. Molecular biology of hair morphogenesis: Development and cycling. J. Exp. Zool. B Mol. Dev. Evol. 2003, 298, 164–180. [Google Scholar] [CrossRef]
- Pummila, M.; Fliniaux, I.; Jaatinen, R.; James, M.J.; Laurikkala, J.; Schneider, P.; Thesleff, I.; Mikkola, M.L. Ectodysplasin has a dual role in ectodermal organogenesis: Inhibition of Bmp activity and induction of Shh expression. Development 2007, 134, 117–125. [Google Scholar] [CrossRef]
- Michon, F.; Forest, L.; Collomb, E.; Demongeot, J.; Dhouailly, D. BMP2 and BMP7 play antagonistic roles in feather induction. Development 2008, 135, 2797–2805. [Google Scholar] [CrossRef] [PubMed]
- Glover, J.D.; Wells, K.L.; Matthäus, F.; Painter, K.J.; Ho, W.; Riddell, J.; Johansson, J.A.; Ford, M.J.; Jahoda, C.A.B.; Klika, V.; et al. Hierarchical patterning modes orchestrate hair follicle morphogenesis. PLoS Biol. 2017, 15, e2002117. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhouailly, D. Evo Devo of the Vertebrates Integument. J. Dev. Biol. 2023, 11, 25. https://doi.org/10.3390/jdb11020025
Dhouailly D. Evo Devo of the Vertebrates Integument. Journal of Developmental Biology. 2023; 11(2):25. https://doi.org/10.3390/jdb11020025
Chicago/Turabian StyleDhouailly, Danielle. 2023. "Evo Devo of the Vertebrates Integument" Journal of Developmental Biology 11, no. 2: 25. https://doi.org/10.3390/jdb11020025
APA StyleDhouailly, D. (2023). Evo Devo of the Vertebrates Integument. Journal of Developmental Biology, 11(2), 25. https://doi.org/10.3390/jdb11020025




