Immunolocalization of Some Epidermal Proteins and Glycoproteins in the Growing Skin of the Australian Lungfish (Neoceratodus forsteri)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Samples
2.2. Methods and Preparation for Microscopy
2.3. Bioinformatics Control
3. Results
3.1. Bioinformatics Control
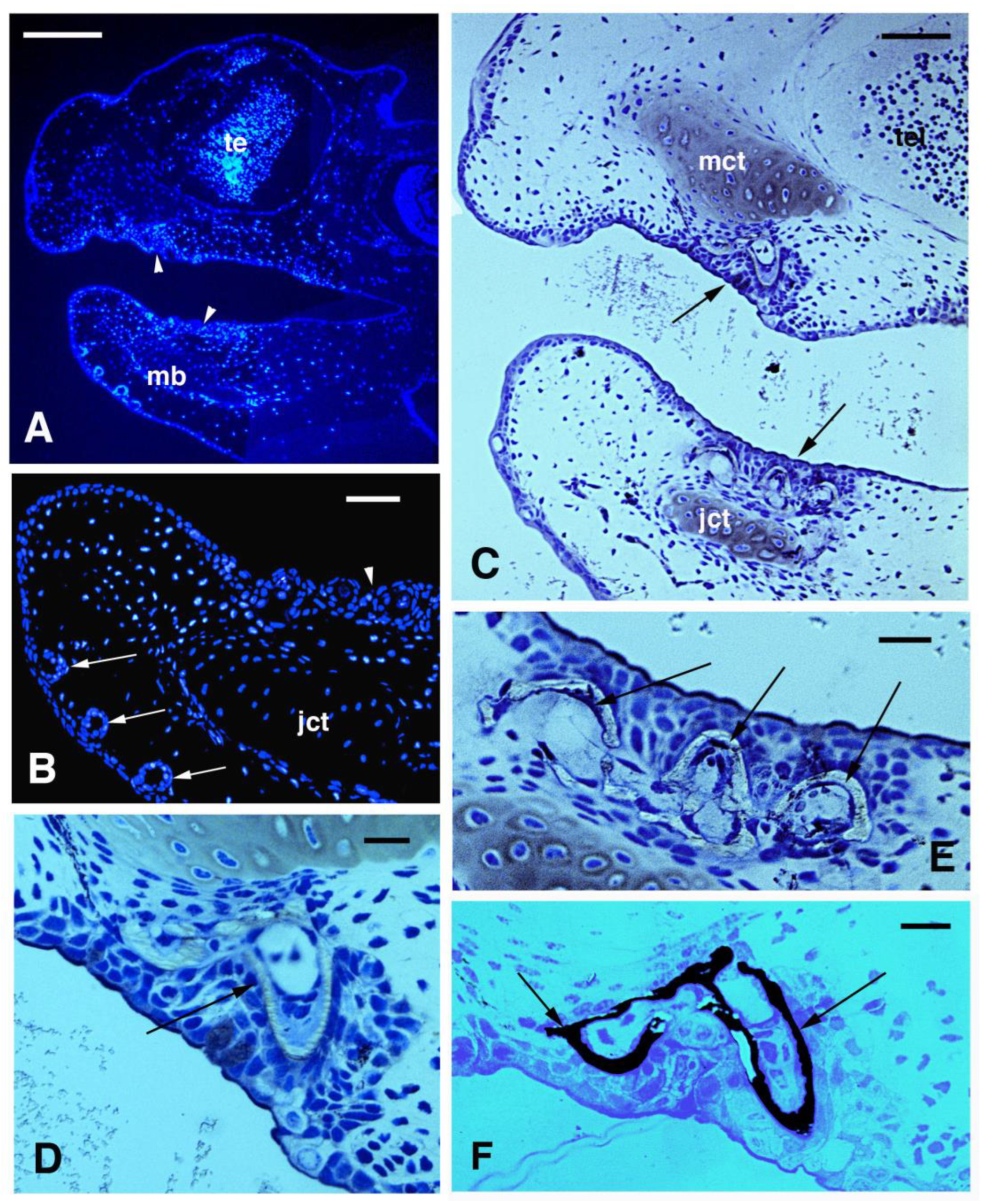
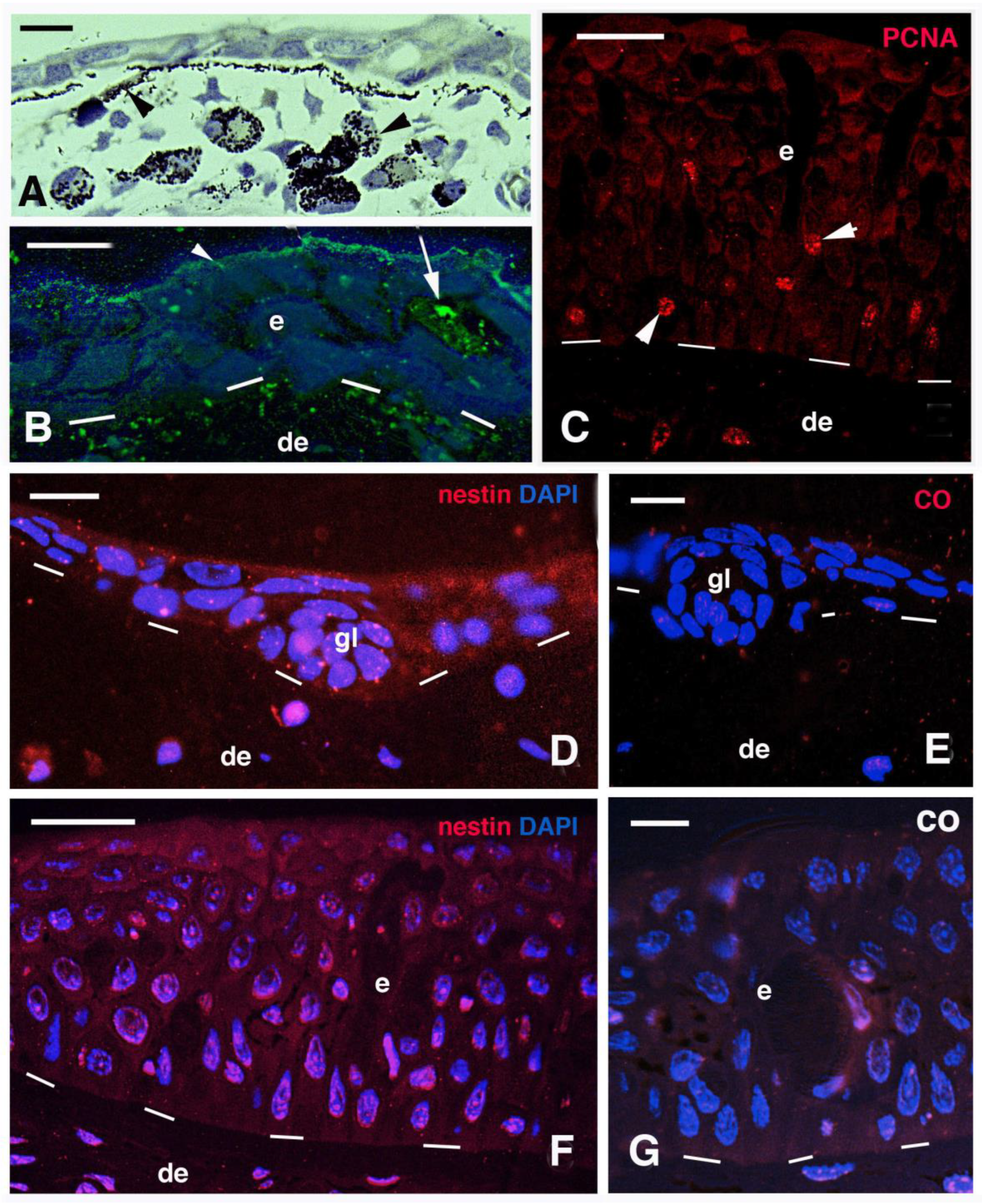
3.2. Skin Histology and Fine Cytology in Growing Juveniles
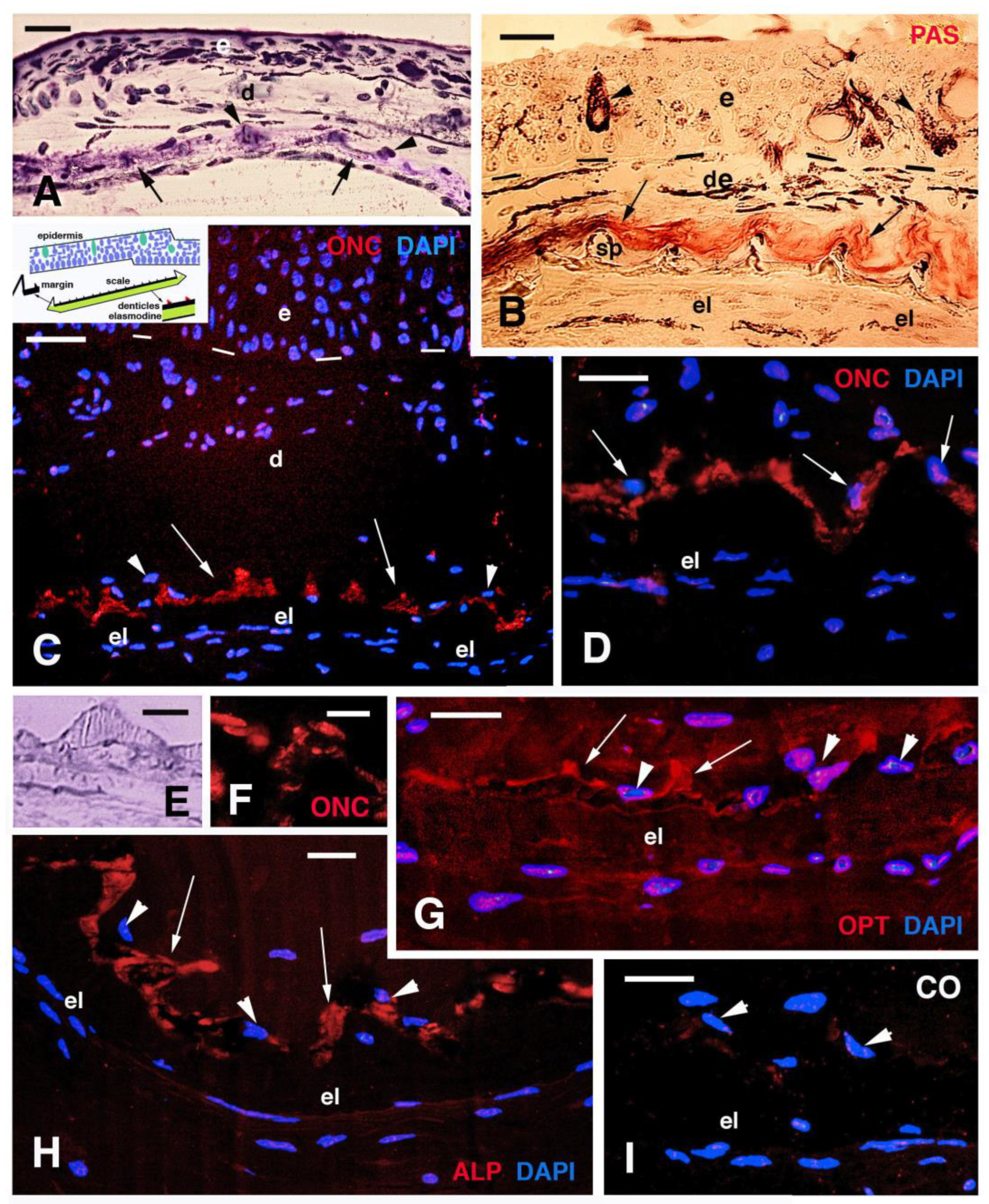
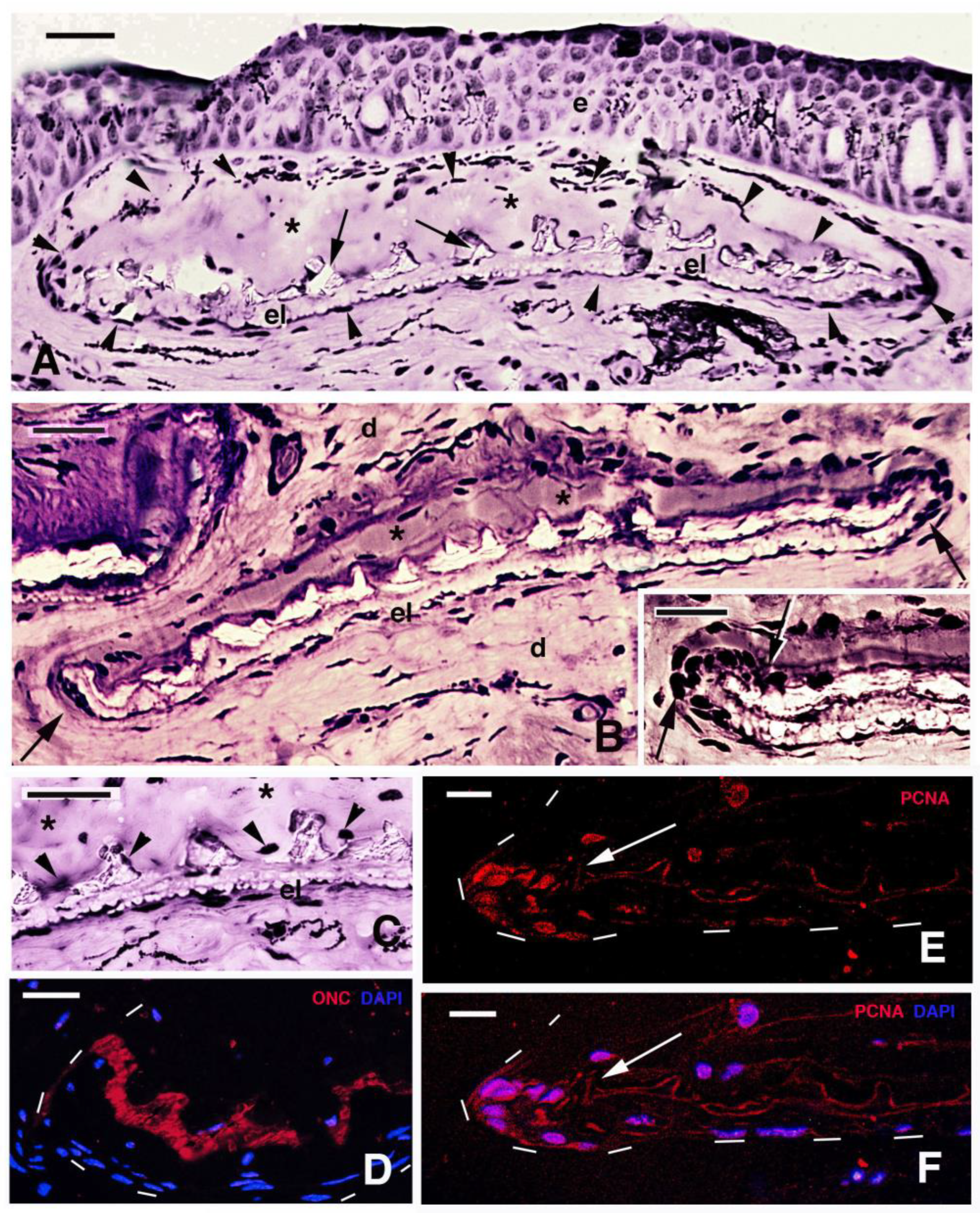
3.3. Scale Histology and Immunofluorescence
4. Discussion
4.1. Epidermal Proteins
4.2. Teeth and Lepidotriches Calcification
4.3. Scale Mineralization
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitear, M. The skin of fishes including cyclostomes. Epidermis. In Biology of the Integument; Bereither-Hahn, J., Matoltsy, G.A., Sylvia-Richards, K., Eds.; Springer: Berlin, Germany, 1986; Volume 2, Vertebrates; pp. 8–38. [Google Scholar]
- Whitear, M. The skin of fishes including cyclostomes. Epidermis. In Biology of the Integument; Bereither-Hahn, J., Matoltsy, G.A., Sylvia-Richards, K., Eds.; Springer: Berlin, Germany, 1986; Volume 2, Vertebrates; pp. 39–55. [Google Scholar]
- Sire, J.Y.; Donoghue, P.C.; Vickaryous, M. Review. Origin and evolution of the integumentary skeleton in non-tetrapod vertebrates. J. Anat. 2009, 214, 409–440. [Google Scholar] [CrossRef] [PubMed]
- Le Guellec, D.; Morvan-Dubois, G.; Sire, J.Y. Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio). Int. J. Dev. Biol. 2004, 48, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Akat, E.; Yenmis, M.; Pombal, M.A.; Molist, P.; Megías, M.; Arman, S.; Vesely, M.; Anderson, R.; Ayaz, D. Comparison of vertebrate skin structure at class level: A review. Anat. Rec. 2022, 305, 3543–3608. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Vertebrate keratinization evolved into cornification mainly by the activity of transglutaminase and sulfhydryl oxidase on epidermal proteins: An immunohistochemical survey. Anat. Rec. 2021, 305, 333–358. [Google Scholar] [CrossRef] [PubMed]
- Fox, H. Tail skin of the dipnoan Neoceratodus larva: Fine structure and differentiation. J. Zool. 1989, 217, 213–226. [Google Scholar] [CrossRef]
- Zaccone, G.; Kapoor, B.G.; Fasulo, S.; Ainis, L. Structural, histochemical and functional aspects of the epidermis of fishes. Adv. Marine Biol. 2001, 40, 255–348. [Google Scholar]
- Sire, Y.J.; Allizard, F.; Babiar, O.; Bourguignon, J.; Quilhac, A. Scale development in zebrafish (Danio rerio). J. Anat. 1997, 190, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Vickaryous, M.; Sire, Y.S. The integumentary skeleton of tetrapods: Origin, evolution, and development. J. Anat. 2009, 214, 441–464. [Google Scholar] [CrossRef]
- Kemp, A. Formation and structure of scales in the Australian lungfish, Neoceratodus forsteri (Osteichtyes: Dipnoi). J. Morph. 2012, 273, 530–540. [Google Scholar] [CrossRef]
- Lee, R.T.; Thiery, J.P.; Camey, T.J. Dermal fin rays and scales derive from mesederm, not neural crest. Curr. Biol. 2013, 23, R336–R337. [Google Scholar] [CrossRef]
- Mongera, A.; Nusslein-Volhard, C. Scales of fish arise from mesoderm. Curr. Biol. 2013, 9, R338–R339. [Google Scholar] [CrossRef] [PubMed]
- Voltering, J.M.; Irisarri, I.; Ericsson, R.; Joss, J.M.P.; Sordino, P.; Meyer, A. Sarcopterygian fin ontogeny elucidates the origin of hands with digits. Sci. Adv. 2020, 6, eabc3510. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A. Skin structure in the snout of the Australian lungfish, Neoceratodus forsteri (Osteichtyes: Dipnoi). Tissue Cell 2014, 46, 397–408. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kemp, A.; Heaslop, M.; Carr, A. Scale structure in the Australian lungfish, Neoceratodus forsteri (Osteichtyes: Dipnoi). J. Morph. 2015, 276, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L. The role of extracellular matrix components in dentin mineralization. Crit. Rev. Oral Biol. Med. 1991, 2, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Murshed, M. Mechanism of bone mineralization. Cold Spring Harb. Perspect. Med. 2018, 8, a031229. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.G.; Laize, V.; Valente, M.S.; Cancela, M.L. Identification of an osteopontin-like protein in fish associated with mineral formation. FEBS J. 2007, 274, 4428–4439. [Google Scholar] [CrossRef] [PubMed]
- Stravi, S.; Zarnescu, O. The expression of alkaline phosphatase, osteopontin, osteocalcin and chondroitin sulphate during pectoral fin regeneration in Carassius auratus gibelio: A combined histochemical and immunohistochemical study. Microsc. Microanal. 2013, 19, 233–242. [Google Scholar]
- Kemp, A. The biology of the australian lungfish, Neoceratodus forsteri (Krefft, 1870). J. Morph. 1987, 190, 181–198. [Google Scholar] [CrossRef]
- Alibardi, L.; Joss, J. Keratinization of the epidermis of the Australian lungfish Neoceratodus forsteri (Dipnoi). J. Morph. 2003, 256, 13–22. [Google Scholar] [CrossRef]
- Meyer, A.; Schloissnig, S.; Franchini, P.; Du, K.; Woltering, J.M.; Isarri, I.; Wong, W.Y.; Nowoshilow, S.; Kneitz, S.; Kawaguchi, A.; et al. Giant lungfish genome elucidates the conquest of land by vertebrates. Nature 2021, 590, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.M.; Coates, C.W. On the histology of the skin of the lungfish Protopterus annectens after experimentally induced aestivation. Quart. J. Microsc. Sci. 1936, 79, 487–491. [Google Scholar] [CrossRef]
- Kitzan, M.; Sweeny, P.R. A light and electron microscopic study of the structure of Protopterus annectens epidermis. I. Mucus production. Can. J. Zool. 1975, 46, 767–779. [Google Scholar] [CrossRef]
- Imaki, H.; Chavin, W. Ultrastructure of mucous cells in the sarcopterigian integument. Scanning Electron Microsc. 1984, 1, 409–422. [Google Scholar]
- Romano, L.A.; Lopez, A.I.H.; Buitrago, J.R.; Pedrosa, V.E. Histology of juvenile skin of Lepidosiren paradoxa Fitzinger, 1837 (Sarcopterygii, Dipnoi). Ann. Bras. Acad. Sci. 2019, 91, e20190822. [Google Scholar] [CrossRef]
- Kemp, A. Rearing of embryos of the Australian lungfish, Neoceratodus forsteri, under laboratory conditions. Copeia 1981, 4, 776–784. [Google Scholar] [CrossRef]
- Joss, J. Lungfish evolution and development. Gen. Comp. Endocrinol. 2006, 148, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L.; Joss, J.; Toni, M. The integument of lungfish: General structure and keratin composition. In The Biology of Lungfishes; Jorgensen, J.M., Joss, J., Eds.; CRC Press: Boca Raton, FL, USA, 2011; Chapter 9; p. 243. [Google Scholar]
- Alibardi, L. Development and structure of the skin in the Australian lungfish (Neoceratodus forsteri) in relation to epidermal adaptation of tetrapods. Acta Zool. 2023. [Google Scholar] [CrossRef]
- Kemp, A. A comparison of the developing dentition of Neoceratodus forsteri and Callorhynchus milii. Proc. Linean Soc. NSW 1984, 107, 245–262. [Google Scholar]
- Kemp, N.E.; Park, J.H. Regeneration of lepidotrichia and acrinotichia in the tailfin of the teleostt Tilapia mossambica. Dev. Biol. 1970, 22, 321–342. [Google Scholar] [CrossRef]
- Shuturminska, K.; Tarakina, N.V.; Azevedo, H.S.; Bushby, A.J.; Mata, A.; Anderson, P.; Al-Jawad, M. Elastin-like protein, with statherin derived peptide formation, controls fluoroapatite formation and morphology. Front. Physiol. 2017, 8, 368. [Google Scholar] [CrossRef] [PubMed]
- Witzmann, F. Morphological and histological changes of dermal scales during the fish-to-tetrapod tansition. Acta Zool. 2011, 92, 281–302. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alibardi, L. Immunolocalization of Some Epidermal Proteins and Glycoproteins in the Growing Skin of the Australian Lungfish (Neoceratodus forsteri). J. Dev. Biol. 2023, 11, 35. https://doi.org/10.3390/jdb11030035
Alibardi L. Immunolocalization of Some Epidermal Proteins and Glycoproteins in the Growing Skin of the Australian Lungfish (Neoceratodus forsteri). Journal of Developmental Biology. 2023; 11(3):35. https://doi.org/10.3390/jdb11030035
Chicago/Turabian StyleAlibardi, Lorenzo. 2023. "Immunolocalization of Some Epidermal Proteins and Glycoproteins in the Growing Skin of the Australian Lungfish (Neoceratodus forsteri)" Journal of Developmental Biology 11, no. 3: 35. https://doi.org/10.3390/jdb11030035
APA StyleAlibardi, L. (2023). Immunolocalization of Some Epidermal Proteins and Glycoproteins in the Growing Skin of the Australian Lungfish (Neoceratodus forsteri). Journal of Developmental Biology, 11(3), 35. https://doi.org/10.3390/jdb11030035





