An Emerging Animal Model for Querying the Role of Whole Genome Duplication in Development, Evolution, and Disease
Abstract
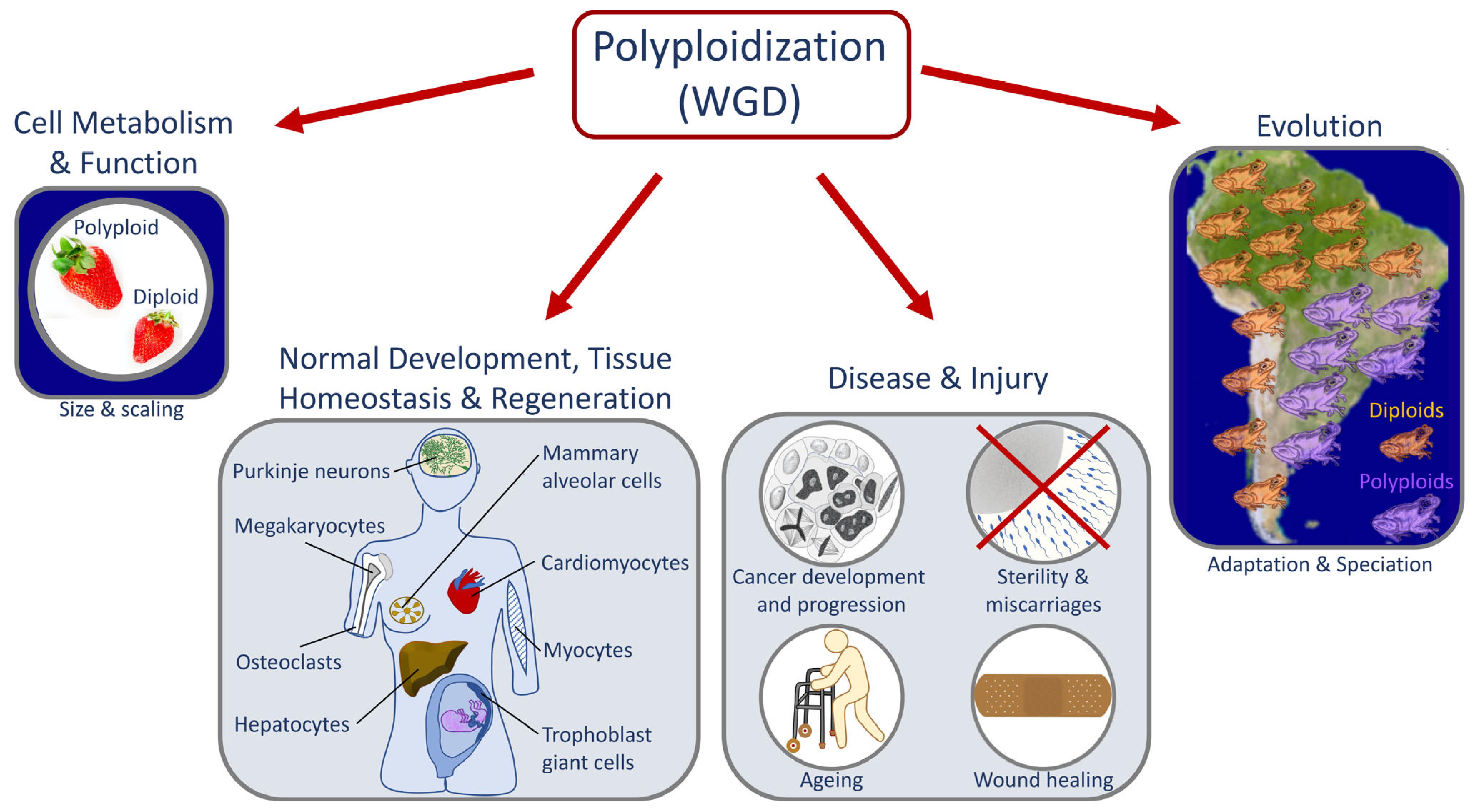
1. Whole Genome Duplication in Development, Evolution, and Disease
1.1. Causes and Downstream Effects of WGD
1.2. Laboratory Multicellular Organism Models for WGD
2. Caenorhabditis Elegans as an Animal Laboratory Model for Understanding WGD
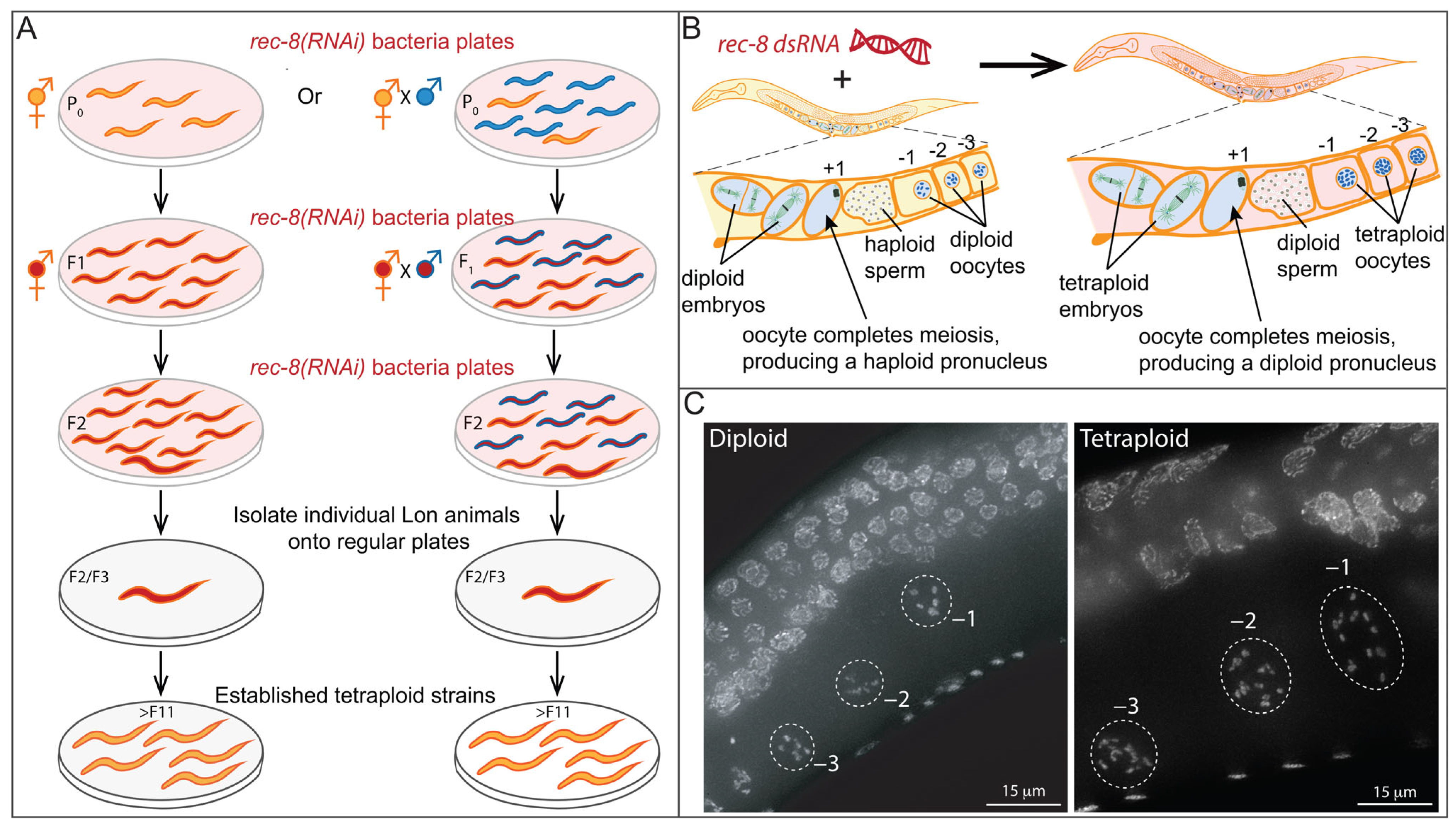
2.1. Polyploidy and Aneuploidy in C. elegans
2.2. Polyploid and Aneuploid Animals Uncover C. elegans Modes of Sex Determination and Dosage Compensation
3. Utilizing Polyploid C. elegans as Tools to Investigate Developmental Processes
3.1. Understanding Early Events of Meiotic Prophase I
3.2. Understanding Meiotic and Early Embryonic Cell Divisions
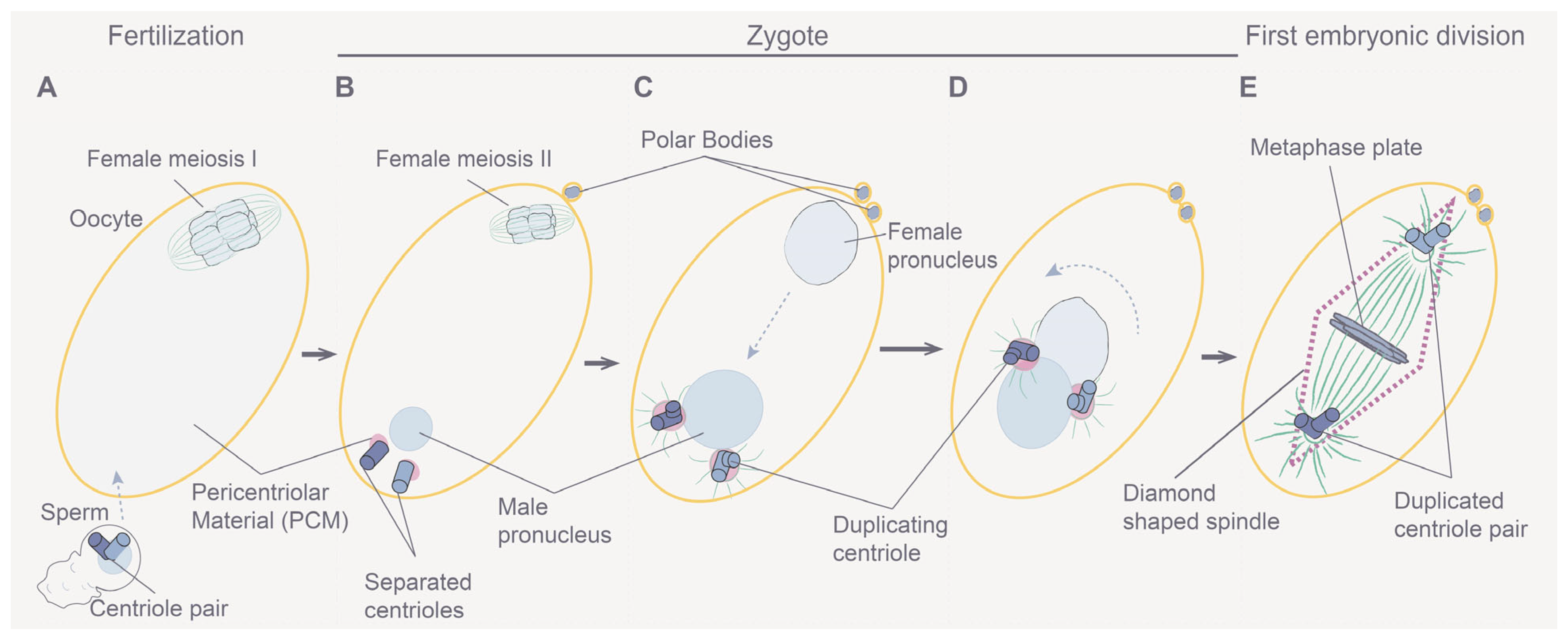
4. Utilizing Caenorhabditis to Understand the Effects of Polyploidization
4.1. Polyploid Tissues in C. elegans
4.2. Biological Size and Scaling (Allometry)
5. Potential Future Queries Utilizing C. elegans Polyploids
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davoli, T.; de Lange, T. The Causes and Consequences of Polyploidy in Normal Development and Cancer. Annu. Rev. Cell Dev. Biol. 2011, 27, 585–610. [Google Scholar] [CrossRef]
- Øvrebø, J.I.; Edgar, B.A. Polyploidy in tissue homeostasis and regeneration. Development 2018, 145, dev156034. [Google Scholar] [CrossRef]
- Donne, R.; Saroul-Aïnama, M.; Cordier, P.; Celton-Morizur, S.; Desdouets, C. Polyploidy in liver development, homeostasis and disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 391–405. [Google Scholar] [CrossRef]
- Bailey, E.C.; Kobielski, S.; Park, J.; Losick, V.P. Polyploidy in Tissue Repair and Regeneration. Cold Spring Harb. Perspect. Biol. 2021, 13, a040881. [Google Scholar] [CrossRef]
- Anatskaya, O.V.; Vinogradov, A.E. Polyploidy as a Fundamental Phenomenon in Evolution, Development, Adaptation and Diseases. Int. J. Mol. Sci. 2022, 23, 3542. [Google Scholar] [CrossRef]
- Berman, J. Ploidy plasticity: A rapid and reversible strategy for adaptation to stress. FEMS Yeast Res. 2016, 16, fow020. [Google Scholar] [CrossRef]
- Wood, T.E.; Takebayashi, N.; Barker, M.S.; Mayrose, I.; Greenspoon, P.B.; Rieseberg, L.H. The frequency of polyploid speciation in vascular plants. Proc. Natl. Acad. Sci. USA 2009, 106, 13875–13879. [Google Scholar] [CrossRef]
- Akagi, T.; Jung, K.; Masuda, K.; Shimizu, K.K. Polyploidy before and after domestication of crop species. Curr. Opin. Plant Biol. 2022, 69, 102255. [Google Scholar] [CrossRef] [PubMed]
- Quinton, R.J.; DiDomizio, A.; Vittoria, M.A.; Kotýnková, K.; Ticas, C.J.; Patel, S.; Koga, Y.; Vakhshoorzadeh, J.; Hermance, N.; Kuroda, T.S.; et al. Whole-genome doubling confers unique genetic vulnerabilities on tumour cells. Nature 2021, 590, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Anatskaya, O.V.; Vinogradov, A.E. Polyploidy and Myc Proto-Oncogenes Promote Stress Adaptation via Epigenetic Plasticity and Gene Regulatory Network Rewiring. Int. J. Mol. Sci. 2022, 23, 9691. [Google Scholar] [CrossRef] [PubMed]
- Orr-Weaver, T.L. When bigger is better: The role of polyploidy in organogenesis. Trends Genet. 2015, 31, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Frawley, L.E.; Orr-Weaver, T.L. Polyploidy. Curr. Biol. 2015, 25, R353–R358. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Z.; Ouseph, M.M.; Li, J.; Pécot, T.; Chokshi, V.; Kent, L.; Bae, S.; Byrne, M.; Duran, C.; Comstock, G.; et al. Canonical and atypical E2Fs regulate the mammalian endocycle. Nature 2012, 14, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.C.; Fu, N.Y.; Jamieson, P.R.; Pal, B.; Whitehead, L.; Nicholas, K.R.; Lindeman, G.J.; Visvader, J.E. Essential role for a novel population of binucleated mammary epithelial cells in lactation. Nat. Commun. 2016, 7, 11400. [Google Scholar] [CrossRef]
- Pandit, S.K.; Westendorp, B.; Nantasanti, S.; van Liere, E.; Tooten, P.C.J.; Cornelissen, P.W.A.; Toussaint, M.J.M.; Lamers, W.H.; de Bruin, A. E2F8 is essential for polyploidization in mammalian cells. Nature 2012, 14, 1181–1191. [Google Scholar] [CrossRef]
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.-D.; Guerquin-Kern, J.-L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013, 493, 433–436. [Google Scholar] [CrossRef]
- Zanet, J.; Freije, A.; Ruiz, M.; Coulon, V.; Sanz, J.R.; Chiesa, J.; Gandarillas, A. A Mitosis Block Links Active Cell Cycle with Human Epidermal Differentiation and Results in Endoreplication. PLoS ONE 2010, 5, e15701. [Google Scholar] [CrossRef]
- Mattia, G.; Vulcano, F.; Milazzo, L.; Barca, A.; Macioce, G.; Giampaolo, A.; Hassan, H.J. Different ploidy levels of megakaryocytes generated from peripheral or cord blood CD34+ cells are correlated with different levels of platelet release. Blood 2002, 99, 888–897. [Google Scholar] [CrossRef]
- Losick, V.P. Wound-Induced Polyploidy Is Required for Tissue Repair. Adv. Wound Care 2016, 5, 271–278. [Google Scholar] [CrossRef]
- Zimmet, J.; Ravid, K. Polyploidy: Occurrence in nature, mechanisms, and significance for the megakaryocyte-platelet system. Exp. Hematol. 2000, 28, 3–16. [Google Scholar] [CrossRef]
- Sladky, V.C.; Eichin, F.; Reiberger, T.; Villunger, A. Polyploidy control in hepatic health and disease. J. Hepatol. 2021, 75, 1177–1191. [Google Scholar] [CrossRef]
- Mazzi, S.; Lordier, L.; Debili, N.; Raslova, H.; Vainchenker, W. Megakaryocyte and polyploidization. Exp. Hematol. 2018, 57, 1–13. [Google Scholar] [CrossRef]
- Anatskaya, O.V.; Sidorenko, N.V.; Vinogradov, A.E.; Beyer, T.V. Impact of neonatal cryptosporidial gastroenteritis on epigenetic programming of rat hepatocytes. Cell Biol. Int. 2007, 31, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Anatskaya, O.V.; Matveev, I.V.; Sidorenko, N.V.; Kharchenko, M.V.; Kropotov, A.V.; Vinogradov, A.E. Changes in the heart of neonatal rats after cryptosporidial gastroenteritis of different degrees of severity. J. Evol. Biochem. Physiol. 2013, 49, 509–518. [Google Scholar] [CrossRef]
- Lazzeri, E.; Angelotti, M.L.; Conte, C.; Anders, H.-J.; Romagnani, P. Surviving Acute Organ Failure: Cell Polyploidization and Progenitor Proliferation. Trends Mol. Med. 2019, 25, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Anatskaya, O.V.; Vinogradov, A.E. Whole-Genome Duplications in Evolution, Ontogeny, and Pathology: Complexity and Emergency Reserves. Mol. Biol. 2021, 55, 813–827. [Google Scholar] [CrossRef]
- Edgar, B.A.; Zielke, N.; Gutierrez, C. Endocycles: A recurrent evolutionary innovation for post-mitotic cell growth. Nat. Rev. Mol. Cell Biol. 2014, 15, 197–210. [Google Scholar] [CrossRef]
- Ohbayashi, R.; Nakamachi, A.; Hatakeyama, T.S.; Watanabe, S.; Kanesaki, Y.; Chibazakura, T.; Yoshikawa, H.; Miyagishima, S.-Y. Coordination of Polyploid Chromosome Replication with Cell Size and Growth in a Cyanobacterium. mBio 2019, 10, e00510-19. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.H.; Afonso, B.; Silver, P.A.; Savage, D.F. Spatial and Temporal Organization of Chromosome Duplication and Segregation in the Cyanobacterium Synechococcus elongatus PCC 7942. PLoS ONE 2012, 7, e47837. [Google Scholar] [CrossRef]
- Watanabe, S.; Ohbayashi, R.; Shiwa, Y.; Noda, A.; Kanesaki, Y.; Chibazakura, T.; Yoshikawa, H. Light-dependent and asynchronous replication of cyanobacterial multi-copy chromosomes. Mol. Microbiol. 2012, 83, 856–865. [Google Scholar] [CrossRef]
- Schoenfelder, K.P.; Fox, D.T. The expanding implications of polyploidy. J. Cell Biol. 2015, 209, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Bomblies, K. When everything changes at once: Finding a new normal after genome duplication. Proc. R. Soc. B Boil. Sci. 2020, 287, 20202154. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Coate, J.E. Polyploidy, the Nucleotype, and Novelty: The Impact of Genome Doubling on the Biology of the Cell. Int. J. Plant Sci. 2019, 180, 1–52. [Google Scholar] [CrossRef]
- Ren, R.; Wang, H.; Guo, C.; Zhang, N.; Zeng, L.; Chen, Y.; Ma, H.; Qi, J. Widespread Whole Genome Duplications Contribute to Genome Complexity and Species Diversity in Angiosperms. Mol. Plant 2018, 11, 414–428. [Google Scholar] [CrossRef]
- Fox, D.T.; Soltis, D.E.; Soltis, P.S.; Ashman, T.-L.; Van de Peer, Y. Polyploidy: A Biological Force from Cells to Ecosystems. Trends Cell Biol. 2020, 30, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Maciver, S.K. Ancestral Eukaryotes Reproduced Asexually, Facilitated by Polyploidy: A Hypothesis. Bioessays 2019, 41, e1900152. [Google Scholar] [CrossRef]
- Nguyen, H.G.; Makitalo, M.; Yang, D.; Chinnappan, D.; St.Hilaire, C.; Ravid, K. Deregulated Aurora-B induced tetraploidy promotes tumorigenesis. FASEB J. 2009, 23, 2741–2748. [Google Scholar] [CrossRef]
- van Rijnberk, L.M.; Barrull-Mascaró, R.; van der Palen, R.L.; Schild, E.S.; Korswagen, H.C.; Galli, M. Endomitosis controls tissue-specific gene expression during development. PLoS Biol. 2022, 20, e3001597. [Google Scholar] [CrossRef]
- Storchova, Z.; Pellman, D. From polyploidy to aneuploidy, genome instability and cancer. Nat. Rev. Mol. Cell Biol. 2004, 5, 45–54. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, Q.; Xu, H.; Zhou, R.; Liu, X. Human cell polyploidization: The good and the evil. Semin. Cancer Biol. 2022, 81, 54–63. [Google Scholar] [CrossRef]
- Otto, S.P. The Evolutionary Consequences of Polyploidy. Cell 2007, 131, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, E.; Angelotti, M.L.; Peired, A.; Conte, C.; Marschner, J.A.; Maggi, L.; Mazzinghi, B.; Lombardi, D.; Melica, M.E.; Nardi, S.; et al. Endocycle-related tubular cell hypertrophy and progenitor proliferation recover renal function after acute kidney injury. Nat. Commun. 2018, 9, 1344. [Google Scholar] [CrossRef] [PubMed]
- Losick, V.P.; Fox, D.T.; Spradling, A.C. Polyploidization and Cell Fusion Contribute to Wound Healing in the Adult Drosophila Epithelium. Curr. Biol. 2013, 23, 2224–2232. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-N.; Wu, Y.-J.; Tsai, H.-W.; Sun, C.-P.; Wu, H.-L.; Pei, Y.-N.; Lu, K.-Y.; Yen, T.T.-C.; Chang, C.-W.; Chan, H.-L.; et al. Intrahepatic hepatitis B virus large surface antigen induces hepatocyte hyperploidy via failure of cytokinesis. J. Pathol. 2018, 245, 502–513. [Google Scholar] [CrossRef] [PubMed]
- David, K.T.; Halanych, K.M. Spatial proximity between polyploids across South American frog genera. J. Biogeogr. 2021, 48, 991–1000. [Google Scholar] [CrossRef]
- Glennon, K.L.; Ritchie, M.E.; Segraves, K.A. Evidence for shared broad-scale climatic niches of diploid and polyploid plants. Ecol. Lett. 2014, 17, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Arnold, B.; Kim, S.-T.; Bomblies, K. Single Geographic Origin of a Widespread Autotetraploid Arabidopsis arenosa Lineage Followed by Interploidy Admixture. Mol. Biol. Evol. 2015, 32, 1382–1395. [Google Scholar] [CrossRef]
- Molina-Henao, Y.F.; Hopkins, R. Autopolyploid lineage shows climatic niche expansion but not divergence in Arabidopsis arenosa. Am. J. Bot. 2019, 106, 61–70. [Google Scholar] [CrossRef]
- Ramsey, J. Polyploidy and ecological adaptation in wild yarrow. Proc. Natl. Acad. Sci. USA 2011, 108, 7096–7101. [Google Scholar] [CrossRef]
- Martin, S.L.; Husband, B.C. Adaptation of diploid and tetraploid chamerion angustifolium to eleva-tion but not local environment: Adaptation of Chamerion angustifolium ploidies. Evolution 2013, 67, 1780–1791. [Google Scholar] [CrossRef]
- Selmecki, A.M.; Maruvka, Y.E.; Richmond, P.A.; Guillet, M.; Shoresh, N.; Sorenson, A.L.; De, S.; Kishony, R.; Michor, F.; Dowell, R.; et al. Polyploidy can drive rapid adaptation in yeast. Nature 2015, 519, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Van De Peer, Y.; Mizrachi, Y.V.D.P.E.; Marchal, K. The evolutionary significance of polyploidy. Nat. Rev. Genet. 2017, 18, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Bielski, C.M.; Zehir, A.; Penson, A.V.; Donoghue, M.T.A.; Chatila, W.; Armenia, J.; Chang, M.T.; Schram, A.M.; Jonsson, P.; Bandlamudi, C.; et al. Genome doubling shapes the evolution and prognosis of advanced cancers. Nat. Genet. 2018, 50, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Zack, T.I.; Schumacher, S.E.; Carter, S.L.; Cherniack, A.D.; Saksena, G.; Tabak, B.; Lawrence, M.S.; Zhang, C.Z.; Wala, J.; Mermel, C.H.; et al. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 2013, 45, 1134–1140. [Google Scholar] [CrossRef]
- Sansregret, L.; Vanhaesebroeck, B.; Swanton, C. Determinants and clinical implications of chromosomal instability in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 139–150. [Google Scholar] [CrossRef]
- Fujiwara, T.; Bandi, M.; Nitta, M.; Ivanova, E.V.; Bronson, R.T.; Pellman, D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 2005, 437, 1043–1047. [Google Scholar] [CrossRef]
- Matsumoto, T.; Wakefield, L.; Peters, A.; Peto, M.; Spellman, P.; Grompe, M. Proliferative polyploid cells give rise to tumors via ploidy reduction. Nat. Commun. 2021, 12, 646. [Google Scholar] [CrossRef]
- Lambuta, R.A.; Nanni, L.; Liu, Y.; Diaz-Miyar, J.; Iyer, A.; Tavernari, D.; Katanayeva, N.; Ciriello, G.; Oricchio, E. Whole-genome doubling drives oncogenic loss of chromatin segregation. Nature 2023, 615, 925–933. [Google Scholar] [CrossRef]
- Dewhurst, S.M.; McGranahan, N.; Burrell, R.A.; Rowan, A.J.; Grönroos, E.; Endesfelder, D.; Joshi, T.; Mouradov, D.; Gibbs, P.; Ward, R.L.; et al. Tolerance of Whole-Genome Doubling Propagates Chromosomal Instability and Accelerates Cancer Genome Evolution. Cancer Discov. 2014, 4, 175–185. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Zhang, S.; Zhu, M.; Lu, T.; Chen, K.; Wen, Z.; Wang, S.; Xiao, G.; Luo, D.; Jia, Y.; et al. Mice with Increased Numbers of Polyploid Hepatocytes Maintain Regenerative Capacity But Develop Fewer Hepatocellular Carcinomas Following Chronic Liver Injury. Gastroenterology 2020, 158, 1698–1712.e14. [Google Scholar] [CrossRef]
- Adams, K.L.; Wendel, J.F. Polyploidy and genome evolution in plants. Curr. Opin. Plant Biol. 2005, 8, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Hanafiah, N.M.; Harikrishna, J.A.; Eem, L.P.; Baisakh, N.; Mispan, M.S. A Reappraisal of Polyploidy Events in Grasses (Poaceae) in a Rapidly Changing World. Biology 2022, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-W.; Bhandari, G.S.; Won, H.; Park, J.H.; Park, D.S. Polyploidy and introgression in invasive giant knotweed (Fallopia sachalinensis) during the colonization of remote volcanic islands. Sci. Rep. 2018, 8, 16021. [Google Scholar] [CrossRef] [PubMed]
- Demin, S.Y.; Berdieva, M.A.; Goodkov, A.V. Cyclic Polyploidy in Obligate Agamic Amoebae. Cell Tissue Biol. 2019, 13, 242–246. [Google Scholar] [CrossRef]
- Storchová, Z.; Breneman, A.; Cande, J.; Dunn, J.; Burbank, K.; O’Toole, E.; Pellman, D. Genome-wide genetic analysis of polyploidy in yeast. Nature 2006, 443, 541–547. [Google Scholar] [CrossRef]
- Albertin, W.; Marullo, P. Polyploidy in fungi: Evolution after whole-genome duplication. Proc. R. Soc. B Boil. Sci. 2012, 279, 2497–2509. [Google Scholar] [CrossRef]
- Soppa, J. Polyploidy in Archaea and Bacteria: About Desiccation Resistance, Giant Cell Size, Long-Term Survival, Enforcement by a Eukaryotic Host and Additional Aspects. Microb. Physiol. 2015, 24, 409–419. [Google Scholar] [CrossRef]
- Markov, A.V.; Kaznacheev, I.S. Evolutionary consequences of polyploidy in prokaryotes and the origin of mitosis and meiosis. Biol. Direct 2016, 11, 28. [Google Scholar] [CrossRef]
- Wertheim, B.; Beukeboom, L.; van de Zande, L. Polyploidy in Animals: Effects of Gene Expression on Sex Determination, Evolution and Ecology. Cytogenet. Genome Res. 2013, 140, 256–269. [Google Scholar] [CrossRef]
- Mable, B.K.; Alexandrou, M.A.; Taylor, M.I. Genome duplication in amphibians and fish: An extended synthesis. J. Zool. 2011, 284, 151–182. [Google Scholar] [CrossRef]
- Mendell, J.E.; Clements, K.D.; Choat, J.H.; Angert, E.R. Extreme polyploidy in a large bacterium. Proc. Natl. Acad. Sci. USA 2008, 105, 6730–6734. [Google Scholar] [CrossRef] [PubMed]
- Sacerdot, C.; Louis, A.; Bon, C.; Berthelot, C.; Crollius, H.R. Chromosome evolution at the origin of the ancestral vertebrate genome. Genome Biol. 2018, 19, 166. [Google Scholar] [CrossRef]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Monnahan, P.; Kolář, F.; Baduel, P.; Sailer, C.; Koch, J.; Horvath, R.; Laenen, B.; Schmickl, R.; Paajanen, P.; Šrámková, G.; et al. Pervasive population genomic consequences of genome duplication in Arabidopsis arenosa. Nat. Ecol. Evol. 2019, 3, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.E.; Rary, J.M. Mosaic tetraploidy in a two-year-old female. Clin. Genet. 1974, 6, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, I.; Jenderny, J.; Kaminsky, E.; Mannhardt, A.; Meinecke, P.; Grozdanova, L.; Gillessen-Kaesbach, G. Mosaic and complete tetraploidy in live-born infants: Two new patients and review of the literature. Clin. Dysmorphol. 2010, 19, 123–127. [Google Scholar] [CrossRef]
- Yamazaki, W.; Takahashi, M.; Kawahara, M. Restricted development of mouse triploid fetuses with disorganized expression of imprinted genes. Zygote 2015, 23, 874–884. [Google Scholar] [CrossRef]
- Menon, T.; Nair, S. Experimental Manipulation of Ploidy in Zebrafish Embryos and Its Application in Genetic Screens. Vertebr. Embryog. Embryol. Cell. Genet. Methods 2019, 1920, 111–128. [Google Scholar] [CrossRef]
- Eakin, G.; Hadjantonakis, A.-K.; Papaioannou, V.; Behringer, R.R. Developmental potential and behavior of tetraploid cells in the mouse embryo. Dev. Biol. 2005, 288, 150–159. [Google Scholar] [CrossRef]
- Clarke, E.K.; Gomez, K.A.R.; Mustachi, Z.; Murph, M.C.; Schvarzstein, M. Manipulation of Ploidy in Caenorhabditis elegans. J. Vis. Exp. 2018, 133, 57296. [Google Scholar] [CrossRef]
- Nigon, V. Les Modalites de La Reproduction et Le Determinisme Du Sexe Chez Quelques Nematodes Libres. Ann. Sci. Nat. Zool. Biol. Anim. 1949, 11, 1–132. [Google Scholar]
- Hodgkin, J. Male phenotypes and mating efficiency in Caenorhabditis elegans. Genetics 1983, 103, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, J. Primary sex determination in the nematode C. elegans. Development 1987, 101, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Nigon, V.M.; Félix, M.-A. History of research on C. elegans and other free-living nematodes as model organisms. Wormbook 2017, 2017, 1–84. [Google Scholar] [CrossRef]
- Brenner, S. The Genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Hedgecock, E.M.; White, J.G. Polyploid tissues in the nematode Caenorhabditis elegans. Dev. Biol. 1985, 107, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Flemming, A.J.; Shen, Z.-Z.; Cunha, A.; Emmons, S.W.; Leroi, A.M. Somatic polyploidization and cellular proliferation drive body size evolution in nematodes. Proc. Natl. Acad. Sci. USA 2000, 97, 5285–5290. [Google Scholar] [CrossRef]
- Hodgkin, J.; Horvitz, H.R.; Brenner, S. Nondisjunction mutants of the nematode Caenorhabditis elegans. Genetics 1979, 91, 67–94. [Google Scholar] [CrossRef]
- Sigurdson, D.C.; Spanier, G.J.; Herman, R.K. Caenorhabditis elegans deficiency mapping. Genetics 1984, 108, 331–345. [Google Scholar] [CrossRef]
- Haack, H.; Hodgkin, J. Tests for parental imprinting in the nematode Caenorhabditis elegans. Mol. Genet. Genom. 1991, 228, 482–485. [Google Scholar] [CrossRef]
- Hodgkin, J. Karyotype, Ploidy, and Gene Dosage. In Wormbook. The C. elegans Research Community, WormBook. 2005. Available online: http://www.wormbook.org/chapters/www_karyotype/karyotype.html (accessed on 30 December 2022).
- Larkin, K.; Tucci, C.; Neiman, M. Effects of polyploidy and reproductive mode on life history trait expression. Ecol. Evol. 2016, 6, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Schoonmaker, A.; Hao, Y.; Bird, D.; Conant, G.C. A Single, Shared Triploidy in Three Species of Parasitic Nematodes. G3 Genes|Genom.|Genet. 2019, 10, 225–233. [Google Scholar] [CrossRef]
- Madl, J.E.; Herman, R.K. Polyploids and sex determination in Caenorhabditis elegans. Genetics 1979, 93, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.; McNally, K.; Friedman, J.A.; Cortes, D.B.; Wang, D.Y.; Korf, I.F.; McNally, F.J. Autosomal Trisomy and Triploidy Are Corrected During Female Meiosis in Caenorhabditis elegans. Genetics 2017, 207, 911–922. [Google Scholar] [CrossRef]
- Nigon, V. Effets de la polyploïdie chez un nématode libre. C. R. Acad. Sci. 1949, 228, 1161–1162. [Google Scholar]
- Nigon, V. Polyploidie Experimentale Chez Un Nematode Libre, Rhabditis Elegans. Maupas. Bull. Biol. Fr. Belg. 1951, 85, 187–225. [Google Scholar]
- Mlynarczyk-Evans, S.; Roelens, B.; Villeneuve, A.M. Evidence That Masking of Synapsis Imperfections Counterbalances Quality Control to Promote Efficient Meiosis. PLoS Genet. 2013, 9, e1003963. [Google Scholar] [CrossRef]
- Roelens, B.; Schvarzstein, M.; Villeneuve, A.M. Manipulation of Karyotype in Caenorhabditis elegans Reveals Multiple Inputs Driving Pairwise Chromosome Synapsis During Meiosis. Genetics 2015, 201, 1363–1379. [Google Scholar] [CrossRef]
- Hodgkin, J. Exploring the Envelope. Systematic Alteration in the Sex-Determination System of the Nematode Caenorhabditis elegans. Genetics 2002, 162, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Meneely, P.M. Sex determination in polyploids of Caenorhabditis elegans. Genetics 1994, 137, 467–481. [Google Scholar] [CrossRef]
- Meyer, B.J. X-Chromosome Dosage Compensation. In Wormbook. The C. elegans Research Community, WormBook. 2005, pp. 1–14. Available online: http://www.wormbook.org/chapters/www_dosagecomp/dosagecomp.html (accessed on 30 December 2022).
- Meyer, B.J. The X chromosome in C. elegans sex determination and dosage compensation. Curr. Opin. Genet. Dev. 2022, 74, 101912. [Google Scholar] [CrossRef] [PubMed]
- Schvarzstein, M.; Wignall, S.M.; Villeneuve, A.M. Coordinating cohesion, co-orientation, and congression during meiosis: Lessons from holocentric chromosomes. Genes Dev. 2010, 24, 219–228. [Google Scholar] [CrossRef]
- MacQueen, A.J.; Phillips, C.; Bhalla, N.; Weiser, P.; Villeneuve, A.M.; Dernburg, A.F. Chromosome Sites Play Dual Roles to Establish Homologous Synapsis during Meiosis in C. elegans. Cell 2005, 123, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Cortes, D.B.; McNally, K.L.; Mains, P.E.; McNally, F.J. The Asymmetry of Female Meiosis Reduces the Frequency of Inheritance of Unpaired Chromosomes. Elife 2015, 4, e06056. [Google Scholar] [CrossRef] [PubMed]
- Meyerzon, M.; Gao, Z.; Liu, J.; Wu, J.-C.; Malone, C.J.; Starr, D.A. Centrosome attachment to the C. elegans male pronucleus is dependent on the surface area of the nuclear envelope. Dev. Biol. 2009, 327, 433–446. [Google Scholar] [CrossRef]
- Schierenberg, E.; Wood, W.B. Control of cell-cycle timing in early embryos of Caenorhabditis elegans. Dev. Biol. 1985, 107, 337–354. [Google Scholar] [CrossRef]
- Sadler, P.L.; Shakes, D.C. Anucleate Caenorhabditis elegans sperm can crawl, fertilize oocytes and direct anterior-posterior polarization of the 1-cell embryo. Development 2000, 127, 355–366. [Google Scholar] [CrossRef]
- Hara, Y.; Kimura, A. An allometric relationship between mitotic spindle width, spindle length, and ploidy in Caenorhabditis elegans embryos. Mol. Biol. Cell 2013, 24, 1411–1419. [Google Scholar] [CrossRef]
- Schierenberg, E. Laser-Induced Cell Fusion. In Cell Fusion; Springer: Boston, MA, USA, 1987; pp. 409–418. [Google Scholar] [CrossRef]
- Oegema, K. Cell Division. In Wormbook. The C. elegans Research Community, WormBook. 2006, pp. 1–40. Available online: http://www.wormbook.org/chapters/www_celldivision/celldivision.html (accessed on 30 December 2022).
- Malone, C.J.; Misner, L.; Le Bot, N.; Tsai, M.-C.; Campbell, J.M.; Ahringer, J.; White, J.G. The C. elegans Hook Protein, ZYG-12, Mediates the Essential Attachment between the Centrosome and Nucleus. Cell 2003, 115, 825–836. [Google Scholar] [CrossRef]
- Gönczy, P.; Pichler, S.; Kirkham, M.; Hyman, A.A. Cytoplasmic Dynein Is Required for Distinct Aspects of Mtoc Positioning, Including Centrosome Separation, in the One Cell Stage Caenorhabditis elegans Embryo. J. Cell Biol. 1999, 147, 135–150. [Google Scholar] [CrossRef]
- Newport, J.; Kirschner, M. A major developmental transition in early xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell 1982, 30, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Sachsenmaier, W.; Remy, U.; Plattner-Schobel, R. Initiation of synchronous mitosis in Physarum polycephalum: A model of the control of cell division in eukariots. Exp. Cell Res. 1972, 73, 41–48. [Google Scholar] [CrossRef] [PubMed]
- A Fantes, P. Isolation of cell size mutants of a fission yeast by a new selective method: Characterization of mutants and implications for division control mechanisms. J. Bacteriol. 1981, 146, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Matson, J.P.; Cook, J.G. Cell cycle proliferation decisions: The impact of single cell analyses. FEBS J. 2017, 284, 362–375. [Google Scholar] [CrossRef]
- Arata, Y.; Takagi, H. Quantitative Studies for Cell-Division Cycle Control. Front. Physiol. 2019, 10, 1022. [Google Scholar] [CrossRef] [PubMed]
- Koreth, J.; Heuvel, S.V.D. Cell-cycle control in Caenorhabditis elegans: How the worm moves from G1 to S. Oncogene 2005, 24, 2756–2764. [Google Scholar] [CrossRef]
- Wang, Z. Regulation of Cell Cycle Progression by Growth Factor-Induced Cell Signaling. Cells 2021, 10, 3327. [Google Scholar] [CrossRef]
- Dumont, S.; Mitchison, T.J. Compression Regulates Mitotic Spindle Length by a Mechanochemical Switch at the Poles. Curr. Biol. 2009, 19, 1086–1095. [Google Scholar] [CrossRef]
- Itabashi, T.; Takagi, J.; Shimamoto, Y.; Onoe, H.; Kuwana, K.; Shimoyama, I.; Gaetz, J.; Kapoor, T.M.; Ishiwata, S. Probing the mechanical architecture of the vertebrate meiotic spindle. Nat. Methods 2009, 6, 167–172. [Google Scholar] [CrossRef]
- E Mains, P.; Kemphues, K.J.; Sprunger, S.A.; Sulston, I.A.; Wood, W.B. Mutations affecting the meiotic and mitotic divisions of the early Caenorhabditis elegans embryo. Genetics 1990, 126, 593–605. [Google Scholar] [CrossRef]
- Quintin, S.; E Mains, P.; Zinke, A.; A Hyman, A. The mbk-2 kinase is required for inactivation of MEI-1/katanin in the one-cell Caenorhabditis elegans embryo. EMBO Rep. 2003, 4, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Segbert, C.; Barkus, R.; Powers, J.; Strome, S.; Saxton, W.M.; Bossinger, O. KLP-18, a Klp2 Kinesin, Is Required for Assembly of Acentrosomal Meiotic Spindles in Caenorhabditis elegans. Mol. Biol. Cell 2003, 14, 4458–4469. [Google Scholar] [CrossRef] [PubMed]
- Maddox, A.S.; Habermann, B.; Desai, A.; Oegema, K. Distinct roles for two C. elegans anillins in the gonad and early embryo. Development 2005, 132, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Dorn, J.F.; Zhang, L.; Paradis, V.; Edoh-Bedi, D.; Jusu, S.; Maddox, P.S.; Maddox, A.S. Actomyosin Tube Formation in Polar Body Cytokinesis Requires Anillin in C. elegans. Curr. Biol. 2010, 20, 2046–2051. [Google Scholar] [CrossRef]
- Fankhauser, G. Cytological studies on egg fragments of the salamander Triton. V. Chromosome number and chromosome individuality in the cleavage mitoses of merogonic fragments. J. Exp. Zool. 1934, 68, 18. [Google Scholar] [CrossRef]
- Hara, Y.; Iwabuchi, M.; Ohsumi, K.; Kimura, A. Intranuclear DNA density affects chromosome condensation in metazoans. Mol. Biol. Cell 2013, 24, 2442–2453. [Google Scholar] [CrossRef]
- Conklin, E.G. Cell size and nuclear size. J. Exp. Zool. 1912, 12, 1–98. [Google Scholar] [CrossRef]
- Di Berardino, M.A. The karyotype of Rana pipiens and investigation of its stability during embryonic differentiation. Dev. Biol. 1962, 5, 101–126. [Google Scholar] [CrossRef]
- Belmont, A.S.; Sedat, J.W.; Agard, D.A. A three-dimensional approach to mitotic chromosome structure: Evidence for a complex hierarchical organization. J. Cell Biol. 1987, 105, 77–92. [Google Scholar] [CrossRef]
- Deppe, U.; Schierenberg, E.; Cole, T.; Krieg, C.; Schmitt, D.; Yoder, B.; von Ehrenstein, G. Cell lineages of the embryo of the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1978, 75, 376–380. [Google Scholar] [CrossRef]
- Sulston, J.E.; Schierenberg, E.; White, J.G.; Thomson, J.N. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 1983, 100, 64–119. [Google Scholar] [CrossRef]
- Greenwald, I. Development of the Vulva, 2nd ed.; C. elegans II.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1997; Chapter 19; pp. 519–541. [Google Scholar]
- Simske, J.S.; Hardin, J. Getting into Shape: Epidermal Morphogenesis in Caenorhabditis elegans Embryos. Bioessays 2001, 23, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.-C.; Schwarzbauer, J.E. Gonad morphogenesis and distal tip cell migration in the Caenorhabditis elegans hermaphrodite. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 519–531. [Google Scholar] [CrossRef]
- Shemer, G.; Podbilewicz, B. Fusomorphogenesis: Cell fusion in organ formation. Dev. Dyn. 2000, 218, 30–51. [Google Scholar] [CrossRef]
- Tsou, M.-F.B.; Stearns, T. Mechanism limiting centrosome duplication to once per cell cycle. Nature 2006, 442, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Tsou, M.-F.B.; Stearns, T. Controlling centrosome number: Licenses and blocks. Curr. Opin. Cell Biol. 2006, 18, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Duensing, A.; Duensing, S. Centrosomes, Polyploidy and Cancer. Adv. Exp. Med. Biol. 2010, 676, 93–103. [Google Scholar] [CrossRef]
- Lu, Y.; Roy, R. Centrosome/Cell Cycle Uncoupling and Elimination in the Endoreduplicating Intestinal Cells of C. elegans. PLoS ONE 2014, 9, e110958-17. [Google Scholar] [CrossRef]
- Song, M.H.; Liu, Y.; Anderson, D.E.; Jahng, W.J.; O’Connell, K.F. Protein Phosphatase 2A-SUR-6/B55 Regulates Centriole Duplication in C. elegans by Controlling the Levels of Centriole Assembly Factors. Dev. Cell 2011, 20, 563–571. [Google Scholar] [CrossRef]
- Kitagawa, D.; Flückiger, I.; Polanowska, J.; Keller, D.; Reboul, J.; Gönczy, P. PP2A Phosphatase Acts upon SAS-5 to Ensure Centriole Formation in C. elegans Embryos. Dev. Cell 2011, 20, 550–562. [Google Scholar] [CrossRef]
- Decker, M.; Jaensch, S.; Pozniakovsky, A.; Zinke, A.; O’Connell, K.F.; Zachariae, W.; Myers, E.; Hyman, A.A. Limiting Amounts of Centrosome Material Set Centrosome Size in C. elegans Embryos. Curr. Biol. 2011, 21, 1259–1267. [Google Scholar] [CrossRef]
- Lee, H.O.; Davidson, J.M.; Duronio, R.J. Endoreplication: Polyploidy with purpose. Genes Dev. 2009, 23, 2461–2477. [Google Scholar] [CrossRef]
- Zhang, L.; Ward, J.D.; Cheng, Z.; Dernburg, A.F. The auxin-inducible degradation (AID) system enables versatile conditional protein depletion in C. elegans. Development 2015, 142, 4374–4384. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, P.; Edgington, N.P.; Schneider, B.L.; Rupeš, I.; Tyers, M.; Futcher, B. The Size of the Nucleus Increases as Yeast Cells Grow. Mol. Biol. Cell 2007, 18, 3523–3532. [Google Scholar] [CrossRef] [PubMed]
- Yahya, G.; Menges, P.; Amponsah, P.S.; Ngandiri, D.A.; Schulz, D.; Wallek, A.; Kulak, N.; Mann, M.; Cramer, P.; Savage, V.; et al. Sublinear scaling of the cellular proteome with ploidy. Nat. Commun. 2022, 13, 6182. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.R.; Nurse, P. Nuclear size control in fission yeast. J. Cell Biol. 2007, 179, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Gillooly, J.F.; Hein, A.; Damiani, R. Nuclear DNA Content Varies with Cell Size across Human Cell Types. Cold Spring Harb. Perspect. Biol. 2015, 7, a019091. [Google Scholar] [CrossRef]
- Katagiri, Y.; Hasegawa, J.; Fujikura, U.; Hoshino, R.; Matsunaga, S.; Tsukaya, H. The coordination of ploidy and cell size differs between cell layers in leaves. Development 2016, 143, 1120–1125. [Google Scholar] [CrossRef]
- Anatskaya, O.V.; E Vinogradov, A. Paraoxical relationship between protein content and nucleolar activity in mammalian cardiomyocytes. Genome 2004, 47, 565–578. [Google Scholar] [CrossRef]
- Robinson, D.O.; Coate, J.E.; Singh, A.; Hong, L.; Bush, M.; Doyle, J.J.; Roeder, A.H. Ploidy and Size at Multiple Scales in the Arabidopsis Sepal. Plant Cell 2018, 30, 2308–2329. [Google Scholar] [CrossRef]
- Hisanaga, T.; Kawade, K.; Tsukaya, H. Compensation: A key to clarifying the organ-level regulation of lateral organ size in plants. J. Exp. Bot. 2015, 66, 1055–1063. [Google Scholar] [CrossRef]
- Kawade, K.; Horiguchi, G.; Tsukaya, H. Non-cell-autonomously coordinated organ size regulation in leaf development. Development 2010, 137, 4221–4227. [Google Scholar] [CrossRef]
- Cadart, C.; Heald, R. Scaling of biosynthesis and metabolism with cell size. Mol. Biol. Cell 2022, 33, pe5. [Google Scholar] [CrossRef]
- Cantwell, H.; Dey, G. Nuclear size and shape control. Semin. Cell Dev. Biol. 2022, 130, 90–97. [Google Scholar] [CrossRef]
- Tsukaya, H. Re-examination of the role of endoreduplication on cell-size control in leaves. J. Plant Res. 2019, 132, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, G.; Tsukaya, H. Organ Size Regulation in Plants: Insights from Compensation. Front. Plant Sci. 2011, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Lozano, E.; Sáez, A.G.; Flemming, A.J.; Cunha, A.; Leroi, A.M. Regulation of Growth by Ploidy in Caenorhabditis elegans. Curr. Biol. 2006, 16, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Nyström, J.; Shen, Z.-Z.; Aili, M.; Flemming, A.J.; Leroi, A.; Tuck, S. Increased or decreased levels of Caenorhabditis elegans lon-3, a gene encoding a collagen, cause reciprocal changes in body length. Genetics 2002, 161, 83–97. [Google Scholar] [CrossRef]
- Morita, K.; Flemming, A.J.; Sugihara, Y.; Mochii, M.; Suzuki, Y.; Yoshida, S.; Wood, W.B.; Kohara, Y.; Leroi, A.M.; Ueno, N. A Caenorhabditis elegans TGF-β, DBL-1, controls the expression of LON-1, a PR-related protein, that regulates polyploidization and body length. EMBO J. 2002, 21, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Chow, K.L.; Ueno, N. Regulation of body length and male tail ray pattern formation of Caenorhabditis elegans by a member of TGF-β family. Development 1999, 126, 1337–1347. [Google Scholar] [CrossRef]
- Hirose, T.; Nakano, Y.; Nagamatsu, Y.; Misumi, T.; Ohta, H.; Ohshima, Y. Cyclic GMP-dependent protein kinase EGL-4 controls body size and lifespan in C. elegans. Development 2003, 130, 1089–1099. [Google Scholar] [CrossRef]
- Nagamatsu, Y.; Ohshima, Y. Mechanisms for the control of body size by a G-kinase and a downstream TGFbeta signal pathway in Caenorhabditis elegans. Genes Cells 2004, 9, 39–47. [Google Scholar] [CrossRef]
- Wang, J.; Tokarz, R.; Savage-Dunn, C. The expression of TGFβ signal transducers in the hypodermis regulates body size in C. elegans. Development 2002, 129, 4989–4998. [Google Scholar] [CrossRef] [PubMed]
- Parisi, T.; Beck, A.R.; Rougier, N.; McNeil, T.; Lucian, L.; Werb, Z.; Amati, B. Cyclins E1 and E2 are required for endoreplication in placental trophoblast giant cells. EMBO J. 2003, 22, 4794–4803. [Google Scholar] [CrossRef]
- Geng, Y.; Yu, Q.; Sicinska, E.; Das, M.; E Schneider, J.; Bhattacharya, S.; Rideout, W.M.; Bronson, R.T.; Gardner, H.; Sicinski, P. Cyclin E Ablation in the Mouse. Cell 2003, 114, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Tuck, S. The control of cell growth and body size in Caenorhabditis elegans. Exp. Cell Res. 2014, 321, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Yandell, M.D.; Roy, P.J.; Krishna, S.; Savage-Dunn, C.; Ross, R.M.; Padgett, R.W.; Wood, W.B. A BMP homolog acts as a dose-dependent regulator of body size and male tail patterning in Caenorhabditis elegans. Development 1999, 126, 241–250. [Google Scholar] [CrossRef]
- Savage-Dunn, C.; Yu, L.; Gill, K.; Awan, M.; Fernando, T. Non-stringent tissue-source requirements for BMP ligand expression in regulation of body size in Caenorhabditis elegans. Genet. Res. 2011, 93, 427–432. [Google Scholar] [CrossRef]
- Savage-Dunn, C. Targets of TGFβ-related signaling in Caenorhabditis elegans. Cytokine Growth Factor Rev. 2001, 12, 305–312. [Google Scholar] [CrossRef]
- Savage-Dunn, C.; Tokarza, R.; Wangb, H.; Cohenb, S.; Giannikasa, C.; Padgett, R.W. SMA-3 Smad Has Specific and Critical Functions in DBL-1/SMA-6 TGFβ-Related Signaling. Dev. Biol. 2000, 223, 70–76. [Google Scholar] [CrossRef]
- Szyperski, T.; Fernández, C.; Mumenthaler, C.; Wüthrich, K. Structure comparison of human glioma pathogenesis-related protein GliPR and the plant pathogenesis-related protein P14a indicates a functional link between the human immune system and a plant defense system. Proc. Natl. Acad. Sci. USA 1998, 95, 2262–2266. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Wang, W.; Gao, Y.; Yang, Y.; Zhu, Z.; Fan, Q. Ce-wts-1 plays important roles in Caenorhabditis elegans development. FEBS Lett. 2009, 583, 3158–3164. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.T.; Greer, E.R.; Pearce, D.; Ashrafi, K. Rictor/TORC2 Regulates Caenorhabditis elegans Fat Storage, Body Size, and Development through sgk-1. PLoS Biol. 2009, 7, e1000060. [Google Scholar] [CrossRef]
- Oldham, S.; Böhni, R.; Stocker, H.; Brogiolo, W.; Hafen, E. Genetic control of size in Drosophila. Philos. Trans. R. Soc. B Biol. Sci. 2000, 355, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Schmelzle, T.; Hall, M.N. TOR, a Central Controller of Cell Growth. Cell 2000, 103, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Watanabe, N.; Nagamatsu, Y.; Gengyo-Ando, K.; Mitani, S.; Ohshima, Y. Control of body size by SMA-5, a homolog of MAP kinase BMK1/ERK5, in C. elegans. Development 2005, 132, 3175–3184. [Google Scholar] [CrossRef]
- Hyun, S. Body size regulation and insulin-like growth factor signaling. Cell. Mol. Life Sci. 2013, 70, 2351–2365. [Google Scholar] [CrossRef]
- McKeown, C.; Praitis, V.; Austin, J. sma-1 encodes a βH-spectrin homolog required for Caenorhabditis elegans morphogenesis. Development 1998, 125, 2087–2098. [Google Scholar] [CrossRef]
- Hayashi, S.; Yokoyama, H.; Tamura, K.; Hayashi, S.; Yokoyama, H.; Tamura, K. Roles of Hippo signaling pathway in size control of organ regeneration. Dev. Growth Differ. 2015, 57, 341–351. [Google Scholar] [CrossRef]
- Goodman, M.B.; Savage-Dunn, C. Reciprocal interactions between transforming growth factor beta signaling and collagens: Insights from C aenorhabditis elegans. Dev. Dyn. 2022, 251, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Ando, A.; Jiang, N.; Ikeda, Y.; Chen, Z.J. Single-cell RNA-seq analysis reveals ploidy-dependent and cell-specific transcriptome changes in Arabidopsis female gametophytes. Genome Biol. 2020, 21, 178. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Rolfe, P.A.; Gifford, D.K.; Fink, G.R. Control of Transcription by Cell Size. PLoS Biol. 2010, 8, e1000523. [Google Scholar] [CrossRef]
- Marguerat, S.; Bähler, J. Coordinating genome expression with cell size. Trends Genet. 2012, 28, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Coate, J.E.; Doyle, J.J. Variation in transcriptome size: Are we getting the message? Chromosoma 2015, 124, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Leitch, I.J.; Bennett, M.D. Genome downsizing in polyploid plants. Biol. J. Linn. Soc. 2004, 82, 651–663. [Google Scholar] [CrossRef]
- Zenil-Ferguson, R.; Ponciano, J.M.; Burleigh, J.G. Evaluating the role of genome downsizing and size thresholds from genome size distributions in angiosperms. Am. J. Bot. 2016, 103, 1175–1186. [Google Scholar] [CrossRef]
- Pellicer, J.; Hidalgo, O.; Dodsworth, S.; Leitch, I.J. Genome Size Diversity and Its Impact on the Evolution of Land Plants. Genes 2018, 9, 88. [Google Scholar] [CrossRef]
- Cheng, F.; Wu, J.; Cai, X.; Liang, J.; Freeling, M.; Wang, X. Gene retention, fractionation and subgenome differences in polyploid plants. Nat. Plants 2018, 4, 258–268. [Google Scholar] [CrossRef]
- Baduel, P.; Quadrana, L.; Hunter, B.; Bomblies, K.; Colot, V. Relaxed purifying selection in autopolyploids drives transposable element over-accumulation which provides variants for local adaptation. Nat. Commun. 2019, 10, 5818. [Google Scholar] [CrossRef]
- Raina, S.N.; Parida, A.; Koul, K.K.; Salimath, S.S.; Bisht, M.S.; Raja, V.; Khoshoo, T.N. Associated chromosomal DNA changes in polyploids. Genome 1994, 37, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Lovén, J.; Orlando, D.A.; Sigova, A.A.; Lin, C.Y.; Rahl, P.B.; Burge, C.B.; Levens, D.L.; Lee, T.I.; Young, R.A. Revisiting Global Gene Expression Analysis. Cell 2012, 151, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, M.; Pirrello, J.; Cheniclet, C.; Coriton, O.; Bourge, M.; Brown, S.; Moïse, A.; Peypelut, M.; Rouyère, V.; Renaudin, J.-P.; et al. Evidence for karyoplasmic homeostasis during endoreduplication and a ploidy-dependent increase in gene transcription during tomato fruit growth. Development 2012, 139, 3817–3826. [Google Scholar] [CrossRef] [PubMed]
- Voichek, Y.; Bar-Ziv, R.; Barkai, N. Expression homeostasis during DNA replication. Science 2016, 351, 1087–1090. [Google Scholar] [CrossRef] [PubMed]

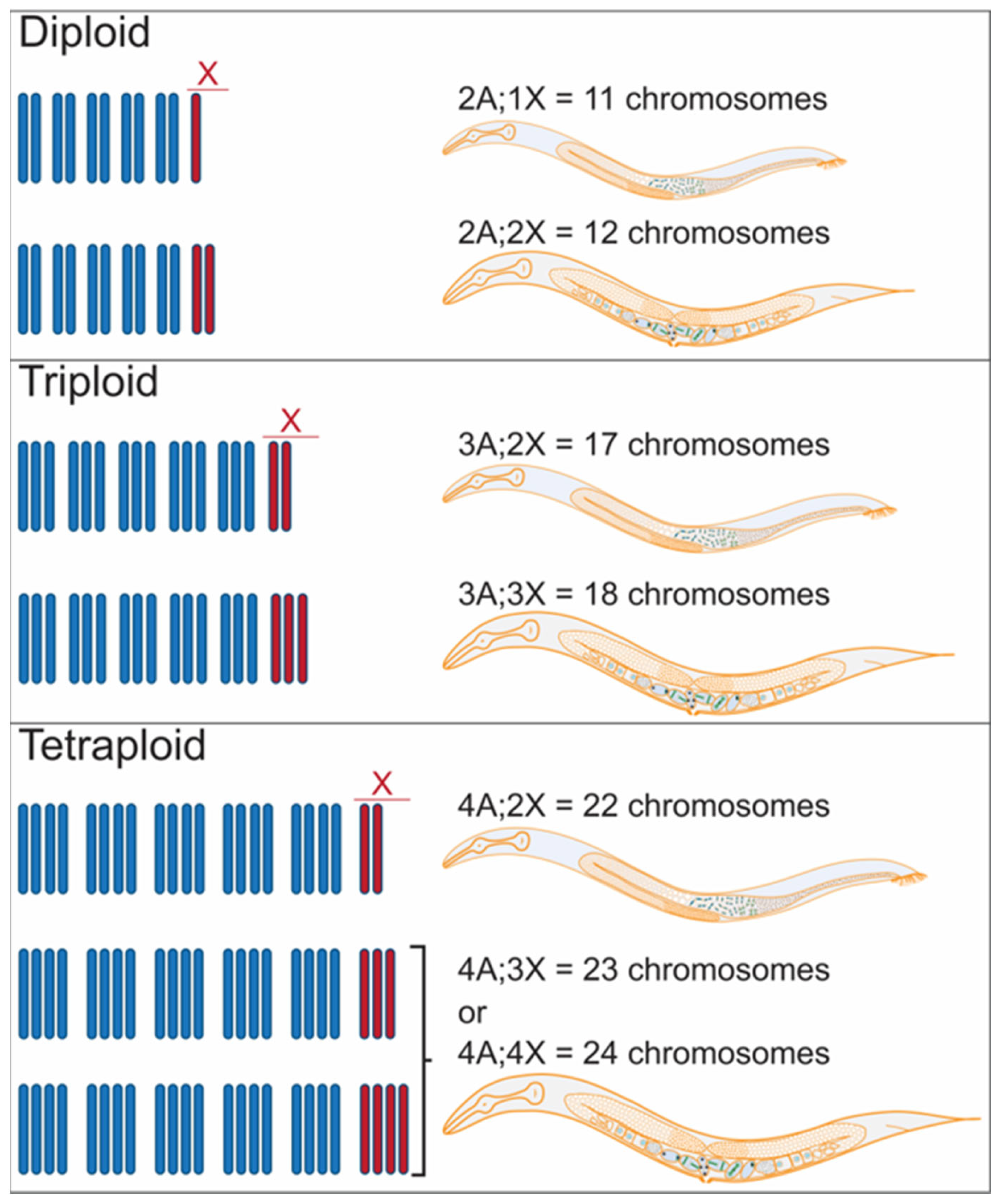

| Karyotype | X/A Ratio | Sexual Fate |
|---|---|---|
| 2A;2X | 1 | female |
| 2A;1X | 0.5 | male |
| 3A;3X | 1 | female |
| 3A;2X | 0.67 * | Male |
| 4A;4X | 1 | female |
| 4A;3X | 0.75 * | female |
| 4A;2X | 0.5 | male |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schvarzstein, M.; Alam, F.; Toure, M.; Yanowitz, J.L. An Emerging Animal Model for Querying the Role of Whole Genome Duplication in Development, Evolution, and Disease. J. Dev. Biol. 2023, 11, 26. https://doi.org/10.3390/jdb11020026
Schvarzstein M, Alam F, Toure M, Yanowitz JL. An Emerging Animal Model for Querying the Role of Whole Genome Duplication in Development, Evolution, and Disease. Journal of Developmental Biology. 2023; 11(2):26. https://doi.org/10.3390/jdb11020026
Chicago/Turabian StyleSchvarzstein, Mara, Fatema Alam, Muhammad Toure, and Judith L. Yanowitz. 2023. "An Emerging Animal Model for Querying the Role of Whole Genome Duplication in Development, Evolution, and Disease" Journal of Developmental Biology 11, no. 2: 26. https://doi.org/10.3390/jdb11020026
APA StyleSchvarzstein, M., Alam, F., Toure, M., & Yanowitz, J. L. (2023). An Emerging Animal Model for Querying the Role of Whole Genome Duplication in Development, Evolution, and Disease. Journal of Developmental Biology, 11(2), 26. https://doi.org/10.3390/jdb11020026






