Osteoderm Development during the Regeneration Process in Eurylepis taeniolata Blyth, 1854 (Scincidae, Sauria, Squamata)
Abstract
1. Introduction
2. Materials and Methods
3. Results
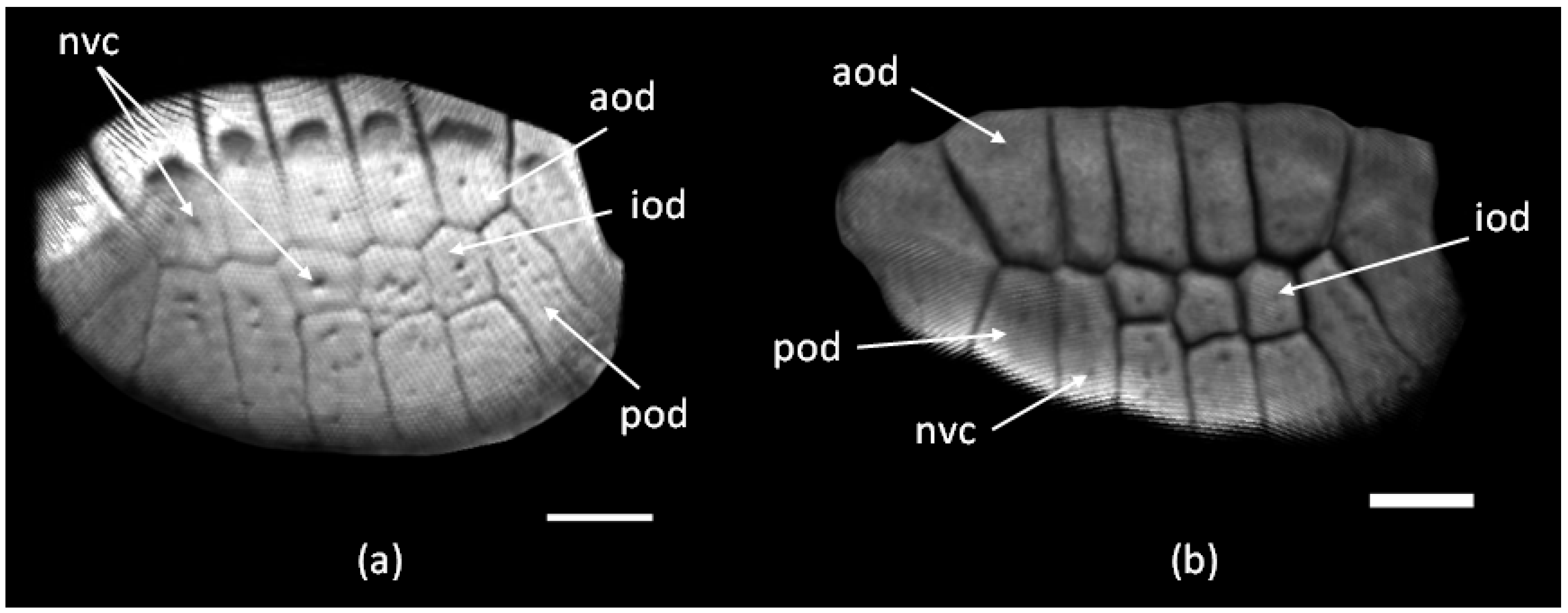
3.1. The Structure of the Original Osteoderms
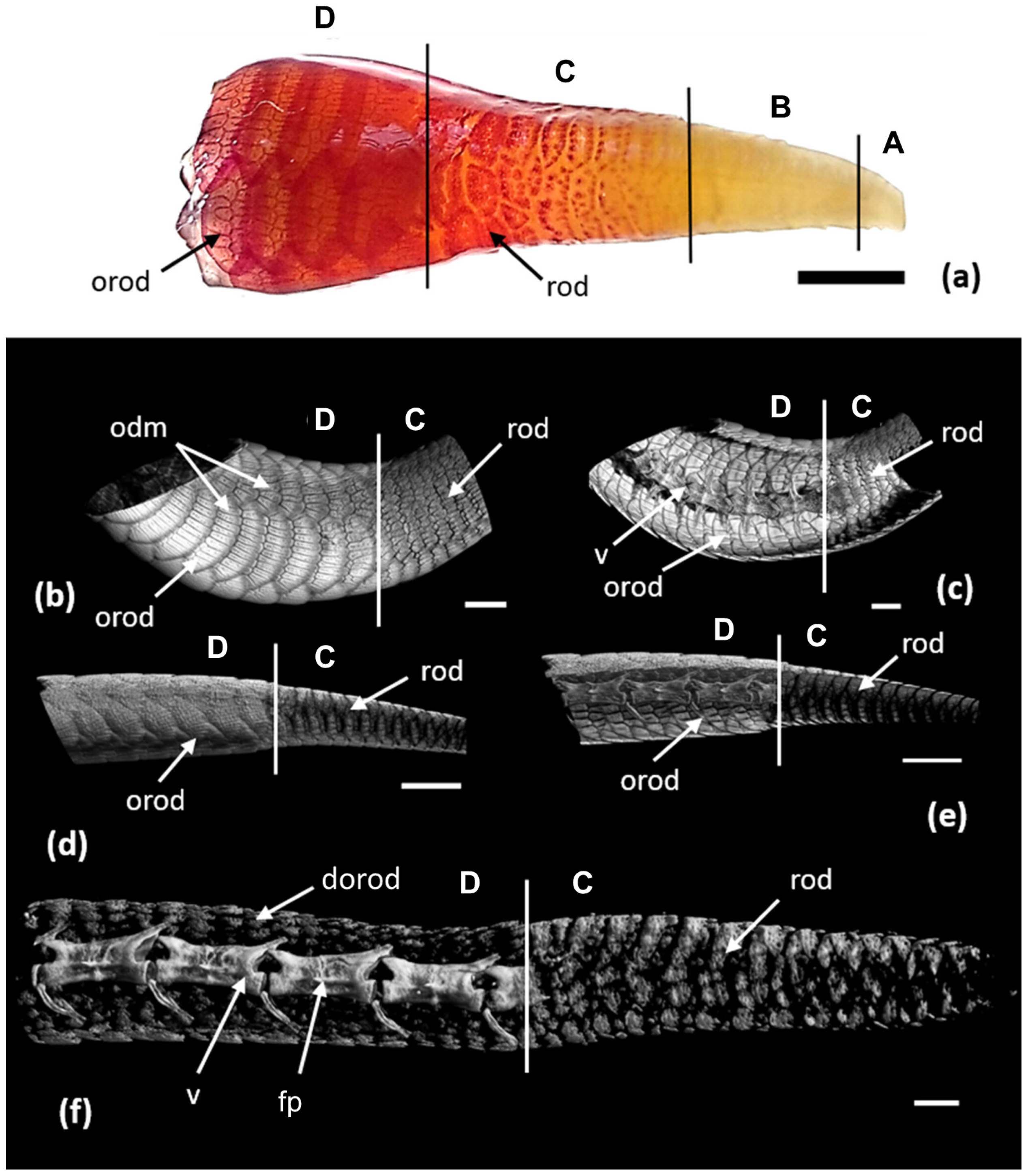
3.2. Development of the Integument during Regeneration and Zoning of the Tail Regenerate
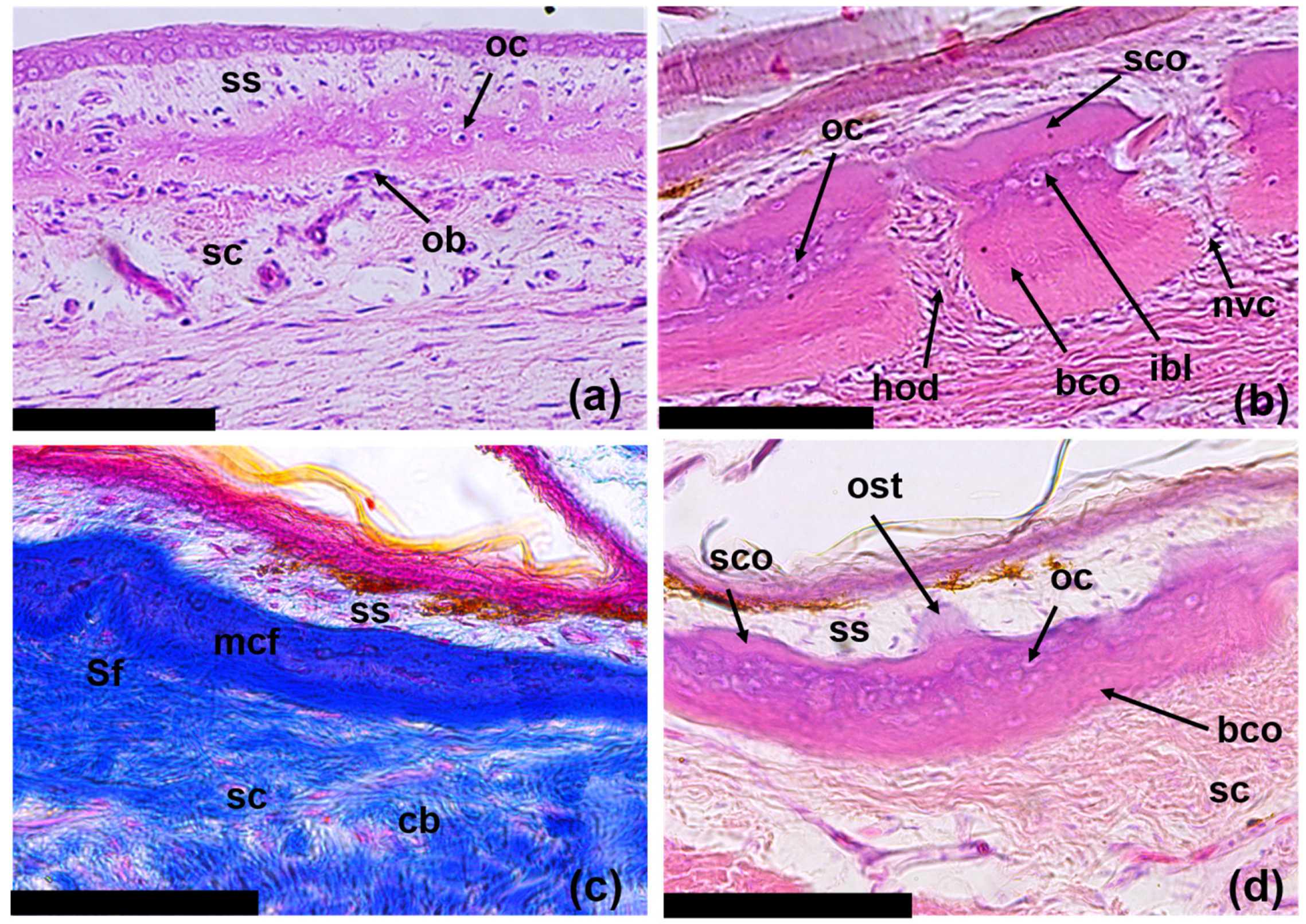
3.3. Regeneration of Osteoderms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paluh, D.J.; Griffing, A.H.; Bauer, A.M. Sheddable Armour: Identification of Osteoderms in the Integument of Geckolepis maculata (Gekkota). Afr. J. Herpetol. 2017, 66, 12–24. [Google Scholar] [CrossRef]
- Marghoub, A.; Williams, C.J.A.; Leite, J.V.; Kirby, A.C.; Kéver, L.; Porro, L.B.; Barrett, P.M.; Bertazzo, S.; Abzhanov, A.; Vickaryous, M.; et al. Unravelling the Structural Variation of Lizard Osteoderms. Acta Biomater. 2022, 146, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Vickaryous, M.; Williams, C.; Willan, G.; Kirby, A.; Herrel, A.; Kever, L.; Moazen, M.; Marghoub, A.; Rai, S.; Abzhanov, A.; et al. Histological Diversity and Evolution of Lizard Osteoderms. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Moss, M.L. Comparative Histology of Dermal Sclerifications in Reptiles. Acta Anat. 1969, 73, 510–533. [Google Scholar] [CrossRef]
- Bochaton, C.; De Buffrenil, V.; Lemoine, M.; Bailon, S.; Ineich, I. Body Location and Tail Regeneration Effects on Osteoderms Morphology—Are They Useful Tools for Systematic, Paleontology, and Skeletochronology in Diploglossine Lizards (Squamata, Anguidae)? J. Morphol. 2015, 276, 1333–1344. [Google Scholar] [CrossRef]
- Kirby, A.; Vickaryous, M.; Boyde, A.; Olivo, A.; Moazen, M.; Bertazzo, S.; Evans, S. A Comparative Histological Study of the Osteoderms in the Lizards Heloderma suspectum (Squamata: Helodermatidae) and Varanus komodoensis (Squamata: Varanidae). J. Anat. 2020, 236, 1035–1043. [Google Scholar] [CrossRef]
- Liang, C.; Marghoub, A.; Kéver, L.; Bertazzo, S.; Abzhanov, A.; Vickaryous, M.; Herrel, A.; Evans, S.; Moazen, M. Lizard Osteoderms-Morphological Characterisation, Biomimetic Design and Manufacturing Based on Three Species. Bioinspir. Biomim. 2021, 16, 066011. [Google Scholar] [CrossRef]
- Williams, C.; Kirby, A.; Marghoub, A.; Kéver, L.; Ostashevskaya-Gohstand, S.; Bertazzo, S.; Moazen, M.; Abzhanov, A.; Herrel, A.; Evans, S.E.; et al. A Review of the Osteoderms of Lizards (Reptilia: Squamata). Biol. Rev. 2022, 97, 1–19. [Google Scholar] [CrossRef]
- Schmidt, W.J. Studien Am Integument Der Reptilien. IV. Die Haut der Gerrhosauridae. Zool. Jahrbucher. Abt. Anat. Ontog. Tiere 1913, 36, 377–464. [Google Scholar]
- Laver, R.J.; Morales, C.H.; Heinicke, M.P.; Gamble, T.; Longoria, K.; Bauer, A.M.; Daza, J.D. The Development of Cephalic Armor in the Tokay Gecko (Squamata: Gekkonidae: Gekko Gecko). J. Morphol. 2020, 281, 213–228. [Google Scholar] [CrossRef]
- Frýdlová, P.; Janovská, V.; Mrzílková, J.; Halašková, M.; Riegerová, M.; Dudák, J.; Tymlová, V.; Žemlička, J.; Zach, P.; Frynta, D. The First Description of Dermal Armour in Snakes. Sci. Rep. 2023, 13, 6405. [Google Scholar] [CrossRef] [PubMed]
- Camp, C.L. Classification of the Lizards. Bull. Am. Mus. Nat. Hist. 1923, 18, 289–481. [Google Scholar]
- Vickaryous, M.K.; Sire, J.-Y. The Integumentary Skeleton of Tetrapods: Origin, Evolution, and Development. J. Anat. 2009, 214, 441–464. [Google Scholar] [CrossRef] [PubMed]
- Canei, J.; Nonclercq, D. Morphological Study of the Integument and Corporal Skeletal Muscles of Two Psammophilous Members of Scincidae (Scincus scincus and Eumeces schneideri). J. Morphol. 2021, 282, 230–246. [Google Scholar] [CrossRef] [PubMed]
- de Buffrénil, V.; Dauphin, Y.; Rage, J.-C.; Sire, J.-Y. An Enamel-like Tissue, Osteodermine, on the Osteoderms of a Fossil Anguid (Glyptosaurinae) Lizard. C. R. Palevol 2011, 10, 427–437. [Google Scholar] [CrossRef]
- Vickaryous, M.K.; Meldrum, G.; Russell, A.P. Armored Geckos: A Histological Investigation of Osteoderm Development in Tarentola (Phyllodactylidae) and Gekko (Gekkonidae) with Comments on Their Regeneration and Inferred Function. J. Morphol. 2015, 276, 1345–1357. [Google Scholar] [CrossRef]
- Iacoviello, F.; Kirby, A.C.; Javanmardi, Y.; Moeendarbary, E.; Shabanli, M.; Tsolaki, E.; Sharp, A.C.; Hayes, M.J.; Keevend, K.; Li, J.-H.; et al. The Multiscale Hierarchical Structure of Heloderma Suspectum Osteoderms and Their Mechanical Properties. Acta Biomater. 2020, 107, 194–203. [Google Scholar] [CrossRef]
- de Buffrénil, V.; Sire, J.-Y.; Rage, J.-C. The Histological Structure of Glyptosaurine Osteoderms (Squamata: Anguidae), and the Problem of Osteoderm Development in Squamates. J. Morphol. 2010, 271, 729–737. [Google Scholar] [CrossRef]
- Schmidt, W.J. Beobactungen an der Haut von Geckolepis und Einigen Anderen Geckoniden. In Reise in Ostafrika in den Jahren 1903–1905 mit Mitteln der Hermann und Elise geb. Hickman Wentzel-Stiftung Ausgeführt Wissenschaftliche Ergebniss von Alfred Voeltzkkow; Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1911; pp. 331–352. [Google Scholar]
- Schmidt, W.J. Studien am Integument der Reptilien. I. Die Haut der Geckoniden. Z. Wiss. Zool. 1912, 101, 139–258. [Google Scholar]
- Schmidt, W.J. Studien am Integument der Reptilien. V. Anguiden. Zool. Jahrbucher. Abt. Anat. Ontog. Tiere 1914, 38, 2–102. [Google Scholar]
- Bryant, S.V.; Bellairs, A.D. Tail Regeneration in the Lizards Anguis fragilis and Lacerta dugesii. J. Linn. Soc. 1967, 46, 297–305. [Google Scholar] [CrossRef]
- Hardaway, T.E.; Williams, K.L. A Procedure for Double Staining Cartilage and Bone. Br. J. Herpetol. 1975, 5, 473–474. [Google Scholar]





| SPbU Numbers | Individual Numbers (Generation Numbers) | Regenerate Length (mm) | Regenerate Age (Month) | Character of Section | Number of Slides |
|---|---|---|---|---|---|
| 1773 | 10 (1) | 3 | 7.5 | Longitudinal | 3 |
| 1774 | 10 (2) | 5 | 2 | Longitudinal | 8 |
| 1775 | 11 (1) | 5.5 | 8 | Longitudinal | 6 |
| 1776 | 7 (1) | 7 | 4 | Longitudinal | 7 |
| 1777 | 5 (1) | 12.5 | 9.5 | Longitudinal | 8 |
| 1779 | 1 (2) | 27 | 3.5 | Transversal | 3 |
| Longitudinal | 10 | ||||
| 1780 | 1 (1) | 49 | 10 | Transverse | 1 |
| 1781 | 2 (1) | 28 | 6 | Longitudinal | 13 |
| 1782 | 4 (1) | 37 | 10 | Longitudinal | 5 |
| ZISP 18967 Numbers | Object to Source (mm) | Source Voltage (kV) | Source Current (uA) | Camera Exposure (ms) | Filter | Image Pixel Size (μm) |
|---|---|---|---|---|---|---|
| R-5328-2, male | 63.071456 | 67 | 59 | 178 | Al 0.5 mm | 12.915298 |
| R-5327-8, female | 50.457308 | 58 | 68 | 127 | Al 0.25 mm | 10.332268 |
| R-5334-5, female | 36.040268 | 58 | 68 | 127 | Al 0.25 mm | 7.380055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherepanov, G.O.; Gordeev, D.A.; Melnikov, D.A.; Ananjeva, N.B. Osteoderm Development during the Regeneration Process in Eurylepis taeniolata Blyth, 1854 (Scincidae, Sauria, Squamata). J. Dev. Biol. 2023, 11, 22. https://doi.org/10.3390/jdb11020022
Cherepanov GO, Gordeev DA, Melnikov DA, Ananjeva NB. Osteoderm Development during the Regeneration Process in Eurylepis taeniolata Blyth, 1854 (Scincidae, Sauria, Squamata). Journal of Developmental Biology. 2023; 11(2):22. https://doi.org/10.3390/jdb11020022
Chicago/Turabian StyleCherepanov, Gennady O., Dmitry A. Gordeev, Daniel A. Melnikov, and Natalia B. Ananjeva. 2023. "Osteoderm Development during the Regeneration Process in Eurylepis taeniolata Blyth, 1854 (Scincidae, Sauria, Squamata)" Journal of Developmental Biology 11, no. 2: 22. https://doi.org/10.3390/jdb11020022
APA StyleCherepanov, G. O., Gordeev, D. A., Melnikov, D. A., & Ananjeva, N. B. (2023). Osteoderm Development during the Regeneration Process in Eurylepis taeniolata Blyth, 1854 (Scincidae, Sauria, Squamata). Journal of Developmental Biology, 11(2), 22. https://doi.org/10.3390/jdb11020022








