A Spatial Agent-Based Model to Assess the Spread of Malaria in Relation to Anti-Malaria Interventions in Southeast Iran
Abstract
:1. Introduction
2. Materials and Methods
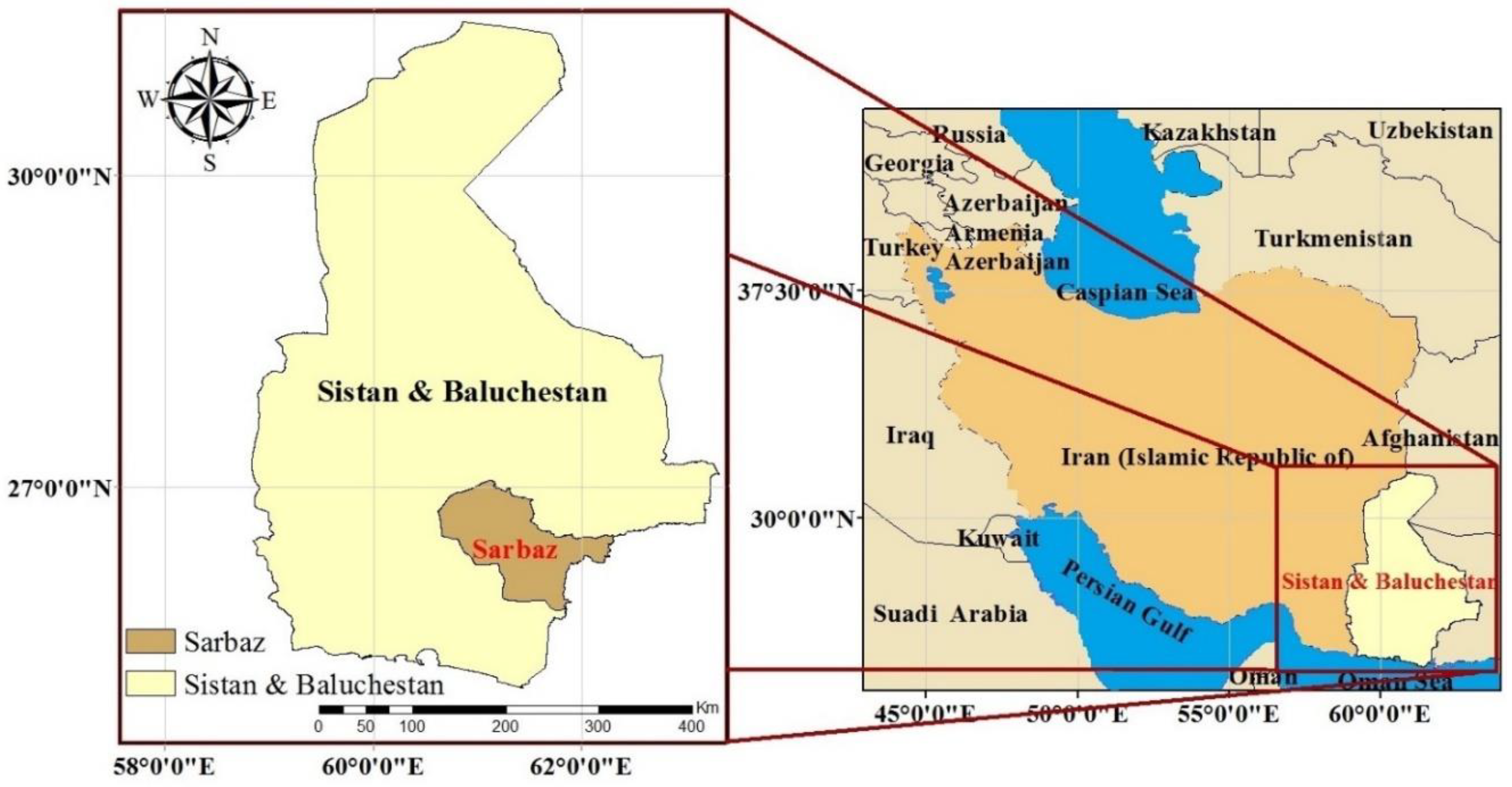
2.1. Study Area
2.2. Data and Preprocessing of the Input Data
2.3. ABM Development
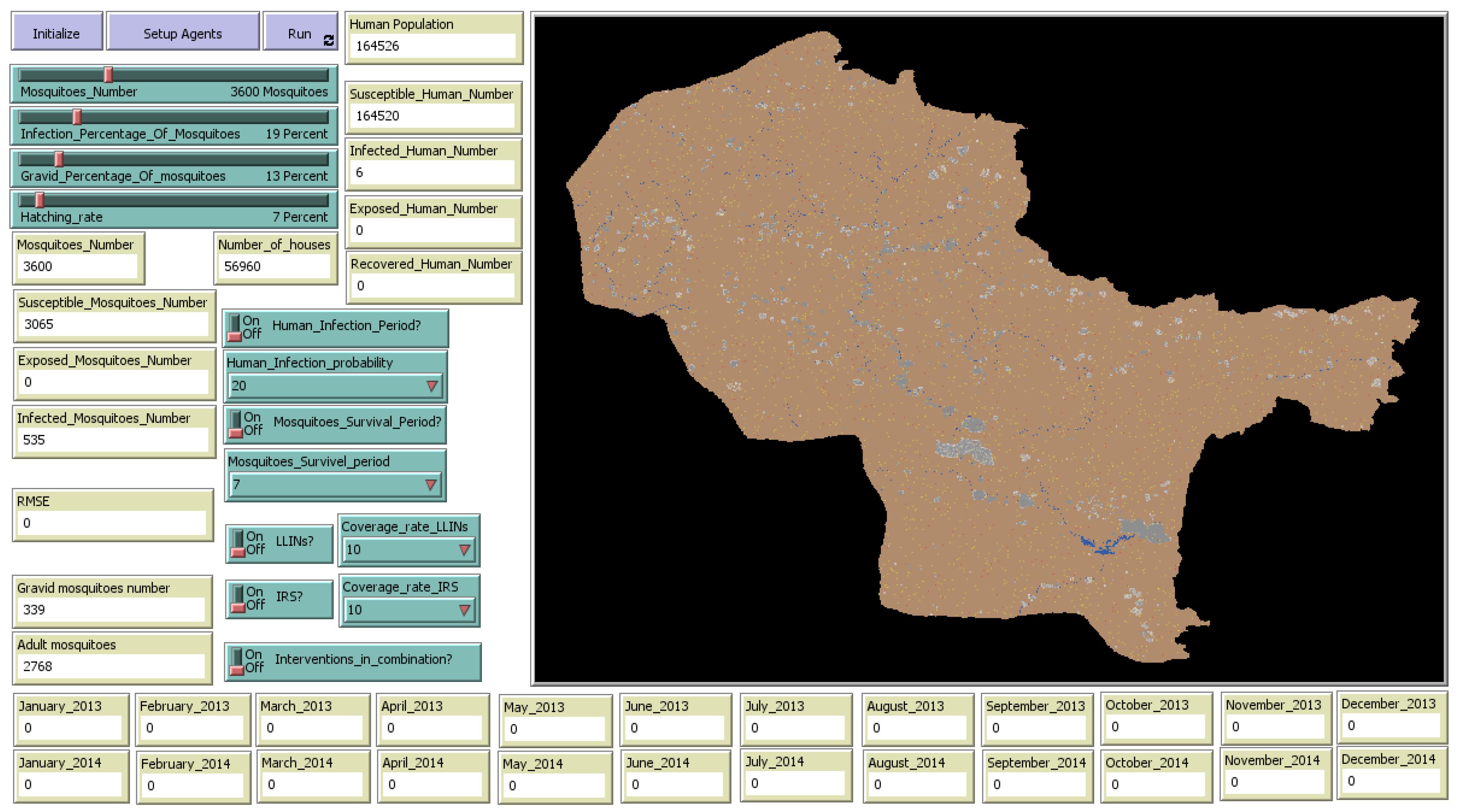
2.3.1. Environment
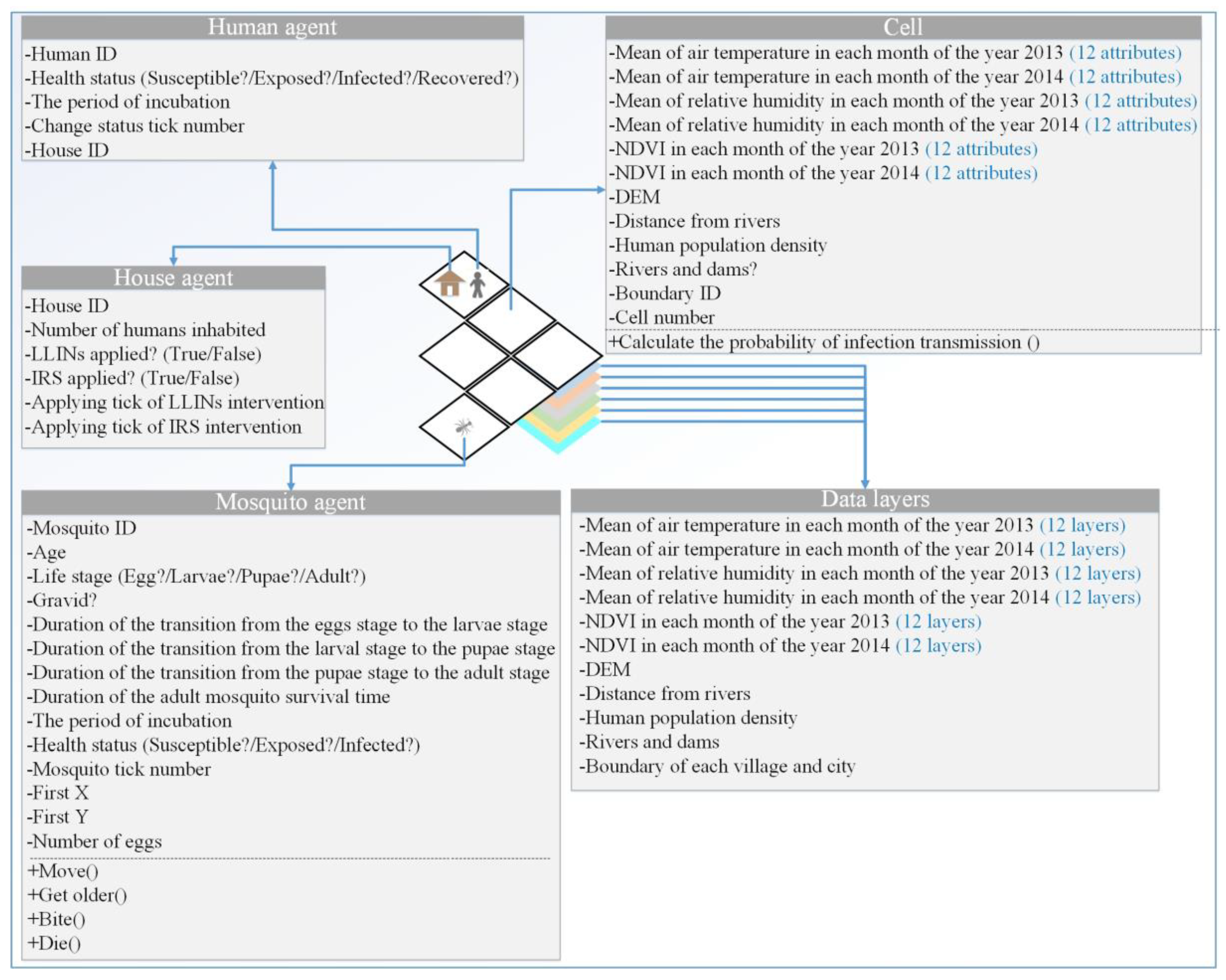
2.3.2. Agents
1. Anopheles Mosquito Agents
2. Human Agents
2.3.3. Transmission of Malaria
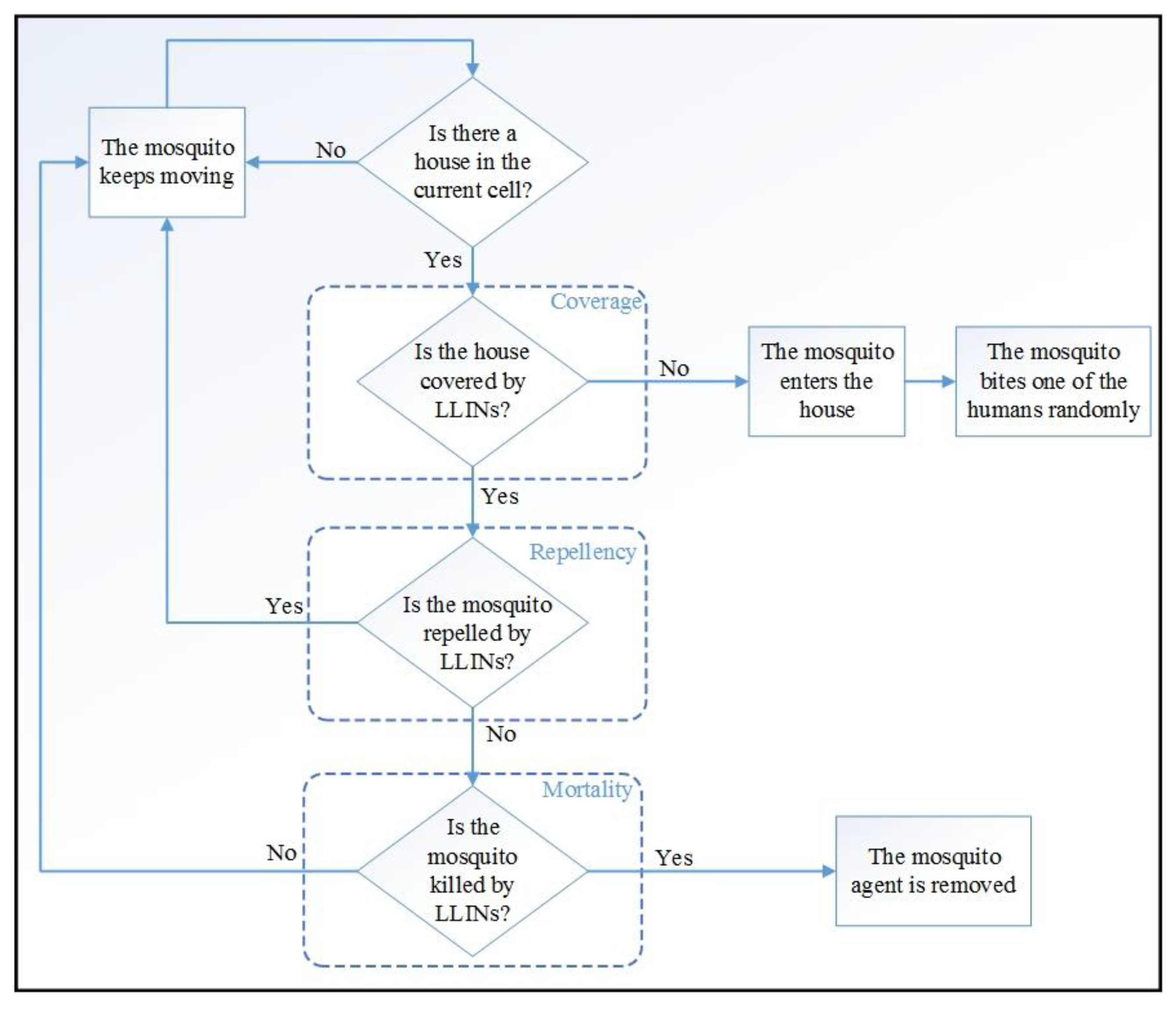
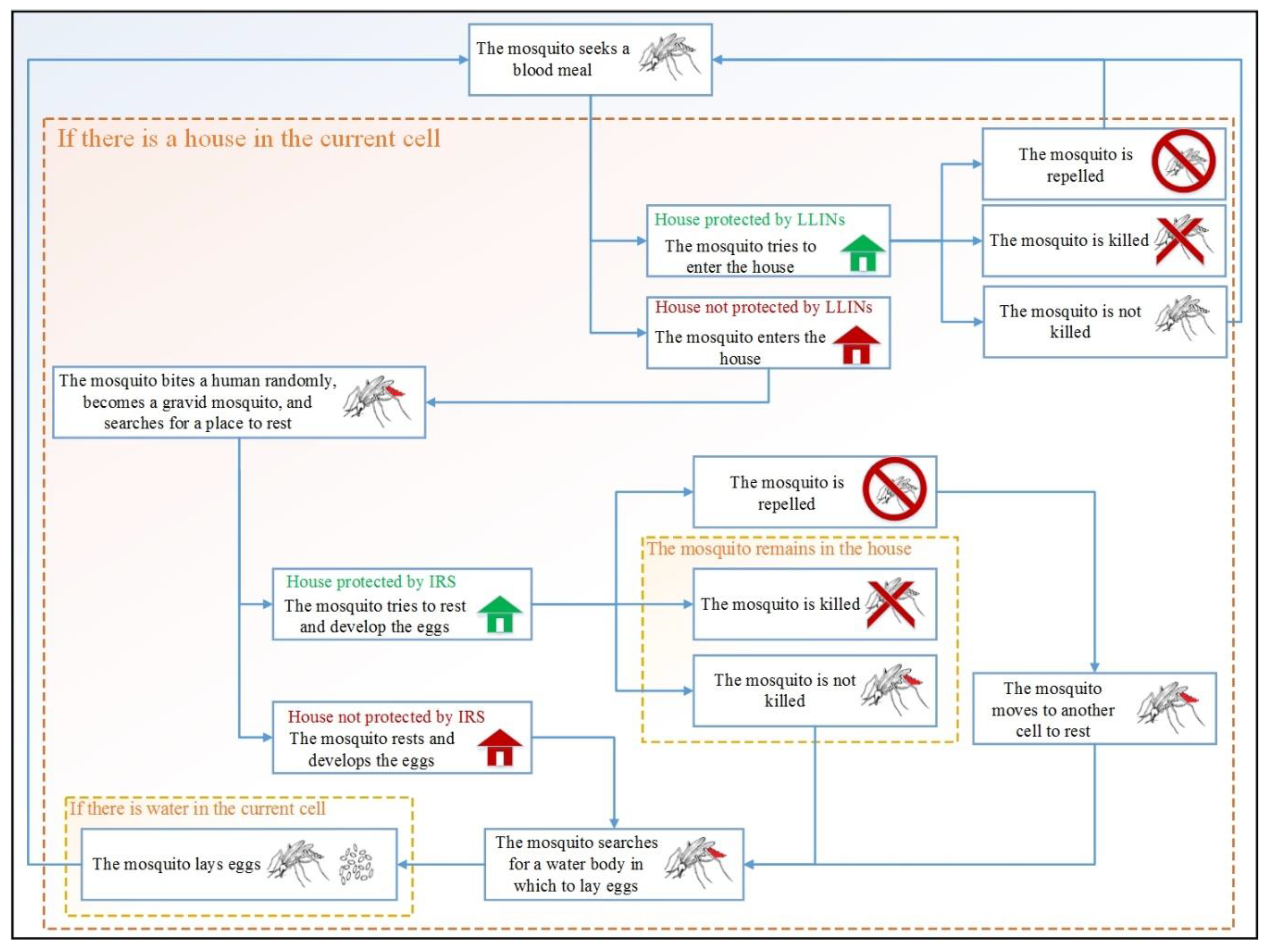
2.3.4. Malaria Interventions
1. Applying Long-Lasting Insecticidal Nets (LLINs) Separately
2. Applying Indoor Residual Spraying (IRS) Separately
3. Applying LLINs and IRS in Combination
2.3.5. Verification, Calibration, and Validation
3. Results
3.1. Model Verification
3.2. Model Calibration and Validation
3.3. Investigating the Number of Infected Human Agents When Applying Control Interventions
3.4. Strengths and Limitations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carter, R.; Mendis, K.N. Evolutionary and Historical Aspects of the Burden of Malaria. Clin. Microbiol. Rev. 2002, 15, 564–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkumar, S.; Jebanesan, A.; Nagarajan, R. Effect of leaf essential oil of Coccinia indica on egg hatchability and different larval instars of malarial mosquito Anopheles stephensi. Asian Pac. J. Trop. Med. 2011, 4, 948–951. [Google Scholar] [CrossRef] [Green Version]
- Akbari, H.; Majdzadeh, R.; Foroushani, A.R.; Raeisi, A. Timeliness of Malaria Surveillance System in Iran. Iran. J. Public Health 2013, 42, 39–47. [Google Scholar] [PubMed]
- World Health Organization (WHO); United Nations Children’s Fund (UNICEF). Achieving the Malaria MDG Target: Reversing the Incidence of Malaria 2000–2015. 2015. Available online: https://www.who.int/malaria/publications/atoz/9789241509442/en/ (accessed on 13 February 2020).
- World Health Organization (WHO). World Malaria Report World Health Organization. 2016. Available online: https://www.who.int/malaria/publications/world-malaria-report-2015/report/en/ (accessed on 13 February 2020).
- McKelvie, W.R.; Haghdoost, A.; Raeisi, A. Defining and detecting malaria epidemics in south-east Iran. Malar. J. 2012, 11, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikhzadeh, K.; Haghdoost, A.; Bahrampour, A.; Raeisi, A.; Zolala, F.; Farzadfar, F.; Kasaeian, A.; Parsaeian, M. Predicting Malaria Transmission Risk in Endemic Areas of Iran: A Multilevel Modeling Using Climate and Socioeconomic Indicators. Iran. Red Crescent Med. J. 2017, 19, 45132. [Google Scholar] [CrossRef]
- Gharakhanlou, N.M.; Mesgari, M.S.; Hooshangi, N. Developing an agent-based model for simulating the dynamic spread of Plasmodium vivax malaria: A case study of Sarbaz, Iran. Ecol. Inform. 2019, 54, 101006. [Google Scholar] [CrossRef]
- Fillinger, U.; Lindsay, S.W. Larval source management for malaria control in Africa: Myths and reality. Malar. J. 2011, 10, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.Z.; Arifin, S.N.; Al-Amin, H.M.; Alam, M.S.; Rahman, M.S. A spatial agent-based model of Anopheles vagus for malaria epidemiology: Examining the impact of vector control interventions. Malar. J. 2017, 16, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbel, V.; Akogbeto, M.; Damien, G.B.; Djènontin, A.; Chandre, F.; Rogier, C.; Moiroux, N.; Chabi, J.; Banganna, B.; Padonou, G.G.; et al. Combination of malaria vector control interventions in pyrethroid resistance area in Benin: A cluster randomised controlled trial. Lancet Infect. Dis. 2012, 12, 617–626. [Google Scholar] [CrossRef]
- Yakob, L.; Yan, G. Modeling the effects of integrating larval habitat source reduction and insecticide treated nets for malaria control. PLoS ONE 2009, 4, e6921. [Google Scholar] [CrossRef]
- Alam, M.S.; Chakma, S.; Khan, W.A.; Glass, G.E.; Mohon, A.N.; Elahi, R.; Norris, L.C.; Podder, M.P.; Ahmed, S.; Haque, R.; et al. Diversity of anopheline species and their Plasmodium infection status in rural Bandarban, Bangladesh. Parasites Vectors 2012, 5, 150. [Google Scholar] [CrossRef] [Green Version]
- Kesteman, T.; Randrianarivelojosia, M.; Raharimanga, V.; Randrianasolo, L.; Piola, P.; Rogier, C. Effectiveness of malaria control interventions in Madagascar: A nationwide case-control survey. Malar. J. 2016, 15, 83. [Google Scholar] [CrossRef] [Green Version]
- Okumu, F.O.; Chipwaza, B.; Madumla, E.P.; Mbeleya, E.; Lingamba, G.; Moore, J.; Ntamatungro, A.J.; Kavishe, D.R.; Moore, S.J. Implications of bio-efficacy and persistence of insecticides when indoor residual spraying and long-lasting insecticide nets are combined for malaria prevention. Malar. J. 2012, 11, 378. [Google Scholar] [CrossRef] [Green Version]
- Chitnis, N.; Schapira, A.; Smith, T.; Steketee, R. Comparing the effectiveness of malaria vector-control interventions through a mathematical model. Am. J. Trop. Med. Hyg. 2010, 83, 230–240. [Google Scholar] [CrossRef] [Green Version]
- Giardina, F.; Kasasa, S.; Sié, A.; Utzinger, J.; Tanner, M.; Vounatsou, P. Effects of vector-control interventions on changes in risk of malaria parasitaemia in sub-Saharan Africa: A spatial and temporal analysis. Lancet Glob. Health 2014, 2, e601–e615. [Google Scholar] [CrossRef] [Green Version]
- Lwin, M.M.; Sudhinaraset, M.; San, A.K.; Aung, T. Improving malaria knowledge and practices in rural Myanmar through a village health worker intervention: A cross-sectional study. Malar. J. 2014, 13, 5. [Google Scholar] [CrossRef] [Green Version]
- Bowen, H.L. Impact of a mass media campaign on bed net use in Cameroon. Malar. J. 2013, 12, 36. [Google Scholar] [CrossRef] [Green Version]
- Fillinger, U.; Ndenga, B.; Githeko, A.; Lindsay, S.W. Integrated malaria vector control with microbial larvicides and insecticide-treated nets in western Kenya: A controlled trial. Bull. World Health Organ. 2009, 87, 655–665. [Google Scholar] [CrossRef]
- Rao, D.M.; Chernyakhovsky, A.; Rao, V. Modeling and analysis of global epidemiology of avian influenza. Environ. Model. Softw. 2009, 24, 124–134. [Google Scholar] [CrossRef]
- Pérez, L.; Dragićević, S. An agent-based approach for modeling dynamics of contagious disease spread. Int. J. Health Geogr. 2009, 8, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajelli, M.; Merler, S. An individual-based model of hepatitis A transmission. J. Theor. Boil. 2009, 259, 478–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchnita, A.; Jebrane, A. A hybrid multi-scale model of COVID-19 transmission dynamics to assess the potential of non-pharmaceutical interventions. Chaos Solitons Fractals 2020, 138, 109941. [Google Scholar] [CrossRef] [PubMed]
- Gharakhanlou, N.M.; Hooshangi, N. Spatio-temporal simulation of the novel coronavirus (COVID-19) outbreak using the agent-based modeling approach (case study: Urmia, Iran). Inform. Med. Unlocked 2020, 20, 100403. [Google Scholar] [CrossRef]
- Linard, C.; Ponçon, N.; Fontenille, D.; Lambin, E.F. A multi-agent simulation to assess the risk of malaria re-emergence in southern France. Ecol. Model. 2009, 220, 160–174. [Google Scholar] [CrossRef]
- Arifin, S.; Arifin, R.R.; Pitts, D.d.A.; Rahman, M.S.; Nowreen, S.; Madey, G.R.; Collins, F.H. Landscape Epidemiology Modeling Using an Agent-Based Model and a Geographic Information System. Land 2015, 4, 378–412. [Google Scholar] [CrossRef] [Green Version]
- Arifin, S.N.; Madey, G.R.; Collins, F.H. Examining the impact of larval source management and insecticide-treated nets using a spatial agent-based model of Anopheles gambiae and a landscape generator tool. Malar. J. 2013, 12, 290. [Google Scholar] [CrossRef]
- Griffin, J.T.; Bhatt, S.; Sinka, M.E.; Gething, P.W.; Lynch, M.; Patouillard, E.; Shutes, E.; Newman, R.D.; Alonso, P.; Cibulskis, R.E.; et al. Potential for reduction of burden and local elimination of malaria by reducing Plasmodium falciparum malaria transmission: A mathematical modelling study. Lancet Infect. Dis. 2016, 16, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Quan-Xing, L.; Zhen, J. Cellular automata modelling of SEIRS. Chin. Phys. 2005, 14, 1370–1377. [Google Scholar] [CrossRef]
- White, S.H.; Del Rey, A.M.; Sánchez, G.R. Modeling epidemics using cellular automata. Appl. Math. Comput. 2007, 186, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Sirakoulis, G.C.; Karafyllidis, I.; Thanailakis, A. A cellular automaton model for the effects of population movement and vaccination on epidemic propagation. Ecol. Model. 2000, 133, 209–223. [Google Scholar] [CrossRef]
- Gu, W.; Novak, R.J. Predicting the impact of insecticide-treated bed nets on malaria transmission: The devil is in the detail. Malar. J. 2009, 8, 256. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Novak, R.J. Agent-based modelling of mosquito foraging behaviour for malaria control. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 1105–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bomblies, A. Agent-based modeling of malaria vectors: The importance of spatial simulation. Parasites Vectors 2014, 7, 308. [Google Scholar] [CrossRef] [Green Version]
- Norouzinejad, F.; Ghaffari, F.; Raeisi, A. Epidemiological status of malaria in Iran, 2011–2014. Asian Pac. J. Trop. Med. 2016, 9, 1055–1061. [Google Scholar] [CrossRef] [Green Version]
- Kelly-Hope, L.A.; Hemingway, J.; McKenzie, F.E. Environmental factors associated with the malaria vectors Anopheles gambiae and Anopheles funestus in Kenya. Malar. J. 2009, 8, 268. [Google Scholar] [CrossRef] [Green Version]
- Patz, J.A.; Graczyk, T.K.; Geller, N.; Vittor, A.Y. Effects of environmental change on emerging parasitic diseases. Int. J. Parasitol. 2000, 30, 1395–1405. [Google Scholar] [CrossRef] [Green Version]
- Kigozi, R.; Zinszer, K.; Mpimbaza, A.; Sserwanga, A.; Kigozi, S.P.; Kamya, M. Assessing temporal associations between environmental factors and malaria morbidity at varying transmission settings in Uganda. Malar. J. 2016, 15, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasyim, H.; Nursafingi, A.; Haque, U.; Montag, D.; Groneberg, D.A.; Dhimal, M.; Kuch, U.; Müller, R. Spatial modelling of malaria cases associated with environmental factors in South Sumatra, Indonesia. Malar. J. 2018, 17, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, M.; Babaeian, E.; Tuller, M.; Jones, S.B. The optical trapezoid model: A novel approach to remote sensing of soil moisture applied to Sentinel-2 and Landsat-8 observations. Remote Sens. Environ. 2017, 198, 52–68. [Google Scholar] [CrossRef] [Green Version]
- Helbich, M. Spatiotemporal Contextual Uncertainties in Green Space Exposure Measures: Exploring a Time Series of the Normalized Difference Vegetation Indices. Int. J. Environ. Res. Public Health 2019, 16, 852. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.R.; Rafique, M.; Rahman, S.U.; Basharat, M.; Shahzadi, C.; Ahmed, I. Geo-spatial analysis of radon in spring and well water using kriging interpolation method. Water Supply 2019, 19, 222–235. [Google Scholar] [CrossRef]
- Binion-Rock, S.M.; Reich, B.J.; Buckel, J.A. A spatial kernel density method to estimate the diet composition of fish. Can. J. Fish. Aquat. Sci. 2019, 76, 249–267. [Google Scholar] [CrossRef]
- Wilensky, U. Center for Connected Learning and Computer-Based Modeling, in NetLogo; Northwestern University: Evanston, IL, USA, 1999. [Google Scholar]
- Mahdizadeh Gharakhanlou, N.; Mesgari, M. Developing a cellular automata model for simulating rainfall-runoff process (case study: Babol catchment). Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2019, 697–702. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention (CDC). Biology of Anopheles Mosquitoes. 2018; Last Updated 14 November 2018. Available online: https://www.cdc.gov/malaria/about/biology/mosquitoes/ (accessed on 11 January 2020).
- Ribeiro, J. Role of saliva in blood-feeding by arthropods. Annu. Rev. Entomol. 1987, 32, 463–478. [Google Scholar] [CrossRef]
- Teknomo, K. Analytic Hierarchy Process (AHP) Tutorial. Available online: https://people.revoledu.com/kardi/tutorial/ (accessed on 24 April 2020).
- Li, J.; Zhao, Y.; Li, S. Fast and Slow dynamics of Malaria model with relapse. Math. Biosci. 2013, 246, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Incubation Period of Malaria. 2019; Last Updated 4 January 2019. Available online: https://www.cdc.gov/malaria/about/disease.html (accessed on 11 January 2020).
- Mbogo, R.W.; Luboobi, L.S.; Odhiambo, J.W. A Stochastic Model for Malaria Transmission Dynamics. J. Appl. Math. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kongmee, M.; Boonyuan, W.; Achee, N.L.; Prabaripai, A.; Lerdthusnee, K.; Chareonviriyaphap, T. Irritant and Repellent Responses of Anopheles harrisoni and Anopheles minimus upon Exposure to Bifenthrin or Deltamethrin Using an Excito-Repellency System and a Live Host. J. Am. Mosq. Control Assoc. 2012, 28, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Khosravani, M.; Rafatpanah, A.; Amiri, S.-A.; Zare, A. The Field Practices of Lambdacyhalothrin and Deltamethrin Insecticides Against Adult Mosquitoes of Anopheles stephensi as the Main Vector of Malaria: Residual Effects. Zahedan J. Res. Med. Sci. 2017, 19, 7629. [Google Scholar] [CrossRef] [Green Version]


| Dataset | Data | Source | ||
|---|---|---|---|---|
| Spatial data | Vector | Point | Mean monthly air temperature in 2013–2014 | Iran Meteorological Organization |
| Mean monthly relative humidity in 2013–2014 | ||||
| Villages and cities in 2013 | National Cartographic Center, Iran | |||
| Polyline | Rivers in 2013 | |||
| Polygon | Boundaries of villages and cities in 2013 | |||
| Dams in 2013 | ||||
| Raster | Monthly normalized differentiated vegetation index (NDVI) in 2013–2014 | Landsat 8 OLI (Operational Land Imager) and TIRS (Thermal Infrared Sensor), downloaded from the website https://earthexplorer.usgs.gov/ | ||
| Digital elevation model (DEM) | Shuttle Radar Topography Mission (SRTM), United States Geological Survey (USGS), downloaded from https://earthexplorer.usgs.gov/ | |||
| Census data | Human population in 2011 | Statistical Center of Iran, downloaded from https://www.amar.org.ir/english/ | ||
| Control interventions notification | Applied malaria control interventions in 2013–2014 | Center for Communicable Disease Management, Ministry of Health and Medical Education, Iran | ||
| Malaria notification | Monthly number of malaria cases in 2012, 2013, and 2014 | Center for Disease Control and Prevention of Sistan and Baluchestan Province, Iran | ||
| Agents | Parameters | Value/Range of Value | Mean | Standard Deviation | Reference |
|---|---|---|---|---|---|
| Anopheles Mosquitoes | Duration of transition from egg stage to larvae stage | 2–3 days | - | - | [47] |
| Duration of transition from larval stage to pupae stage | 5–8 days | 6.5 days | (6.5/8) days | ||
| Duration of transition from pupae stage to adult stage | 2–3 days | - | - | ||
| Duration of adult mosquito’s lifespan | 7–30 days | 18.5 days | (18.5/4) days | ||
| Number of eggs per reproduction | 50–200 eggs | 125 eggs | (125/4) eggs | ||
| Total number of eggs laid by a mosquito during its lifespan | 500 | - | - | ||
| Duration of digesting blood meal and eggs development in mosquito’s body | 2–3 days | - | - | ||
| Duration of exposed state (incubation period) | 10–21 days | 15.5 days | (15.5/4) days | ||
| Probability of becoming exposed | 2% | - | - | [50] | |
| Maximum daily range of movement | 250 m ≈ 10 cell | - | - | [27] | |
| Maximum distance that Anopheles mosquitoes can move away from their habitats (flight range) | 2100 m = 60 cells | - | - | [26] | |
| Humans | Duration of exposed state (incubation period) | 7–30 days | 18.5 days | (18.5/4) days | [51] |
| Probability of a human agent becoming susceptible after having recovered (recovery probability) | (0.01)*(Number of days since human agent recovered) | - | - | [52] | |
| Probability of a human agent recovering (treatment probability) | (0.037)*(Number of days since human agent infected) | - | - |
| Number of Days Elapsed | Mortality Rate Parameter | ||
|---|---|---|---|
| Range of Value [54] | Mean | Standard Deviation | |
| 1–35 | [97.5–100%] | 98.75% | 0.77% |
| 36–49 | [95–97.5%] | 96.25% | 0.75% |
| 50–63 | [89–95%] | 92% | 1.44% |
| 64–77 | [79–89%] | 84% | 1.31% |
| 78–91 | [71.5–79%] | 75.25% | 1.18% |
| 92–105 | [64.5–71.5%] | 68% | 1.06% |
| 106–120 | [58.5–64.5%] | 61.5% | 0.96% |
| >120 | [0–58.5%] | 29.25% | 7.31% |
| Separate Interventions | Coverage Rate | Average Number of Infected Human Agents | Percentage Reduction in the Average Number of Infected Human Agents |
|---|---|---|---|
| LLINs | 10% | 830.02 | 18.957% |
| 25% | 584.81 | 42.899% | |
| 40% | 100.46 | 90.191% | |
| IRS | 10% | 949.31 | 7.309% |
| 25% | 837.54 | 18.222% | |
| 40% | 685.94 | 33.025% |
| Two Interventions in Combination | Coverage Rates | Average Number of Infected Human Agents in 100 Runs | Percentage Reduction in the Average Number of Infected Human Agents after LLIN and IRS Implementation | |
|---|---|---|---|---|
| LLINs | IRS | |||
| LLINs and IRS | 10% | 10% | 769.79 | 24.838% |
| 25% | 678.62 | 33.740% | ||
| 40% | 570.52 | 44.294% | ||
| 25% | 10% | 542.35 | 47.045% | |
| 25% | 463.28 | 54.765% | ||
| 40% | 388.91 | 62.027% | ||
| 40% | 10% | 85.76 | 91.626% | |
| 25% | 59.33 | 94.207% | ||
| 40% | 46.93 | 95.418% | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharakhanlou, N.M.; Hooshangi, N.; Helbich, M. A Spatial Agent-Based Model to Assess the Spread of Malaria in Relation to Anti-Malaria Interventions in Southeast Iran. ISPRS Int. J. Geo-Inf. 2020, 9, 549. https://doi.org/10.3390/ijgi9090549
Gharakhanlou NM, Hooshangi N, Helbich M. A Spatial Agent-Based Model to Assess the Spread of Malaria in Relation to Anti-Malaria Interventions in Southeast Iran. ISPRS International Journal of Geo-Information. 2020; 9(9):549. https://doi.org/10.3390/ijgi9090549
Chicago/Turabian StyleGharakhanlou, Navid Mahdizadeh, Navid Hooshangi, and Marco Helbich. 2020. "A Spatial Agent-Based Model to Assess the Spread of Malaria in Relation to Anti-Malaria Interventions in Southeast Iran" ISPRS International Journal of Geo-Information 9, no. 9: 549. https://doi.org/10.3390/ijgi9090549
APA StyleGharakhanlou, N. M., Hooshangi, N., & Helbich, M. (2020). A Spatial Agent-Based Model to Assess the Spread of Malaria in Relation to Anti-Malaria Interventions in Southeast Iran. ISPRS International Journal of Geo-Information, 9(9), 549. https://doi.org/10.3390/ijgi9090549






