Contribution of Connectivity Assessments to Green Infrastructure (GI)
Abstract
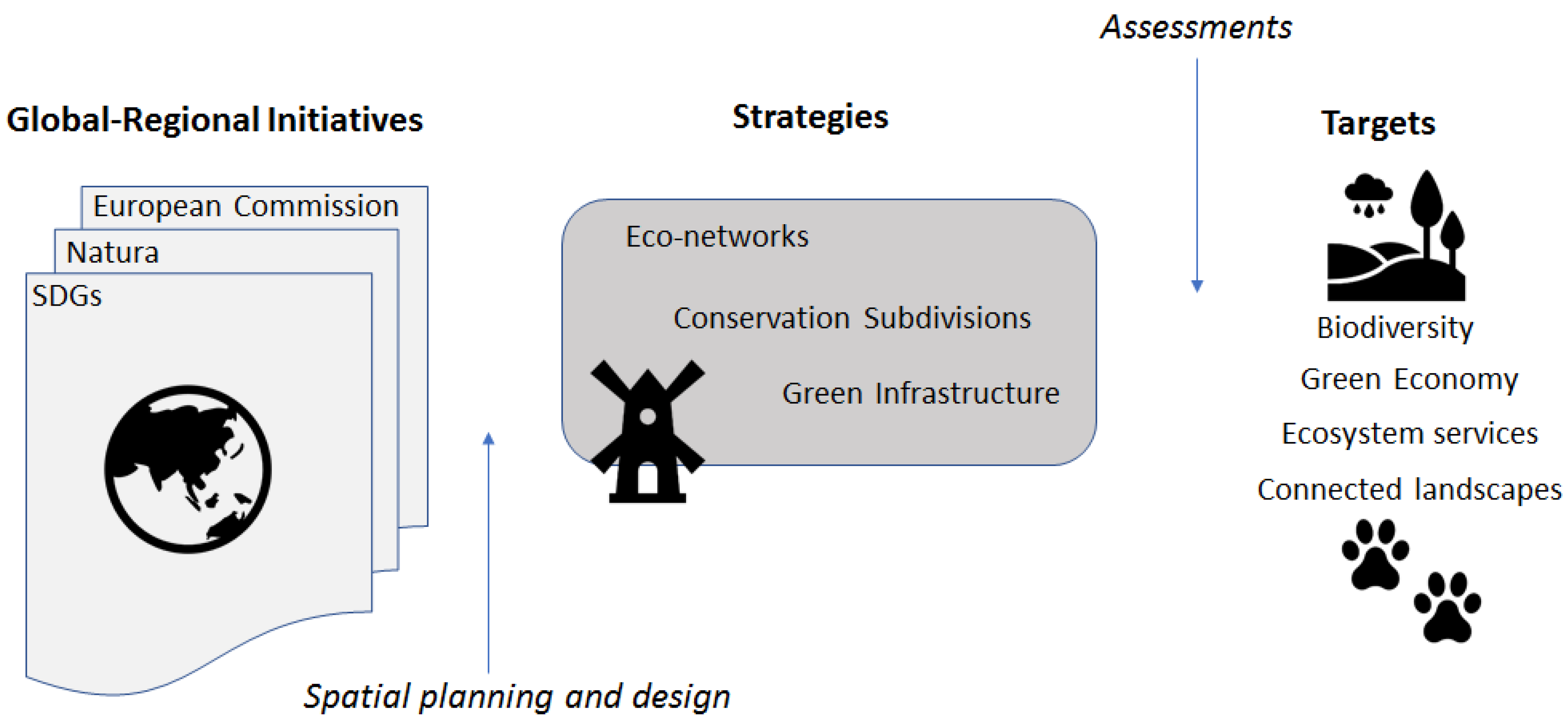
1. Introduction
2. Characterizing Functional Connectivity
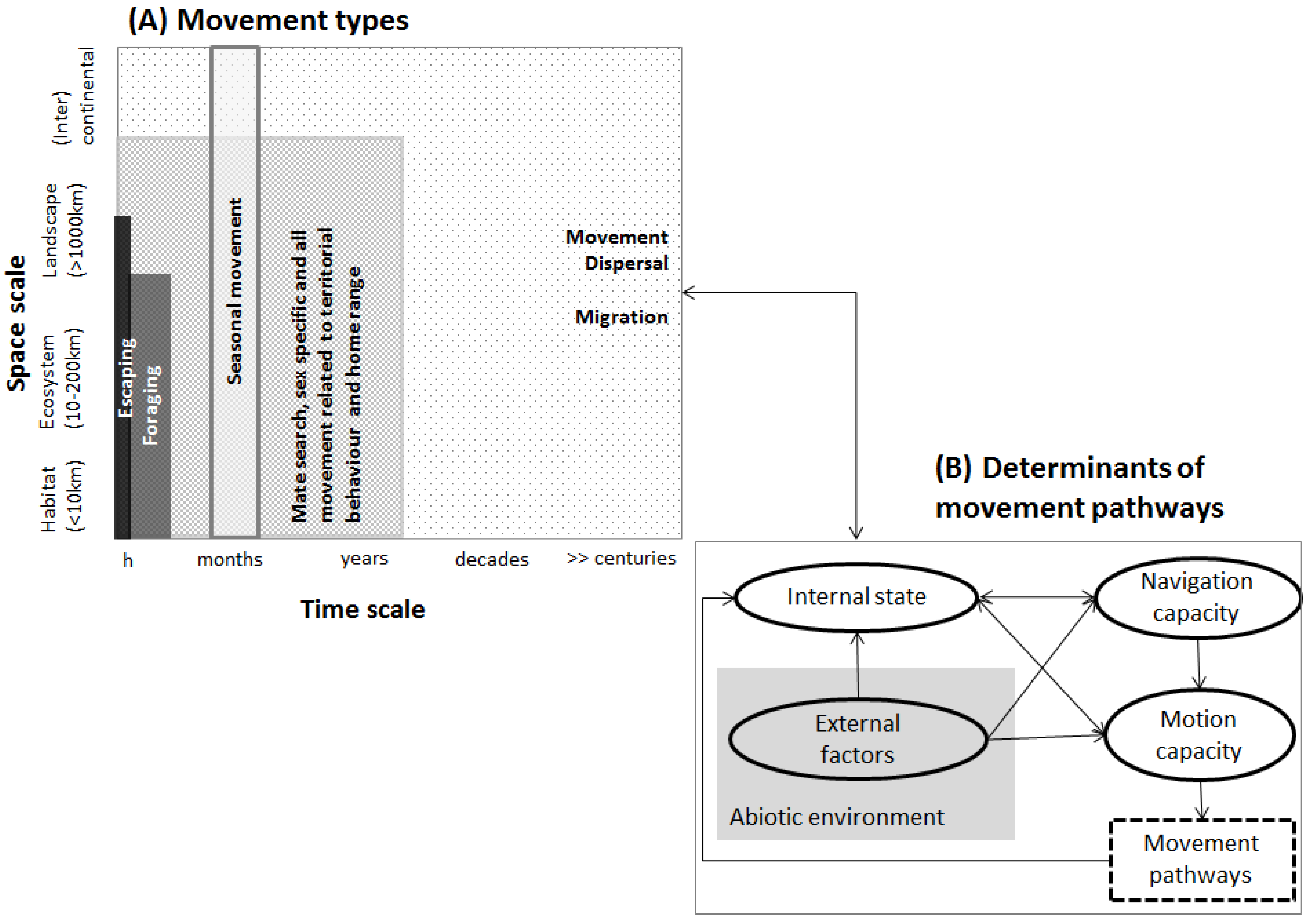
2.1. Movement
2.2. Data, Space and Time Scale for Movement
3. Trends in Quantifying Connectivity
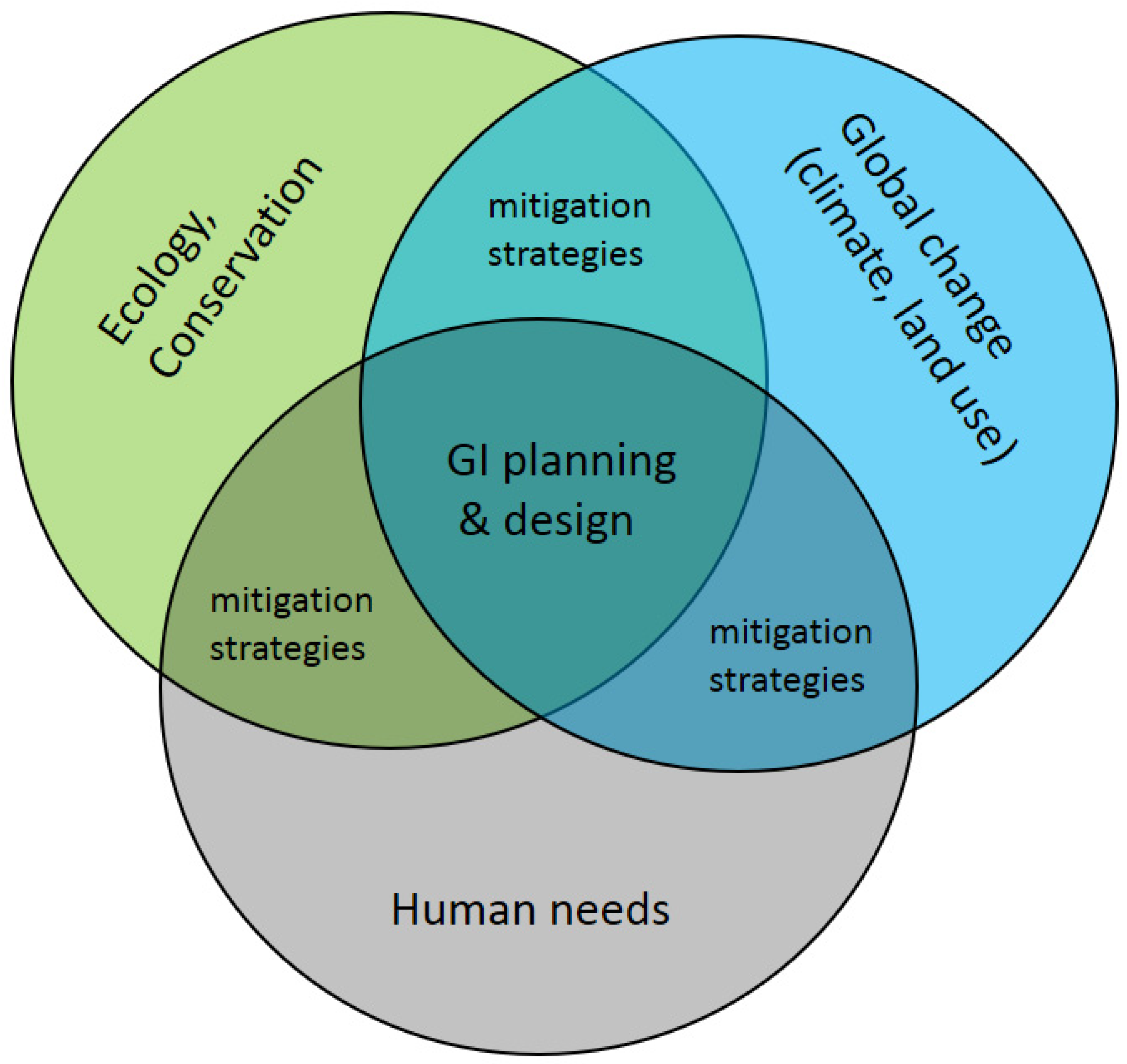
4. Planning and Design Strategies for Green Infrastructure: Approaches, Considerations, Tools
5. Avenues Towards Connectivity Assessments for Sustainable Future Green Infrastructures
- (1)
- Connectivity assessments would profit from continuous 3D landscape data. The past decade has seen remote sensing advance, and three-dimensional (3D) landscape structure is now available [129,130], UAV (unmanned aerial vehicles) and active remote sensors e.g., LiDAR, a laser detection system [131,132]. Compelling empirical evidence suggests that connectivity assessments could be supplemented with continuous 3D vegetation structure to provide functionally relevant landscape features in connectivity assessments.
- (2)
- Connectivity assessments could account for dynamic environments as static approaches are only of limited use for decision-makers, given expected significant environmental change in the future [110,133,134,135]. Accounting for dynamic processes such as urbanization and providing projections of future connectivity assessments in a changing environment may be of great value for future GI planning.
- (3)
- (4)
- Multi-species connectivity could be important for conservation management. While most connectivity analyses focus on a single species, conservation planners with limited resources could benefit greatly from models that predict the movement patterns of multiple species [59] as they are more efficient for conservation and restoration [138].
- (5)
- Finally, we see that spatial planning and design for GI is still challenged by how to integrate multiple demands and reconcile the trade-offs between functions that GI serves in natural and human systems. Planners and designers could do well to converge on a more unified perspective and approach to integrate multifunctional goals to the extent possible, and to use tools to measure the performance and trade-offs of different spatial plans in meeting those goals. In fact, geodesign processes are rapidly advancing to meet these needs [139]. Municipalities and regional governing boards could then use these decision-support tools to move toward GI implementation (as in Stessens et al. [31].
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Otto, S.P. Adaptation, speciation and extinction in the Anthropocene. Proc. R. Soc. B Biol. Sci. 2018, 285, 20182047. [Google Scholar] [CrossRef] [PubMed]
- Steffen, W.; Broadgate, W.; Deutsch, L.; Gaffney, O.; Ludwig, C. The trajectory of the Anthropocene: The Great Acceleration. Anthr. Rev. 2015, 2, 81–98. [Google Scholar] [CrossRef]
- Irwin, A. The dark side of light. Nature 2018, 553, 268. [Google Scholar] [CrossRef] [PubMed]
- Krauss, J.; Bommarco, R.; Guardiola, M.; Heikkinen, R.K.; Helm, A.; Kuussaari, M.; Lindborg, R.; Öckinger, E.; Pärtel, M.; Pino, J.; et al. Habitat fragmentation causes immediate and time-delayed biodiversity loss at different trophic levels. Ecol. Lett. 2010, 12, 597–605. [Google Scholar]
- Farneda, F.Z.; Grelle, C.E.V.; Rocha, R.; Ferreira, D.F.; Lopez-Baucells, A.; Meyer, C.F.J. Predicting biodiversity loss in island and countryside ecosystems through the lens of taxonomic and functional biogeography. Ecography 2019. [Google Scholar] [CrossRef]
- Fletcher, R.; Didham, R.; Banks-Leite, C.; Barlow, J.; Ewers, R.M.; Rosindell, J.; Holt, R.D.; Gonzalez, A.; Pardini, R.; Damschen, E.I.; et al. Is habitat fragmentation good for biodiversity? Biol. Conserv. 2018, 226, 9–15. [Google Scholar] [CrossRef]
- Verburg, R.W.; Osseweijer, F. A framework to estimate biodiversity loss and associated costs due to nitrogen emissions from single power plants. J. Clean. Prod. 2019, 239. [Google Scholar] [CrossRef]
- Sauter, I.; Kienast, F.; Bolliger, J.; Winter, B.; Pazur, R. Changes in demand and supply of ecosystem services under scenarios of future land use in Vorarlberg, Austria. J. Mt. Sci. 2019, in press. [Google Scholar] [CrossRef]
- Fahrig, L. Ecological Responses to Habitat Fragmentation Per Se. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Fahrig, L.; Arroyo-Rodríguez, V.; Bennett, J.; Boucher-Lalonde, V.; Cazetta, E.; Currie, D.J.; Eigenbrod, F.; Ford, A.T.; Harrison, S.P.; Jaeger, J.A.G.; et al. Is habitat fragmentation bad for biodiversity? Biol. Conserv. 2019, 230, 179–186. [Google Scholar] [CrossRef]
- Burkart, S.; Gugerli, F.; Senn, J.; Kuehn, R.; Bolliger, J. Evaluating the functionality of expert-assessed wildlife corridors with genetic data: Setting priorities for management measures in roe deer (Capreolus capreolus). Basic Appl. Ecol. 2016, 17, 52–60. [Google Scholar] [CrossRef]
- Luqman, H.; Muller, R.; Vaupel, A.; Brodbeck, S.; Bolliger, J.; Gugerli, F. No distinct barrier effect of highways and wide river on genetic structure of the Alpine newt (Ichthyosaura alpestris) in densely settled landscapes. Conserv. Genet. 2018, 19, 673–685. [Google Scholar] [CrossRef]
- Bolliger, J.; Edwards, T.C.; Eggenberg, S.; Ismail, S.; Seidl, I.; Kienast, F. Balancing forest-regeneration probabilities and maintenance costs in dry grassland meadows of high conservation priority. Conserv. Biol. 2011, 25, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.B.; Cabeza, M.; Thuiller, W.; Hannah, L.; Williams, P.H. Would climate change drive species out of reserves? An assessment of existing reserve-selection methods. Glob. Chang. Biol. 2004, 10, 1618–1626. [Google Scholar] [CrossRef]
- Wang, J.X.; Banzhaf, E. Towards a better understanding of Green Infrastructure: A critical review. Ecol. Indic. 2018, 85, 758–772. [Google Scholar] [CrossRef]
- Snäll, T.; Lehtomäki, J.; Arponen, A.; Elith, J.; Moilanen, A. Green infrastructure design based on spatial conservation prioritization and modeling of biodiversity features and ecosystem services. Environ. Manag. 2016, 57, 251–256. [Google Scholar]
- European Commission. Green Infrastructure (GI)—Enhancing Europe’s Natural Capital; EEA: Brussels, Belgium, 2013; p. 149. [Google Scholar]
- EEA. What Is Green Infrastructure? Available online: https://www.eea.europa.eu/themes/sustainability-transitions/urban-environment/urban-green-infrastructure/what-is-green-infrastructure (accessed on 27 March 2020).
- Privitera, R.; La Rosa, D. Reducing Seismic Vulnerability and Energy Demand of Cities through Green Infrastructure. Sustainability 2018, 10. [Google Scholar] [CrossRef]
- Lanzas, M.; Hermoso, V.; de-Miguel, S.; Bota, G.; Brotons, L. Designing a network of green infrastructure to enhance the conservation value of protected areas and maintain ecosystem services. Sci. Total Environ. 2019, 651, 541–550. [Google Scholar] [CrossRef]
- Brink, E.; Aalders, T.; Adam, D.; Feller, R.; Henselek, Y.; Hoffmann, A.; Ibe, K.; Matthey-Doret, A.; Meyer, M.; Negrut, N.L.; et al. Cascades of green: A review of ecosystem-based adaptation in urban areas. Glob. Environ. Chang. Hum. Policy Dimens. 2016, 36, 111–123. [Google Scholar] [CrossRef]
- Derkzen, M.L.; van Teeffelen, A.J.A.; Verburg, P.H. Green infrastructure for urban climate adaptation: How do residents’ views on climate impacts and green infrastructure shape adaptation preferences? Landsc. Urban Plan. 2017, 157, 106–130. [Google Scholar] [CrossRef]
- Demuzere, M.; Orru, K.; Heidrich, O.; Olazabal, E.; Geneletti, D.; Orru, H.; Bhave, A.G.; Mittal, N.; Feliu, E.; Faehnle, M. Mitigating and adapting to climate change: Multi-functional and multi-scale assessment of green urban infrastructure. J. Environ. Manag. 2014, 146, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Carlier, J.; Moran, J.; Aughney, T.; Roche, N. Effects of greenway development on functional connectivity for bats. Glob. Ecol. Conserv. 2019, 18, e00613. [Google Scholar] [CrossRef]
- Bartesaghi-Koc, C.; Osmond, P.; Peters, A. Spatio-temporal patterns in green infrastructure as driver of land surface temperature variability: The case of Sydney. Int. J. Appl. Earth Obs. Geoinf. 2019, 83. [Google Scholar] [CrossRef]
- Liu, X.L.; Wang, Y.; Li, Y.; Wu, J.S. Quantifying the Spatio-Temporal Process of Township Urbanization: A Large-Scale Data-Driven Approach. Isprs Int. J. Geo-Inf. 2019, 8. [Google Scholar] [CrossRef]
- Tischendorf, L.; Fahrig, L. On the usage and measurement of landscape connectivity. Oikos 2001, 90, 7–19. [Google Scholar] [CrossRef]
- Tischendorf, L. Can landscape indices predict ecological processes consistently? Landsc. Ecol. 2001, 16, 235–254. [Google Scholar] [CrossRef]
- Hrdalo, I.; Tomić, D.; Pereković, P. Implementation of Green Infrastructure principles in Dubrovnik, Croatia to minimize cimate change problems. Urbani Izziv 2015, 26, S38–S49. [Google Scholar] [CrossRef]
- Manna, P.; Bonfante, A.; Colandrea, M.; Di Vaio, C.; Langella, G.; Marotta, L.; Mileti, F.A.; Minieri, L.; Terribile, F.; Vingiani, S.; et al. A geospatial decision support system to assist olive growing at the landscape scale. Comput. Electron. Agric. 2020, 168. [Google Scholar] [CrossRef]
- Stessens, P.; Khan, A.Z.; Huysmans, M.; Canters, F. Analysing urban green space accessibility and quality: A GIS-based model as spatial decision support for urban ecosystem services in Brussels. Ecosyst. Serv. 2017, 28, 328–340. [Google Scholar] [CrossRef]
- Williams, A.E.; Worsley-Tonks, K.E.L.; Ezenwa, V.O. Drivers and consequences of variation in individual social connectivity. Anim. Behav. 2017, 133, 1–9. [Google Scholar] [CrossRef]
- Holyoak, M.; Casagrandi, R.; Nathan, R.; Revilla, E.; Spiegel, O. Trends and missing parts in the study of movement ecology. Proc. Natl. Acad. Sci. USA 2008, 105, 19060–19065. [Google Scholar] [CrossRef] [PubMed]
- LaPoint, S.; Gallery, P.; Wikelski, M.; Kays, R. Animal behavior, cost-based corridor models, and real corridors. Landsc. Ecol. 2013, 28, 1615–1630. [Google Scholar] [CrossRef]
- McClure, M.L.; Hansen, A.J.; Inman, R.M. Connecting models to movements: Testing connectivity model predictions against empirical migration and dispersal data. Landsc. Ecol. 2016, 31, 1419–1432. [Google Scholar] [CrossRef]
- Jaquiéry, J.; Broquet, T.; Hirzel, A.; Yearsley, J.; Perrin, N. Inferring landscape effects on dispersal from genetic distances: How far can we go? Mol. Ecol. 2011, 29, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Lee-Yaw, J.A.; Davidson, A.; McRae, B.H.; Green, D.M. Do landscape processes predict phylogeographic patterns in the wood frog? Mol. Ecol. 2009, 18, 1863–1874. [Google Scholar] [CrossRef]
- Dickson, B.G.; Albano, C.M.; Anantharaman, R.; Beier, P.; Fargione, J.; Graves, T.A.; Gray, M.E.; Hall, K.R.; Lawler, J.J.; Leonard, P.B.; et al. Circuit-theory applications to connectivity science and conservation. Conserv. Biol. 2019, 33, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.W.; Brown, W.S.; Stechert, R.; Zamudio, K.R. Integrating individual behaviour and landscape genetics: The population structure of timber rattlesnake hibernacula. Mol. Ecol. 2008, 17, 719–730. [Google Scholar] [CrossRef]
- Andreasen, A.M.; Stewart, K.M.; Longland, W.S.; Beckmann, J.P.; Forister, M.L. Identification of source-sink dynamics in mountain lions of the Great Basin. Mol. Ecol. 2012, 21, 5689–5701. [Google Scholar] [CrossRef]
- Reding, D.M.; Cushman, S.A.; Gosselink, T.E.; Clark, W.R. Linking movement behavior and fine-scale genetic structure to model landscape connectivity for bobcats (Lynx rufus). Landsc. Ecol. 2013, 28, 471–486. [Google Scholar] [CrossRef]
- Nathan, R.; Getz, W.M.; Revilla, E.; Holyoak, M.; Kadmon, R.; Saltz, D.; Smouse, P.E. A movement ecology paradigm for unifying organismal movement research. Proc. Natl. Acad. Sci. USA 2008, 105, 19052–19059. [Google Scholar] [CrossRef]
- Cushman, S.A.; Lewis, J.S. Movement behavior explains genetic differentiation in American black bears. Landsc. Ecol. 2010, 25, 1613–1625. [Google Scholar] [CrossRef]
- Fletcher, R.; Young, J.; Hutto, R.; Noson, A.; Rota, C. Insights from ecological theory on temporal dynamics and species distribution modeling. In Predictive Species and Habitat Modeling in Landscape Ecology: Concepts and Applications; Drew, A., Wiersma, Y., Huettmann, F., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Fattebert, J.; Robinson, H.S.; Balme, G.; Slotow, R.; Hunter, L. Structural habitat predicts functional dispersal habitat of a large carnivore: How leopards change spots. Ecol. Appl. 2015, 25, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Baguette, M.; Van Dyck, H. Landscape connectivity and animal behavior: Functional grain as a key determinant for dispersal. Landsc. Ecol. 2007, 22, 1117–1129. [Google Scholar] [CrossRef]
- Nixon, K.; Silbernagel, J.; Price, J.; Miller, N.; Swaty, R. Habitat availability for multiple avian species under modeled alternative conservation scenarios in the Two Hearted River watershed in Michigan, USA. J. Nat. Conserv. 2014, 22, 302–317. [Google Scholar] [CrossRef]
- Peterman, W.E.; Connette, G.M.; Semlitsch, R.D.; Eggert, L.S. Ecological resistance surfaces predict fine-scale genetic differentiation in a terrestrial woodland salamander. Mol. Ecol. 2014, 23, 2402–2413. [Google Scholar] [CrossRef]
- Jackson, C.R.; Marnewick, K.; Lindsey, P.A.; Roskaft, E.; Robertson, M.P. Evaluating habitat connectivity methodologies: A case study with endangered African wild dogs in South Africa. Landsc. Ecol. 2016, 31, 1433–1447. [Google Scholar] [CrossRef]
- Brodie, J.F.; Giordano, A.J.; Dickson, B.G.; Hebblewhite, M.; Bernard, H.; Mohd-Azlan, J.; Anderson, J.; Ambu, L. Evaluating multispecies landscape connectivity in a threatened tropical mammal community. Conserv. Biol. 2015, 29, 122–132. [Google Scholar] [CrossRef]
- Bond, M.L.; Bradley, C.M.; Kiffner, C.; Morrison, T.A.; Lee, D.E. A multi-method approach to delineate and validate migratory corridors. Landsc. Ecol. 2017, 32, 1705–1721. [Google Scholar] [CrossRef]
- Abrahms, B.; DiPietro, D.; Graffis, A.; Hollander, A. Managing biodiversity under climate change: Challenges, frameworks, and tools for adaptation. Biodivers. Conserv. 2017, 26, 2277–2293. [Google Scholar] [CrossRef]
- Lechner, A.M.; Doerr, V.; Harris, R.M.B.; Doerr, E.; Lefroy, E.C. A framework for incorporating fine-scale dispersal behaviour into biodiversity conservation planning. Landsc. Urban Plan. 2015, 141, 11–23. [Google Scholar] [CrossRef]
- Reed, G.C.; Litvaitis, J.A.; Callahan, C.; Carroll, R.P.; Litvaitis, M.K.; Broman, D.J.A. Modeling landscape connectivity for bobcats using expert-opinion and empirically derived models: How well do they work? Anim. Conserv. 2017, 20, 308–320. [Google Scholar] [CrossRef]
- Charney, N.D. Evaluating expert opinion and spatial scale in an amphibian model. Ecol. Model. 2012, 242, 37–45. [Google Scholar] [CrossRef]
- Milanesi, P.; Holderegger, R.; Caniglia, R.; Fabbri, E.; Galaverni, M.; Randi, E. Expert-based versus habitat-suitability models to develop resistance surfaces in landscape genetics. Oecologia 2017, 183, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Koen, E.L.; Bowman, J.; Sadowski, C.; Walpole, A.A. Landscape connectivity for wildlife: Development and validation of multispecies linkage maps. Methods Ecol. Evol. 2014, 5, 626–633. [Google Scholar] [CrossRef]
- Schultz, A.; Klenke, R.; Lutze, G.; Voss, M.; Wieland, R.; Wilkening, B. Habitat models as tools for situation evaluation and planning dupport in agricultural landscapes. In Landscape Theory and Resource Management: Linking Theory with Practice; Bissonette, J., Storch, I., Eds.; Island Press: Washington, DC, USA, 2003; pp. 261–282. [Google Scholar]
- Keller, D.; Holderegger, R.; Van Strien, M.J.; Bolliger, J. How to make landscape genetics beneficial for conservation management? Conserv. Genet. 2015, 16, 503–512. [Google Scholar] [CrossRef]
- Van Strien, M.J.; Keller, D.; Holderegger, R.; Ghazoul, J.; Kienast, F.; Bolliger, J. Landscape genetics as a tool for conservation planning: Predicting the effects of landscape change on gene flow. Ecol. Appl. 2014, 24, 327–339. [Google Scholar] [CrossRef]
- Bolliger, J.; Keller, D.; Holderegger, R. When landscape variables do not explain migration rates: An example from an endangered dragonfly (Leucorrhinia caudalis). Eur. J. Entomol. 2011, 108, 327–330. [Google Scholar] [CrossRef]
- Le Lay, G.; Angelone, S.; Flory, C.; Holderegger, R.; Bolliger, J. Increasing pond density to maintain a patchy habitat network of the European tree frog (Hyla arborea). J. Herpetol. 2015, 49, 217–221. [Google Scholar] [CrossRef]
- Pfeiffer, V.; Silbernagel, J.; Guédot, C.; Zalapa, J. Woodland and floral richness boost bumble bee density in cranberry resource pulse landscapes. Landsc. Ecol. 2019, 34, 979–996. [Google Scholar] [CrossRef]
- Row, J.R.; Blouin-Demers, G.; Lougheed, S.C. Habitat distribution influences dispersal and fine-scale genetic population structure of eastern foxsnakes (Mintonius gloydi) across a fragmented landscape. Mol. Ecol. 2010, 19, 5157–5171. [Google Scholar] [CrossRef] [PubMed]
- Zeller, K.A.; McGarigal, K.; Whiteley, A.R. Estimating landscape resistance to movement: A review. Landsc. Ecol. 2012, 27, 777–797. [Google Scholar] [CrossRef]
- Yumnam, B.; Jhala, Y.V.; Qureshi, Q.; Maldonado, J.E.; Gopal, R.; Saini, S.; Srinivas, Y.; Fleischer, R.C. Prioritizing tiger conservation through landscape genetics and habitat linkages. PLoS ONE 2014, 9, e111207. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Gryseels, S.; de Bellocq, J.G.; Makundi, R.; Vanmechelen, K.; Broeckhove, J.; Mazoch, V.; Sumbera, R.; Zima, J.; Leirs, H.; Baird, S.J.E. Genetic distinction between contiguous urban and rural multimammate mice in Tanzania despite gene flow. J. Evol. Biol. 2016, 29, 1952–1967. [Google Scholar] [CrossRef]
- Harrisson, K.A.; Pavlova, A.; Amos, J.N.; Radford, J.Q.; Sunnucks, P. Does reduced mobility through fragmented landscapes explain patch extinction patterns for three honeyeaters? J. Anim. Ecol. 2014, 83, 616–627. [Google Scholar] [CrossRef]
- Harrisson, K.A.; Pavlova, A.; Amos, J.N.; Takeuchi, N.; Lill, A.; Radford, J.Q.; Sunnucks, P. Disrupted fine-scale population processes in fragmented landscapes despite large-scale genetic connectivity for a widespread and common cooperative breeder: The superb fairy-wren (Malurus cyaneus). J. Anim. Ecol. 2013, 82, 322–333. [Google Scholar] [CrossRef]
- Frei, M.; Csencsics, C.; Brodbeck, S.; Schweizer, E.; Bühler, C.; Gugerli, F.; Bolliger, J. Combining landscape genetics, radio-tracking and long-term monitoring to derive management implications for Natterjack toads (Epidalea calamita) in agricultural landscapes. J. Nat. Conserv. 2016, 32, 22–34. [Google Scholar] [CrossRef]
- Naidoo, R.; Kilian, J.W.; Du Preez, P.; Beytell, P.; Aschenborn, O.; Taylor, R.D.; Stuart-Hill, G. Evaluating the effectiveness of local- and regional-scale wildlife corridors using quantitative metrics of functional connectivity. Biol. Conserv. 2018, 217, 96–103. [Google Scholar] [CrossRef]
- Wasserman, T.N.; Cushman, S.A.; Schwartz, M.K.; Wallin, D.O. Spatial scaling and multi-model inference in landscape genetics: Martes americana in northern Idaho. Landsc. Ecol. 2010, 25, 1601–1612. [Google Scholar] [CrossRef]
- Squires, J.R.; DeCesare, N.J.; Olson, L.E.; Kolbe, J.A.; Hebblewhite, M.; Parks, S.A. Combining resource selection and movement behavior to predict corridors for Canada lynx at their southern range periphery. Biol. Conserv. 2013, 157, 187–195. [Google Scholar] [CrossRef]
- Parks, L.C.; Wallin, D.O.; Cushman, S.A.; McRae, B.H. Landscape-level analysis of mountain goat population connectivity in Washington and southern British Columbia. Conserv. Genet. 2015, 16, 1195–1207. [Google Scholar] [CrossRef]
- Moran-Ordonez, A.; Pavlova, A.; Pinder, A.M.; Sim, L.; Sunnucks, P.; Thompson, R.M.; Davis, J. Aquatic communities in arid landscapes: Local conditions, dispersal traits and landscape configuration determine local biodiversity. Divers. Distrib. 2015, 21, 1230–1241. [Google Scholar] [CrossRef]
- Bleyhl, B.; Baumann, M.; Griffiths, P.; Heidelberg, A.; Manvelyan, K.; Radeloff, V.C.; Zazanashvili, N.; Kuemmerle, T. Assessing landscape connectivity for large mammals in the Caucasus using Landsat 8 seasonal image composites. Remote Sens. Environ. 2017, 193, 193–203. [Google Scholar] [CrossRef]
- Xiu, N.; Ignatieva, M.; van den Bosch, C.K.; Chai, Y.Y.; Wang, F.; Cui, T.F.; Yang, F.P. A socio-ecological perspective of urban green networks: The Stockholm case. Urban Ecosyst. 2017, 20, 729–742. [Google Scholar] [CrossRef]
- Adriaensen, F.; Chardon, J.P.; De Blust, G.; Swinnen, E.; Villalba, S.; Gulnick, H.; Matthysen, E. The application of “least cost” modelling as functional landscape models. Landsc. Urban Plan. 2003, 64, 233–247. [Google Scholar] [CrossRef]
- McRae, B.H.; Dickson, B.G.; Keitt, T.H.; Shah, V.B. Using circuit theory to model connectivity in ecology and conservation. Ecology 2008, 10, 2712–2724. [Google Scholar] [CrossRef]
- Moilanen, A.; Wilson, K.A.; Possingham, H. Spatial Conservation Prioritization: Quantitative Methods and Computational Tools; University Press: Oxford, UK, 2009. [Google Scholar]
- Pinto, N.; Keitt, T.H. Beyond the least-cost path: Evaluating corridor redundancy using a graph-theoretic approach. Landsc. Ecol. 2009, 24, 253–266. [Google Scholar] [CrossRef]
- Fattebert, J.; Baubet, E.; Slotow, R.; Fischer, C. Landscape effects on wild boar home range size under contrasting harvest regimes in a human-dominated agro-ecosystem. Eur. J. Wildl. Res. 2017, 63. [Google Scholar] [CrossRef]
- Bolliger, J.; Lander, T.; Balkenhol, N. Landscape genetics since 2003: Status, challenges and future directions. Landsc. Ecol. 2014, 29, 361–366. [Google Scholar] [CrossRef]
- Rayfield, B.; Fortin, M.J.; Fall, A. The sensitivity of least-cost habitat graphs to relative cost surface values. Landsc. Ecol. 2010, 25, 519–532. [Google Scholar] [CrossRef]
- Panzacchi, M.; Van Moorter, B.; Strand, O.; Saerens, M.; Ki, I.K.; St Clair, C.C.; Herfindal, I.; Boitani, L. Predicting the continuum between corridors and barriers to animal movements using Step Selection Functions and Randomized Shortest Paths. J. Anim. Ecol. 2016, 85, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Grafius, D.R.; Corstanje, R.; Siriwardena, G.M.; Plummer, K.E.; Harris, J.A. A bird’s eye view: Using circuit theory to study urban landscape connectivity for birds. Landsc. Ecol. 2017, 32, 1771–1787. [Google Scholar] [CrossRef] [PubMed]
- Lechner, A.M.; Sprod, D.; Carter, O.; Lefroy, E.C. Characterising landscape connectivity for conservation planning using a dispersal guild approach. Landsc. Ecol. 2017, 32, 99–113. [Google Scholar] [CrossRef]
- Bani, L.; Pisa, G.; Luppi, M.; Spilotros, G.; Fabbri, E.; Randi, E.; Orioli, V. Ecological connectivity assessment in a strongly structured fire salamander (Salamandra salamandra) population. Ecol. Evol. 2015, 5, 3472–3485. [Google Scholar] [CrossRef] [PubMed]
- Braaker, S.; Moretti, M.; Boesch, R.; Ghazoul, J.; Obrist, M.K.; Bontadina, F. Assessing habitat connectivity for ground-dwelling animals in an urban environment. Ecol. Appl. 2014, 24, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.J.; Veiman-Echeverria, M.; Kurz, D.J.; Donnelly, M.A. Evaluating connectivity for tropical amphibians using empirically derived resistance surfaces. Ecol. Appl. 2015, 25, 928–942. [Google Scholar] [CrossRef]
- Zeller, K.A.; Jennings, M.K.; Vickers, T.W.; Ernest, H.B.; Cushman, S.A.; Boyce, W.M. Are all data types and connectivity models created equal? Validating common connectivity approaches with dispersal data. Divers. Distrib. 2018, 24, 868–879. [Google Scholar] [CrossRef]
- Graves, T.; Chandler, R.B.; Royle, J.A.; Beier, P.; Kendall, K.C. Estimating landscape resistance to dispersal. Landsc. Ecol. 2014, 29, 1201–1211. [Google Scholar] [CrossRef]
- Koen, E.L.; Bowman, J.; Walpole, A.A. The effect of cost surface parameterization on landscape resistance estimates. Mol. Ecol. Resour. 2012, 12, 686–696. [Google Scholar] [CrossRef]
- Dilts, T.E.; Weisberg, P.J.; Leitner, P.; Matocq, M.D.; Inman, R.D.; Nussear, K.E.; Esque, T.C. Multiscale connectivity and graph theory highlight critical areas for conservation under climate change. Ecol. Appl. 2016, 26, 1223–1237. [Google Scholar] [CrossRef]
- Garrido-Garduno, T.; Tellez-Valdes, O.; Manel, S.; Vazquez-Dominguez, E. Role of habitat heterogeneity and landscape connectivity in shaping gene flow and spatial population structure of a dominant rodent species in a tropical dry forest. J. Zool. 2016, 298, 293–302. [Google Scholar] [CrossRef]
- Churko, G.; Kienast, F.; Bolliger, J. A multispecies assessment to identify functional connectivity in a human-dominated landscape. Int. J. Geogr. Inf. Syst. 2020, in press. [Google Scholar]
- Cushman, S.A.; McKelvey, K.S.; Hayden, J.; Schwartz, M.K. Gene flow in complex landscapes: Testing multiple hypotheses with causal modeling. Am. Nat. 2006, 168, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.A.; Cushman, S.A.; Srivastava, A.; Sarkar, M.S.; Shivaji, S. Tiger abundance and gene flow in Central India are driven by disparate combinations of topography and land cover. Divers. Distrib. 2017, 23, 863–874. [Google Scholar] [CrossRef]
- Vergara, M.; Cushman, S.A.; Ruiz-Gonzalez, A. Ecological differences and limiting factors in different regional contexts: Landscape genetics of the stone marten in the Iberian Peninsula. Landsc. Ecol. 2017, 32, 1269–1283. [Google Scholar] [CrossRef]
- Landguth, E.L.; Hand, B.K.; Glassy, J.; Cushman, S.A.; Sawaya, M.A. UNICOR: A species connectivity and corridor network simulator. Ecography 2012, 35, 9–14. [Google Scholar] [CrossRef]
- Koen, E.L.; Ellington, E.H.; Bowman, J. Mapping landscape connectivity for large spatial extents. Landsc. Ecol. 2019, 34, 2421–2433. [Google Scholar] [CrossRef]
- Leibovici, D.G.; Claramunt, C. On Integrating Size and Shape Distributions into a Spatio-Temporal Information Entropy Framework. Entropy 2019, 21. [Google Scholar] [CrossRef]
- Perkl, R.; Norman, L.M.; Mitchell, D.; Feller, M.; Smith, G.; Wilson, N.R. Urban growth and landscape connectivity threats assessment at Saguaro National Park, Arizona, USA. J. Land Use Sci. 2018, 13, 102–117. [Google Scholar] [CrossRef]
- Krosby, M.; Breckheimer, I.; Pierce, D.J.; Singleton, P.H.; Hall, S.A.; Halupka, K.C.; Gaines, W.L.; Long, R.A.; McRae, B.H.; Cosentino, B.L.; et al. Focal species and landscape “naturalness” corridor models offer complementary approaches for connectivity conservation planning. Landsc. Ecol. 2015, 30, 2121–2132. [Google Scholar] [CrossRef]
- Freeman, C.F.; Bell, K.P. Conservation versus cluster subdivisions and implications for habitat connectivity. Landsc. Urban Plan. 2011, 10, 30–42. [Google Scholar] [CrossRef]
- Sawyer, S.C.; Epps, C.W.; Brashares, J.S. Placing linkages among fragmented habitats: Do least-cost models reflect how animals use landscapes? J. Appl. Ecol. 2011, 48, 668–678. [Google Scholar] [CrossRef]
- Marrotte, R.R.; Bowman, J.; Brown, M.G.C.; Cordes, C.; Morris, K.Y.; Prentice, M.B.; Wilson, P.J. Multi-species genetic connectivity in a terrestrial habitat network. Mov. Ecol. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Vukomanovic, J.; Skrip, M.; Meentenmeyer, R. Making It Spatial Makes It Personal: Engaging Stakeholders with Geospatial Participatory Modeling. Land 2019, 8, 38. [Google Scholar] [CrossRef]
- Lechner, A.M.; Harris, R.M.S.; Doerr, V.; Doerr, E.; Drielsma, M.; Lefroy, E.C. From static connectivity modelling to scenario-based planning at local and regional scales. J. Nat. Conserv. 2015, 28, 78–88. [Google Scholar] [CrossRef]
- Zhang, Z.; Meerow, S.; Newell, J.; Lindquist, M. Enhancing landscape connectivity through multifunctional green infrastructure corridor modeling and design. Urban For. Urban Green. 2019, 38, 305–317. [Google Scholar] [CrossRef]
- Perkl, R.M. Geodesigning landscape linkages: Coupling GIS with wildlife corridor design in conservation planning. Landsc. Urban Plan. 2016, 156, 44–58. [Google Scholar] [CrossRef]
- Firehock, K.E.; Walker, R.A. Green Infrastructure: Map and Plan the Natural World with GIS; Esri Press: Redlands, CA, USA, 2019. [Google Scholar]
- Wisconsin, G. Tackling Barriers to Green Infrastructure: An Audit of Local Codes and Ordinances; WI, USA, 2017; Available online: https://www.seagrant.wisc.edu/our-work/focus-areas/coastal-communities/green-infrastructure/ (accessed on 1 January 2020).
- Benedict, M.; McMahon, E.; Bergen, L. Green Infrastructure: Linking Landscapes and Communities; Island Press: Washington, DC, USA, 2006. [Google Scholar]
- Lynch, A. Is it good to be green? Assessing the ecological results of county green infrastructure planning. J. Plan. Educ. Res. 2016, 36, 90–104. [Google Scholar] [CrossRef]
- Szulczewska, B.; Giedych, R.; Maksymiuk, G. Can we face the challenge: How to implement a theoretical concept of green infrastructure into planning practice? Warsaw case study. Landsc. Res. 2017, 42, 76–194. [Google Scholar] [CrossRef]
- Meerow, S.; Newell, J. Spatial planning for multifunctional green infrastructure: Growing resilience in Detroit. Landsc. Urban Plan. 2017, 159, 62–75. [Google Scholar] [CrossRef]
- Lai, S.; Leone, F. Bridging Biodiversity Conservation Objectives with Landscape Planning Through Green Infrastructures: A Case Study from Sardinia, Italy. In Lecture Notes in Computer Science, Proceedings of theComputational Science and Its Applications, Trieste, Italy, 3–6 July 2017; Springer: Cham, Switzerland, 2017; Volume 10409, pp. 10456–10472. [Google Scholar]
- Lafortezza, R.; Davies, C.; Sanesi, G.; Konijnendijk, C. Green Infrastructure as a tool to support spatial planning in European urban regions. iForest Biogeosci. For. 2013, 6, 102–108. [Google Scholar] [CrossRef]
- Reimer, M.; Rusche, K. Green infrastructure under pressure. A global narrative between regional vision and local implementation. Eur. Plan. Stud. 2019, 27, 1542–1563. [Google Scholar] [CrossRef]
- Liquete, C.; Kleeschulte, S.; Dige, G.; Maes, J.; Grizzetti, B.; Olah, B.; Zulian, G. Mapping green infrastructure based on ecosystem services and ecological networks: A Pan-European case study. Environ. Sci. Policy 2015, 54, 268–280. [Google Scholar] [CrossRef]
- Vasiljevic, N.; Radic, B.; Gavrilovic, S.; Sljukic, B.; Medarevic, M.; Ristic, R. The concept of green infrastructure and urban landscape planning: A challenge for urban forestry planning in Belgrade, Serbia. iForest Biogeosci. For. 2018, 11, 491–498. [Google Scholar] [CrossRef]
- Liu, S.; Yin, Y.; Li, J.; Cheng, F.; Dong, S.; Zhang, Y. Using cross-scale landscape connectivity indices to identify key habitat resource patches for Asian elephants in Xishuangbanna, China. Landsc. Urban Plan. 2018, 171, 80–87. [Google Scholar] [CrossRef]
- United Nations. About the Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 15 May 2009).
- Bai, X.M.; van der Leeuw, S.; O’Brien, K.; Berkhout, F.; Biermann, F.; Brondizio, E.S.; Cudennec, C.; Dearing, J.; Duraiappah, A.; Glaser, M.; et al. Plausible and desirable futures in the Anthropocene: A new research agenda. Glob. Environ. Chang. Hum. Policy Dimens. 2016, 39, 351–362. [Google Scholar] [CrossRef]
- Future Earth. Research Agenda. 2014. Available online: http://www.futureearth.org/sites/default/files/strategic_research_agenda_2014.pdf (accessed on 27 March 2020).
- Van Strien, M.J.; Keller, D.; Holderegger, R. A new analytical approach to landscape genetic modelling: Least-cost transect analysis and linear mixed models. Mol. Ecol. 2012, 21, 4010–4023. [Google Scholar] [CrossRef]
- Ginzler, C.; Hobi, M.L. Countrywide stereo-image matching for updating digital surface models in the framework of the Swiss National Forest Inventory. Remote Sens. 2015, 7, 4343–4370. [Google Scholar] [CrossRef]
- Randin, C.F.; Ashcroft, M.; Bolliger, J.; Cavender-Bares, J.; Coops, N.; Dullinger, S.; Dirnböck, T.; Eckert, S.; Ellis, E.; Giuliani, G.; et al. Towards improved monitoring of terrestrial ecosystems in the Anthropocene through the closer integration of remotely sensed data into species distribution models. Remote Sens. Environ. 2020, in press. [Google Scholar]
- Bergen, K.M.; Goetz, S.J.; Dubayah, R.O.; Henebry, G.M.; Hunsaker, C.T.; Imhoff, M.L.; Nelson, R.F.; Parker, G.G.; Radeloff, V.C. Remote sensing of vegetation 3-D structure for biodiversity and habitat: Review and implications for lidar and radar spaceborne missions. J. Geophys. Res. 2009, 114, G00E06. [Google Scholar] [CrossRef]
- Merrick, M.J.; Koprowski, J.L. Circuit theory to estimate natal dispersal routes and functional landscape connectivity for an endangered small mammal. Landsc. Ecol. 2017, 32, 1163–1179. [Google Scholar] [CrossRef]
- Maggini, R.; Lehmann, A.; Zbinden, N.; Zimmermann, N.E.; Bolliger, J.; Schroder, B.; Foppen, R.; Schmid, H.; Beniston, M.; Jenni, L. Assessing species vulnerability to climate and land use change: The case of the Swiss breeding birds. Divers. Distrib. 2014, 20, 708–719. [Google Scholar] [CrossRef]
- Leonard, P.; Sutherland, R.; Baldwin, R.; Fedak, D.; Carnes, R.; Montgomery, A. Landscape connectivity losses due to sea levelrise and land use change. Anim. Conserv. 2017, 20, 80–90. [Google Scholar] [CrossRef]
- Nor, A.N.M.; Corstanje, R.; Harris, J.A.; Grafius, D.R.; Siriwardena, G.M. Ecological connectivity networks in rapidly expanding cities. Heliyon 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Kanagaraj, R.; Wiegand, T.; Kramer-Schadt, S.; Goyal, S.P. Using individual-based movement models to assess inter-patch connectivity for large carnivores in fragmented landscapes. Biol. Conserv. 2013, 167, 298–309. [Google Scholar] [CrossRef]
- Polansky, L.; Kilian, W.; Wittemyer, G. Elucidating the significance of spatial memory on movement decisions by African savannah elephants using state-space models. Proc. R. Soc. B Biol. Sci. 2015, 282, 20143042. [Google Scholar] [CrossRef]
- Fleishman, E.; Anderson, J.; Dickson, B.G. Single-species and multiple-species connectivity models for large mammals on the Navajo nation. West. N. Am. Nat. 2017, 77, 237–251. [Google Scholar] [CrossRef]
- Rodriguez-Espinos, V.M.; Aguilera-Benavente, F.; Gimez-Delgado, M. Green infrastructure design using GIS and spatial analysis: A proposal for the Henares Corridor (Madrid-Guadalajara, Spain). Landsc. Res. 2020, 45, 26–43. [Google Scholar] [CrossRef]
| Data Geometry | Data Type and Origin | Temporal Depth | Data Represents (sensu stricto) | Information on Movement |
|---|---|---|---|---|
| Points | Presence data originating from genetic clustering | Contemporary, recent or long-term | Genetic groups representing kinship | Indirect movement: no direct information on spatial movement pathways, but information on genetic kinship (i.e., gene flow). |
| Points | Presence data from monitoring, photo traps, mark-recapture studies | Days-years-decades | The organisms were present at the coordinates at the time of the survey | Indirect assumed movement: no direct information on spatial movement pathway. |
| Vector (polygon, line) /raster | Monitoring or survey polygons or transects; modeled habitat suitability/probability of occurrence) | Days-years-decades | The organisms were present in the area at the time of the survey; present as modeled | Indirect assumed movement: no direct information on spatial movement pathway. |
| Vector (polygons/line) | Expert knowledge | Years-decades | Areas of realized local wildlife corridors, underpasses, etc. | Direct movement: usually local and likely selected subjective information on spatial movement pathways, but well covered by long-term in-depth expert experience. |
| Line | GPS tracking, telemetry | Days-years-decades | Spatially and temporally explicit realized pathways | Direct movement: allows to infer a broad range of realized movement spatially and temporally explicitly including aspects of behavior. No indication whether movement has an effect on reproduction. Applied often short-term for a limited number of organisms as the approach is costly. |
| Line | Genetic first-generation migrants | Contemporary (< 1 year) | Realized gene flow | Indirect movement: allows to infer short-term realized movement of organisms as assessed by gene flow, for which analyses allow to deduce movement direction [61]. No direct inference on spatial movement pathways. |
| Line | Genetic assignment tests | Contemporary, recent or long-term | Realized gene flow | Indirect movement: allows to infer realized movement of organisms as assessed by gene flow at various temporal depths including the directionality of movement. No direct indication on spatial movement pathways. |
| Line | Genetic differentiation FST | Historic (>20 generations) | Realized gene flow | Indirect movement: allows to deduce past realized movement of organisms as assessed by gene flow but does not directly relate to spatial movement pathways. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolliger, J.; Silbernagel, J. Contribution of Connectivity Assessments to Green Infrastructure (GI). ISPRS Int. J. Geo-Inf. 2020, 9, 212. https://doi.org/10.3390/ijgi9040212
Bolliger J, Silbernagel J. Contribution of Connectivity Assessments to Green Infrastructure (GI). ISPRS International Journal of Geo-Information. 2020; 9(4):212. https://doi.org/10.3390/ijgi9040212
Chicago/Turabian StyleBolliger, Janine, and Janet Silbernagel. 2020. "Contribution of Connectivity Assessments to Green Infrastructure (GI)" ISPRS International Journal of Geo-Information 9, no. 4: 212. https://doi.org/10.3390/ijgi9040212
APA StyleBolliger, J., & Silbernagel, J. (2020). Contribution of Connectivity Assessments to Green Infrastructure (GI). ISPRS International Journal of Geo-Information, 9(4), 212. https://doi.org/10.3390/ijgi9040212





