Analyzing the Correlation between Deer Habitat and the Component of the Risk for Lyme Disease in Eastern Ontario, Canada: A GIS-Based Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area

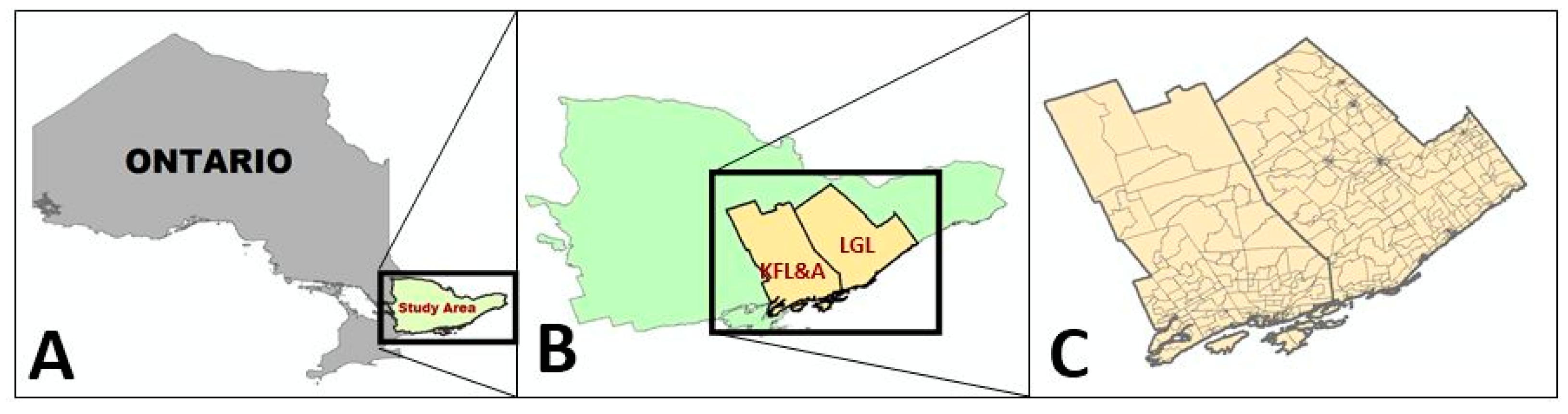
2.2. Data Collection
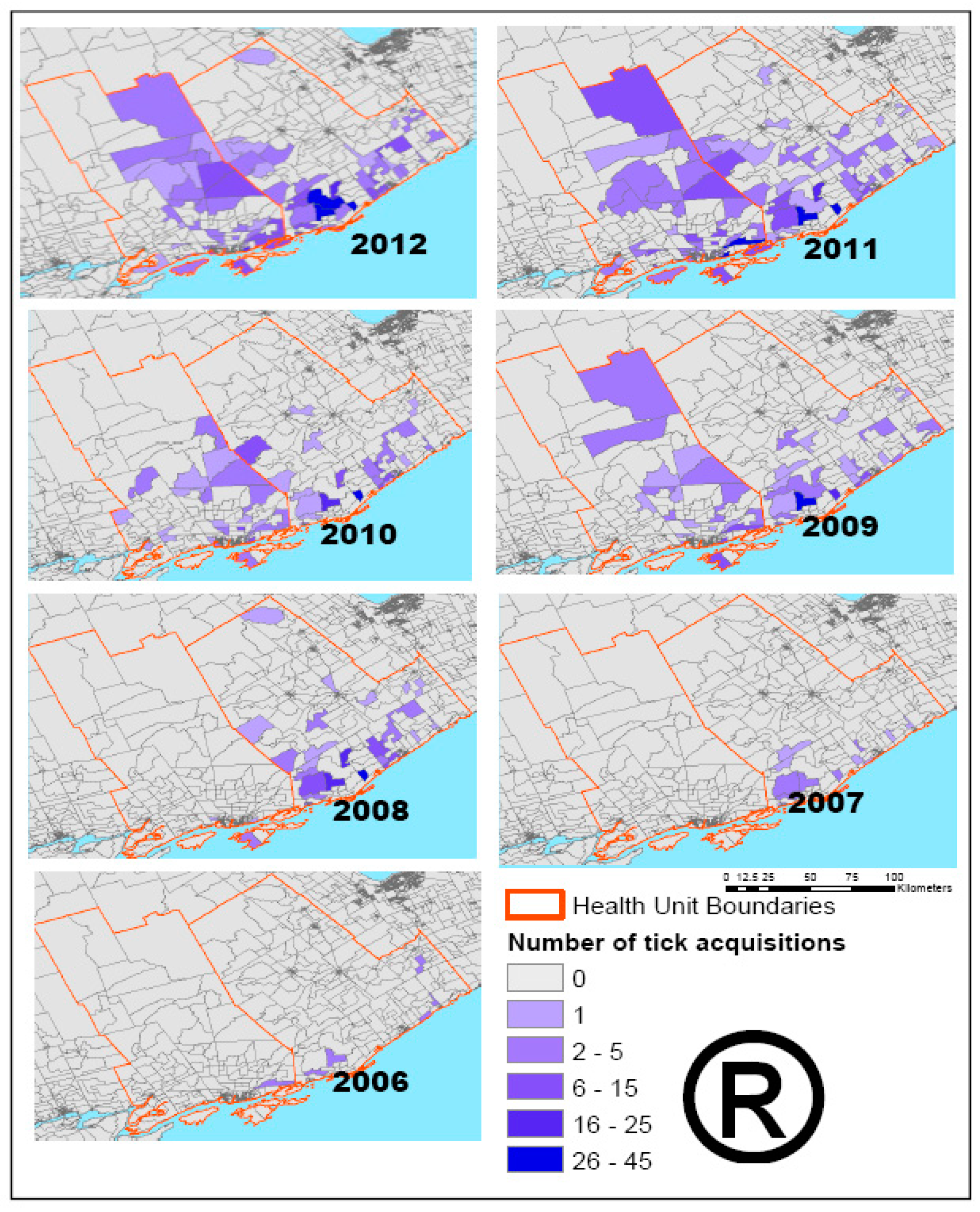
2.2.1. Tick Data
2.2.2. Deer Harvest Data
2.2.3. Deer Habitat Data
2.3. Procedures
2.3.1. Multi-Criterial Decision Making Model for Deer Habitat

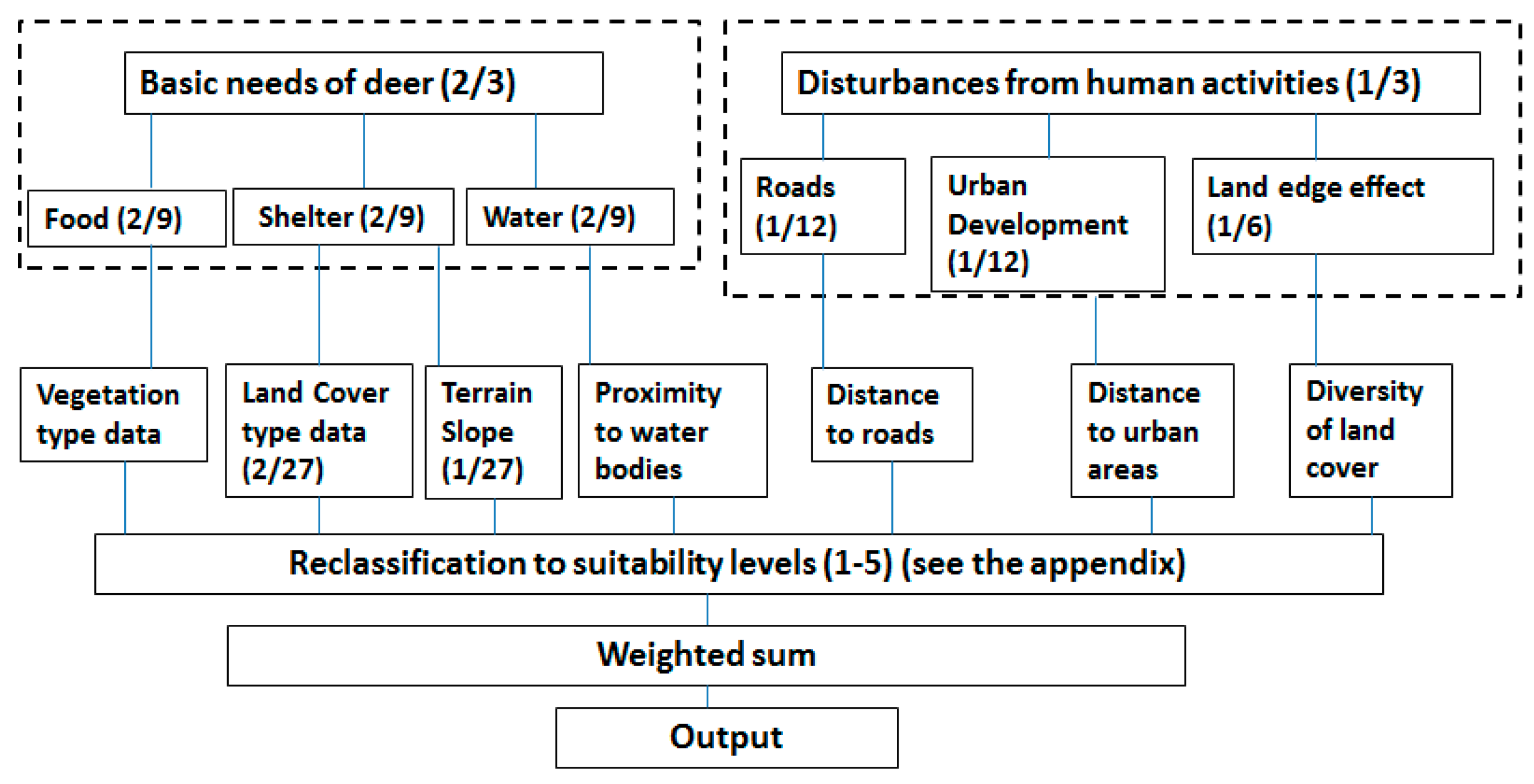
| Variables | Equal Weights | Weights from Expected Value Method | Weights from Rank Sum Method |
|---|---|---|---|
| Vegetation (food) | 1/7 | 1/4 | 2/9 |
| Land cover (shelter) | 1/7 | 3/16 | 2/27 |
| Terrain slope (shelter) | 1/7 | 1/16 | 1/27 |
| Proximity to water | 1/7 | 1/4 | 2/9 |
| Distance to roads | 1/7 | 1/16 | 1/12 |
| Distance to urban areas | 1/7 | 1/16 | 1/12 |
| Diversity of land cover | 1/7 | 1/8 | 1/6 |
2.3.2. Deer Habitat Suitability and Deer Harvest Data Analysis
2.3.3. Deer Habitat Suitability and Tick Data Analysis
3. Results and Discussions
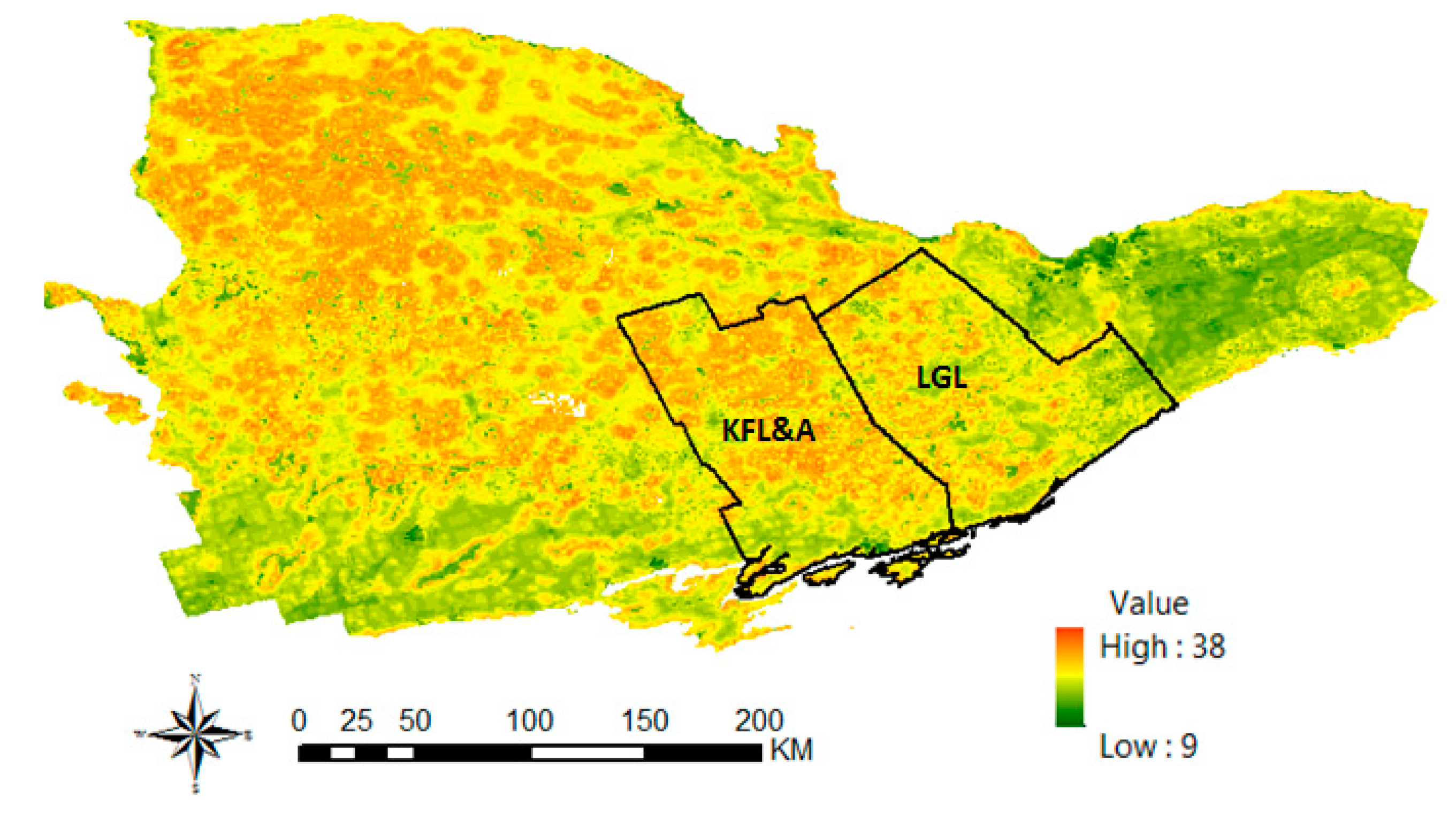
| Variables | Correlation Coefficient * |
|---|---|
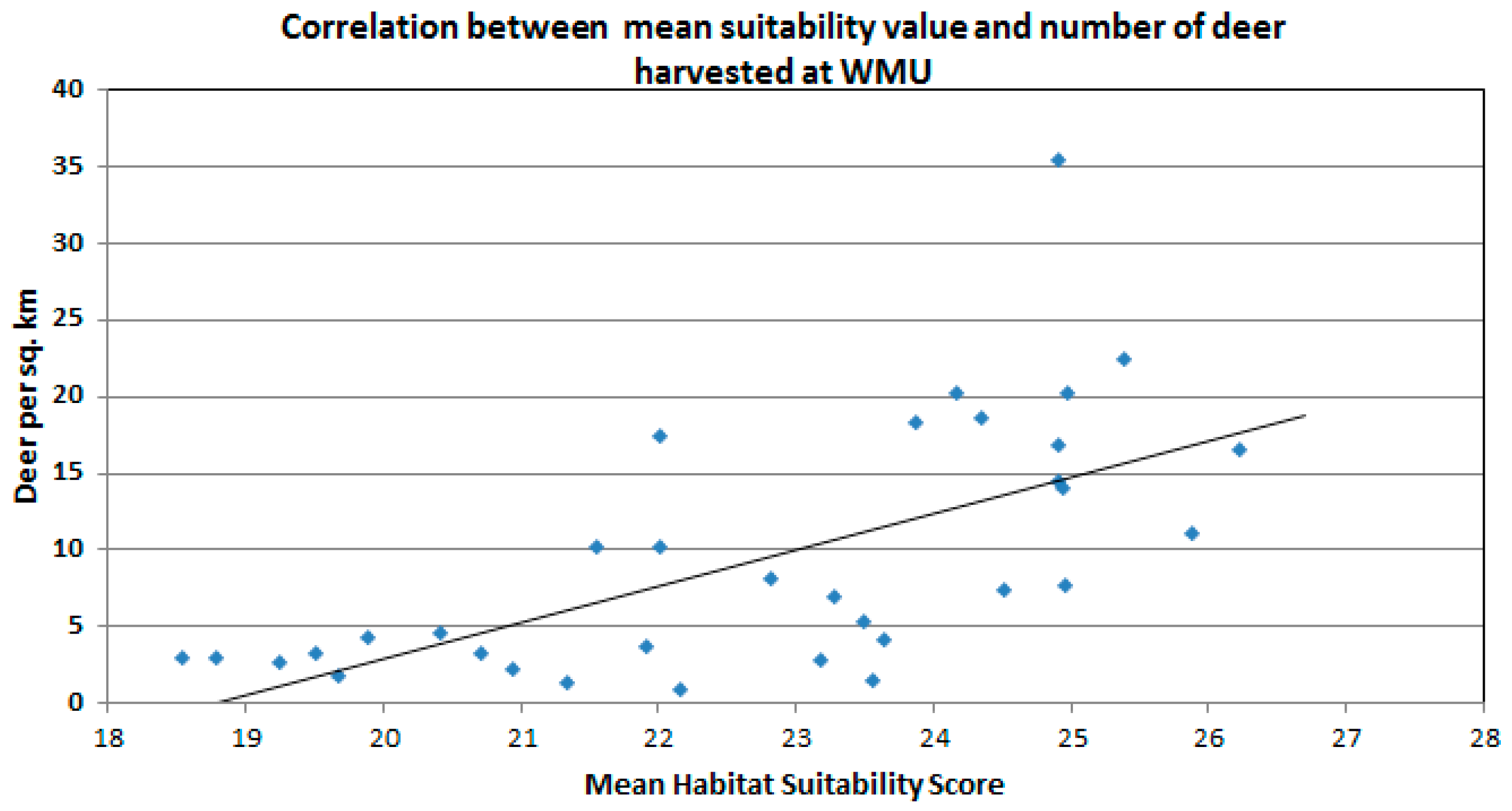
| Mean habitat suitability and adjusted deer harvest for all WMUs | 0.645 |
| Mean habitat suitability and the number of positive ticks for all DAs in the KFL&A and LGL Health Units | 0.609 |
| The number of tick acquisition and the number of positive ticks for all Das in KFL&A and LGL Health Units | 0.892 |
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
Appendix
| Layers (Weights) | Original Values | Recoding Values * |
|---|---|---|
| Food sources (2/9) | Water | 1 |
| Barren or sparsely vegetated | 2 | |
| Build-up, wetland | ||
| Deciduous needleleaf forest | 3 | |
| Deciduous broadleaf forest | ||
| Evergreen broadleaf forest | 4 | |
| Evergreen needleleaf forest | ||
| Wood savannah | ||
| Crops, shrubs, grass, mixed vegetation | 5 | |
| Land cover shelter (2/27) | Water | 1 |
| Settlement and developed land | 2 | |
| Mine tailings, quarries, redrocks | ||
| Marshes, open wetlands | ||
| Treed wetlands | 3 | |
| Agriculture | ||
| Agriculture/natural vegetation | ||
| Early successional forest | 4 | |
| Successional forest | ||
| Sparse forest | ||
| Dense coniferous forest | 5 | |
| Dense deciduous forest | ||
| Mixed forest | ||
| Terrain slope (1/27) | Water | 0 |
| 13.41°–20.89° | 1 | |
| 8.44°–13.41° | 2 | |
| 5.02°–8.44° | 3 | |
| 2.36 °–5.02° | 4 | |
| 0°–2.36° | 5 | |
| Proximity to water bodies (2/9) | >8 miles | 1 |
| 2.4 to 8 miles | 2 | |
| 1.5 to 2.4 miles | 3 | |
| 1 to 1.5 miles | 4 | |
| 0–1 miles | 5 | |
| Distance to roads (1/12) | 0–0.8 miles | 1 |
| 0.8 to 1.6 miles | 2 | |
| 1.6 to 2.4 miles | 3 | |
| 2.4 to 3.1 miles | 4 | |
| >3.1 miles | 5 | |
| Distance to urban areas (1/12) | 0–0.8 miles | 1 |
| 0.8 to 1.6 miles | 2 | |
| 1.6 to 2.4 miles | 3 | |
| 2.4 to 3.1 miles | 4 | |
| 3.1 miles | 5 | |
| Diversity of land cover (1/6) | 1 | 1 |
| 2 | 2 | |
| 3 | 3 | |
| 4 | 4 | |
| >5 | 5 |
References
- Ogden, N.H.; Maarouf, A.; Barker, I.K.; Poulin, M.B.; Lindsay, L.R.; Morshed, M.G.; O’Callaghan, C.J.; Ramay, F.; Waltner-Toews, D.; Charron, D.F. Climate change and the potential for range expansion of the lyme disease vector Ixodes scapularis in Canada. Int. J. Parasitol. 2005, 36, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Canada. Lyme Disease Fact Sheet; Government of Canada: Ottawa, ON, Canada, 2012. [Google Scholar]
- Ogden, N.H.; Bigras-Poulin, M.; O’Callaghan, C.J.; Barker, I.K.; Lindsay, L.R.; Maarouf, A.; Smoyer-Tomic, K.E.; Waltner-Toews, D.; Charron, D. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. Int. J. Parasitol. 2005, 35, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.G.; Anderson, R.C. Ixodes scapularis say on white-tailed deer (Odocoileus virginianus) from Long Point, Ontario. J. Wildl. Dis. 1976, 12, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Warden, L. Factors Affecting the Abundance of Blacklegged Ticks (Ixodes scapularis) and the PREVALENCE of Borrelia burgdorferi in Ticks and Small Mammals in the Thousand Islands Region. Master’s Thesis, The University of Guelph, Guelph, ON, Canada, 2012. [Google Scholar]
- Spielman, A. The emergence of lyme disease and human babesiosis in a changing environment. Ann. N. Y. Acad. Sci. 1994, 740, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Odgen, N.H.; St-Onge, L.; Barker, I.K.; Brazeau, S.; Bigras-Poulin, M.; Charron, D.F.; Francis, C.M.; Heagy, A.; Lindsay, L.R.; Maarouf, A.; et al. Risk maps for range expansion of the Lyme disease vector, Ixodes scapularis, in Canada now and with climate change. Int. J. Health Geogr. 2008, 7. [Google Scholar] [CrossRef]
- Scott, J.D.; Fernado, K.; Durden, L.A.; Morshed, M.G. Lyme disease spirochete, Borrelia burgdorferi, endemic in epicentre at Turkey Point, Ontario. J. Med. Entomol. 2004, 41, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Rand, P.W.; Lubelczyk, C.; Lavigne, G.R.; Elias, S.; Holman, M.S.; Lacombe, E.H.; Smith, R.P. Deer density and the abundance of Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 2003, 40, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G.; Ostfeld, R.S.; Richard, M.P.; Schauber, E.M.; Wolff, J.O. Chain reactions linking acorns to gypsy moth outbreaks and Lyme disease risk. Science 1998, 279, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Bosler, E.M.; Ormiston, B.G.; Coleman, J.L.; Hanrahan, J.P.; Benach, J.L. Prevalance of the Lyme disease spirochete in populations of white-tailed deer and white-footed mice. Yale J. Biol. Med. 1984, 57, 651–659. [Google Scholar] [PubMed]
- Rand, P.W.; Lubelczyk, C.; Holman, M.S.; Lacombe, E.H.; Smith, R.P. Abundance of Ixodes scapularis (Acari: Ixodidae) after complete removal of deer from an isolated offshore island, endemic for Lyme disease. J. Med. Entomol. 2004, 41, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Spielman, A.; Krause, P.J. Coninfecting deer-associated zoonoses: Lyme disease, babesiosis, and ehrlichiosis. CID 2001, 33, 676–685. [Google Scholar] [CrossRef]
- Garnett, J.M.; Connally, N.P.; Stafford, K.C.; Cartter, M.L. Evaluation of deer-targeted interventions on Lyme disease incidence in Connecticut. Public Health Rep. 2011, 126, 446–454. [Google Scholar] [PubMed]
- Fish, D.; Childs, J.E. Community-based prevention of Lyme disease and other tick-borne diseases through topical application of acaricide to white-tailed deer: Background and rationale. Vector-Borne Zoonotic Dis. 2009, 9, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Piesman, J. Ecology of Borrelia burgdorferi sensu lato in North America. In Lyme Borreliosis: Biology, Epidemiology and Control; Gray, J.S., Kahl, O., Lane, R.S., Stanek, G., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 223–249. [Google Scholar]
- Shope, R. Global climate change and infectious diseases. Environ. Health Perspect. 1991, 96, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, J.E.; Price, S.D.; Das, A.; Shields, T.; Glass, G.E. Geographic information system and spatial analysis of adult Ixodes scapularis (Acari: Ixodidae) in the Middle Atlantic region of the U.S.A. J. Med. Entomol. 2003, 40, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Lindsay, L.R.; Beauchamp, G.; Charron, D.; Maarout, A.; O’Callaghan, C.J.; Walternet-Toews, D.; Barker, I.K. Investigation of the relationships between temperature and development rates of the tick Ixodes scapularis (Acari: Ixodidae) in the laboratory and field. J. Med. Entomol. 2004, 41, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, J.S.; Holford, T.R.; Fish, D. A climate-based model predicts the spatial distribution of the Lyme disease vector Ixodes scapularis in the United States. Environ. Health Perspect. 2003, 111, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, J.S.; Holdford, T.R.; Fish, D. Effect of climate change on Lyme disease risk in North America. EcoHealth 2005, 2, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Trudel, L.; Artsob, H.; Barker, I.K.; Beauchamp, G.; Charron, D.F.; Drebot, M.A.; Galloway, T.D.; O’Handley, R.; Thompson, R.A.; et al. Ixodes scapularis ticks collected by passive surveillance in Canada: Analysis of geographic distribution and infection with Lyme borreliosis agent Borrelia burgdorferi. J. Med. Entomol. 2006, 43, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Phua, M.H.; Minowa, M. A GIS-based multi-criteria decision making approach to forest conservation planning at a landscape scale: A case study in the Kinabalu Area, Sabah, Malaysia. Landsc. Urban Plan. 2004, 71, 207–222. [Google Scholar] [CrossRef]
- Keisler, J.M.; Sundell, R.C. Combining multi-attribute utility and geographic information for boundary decision: An application to park planning. J. Geogr. Inf. Decis. Anal. 1997, 1, 101–118. [Google Scholar]
- Eastman, J.R.; Jin, W.; Kyem, P.A.K.; Toledano, J. Raster procedures for multi-criteria/multi-objective decisions. Photogramm. Eng. Remote Sens. 1995, 61, 539–547. [Google Scholar]
- Land Cover, Circa 2000—Vector. Available online: http://www.geobase.ca/geobase/en/data/landcover/csc2000v/description.html (accessed on 10 October 2014).
- Compton, B.; Mackie, R.; Dusek, G.L. Factors influencing distribution of white-tailed deer in Riparian Habitats. J. Wildl. Manag. 1988, 52, 542–548. [Google Scholar] [CrossRef]
- Ministry of Natural Resources. White-Tailed Deer Biology. Ontario Ministry of Natural Resources. 2012. Available online: http://www.mnr.gov.on.ca/en/Business/FW/2ColumnSubPage/STDPROD_097096.html (accessed on 3 April 2014). [Google Scholar]
- Moen, A.N. Energy conservation by white-tailed deer in the winter. Ecology 1976, 57, 192–198. [Google Scholar] [CrossRef]
- Clef, M.V. Review of the Ecological Effects and Management of White-Tailed Deer in New Jersey. The Nature Conservancy, New Jersey Chapter, Oct. 2004. Avilable online: http://deerinbalance.files.wordpress.com/2010/01/review-of-the-ecological-effects-and-management-of.pdf (accessed on 20 November 2014).
- Rodgers, K.J.; Ffolliott, P.F.; Patton, D.R. Home range and movement of five mule deer in a semidesert grass-shrub community. In Rocky Mountain Forest and Range Experiment Station; Forest Service US, Department of Agriculture: Fort Collins, CO, USA, 1978; Volume 355, pp. 1–6. [Google Scholar]
- Beier, P.; McCullough, D.R. Factors influencing white-tailed deer activity patterns and habitat use. Wildl. Monogr. 1990, 109, 3–51. [Google Scholar]
- Richardson, C.L. Brush Management Effects on Deer Habitat. Texas A & M AgriLife Extension. 1914. E-129. Available online: http://gillespie.agrilife.org/files/2013/02/Brush-Management-Effects-on-Deer-Habitats.pdf (accessed on 20 November 2014).
- Janssen, R.; van Herwijnen, M. Multiobjective Decision Support for Environmental Management + DEFINITE DEcisions on an FINITE Set of Alternatives: Demonstration Disks and Instruction; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; p. 232. [Google Scholar]
- Ogden, N.H.; Bouchard, C.; Kurtenbach, K.; Margos, G.; Lindsay, L.R.; Trudel, L.; Nguon, S.; Milord, F. Active and passive surveillance and phylogenetic analysis of Borrelia burgdorferi elucidate the process of Lyme disease risk emergence in Canada. Environ. Health Perspect. 2010, 118, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Pena, A. Increasing habitat suitability in the United States for the tick that transmits Lyme disease: A remote sensing approach. Environ. Health Perspect. 2002, 110, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Kitron, U.; Kazmierczak, J.J. Spatial analysis of the distribution of Lyme disease in Wisconsin. Am. J. Epidemiol. 1997, 145, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J.; Reiter, P.; Ebi, K.L.; Yap, W.; Nasci, R.; Patz, J.A. Climate variability and change in the United States: Potential impacts on vector and rodent-borne diseases. Environ. Health Perspect. 2001, 109, 223–233. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Wong, H.; Belanger, P.; Moore, K.; Peterson, M.; Cunningham, J. Analyzing the Correlation between Deer Habitat and the Component of the Risk for Lyme Disease in Eastern Ontario, Canada: A GIS-Based Approach. ISPRS Int. J. Geo-Inf. 2015, 4, 105-123. https://doi.org/10.3390/ijgi4010105
Chen D, Wong H, Belanger P, Moore K, Peterson M, Cunningham J. Analyzing the Correlation between Deer Habitat and the Component of the Risk for Lyme Disease in Eastern Ontario, Canada: A GIS-Based Approach. ISPRS International Journal of Geo-Information. 2015; 4(1):105-123. https://doi.org/10.3390/ijgi4010105
Chicago/Turabian StyleChen, Dongmei, Haydi Wong, Paul Belanger, Kieran Moore, Mary Peterson, and John Cunningham. 2015. "Analyzing the Correlation between Deer Habitat and the Component of the Risk for Lyme Disease in Eastern Ontario, Canada: A GIS-Based Approach" ISPRS International Journal of Geo-Information 4, no. 1: 105-123. https://doi.org/10.3390/ijgi4010105
APA StyleChen, D., Wong, H., Belanger, P., Moore, K., Peterson, M., & Cunningham, J. (2015). Analyzing the Correlation between Deer Habitat and the Component of the Risk for Lyme Disease in Eastern Ontario, Canada: A GIS-Based Approach. ISPRS International Journal of Geo-Information, 4(1), 105-123. https://doi.org/10.3390/ijgi4010105







