Quantitative Study on American COVID-19 Epidemic Predictions and Scenario Simulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Region and Data
2.2. Research Methods
2.2.1. Correlation Analysis and the Multicollinearity Test
2.2.2. The GTNNWR Model
2.2.3. The SEIR Model
2.2.4. Scenario Simulation Methods
2.3. Experiment Implementation
2.3.1. Experiment Design
2.3.2. Performance Evaluation
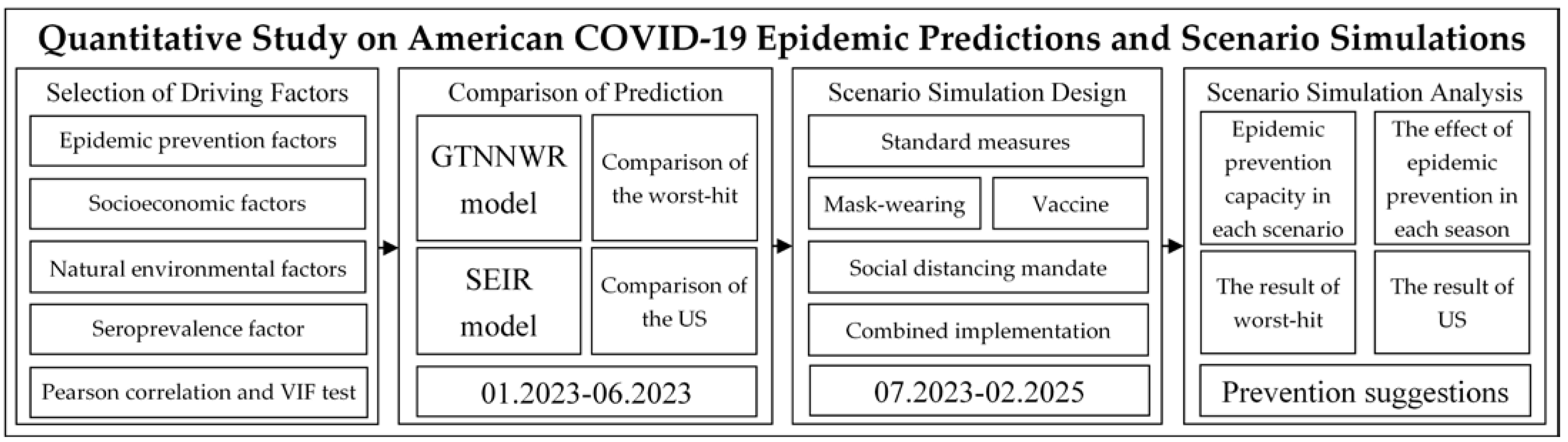
2.3.3. Research Framework
- (1)
- Selection of Driving Factors: After reviewing the relevant literature, this study selected four categories of driving factors as independent variables. Through Pearson correlation testing and multicollinearity testing, variables suitable for subsequent experiments were chosen.
- (2)
- Comparison of Prediction: This study designed comparative experiments with the commonly used SEIR model in COVID-19 prediction. It calculated the prediction accuracy of the GTNNWR model and the SEIR model relative to the actual values from January 2023 to June 2023. Comparative analyses were conducted for the 12 states heavily affected by the pandemic and the continental United States.
- (3)
- Scenario Simulation Design: Based on three health and epidemic prevention factors (mask wearing, vaccination, social distancing mandate), this study designed five scenarios. These scenarios were used as inputs for the GTNNWR model, and the death toll under different scenarios in each state was calculated.
- (4)
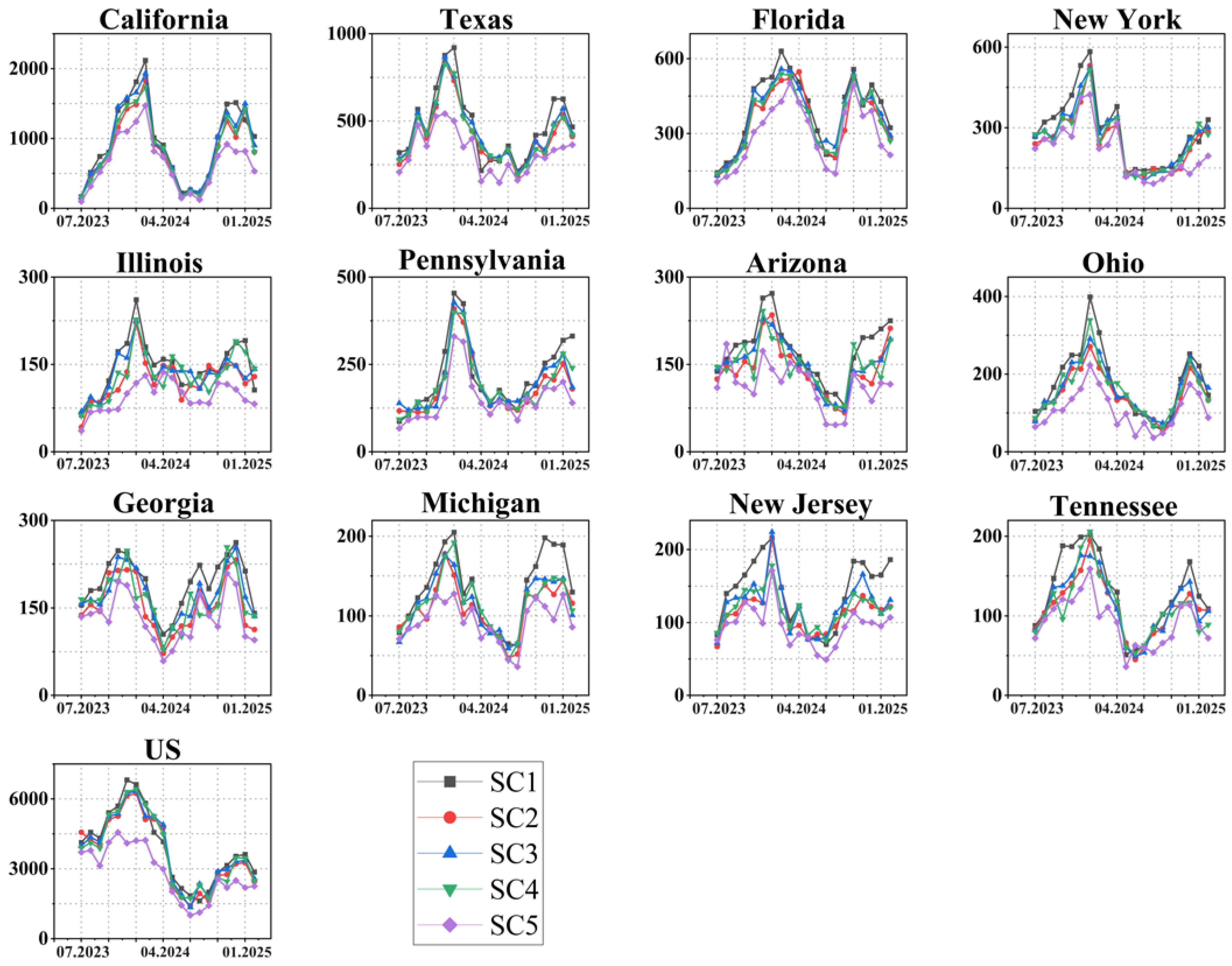
- Scenario Simulation Analysis: This step involved comparing the effectiveness of different scenario prevention measures, comparing the effectiveness of prevention measures in different seasons under the same scenario, and analyzing changes in the number of deaths in worst-hit areas and the continental United States under different scenarios.
3. Results and Discussion
3.1. Data Description and Analysis
3.2. Comparison and Analysis of the Accuracy during the Validation Period
3.3. Prediction and Analysis of COVID-19 during the Predicted Period
3.4. The COVID-19 Scenario Simulation and Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Modeling COVID-19 scenarios for the United States. Nat. Med. 2021, 27, 94–105. [CrossRef]
- Ciotti, M.; Angeletti, S.; Minieri, M.; Giovannetti, M.; Benvenuto, D.; Pascarella, S.; Sagnelli, C.; Bianchi, M.; Bernardini, S.; Ciccozzi, M. COVID-19 outbreak: An overview. Chemotherapy 2020, 64, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.-C.; Wang, C.-B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef]
- Coronavirus Disease 2019 (COVID-19). p. CDC Provides Credible COVID-19 Health Information to the U.S. Available online: https://www.cdc.gov/coronavirus/2019-ncov/index.html (accessed on 9 January 2024).
- Betsch, C.; Korn, L.; Sprengholz, P.; Felgendreff, L.; Eitze, S.; Schmid, P.; Böhm, R. Social and behavioral consequences of mask policies during the COVID-19 pandemic. Proc. Natl. Acad. Sci. USA 2020, 117, 21851–21853. [Google Scholar] [CrossRef]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Cohn, B.A.; Cirillo, P.M.; Murphy, C.C.; Krigbaum, N.Y.; Wallace, A.W. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science 2022, 375, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Cohen, C.; A Hernán, M.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Mccafferty, S.; Ashley, S. Covid-19 Social Distancing Interventions by Statutory Mandate and Their Observational Correlation to Mortality in the United States and Europe. Pragmat. Obs. Res. 2021, 12, 15–24. [Google Scholar] [CrossRef]
- Ganesapillai, M.; Mondal, B.; Sarkar, I.; Sinha, A.; Ray, S.S.; Kwon, Y.-N.; Nakamura, K.; Govardhan, K. The face behind the Covid-19 mask-A comprehensive review. Environ. Technol. Innov. 2022, 28, 102837. [Google Scholar] [CrossRef]
- Notarte, K.I.; Catahay, J.A.; Velasco, J.V.; Pastrana, A.; Ver, A.T.; Pangilinan, F.C.; Peligro, P.J.; Casimiro, M.; Guerrero, J.J.; Gellaco, M.M.L.; et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. EClinicalMedicine 2022, 53, 101624. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Samanta, G.; Nieto, J.J. Impact of optimal vaccination and social distancing on COVID-19 pandemic. Math. Comput. Simul. 2022, 200, 285–314. [Google Scholar] [CrossRef] [PubMed]
- Feyisa, H.L. The World Economy at COVID-19 quarantine: Contemporary review. Int. J. Econ. Financ. Manag. Sci. 2020, 8, 63–74. [Google Scholar]
- Chakraborty, I.; Maity, P. COVID-19 outbreak: Migration, effects on society, global environment and prevention. Sci. Total Environ. 2020, 728, 138882. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Chen, J.H.; Xu, Y.F. Patients with mental health disorders in the COVID-19 epidemic. Lancet Psychiatry 2020, 7, e21. [Google Scholar] [CrossRef]
- Flores, A.; Cole, J.C.; Dickert, S.; Eom, K.; Jiga-Boy, G.M.; Kogut, T.; Loria, R.; Mayorga, M.; Pedersen, E.J.; Pereira, B.; et al. Politicians polarize and experts depolarize public support for COVID-19 management policies across countries. Proc. Natl. Acad. Sci. USA 2022, 119, e2117543119. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.B.; Aghagoli, G.; Lavine, K.; Yang, L.; Siff, E.J.; Chiang, S.S.; Salazar-Mather, T.P.; Dumenco, L.; Savaria, M.C.; Aung, S.N.; et al. Predictors of COVID-19 severity: A literature review. Rev. Med. Virol. 2021, 31, 1–10. [Google Scholar] [CrossRef]
- Rahman, M.; Paul, K.C.; Hossain, A.; Ali, G.G.M.N.; Rahman, S.; Thill, J.-C. Machine Learning on the COVID-19 Pandemic, Human Mobility and Air Quality: A Review. IEEE Access 2021, 9, 72420–72450. [Google Scholar] [CrossRef]
- Wynants, L.; Van Calster, B.; Collins, G.S.; Riley, R.D.; Heinze, G.; Schuit, E.; Bonten, M.M.J.; Dahly, D.L.; Damen, J.A.; Debray, T.P.A.; et al. Prediction models for diagnosis and prognosis of covid-19: Systematic review and critical appraisal. BMJ 2020, 369, m1328. [Google Scholar] [CrossRef]
- Swapnarekha, H.; Behera, H.S.; Nayak, J.; Naik, B. Role of intelligent computing in COVID-19 prognosis: A state-of-the-art review. Chaos Solitons Fractals 2020, 138, 109947. [Google Scholar] [CrossRef]
- Xiang, Y.; Jia, Y.; Chen, L.; Guo, L.; Shu, B.; Long, E. COVID-19 epidemic prediction and the impact of public health interventions: A review of COVID-19 epidemic models. Infect. Dis. Model. 2021, 6, 324–342. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Liu, X.; Wang, J.; Yao, L.; Zhou, C. A Systematic Review of COVID-19 Geographical Research: Machine Learning and Bibliometric Approach. Ann. Am. Assoc. Geogr. 2023, 113, 581–598. [Google Scholar] [CrossRef]
- Isazade, V.; Qasimi, A.B.; Dong, P.; Kaplan, G.; Isazade, E. Integration of Moran’s I, geographically weighted regression (GWR), and ordinary least square (OLS) models in spatiotemporal modeling of COVID-19 outbreak in Qom and Mazandaran provinces, Iran. Model. Earth Syst. Environ. 2023, 9, 3923–3937. [Google Scholar] [CrossRef] [PubMed]
- Ardiyanto, R.; Indra, T.L.; Manesa, M.D.M. Geospatial approach to accessibility of referral hospitals using geometric network analysts and spatial distribution models of Covid-19 spread cases based on GIS in Bekasi City, West Java. Indones. J. Geogr. 2022, 54, 173–184. [Google Scholar] [CrossRef]
- Rasul, A.; Balzter, H. The Role of Climate in the Spread of COVID-19 in Different Latitudes across the World. COVID 2022, 2, 1183–1192. [Google Scholar] [CrossRef]
- Qiao, M.; Huang, B. Studying the spatiotemporal impacts of socio-demographic and mobility-related factors to predict the spread of COVID-19. Cities 2023, 138, 104360. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Huang, B. COVID-19 spread prediction using socio-demographic and mobility-related data. Cities 2023, 138, 104360. [Google Scholar] [CrossRef] [PubMed]
- Sifriyani, S.; Rasjid, M.; Rosadi, D.; Anwar, S.; Wahyuni, R.D.; Jalaluddin, S. Spatial-temporal epidemiology of COVID-19 Using a geographically and temporally weighted regression model. Symmetry 2022, 14, 742. [Google Scholar] [CrossRef]
- Manyangadze, T.; Chimbari, M.J.; Gebreslasie, M.; Mukaratirwa, S. Risk factors and micro-geographical heterogeneity of Schistosoma haematobium in Ndumo area, uMkhanyakude district, KwaZulu-Natal, South Africa. Acta Trop. 2016, 159, 176–184. [Google Scholar] [CrossRef]
- Mohammadinia, A.; Saeidian, B.; Pradhan, B.; Ghaemi, Z. Prediction mapping of human leptospirosis using ANN, GWR, SVM and GLM approaches. BMC Infect. Dis. 2019, 19, 971. [Google Scholar] [CrossRef]
- Halim, S.; Octavia, T.; Handojo, A. Dengue fever outbreak prediction in Surabaya using a geographically weighted regression. In Proceedings of the 2019 4th Technology Innovation Management and Engineering Science International Conference (TIMES-iCON), Bangkok, Thailand, 11–13 December 2019. [Google Scholar]
- Ahangarcani, M.; Farnaghi, M.; Shirzadi, M.R. Predictive Map of Spatio-Temporal Distribution of Leptospirosis Using Geographical Weighted Regression and Multilayer Perceptron Neural Network Methods. J. Geomat. Sci. Technol. 2016, 6, 79–98. [Google Scholar]
- Sollers, K.; Liu, X.; Martínez-López, B. An implementation of Geographical and Temporal Weighted Regression in modeling occurrences of Porcine Reproductive and Respiratory Syndrome in the US swine industry. Front. Vet. Sci. 2019. [Google Scholar] [CrossRef]
- Kolesnikov, A.A.; Kikin, P.M.; Portnov, A.M. Diseases spread prediction in tropical areas by machine learning methods ensembling and spatial analysis techniques. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2019, 42, 221–226. [Google Scholar] [CrossRef]
- Du, Z.; Wang, Z.; Wu, S.; Zhang, F.; Liu, R. Geographically neural network weighted regression for the accurate estimation of spatial non-stationarity. Int. J. Geogr. Inf. Sci. 2020, 34, 1353–1377. [Google Scholar] [CrossRef]
- Miao, L.; Tang, S.; Li, X.; Yu, D.; Deng, Y.; Hang, T.; Yang, H.; Liang, Y.; Kwan, M.-P.; Huang, L. Estimating the CO2 emissions of Chinese cities from 2011 to 2020 based on SPNN-GNNWR. Environ. Res. 2023, 218, 115060. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Zhang, L.; Wu, S.; Zhu, Y.; Dai, Z.; Wang, Y.; Qi, J.; Chen, Y.; Du, Z. A High-Resolution Land Surface Temperature Downscaling Method Based on Geographically Weighted Neural Network Regression. Remote Sens. 2023, 15, 1740. [Google Scholar] [CrossRef]
- Qi, J.; Du, Z.; Wu, S.; Chen, Y.; Wang, Y. A spatiotemporally weighted intelligent method for exploring fine-scale distributions of surface dissolved silicate in coastal seas. Sci. Total Environ. 2023, 886, 163981. [Google Scholar] [CrossRef]
- Liu, C.; Wu, S.; Dai, Z.; Wang, Y.; Du, Z.; Liu, X.; Qiu, C. High-Resolution Daily Spatiotemporal Distribution and Evaluation of Ground-Level Nitrogen Dioxide Concentration in the Beijing-Tianjin-Hebei Region Based on TROPOMI Data. Remote Sens. 2023, 15, 3878. [Google Scholar] [CrossRef]
- Fanelli, D.; Piazza, F. Analysis and forecast of COVID-19 spreading in China, Italy and France. Chaos Solitons Fractals 2020, 134, 109761. [Google Scholar] [CrossRef]
- Hou, X.; Gao, S.; Li, Q.; Kang, Y.; Chen, N.; Chen, K.; Rao, J.; Ellenberg, J.S.; Patz, J.A. Intracounty modeling of COVID-19 infection with human mobility: Assessing spatial heterogeneity with business traffic, age, and race. Proc. Natl. Acad. Sci. USA 2021, 118, e2020524118. [Google Scholar] [CrossRef]
- Tang, B.; Bragazzi, N.L.; Li, Q.; Tang, S.; Xiao, Y.; Wu, J. An updated estimation of the risk of transmission of the novel coronavirus (2019-nCov). Infect. Dis. Model. 2020, 5, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Lin, Q.; Ran, J.; Musa, S.S.; Yang, G.; Wang, W.; Lou, Y.; Gao, D.; Yang, L.; He, D.; et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreak. Int. J. Infect. Dis. 2020, 92, 214–217. [Google Scholar] [CrossRef]
- Choi, S.; Ki, M. Estimating the reproductive number and the outbreak size of COVID-19 in Korea. Epidemiol. Health 2020, 42, e2020011. [Google Scholar] [CrossRef]
- Chimmula, V.; Zhang, L. Time series forecasting of COVID-19 transmission in Canada using LSTM networks. Chaos Solitons Fractals 2020, 135, 109864. [Google Scholar] [CrossRef] [PubMed]
- Soures, N.; Chambers, D.; Carmichael, Z.; Daram, A.; Shah, D.P.; Clark, K.; Potter, L.; Kudithipudi, D. SIRNet: Understanding social distancing measures with hybrid neural network model for COVID-19 infectious spread. arXiv 2020, arXiv:2004.10376. [Google Scholar]
- Wang, D.; Wang, D.; Zuo, F.; Gao, J.; He, Y.; Bian, Z.; Bernardes, S.D.; Na, C.; Wang, J.; Petinos, J.; et al. Agent-based simulation model and deep learning techniques to evaluate and predict transportation trends around COVID-19. arXiv 2020, arXiv:2010.09648. [Google Scholar]
- Kai, D.; Kai, D.; Goldstein, G.-P.; Morgunov, A.; Nangalia, V.; Rotkirch, A. Universal masking is urgent in the COVID-19 pandemic: SEIR and agent based models, empirical validation, policy recommendations. arXiv 2020, arXiv:2004.13553. [Google Scholar]
- Panovska-Griffiths, J.; Kerr, C.C.; Waites, W.; Stuart, R.M.; Mistry, D.; Foster, D.; Klein, D.J.; Viner, R.M.; Bonell, C. Modelling the potential impact of mask use in schools and society on COVID-19 control in the UK. Sci. Rep. 2021, 11, 8747. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Rothman, K.J.; Ferrari, F.; Goffi, A.; Maffeis, G.; Orsini, N. Lockdown timing and efficacy in controlling COVID-19 using mobile phone tracking. EClinicalMedicine 2020, 25, 100457. [Google Scholar] [CrossRef]
- Spada, A.; Tucci, F.A.; Ummarino, A.; Ciavarella, P.P.; Calà, N.; Troiano, V.; Caputo, M.; Ianzano, R.; Corbo, S.; de Biase, M.; et al. Structural equation modeling to shed light on the controversial role of climate on the spread of SARS-CoV-2. Sci. Rep. 2021, 11, 8358. [Google Scholar] [CrossRef]
- Dutta, B.K.; King, W.R. A competitive scenario modeling system. Manag. Sci. 1980, 26, 261–273. [Google Scholar] [CrossRef]
- Lemaitre, J.C.; Grantz, K.H.; Kaminsky, J.; Meredith, H.R.; Truelove, S.A.; Lauer, S.A.; Keegan, L.T.; Shah, S.; Wills, J.; Kaminsky, K.; et al. A scenario modeling pipeline for COVID-19 emergency planning. Sci. Rep. 2021, 11, 7534. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.D.; Rosenstrom, E.; Ivy, J.S.; Mayorga, M.E.; Keskinocak, P.; Boyce, R.M.; Lich, K.H.; Smith, R.L., 3rd; Johnson, K.T.; Delamater, P.L.; et al. Association of simulated COVID-19 vaccination and nonpharmaceutical interventions with infections, hospitalizations, and mortality. JAMA Netw. Open 2021, 4, e2110782. [Google Scholar] [CrossRef]
- Francesco, P.; Lorenzo, Z.; Maurizio, P.; Alessandro, R. Modelling and predicting the effect of social distancing and travel restrictions on COVID-19 spreading. J. R. Soc. Interface 2021, 18, 20200875. [Google Scholar]
- Zhang, Y.; Yu, X.; Sun, H.; Tick, G.R.; Wei, W.; Jin, B. COVID-19 infection and recovery in various countries: Modeling the dynamics and evaluating the non-pharmaceutical mitigation scenarios. arXiv 2020, arXiv:2003.13901. [Google Scholar]
- Leung, N.H.L.; Chu, D.K.W.; Shiu, E.Y.C.; Chan, K.-H.; McDevitt, J.J.; Hau, B.J.P.; Yen, H.-L.; Li, Y.; Ip, D.K.M.; Peiris, J.S.M.; et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020, 26, 676–680. [Google Scholar] [CrossRef]
- Wake, A.D. The Willingness to Receive COVID-19 Vaccine and Its Associated Factors: “Vaccination Refusal Could Prolong the War of This Pandemic”—A Systematic Review. Risk Manag. Healthc. Policy 2021, 14, 2609–2623. [Google Scholar] [CrossRef]
- Wheeler, D.; Tiefelsdorf, M.; Fischer, M.M. Multicollinearity and correlation among local regression coefficients in geographically weighted regression. J. Geogr. Syst. 2005, 7, 161–187. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Z.; Du, Z.; Huang, B.; Zhang, F.; Liu, R. Geographically and temporally neural network weighted regression for modeling spatiotemporal non-stationary relationships. Int. J. Geogr. Inf. Sci. 2021, 35, 582–608. [Google Scholar] [CrossRef]
- Tirupathi, R.; Bharathidasan, K.; Palabindala, V.; Salim, S.A.; Al-Tawfiq, J.A. Comprehensive review of mask utility and challenges during the COVID-19 pandemic. Infez. Med. 2020, 28 (Suppl. S1), 57–63. [Google Scholar]
- Fotheringham, A.S.; Brunsdon, C.; Charlton, M. Geographically Weighted Regression: The Analysis of Spatially Varying Relationships; John Wiley & Sons: Chichester, UK, 2003. [Google Scholar]
- Brunsdon, C.; Fotheringham, S.; Charlton, M. Geographically weighted regression. J. R. Stat. Soc. Ser. D Stat. 1998, 47, 431–443. [Google Scholar] [CrossRef]
- Brunsdon, C.; Fotheringham, A.S.; Charlton, M.E. Geographically weighted regression: A method for exploring spatial nonstationarity. Geogr. Anal. 1996, 28, 281–298. [Google Scholar] [CrossRef]
- Ngonghala, C.N.; Knitter, J.R.; Marinacci, L.; Bonds, M.H.; Gumel, A.B. Assessing the impact of widespread respirator use in curtailing COVID-19 transmission in the USA. R. Soc. Open Sci. 2021, 8, 210699. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Cotrina, J.J.; Marengo-Coronel, N.; Atoche-Socola, K.J.; Peña-Soto, C.; Arriola-Guillén, L.E. Effectiveness and Recommendations for the Use of Dental Masks in the Prevention of COVID-19: A Literature Review. Disaster Med. Public Health Prep. 2021, 15, e43–e48. [Google Scholar] [CrossRef] [PubMed]
- IHME|COVID-19 Projections. p. Explore Forecasts of COVID-19 Cases, Deaths, and Hospital Resource Use. Available online: https://covid19.healthdata.org/united-states-of-america?view=mask-use&tab=trend (accessed on 9 January 2024).
- Fan, Y.; Li, X.; Zhang, L.; Wan, S.; Zhang, L.; Zhou, F. SARS-CoV-2 Omicron variant: Recent progress and future perspectives. Signal Transduct. Target. Ther. 2022, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Li, R.; Liu, H.; Fairley, C.K.; Zou, Z.; Xie, L.; Li, X.; Shen, M.; Li, Y.; Zhang, L. Cost-effectiveness analysis of BNT162b2 COVID-19 booster vaccination in the United States. Int. J. Infect. Dis. 2022, 119, 87–94. [Google Scholar] [CrossRef]
- Chi, Y.; Wang, Q.; Chen, G.; Zheng, S. The Long-Term Presence of SARS-CoV-2 on Cold-Chain Food Packaging Surfaces Indicates a New COVID-19 Winter Outbreak: A Mini Review. Front. Public Health 2021, 9, 650493. [Google Scholar] [CrossRef]



| Variables | Representations of Variables | Units | Abbreviations |
|---|---|---|---|
| Dependent variable | Number of deaths | - | - |
| Epidemic prevention factors | The outdoor mask usage rate | % | Masks-p |
| Social distancing index | - | SDI | |
| Total vaccination coverage rate | % | Vaccinate-p | |
| Natural environmental factors | Temperature | °C | Temp |
| Wind speed | m/s | WS | |
| Surface pressure | % | SP | |
| Precipitation | millimeter | Prec | |
| The poverty rate | % | Poverty-p | |
| The proportion of elderly population | % | Elder-p | |
| The unemployment rate | % | Unemp-p | |
| Socioeconomic factors | The median household income | dollar | m-Income |
| The percentage of population with a college degree | % | College-p | |
| The percentage of population that did not complete high school | % | High-p | |
| Seroprevalence | The percentage of population that had been infected by COVID-19 | % | Sero-p |
| Independent | Masks-p | SDI | Vaccinate-p | Temp | WS | SP | Prec | Poverty-p | Elder-p | Unemp-p | m-Income | College-p | High-p | Sero-p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dependent | |||||||||||||||
| 04.2020–06.2023 | −0.268 ** | 0.316 ** | −0.378 ** | −0.209 ** | −0.264 * | 0.242 ** | 0.201 ** | 0.313 ** | 0.358 * | 0.326 ** | −0.247 ** | −0.177 ** | 0.254 ** | −0.439 ** | |
| Independent | Masks-p | SDI | Vaccinate-p | Temp | WS | SP | Prec | Poverty-p | Elder-p | Unemp-p | m-Income | College-p | High-p | Sero-p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dependent | |||||||||||||||
| 04.2020–06.2023 | 8.654 | 4.223 | 6.321 | 9.336 | 6.235 | 5.681 | 2.398 | 2.314 | 8.227 | 5.312 | 2.693 | 8.361 | 1.684 | 5.255 | |
| Models | Months | CA | TX | FL | NY | IL | PA | AZ | OH | GA | MI | NJ | TN | US |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01.2023 | 92.1% | 95.8% | 91.1% | 95.1% | 90.7% | 91.1% | 88.6% | 87.6% | 86.8% | 87.7% | 87.4% | 86.6% | 91.0% | |
| 02.2023 | 85.6% | 84.6% | 82.8% | 81.8% | 76.6% | 83.9% | 92.2% | 75.7% | 81.0% | 75.0% | 89.5% | 81.2% | 91.5% | |
| GTNNWR | 03.2023 | 81.9% | 82.0% | 87.1% | 76.0% | 89.5% | 76.0% | 84.7% | 81.1% | 78.4% | 79.1% | 89.6% | 85.3% | 87.2% |
| 04.2023 | 72.7% | 78.8% | 83.5% | 81.0% | 78.2% | 86.5% | 83.9% | 74.4% | 69.0% | 83.3% | 79.8% | 75.6% | 87.4% | |
| 05.2023 | 59.6% | 69.5% | 83.2% | 72.9% | 69.6% | 80.0% | 77.3% | 81.5% | 77.9% | 72.3% | 75.7% | 72.2% | 74.7% | |
| 06.2023 | 68.6% | 73.8% | 74.4% | 79.0% | 79.2% | 78.4% | 84.8% | 67.7% | 66.7% | 68.7% | 80.0% | 77.6% | 82.6% | |
| 01.2023 | 80.30% | 83.80% | 63.00% | 74.30% | 78.20% | 88.40% | 79.70% | 78.30% | 72.90% | 76.60% | 82.40% | 78.10% | 75.80% | |
| 02.2023 | 32.10% | 62.70% | 62.70% | 71.20% | 49.20% | 58.10% | 79.10% | 40.10% | 59.30% | 49.30% | 82.90% | 76.00% | 76.10% | |
| SEIR | 03.2023 | 47.90% | 65.10% | 81.30% | 75.40% | 71.90% | 62.80% | 77.10% | 75.00% | 44.20% | 53.70% | 71.00% | 74.50% | 80.60% |
| 04.2023 | 52.10% | 52.00% | 68.70% | 64.10% | 56.90% | 79.60% | 76.60% | 60.40% | 31.00% | 72.40% | 37.10% | 71.20% | 83.00% | |
| 05.2023 | 26.50% | 58.70% | 59.60% | 53.50% | 41.30% | 65.90% | 52.10% | 33.80% | 32.60% | 50.70% | 44.30% | 56.70% | 67.70% | |
| 06.2023 | 31.00% | 59.80% | 53.40% | 63.00% | 58.50% | 41.20% | 62.10% | 58.30% | 23.50% | 50.60% | 20.00% | 65.50% | 78.70% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Qi, J.; Yan, Z.; Li, Y.; Liang, J.; Wu, S. Quantitative Study on American COVID-19 Epidemic Predictions and Scenario Simulations. ISPRS Int. J. Geo-Inf. 2024, 13, 31. https://doi.org/10.3390/ijgi13010031
Sun J, Qi J, Yan Z, Li Y, Liang J, Wu S. Quantitative Study on American COVID-19 Epidemic Predictions and Scenario Simulations. ISPRS International Journal of Geo-Information. 2024; 13(1):31. https://doi.org/10.3390/ijgi13010031
Chicago/Turabian StyleSun, Jingtao, Jin Qi, Zhen Yan, Yadong Li, Jie Liang, and Sensen Wu. 2024. "Quantitative Study on American COVID-19 Epidemic Predictions and Scenario Simulations" ISPRS International Journal of Geo-Information 13, no. 1: 31. https://doi.org/10.3390/ijgi13010031
APA StyleSun, J., Qi, J., Yan, Z., Li, Y., Liang, J., & Wu, S. (2024). Quantitative Study on American COVID-19 Epidemic Predictions and Scenario Simulations. ISPRS International Journal of Geo-Information, 13(1), 31. https://doi.org/10.3390/ijgi13010031






