Abstract
With the continuous increase in the global aging population, stroke has become one of the major diseases affecting the health of the elderly, and the upper limb motor dysfunction it causes often requires long-term rehabilitation. To improve rehabilitation outcomes for hemiplegic patients and alleviate the shortage of rehabilitation physicians, upper limb rehabilitation robots have shown great potential in enhancing motor function and improving stroke patients’ rehabilitation outcomes in clinical research. This paper first classifies rehabilitation robots based on their driving mechanisms and interaction modes, describing the application of their structural features in various scenarios. It then analyzes the optimization methods used in the trajectory planning process of rehabilitation robots at different stages. Finally, based on existing shortcomings, the paper summarizes the future development directions of upper limb rehabilitation robots, providing prospects for the development of upper limb rehabilitation robots in the areas of artificial intelligence and compliant control, multi-sensory feedback and interactive training, ergonomics and new driving technologies, modular and customizable designs, and multi-modal brain stimulation techniques.
1. Introduction
With advancements in medical technology and declining birth rates, the number of elderly people worldwide is steadily increasing. According to reports from the United Nations Population Fund and the World Health Organization, by 2024, the global population aged 60 and above is expected to account for about 13% of the total population. This proportion is expected to continue rising in the coming decades, with the elderly population estimated to reach 22% by 2050 [1]. Stroke, with its high incidence, disability rate, and recurrence rate, has become one of the major diseases affecting the health of the elderly. Age-related diseases have increasingly become a major focus of global health issues [2]. Stroke often leads to varying degrees of motor dysfunction, including both upper and lower limb disabilities. The heavy burden of aging and declining birth rates is putting increasing pressure on societies, economies, and families, along with the shortage of rehabilitation therapists. As a result, the development of effective rehabilitation methods has become an important direction in clinical research [3]. The Brunnstrom stroke rehabilitation method emphasizes the gradual restoration of motor function through upper limb rehabilitation devices to help patients recover the use of their upper limbs. Modern upper limb rehabilitation devices, such as robotic-assisted devices and virtual reality systems, are applied within this theoretical framework to promote the gradual recovery of patients at various stages of movement through simulation and assisted motion. These upper limb rehabilitation devices not only provide targeted exercise training but also adjust the intensity of the training based on the patient’s recovery progress, thereby more effectively complementing the Brunnstrom method for rehabilitation. Consequently, robot-assisted therapy has been widely applied in clinical settings and has proven effective in enhancing limb recovery and improving patients’ ability to return to productive lives.
This paper first introduces the physiological structure of the upper limb, explaining the rehabilitation training requirements based on the principles of neuroplasticity. It then classifies upper limb rehabilitation robots according to their driving mechanisms and interaction modes, reviews mainstream methods for evaluating upper limb rehabilitation training and rehabilitation trajectories, and examines various path planning methods. Finally, based on existing shortcomings, the paper summarizes the future development directions of upper limb rehabilitation robots.
2. Upper Limb Rehabilitation Training Requirements
2.1. Components of the Upper Limb
The purpose of a rehabilitation robot is to replicate the kinematics or dynamics of a human musculoskeletal structure to support the movement of the limbs. Due to the complex anatomy of the human body, there has been no unified upper limb kinematics model in past studies. Therefore, we need to analyze the anatomy of the human upper limb in order to consider the application needs of the end user. As we all know, the human upper limb has complex skeletal joints and rich muscle groups, and the upper limb movement space depends on the range of motion of the shoulder joint, elbow joint, and wrist joint, ensuring that upper limb rehabilitation robot conforms to the movement of the human upper limb is another focus of scholars’ research.
According to the theory of human upper limb anatomy [4], the upper limb is composed of a shoulder complex, an elbow complex, and a wrist complex, and the anatomy of the upper limb is shown in Figure 1. The shoulder compound joint is a joint with high degrees of freedom of movement, and is multi-functional, composed of multiple bones and muscles, which plays a vital role in the movement of the upper limbs during rehabilitation. The shoulder joint complex is composed of the scapulohumeral joint, acromioclavicular joint, sternoclavicular joint, and scapulothoracic joint, respectively, and is the joint with the most degrees of freedom in the human body. The scapulohumeral joint with an instantaneous center of rotation (COR) is often referred to as the ball-and-socket joint and forms between the joints of the humerus and glenoid [5]. The elbow compound joint is a synovial fluid compound joint consisting of the brachioradial and humeral–ulnar joints, which are hinge joints that connect the humerus of the upper arm to the radius of the forearm. Specific cubital structures include the humerus, radius, and ulna, as well as the radius and ulnar collateral ligaments and annular ligaments. The carpal compound joint consists of eight carpal bones, the distal ends of the radius and ulna, and multiple ligaments that hold the bones together. The specific bone and joint structures and specific muscles used in the upper limb rehabilitation of the shoulder, elbow, and wrist joints work together in a coordinated manner to produce different upper limb movements, and the study of the specific functions of these muscles and their coordination is essential to design effective rehabilitation training tasks and help patients achieve efficient rehabilitation training tasks [6]. By completing shoulder abduction and adduction exercises, shoulder flexion and extension exercises, elbow flexion and extension exercises, and wrist abduction and adduction exercises, the flexibility of the shoulder, elbow, and wrist joints can be maintained, the recovery of back and upper limb muscles can be stimulated, the range of motion of the affected joints can be improved, and stroke patients can regain the ability to perform daily activities, such as reaching, lifting, and grasping, while also reducing muscle spasms and preventing shoulder, elbow, and wrist pain.
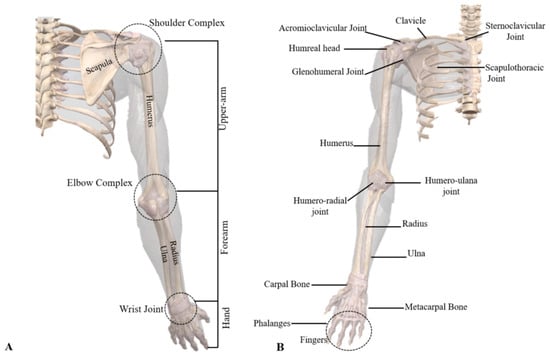
Figure 1.
Composition of the human upper limb: (A) upper limb and (B) skeletal structure of the shoulder, elbow, and wrist [5].
2.2. Principles of Neuroplasticity
As early as the beginning of the 20th century, scientists recognized that certain areas of the brain and spinal cord possessed a high degree of adaptability, although at that time, this adaptability was mainly limited to the restoration of basic functions [7]. This awareness led to the gradual development of rehabilitation techniques and methods for motor dysfunction after stroke, such as Bobath Neurodevelopmental Therapy and Brunnstrom Movement Therapy [8]. In the field of motor neuron rehabilitation, the concept of neuroplasticity was introduced [9,10]. Neuroplasticity includes various forms of regeneration in central nervous pathways, regeneration of damaged nerve axons, and compensation by the central nervous system [11]. A schematic diagram of neuroplasticity is shown in Figure 2.
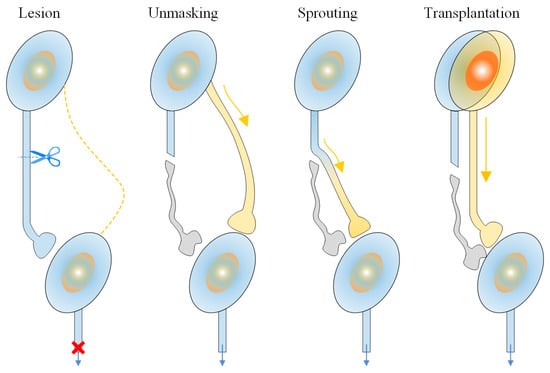
Figure 2.
A schematic diagram of neuroplasticity. The figure illustrates the regeneration of central nervous pathways, the regeneration of damaged nerve axons, and the compensation process of the central nervous system.
Neuroplasticity refers to the adaptive changes in the nervous system in response to the environment, experience, and training. It involves processes such as the reorganization of neurons, strengthening of synapses, and changes in neural networks. It is the process by which the brain adjusts its coding and learns new behaviors based on experience [12], as shown in Figure 3.
Neuroplasticity primarily includes spontaneous neuroplasticity and experience-dependent plasticity. The factors that induce spontaneous neuroplasticity are endogenous triggers, such as vascular regeneration, brain edema repair, and the repair of the peri-infarct zone. Experience-dependent plasticity is driven by exogenous factors, including the learning and acquisition of motor skills, central and peripheral nerve stimulation, and behavioral training.
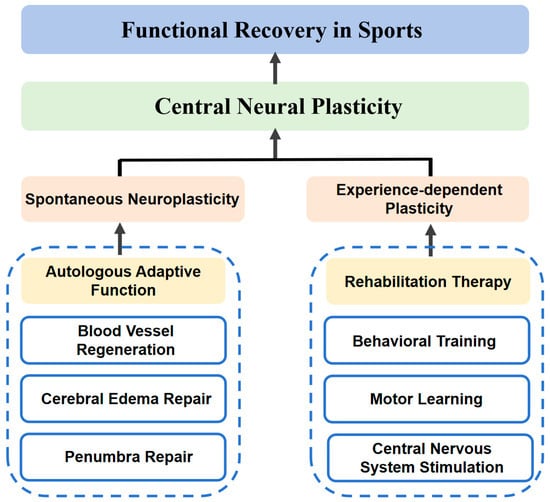
Figure 3.
Schematic diagram of the mechanism of central nervous system plasticity principles.
Based on the principles of neuroplasticity, researchers have found that neuronal synaptic responses depend on repetitive movements. The more frequently neurons are stimulated, the higher the efficiency of synaptic responses. Veerbeek [13] proposed that most rehabilitation therapies are achieved through repetitive rehabilitation training and motor learning, with clear rehabilitation goals and content. Among these, constraint-induced movement therapy, which involves restraining the affected limb using a rehabilitation device and utilizing the device to drive the affected limb through therapeutic trajectories, is one of the most effective rehabilitation techniques. By introducing the principle of neuroplasticity, positive feedback can be generated for the brain through sensory and motor input, mainly through interventions such as motor manipulation therapy, physical factor treatments, and intelligent rehabilitation robots [14,15]. These rehabilitation therapies can promote neuroplasticity through either active or passive training. Among them, motor manipulation therapy includes methods such as mirror therapy and task-oriented training, which can be conducted under the guidance of a rehabilitation therapist to guide the patient’s rehabilitation process. Physical factor treatments utilize physical stimuli, such as electrical stimulation and light stimulation, to stimulate the neuromuscular system of the affected limb for rehabilitation purposes. Intelligent rehabilitation robots are one of the most popular rehabilitation methods today. By combining robotic technology, they can provide patients with more precise repetitive training and, based on the recovery progress, generate better rehabilitation treatment methods, offering stronger adaptability. The theory of motor neuron rehabilitation technology has undergone several important developments, from early physical therapy to later advances based on neuroplasticity and modern neuroengineering technologies. Therefore, using upper limb rehabilitation robots to guide patients with upper limb motor dysfunction along pre-set rehabilitation trajectories is of great significance for promoting the recovery of upper limb motor function.
3. Classification of Upper Limb Rehabilitation Robots
According to the degree of freedom, rehabilitation robots can be divided into single-degree-of-freedom robots and multi-degree-of-freedom robots. According to their configuration characteristics, rehabilitation robots are mainly classified into two types: end-effector robots and exoskeleton robots, as shown in Figure 4. End-effector robots typically train the affected limb along a predetermined trajectory through traction. Since the robot is only connected to the distal end of the patient’s limb, there is no need for the patient to align with the robot’s joints during movement. This makes the structure of the rehabilitation robot simple, easy to control, with lower design and maintenance costs, and it is well-suited to patients. On the other hand, exoskeleton robots involve the patient wearing a multi-degree-of-freedom robot, which can fit more complex three-dimensional spatial trajectories. Exoskeleton robots can be divided into motor drive, pneumatic drive, and elastic actuator drive according to the driving mechanism. These different configuration features and design methods provide rehabilitation robots with distinctive capabilities in movement assistance, human–machine interaction, and clinical rehabilitation training effectiveness.
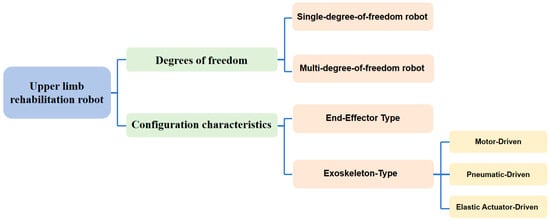
Figure 4.
Classification of upper limb rehabilitation robots.
3.1. End-Effector Type
In recent years, rehabilitation robots have developed rapidly, especially in the field of stroke rehabilitation. End-effector type upper limb rehabilitation robots connect the robot’s end effector to the patient’s hand or forearm, assisting in limb movement training through different control strategies. Traditional methods usually drive the patient’s limbs along a predetermined trajectory for rehabilitation training, while new control strategies (such as velocity field control and adaptive assistance) can provide more adaptive assistance based on the patient’s motor ability, offering better support outcomes. The most representative end-effector upper limb rehabilitation robot is the MIT-MANUS, developed by Professor Hogan’s team at the Massachusetts Institute of Technology [16]. It uses a five-bar linkage structure to pull the patient’s shoulder and elbow joints for two-dimensional planar motion. MIT-MANUS increases the patient’s effort during rehabilitation, reduces the risk of muscle injury and discomfort, and can also add appropriate damping to assist in muscle strength training. Stanford University [17] developed the MIME, an upper limb rehabilitation robot based on the PUMA560 industrial robotic arm. It binds the affected limbs using splints and assists the affected side in performing mirror movements to achieve upper limb training in a larger range of motion. The Fatronik Foundation [18] developed the low-cost home rehabilitation device ARMassist, equipped with optical and force sensors to capture the patient’s arm position and gravity in the horizontal plane. It provides remote assistance and collects motion data. Its base has driving wheels that can move the patient’s forearm in the horizontal plane and the feedback rehabilitation status on a PC. The Free University of Berlin [19] developed the Bi-Manu-Track upper limb rehabilitation robot, which enables rehabilitation training for the forearm and wrist. It can flexibly adjust the intensity, speed, and resistance of the assistance. The ReoGo robot, developed by Motorika in Israel [20,21,22], is a three-dimensional end-effector upper limb rehabilitation robot that targets shoulder and elbow joint rehabilitation. Additionally, Tsinghua University [23] designed a two-degree-of-freedom end-effector upper limb rehabilitation robot, UECM, which has various training modes, including passive, active, and assistive modes. The iPAM robot, developed by the University of Leeds [24], provides a dual-robot system with intelligent pneumatic arm movements to assist upper limb training. The robotic arms are connected to the upper limb via specially designed correctors and are equipped with rotational sensors and force sensors to measure and record movement data.
Among end-effector rehabilitation robots, there is also a special configuration—the suspension-type rehabilitation robot. This type usually uses multiple suspension cables to connect the base to fixed anchor points. It is a special cable-driven parallel robot, where the spatial position of the anchor points is adjusted by controlling the length of the cables. The cables, as the medium for transmission and traction, have excellent properties, with a motion conduction mode similar to that of biological muscles. This design overcomes the limitation of end-effector rehabilitation robots that only assist the distal connection point and allows free movement within a certain range from the preset path while the robot provides motion assistance. In addition, the inertia of the components connected to the patient’s arm is minimized, eliminating the need for complex control methods to achieve the robot’s low inertia characteristics. Suspension-type rehabilitation robots have been widely used and have shown good rehabilitation effects, especially in lower-limb rehabilitation [25]. For patients with upper limb weakness, suspension-type robots can share the weight of the upper limb through the suspension system, helping to avoid secondary fatigue or injury due to muscle weakness during rehabilitation. The NeReBot, a three-degree-of-freedom cable-driven upper limb rehabilitation robot developed by the University of Padua [26], consists of three independent motors driving three cables, which are connected to the patient’s upper limb through splints. The length of the cables is controlled to carry out rehabilitation training. GENTLE/S, developed by the University of Reading [27], is a cable-driven traction robot combined with a wheeled assistance device, mainly helping patients to perform rehabilitation movements in the horizontal plane. Cable transmission gives suspension-type rehabilitation robots the flexibility in movement, but it also causes low movement precision and the inability to accurately apply assistive torques to specific joints [28]. Its low inertia characteristics and unique gravity compensation function have laid the theoretical and practical foundation for the development of many exoskeleton rehabilitation robots. Among them, Figure 5 shows the end-guided upper limb rehabilitation robots mentioned in the text.

Figure 5.
The end-guided upper limb rehabilitation robots.
The specific information of the end-effector type upper limb rehabilitation robot mentioned in the text is shown in Table 1.

Table 1.
Classification and characteristics of the upper limb rehabilitation robot of end-effector type.
3.2. Exoskeleton Type
Exoskeleton robots are wearable assistive devices. Due to their more complex structure and control requirements, they can support multi-degree-of-freedom movement and facilitate training of complex motion trajectories in three-dimensional space. Exoskeleton robots help users reduce muscle fatigue, improve movement efficiency, and provide support and protection during prolonged physical labor [29]. Some examples of exoskeleton robots are shown in Figure 6. The specific information of the exoskeleton-type upper limb rehabilitation robot mentioned in the text is shown in Table 2.
3.2.1. Motor-Driven
The University of Arizona [30] developed a parallel exoskeleton robot, SPM, which is a new type of three-dimensional spherical parallel mechanism. It decouples and controls the shoulder’s roll, pitch, and yaw using three four-bar linkages. It offers high acceleration, which is sufficient to meet the speed requirements needed to characterize shoulder neuromuscular properties. The ARMin robot, developed by Professor Rainer at ETH Zurich [31], has six degrees of freedom and supports exercise for the forearm, elbow, and shoulder simultaneously. The developer, Hocoma, built commercial versions of the Armeo Power [32] and Armeo Spring upper limb exoskeleton robots based on ARMin. These are among the most successful commercial exoskeleton rehabilitation robots globally, equipped with interactive games and virtual reality interfaces to provide motion feedback and adjust assistive force, thereby helping patients perform active rehabilitation training in a 3D workspace [33,34]. The Harmony robot developed by the University of Texas [35] is designed with five degrees of freedom, enabling training for both arms simultaneously. It allows for customized, precise control and feedback, supports active, passive, and assisted therapy, and improves patient adherence through interaction and data recording. The five-degree-of-freedom rehabilitation exoskeleton arm developed by Cheng Yang and others from Tsinghua University [36] ensures the safety and effectiveness of rehabilitation training for stroke patients by eliminating the wrist joint and applying virtual decomposition control theory. This approach decomposes the complex system into four-degree-of-freedom subsystems for precise control. The Nanyang Technological University in Singapore [37] developed a flexible and lightweight wearable robot with three inertial measurement units (IMUs) on the forearm, upper arm, and back supports to determine the assistive torque against gravity. Adjustable parameters can be modified by rotating knobs on the joystick, making the device adaptable to individuals with different body parameters. This robot aids in comprehensive rehabilitation tasks for the shoulder, elbow, wrist, and hand. The LIMPACT exoskeleton device, developed by ETH Zurich [38], combines a lightweight frame with high-power-to-weight ratio actuators, offering dynamic transparency. The passive balancing mechanism compensates for the weight of both the exoskeleton and the human arm. Various self-aligning mechanisms ensure that the joint axes of the human body align with those of the exoskeleton, ensuring safety and reducing the time required to don and doff the device.
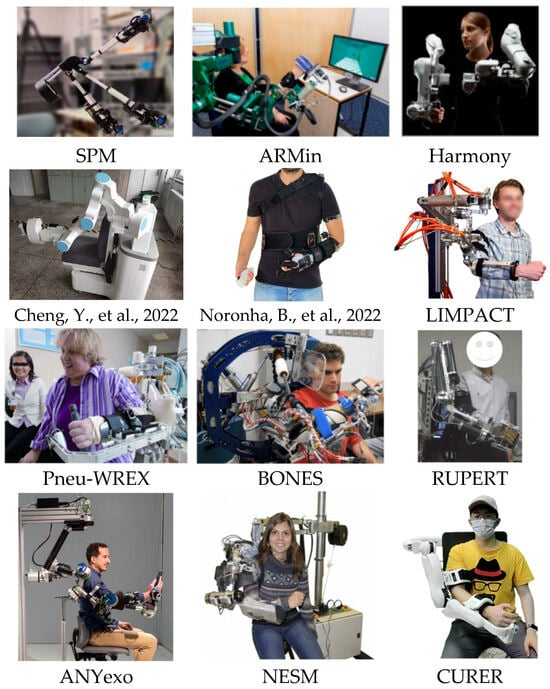
Figure 6.
Partial exoskeleton-type upper limb rehabilitation robot [36,37].
3.2.2. Pneumatic-Driven
Pneumatic actuators are a type of flexible drive system known for their lightweight, fast response, high adjustment precision, low energy consumption, and ability to simulate a range of natural movements [39]. These advantages make pneumatic actuators an ideal drive solution for rehabilitation robots. Their compliant nature also helps prevent secondary injuries during patient use, providing a more comfortable, natural, and safe rehabilitation experience.
The Pneu-WREX robot, developed by Reinkensmeyer and colleagues at the University of California [40], is a pneumatic flexible upper limb exoskeleton. It has four degrees of freedom at the shoulder and one at the elbow, using passive devices to balance the weight of the exoskeleton and the arm. Its prototype was the T-WREX rehabilitation robot [41]. However, neither of these robots allows for internal and external rotation of the shoulder joint, limiting shoulder mobility. To address this limitation, the University of California later developed the BONES pneumatic exoskeleton robot [42,43], which enables full-range shoulder motion. It uses parallel cylinders to drive a pushrod that moves the upper arm during rehabilitation training. This mechanism can generate large torque throughout most of the arm’s movement range while remaining lightweight. Tu et al. [44] developed the RUPERT exoskeleton rehabilitation robot for stroke patients with high tone in the arm flexors. The joints use pneumatic artificial muscles for unidirectional driving, while functional electrical stimulation is used to activate the paralyzed arm muscles, enabling the patient to perform active grasping training. Several pneumatic exoskeleton robots aimed at hand and wrist rehabilitation have also been developed [45,46,47,48,49], showing promising potential for future applications.
3.2.3. Elastic Actuator-Driven
Elastic actuators are drive systems with compliant characteristics. They provide mechanical flexibility through elastic components such as springs, rubber, and other elastic materials, reducing the impact forces on the patient. These actuators offer force feedback and compliance different from traditional rigid electric actuators, making them widely used in fields like rehabilitation robotics. Common types of elastic actuators include series elastic actuators (SEAs), parallel elastic actuators (PEAs), elastic muscle actuators (EMDs), and super-elastic actuators. Among them, series elastic actuators (SEAs) are the most popular research topic, known for their shock absorption, strong compliance, good adaptability, and high precision in force control.
The ANYexo 1.0, developed by ETH Zurich [50,51], is a seven-degree-of-freedom upper limb exoskeleton driven by low-impedance torque-controllable series elastic actuators. This allows it to closely simulate the therapist’s compliant and precise tactile interaction and supports a wide range of upper limb rehabilitation movements. The optimized and upgraded ANYexo 2.0 features nine degrees of freedom, enhancing the range of motion, speed, force, and tactile feedback. It also improves the kinematic structure and shoulder coupling design, offering better support for daily living activity training and providing more accurate force and speed control to accommodate a broader patient population and training types. The University of Pisa, Italy [52,53], designed the NESM upper limb rehabilitation robot for shoulder and elbow joint rehabilitation, featuring four active degrees of freedom, all using series elastic actuators. The shoulder joint has three degrees of freedom, and the robot includes eight passive joints to adjust parameters and meet the rehabilitation needs of patients with different body parameters. The optimized version, NESM-γ [54], features a quick-reversal structure that allows for both left- and right-hand use, addressing the limitation of the NESM robot, which was only suitable for unilateral arm rehabilitation. This version also improves torque and angle tracking stability. Wuhan University [55] designed a series elastic actuator for a seven-degree-of-freedom upper limb exoskeleton rehabilitation robot, CURER, which is used for training shoulder and elbow joints. Four degrees of freedom are driven by the series elastic actuators, covering most of the human arm’s motion trajectory during rehabilitation training, with precise force control and position adjustment capabilities.

Table 2.
Classification and characteristics of the upper limb rehabilitation robot of exoskeleton-type.
Table 2.
Classification and characteristics of the upper limb rehabilitation robot of exoskeleton-type.
| Robot | Type of Actuators | Number of Degrees of Freedom | Features | Supported Movements | References |
|---|---|---|---|---|---|
| SPM | DC motor | 4 | High acceleration, Sensitive activities | Shoulder, elbow, wrist | [30] |
| ARMin | DC motor | 6 | High commercial value | Shoulder, elbow, wrist | [31] |
| Harmony | DC motor | 5 | Good flexibility, easy to use | Shoulder, elbow, wrist, | [35] |
| / | DC motor | 4 | Safe and convenient | elbow, wrist | [36] |
| / | DC motor | 8 | Adapted to different body parameters | Shoulder, elbow, wrist | [37] |
| LIMPACT | DC motor | 5 | Lightweight, safe | Shoulder, elbow, wrist | [38] |
| Pneu-WREX | Pneumatic actuator | 5 | Flexible exoskeleton, safe | Shoulder, elbow, wrist | [40] |
| BONES | Pneumatic actuator | 4 | Shoulders are fully directional, light | Shoulder, elbow, wrist, fingers | [42,43] |
| RUPERT | Pneumatic actuator | 5 | Hand grip training was performed using electrical stimulation | Shoulder, elbow, wrist, fingers | [44] |
| ANYexo | Elastic actuator | 7 | Strong shock absorption and flexibility | Shoulder, elbow, wrist | [50,51] |
| NESM | Elastic actuator | 7 | Force control precision is higher | Shoulder, elbow, wrist | [52,53] |
| CURER | Elastic actuator | 7 | Precise power control and position regulation capabilities | Shoulder, elbow, wrist | [55] |
4. Rehabilitation Modes of Upper Limb Rehabilitation Robots
The recovery process for stroke patients typically consists of three stages: the passive assisted therapy phase during the subacute period, the active therapy phase during the recovery phase, and the bilateral therapy phase with some motor ability [56]. These rehabilitation methods help restore the patient’s limb mobility and coordination. The main rehabilitation modes are shown in Figure 7. Table 3 presents the summary of target patients, therapy procedure, and treatment types in terms of advantages. Depending on the specific needs of each rehabilitation stage, the rehabilitation modes of upper limb rehabilitation robots are divided into assistive mode, corrective mode, and resistance mode, with the assistive mode being the primary mode of the upper limb rehabilitation robot.
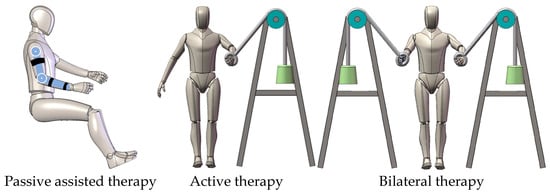
Figure 7.
Rehabilitation modes of the upper limb rehabilitation robot.

Table 3.
Summary of targeted patients, therapy procedure, and treatment types in terms of advantages.
4.1. Assistive Mode
The assistive mode of upper limb rehabilitation robots can be divided into passive assistive mode and partial assistive mode. The difference between the two lies in whether the patient actively participates in the therapy. Active therapy refers to the patient’s active involvement in the rehabilitation process, where they control and complete movements themselves. During this process, the patient uses their own muscle strength and neural control to perform tasks. This approach helps promote the recovery of muscle strength, endurance, and motor coordination, while also enhancing the adaptability of the nervous system [4].
4.1.1. Passive Assistive Mode
In rehabilitation therapy, passive assistive mode refers to a scenario where the patient’s upper limb is moved by the rehabilitation device, with no active involvement of the patient’s voluntary movement intention in the control. The purpose of passive movement is to maintain joint mobility, prevent joint stiffness and muscle atrophy, promote blood circulation, and reduce muscle tension. Passive therapy is mainly used in the early stages of recovery when the patient is unable to actively control the limb or when motor function is severely limited. Typically, during the early recovery phase of stroke patients, when muscle strength is graded 0–2, they are unable to support the weight of the upper limb [57]. In such cases, the patient is unable to actively participate in therapy and requires the rehabilitation device to provide full assistance, guiding the affected limb through the rehabilitation training along a pre-determined trajectory. The most commonly used robotic trajectory tracking control method is PID (Proportional–Integral–Derivative) control, which adjusts the three control parameters—proportional (P), integral (I), and derivative (D)—to minimize errors and maintain the robot’s motion along the desired trajectory. Due to its fast response, disturbance resistance, and ease of parameter tuning, PID control and its derivative controllers are widely applied in upper limb rehabilitation robots [58,59,60]. A typical example is the work of the Rahman team [61], which used linear PID control to operate an upper limb exoskeleton robot along a pre-set trajectory, effectively tracking the target rehabilitation path and providing passive assistive therapy to the patient’s wrist and elbow.
A key issue in passive assistive mode is how to obtain the target trajectory. There are several methods for acquiring the target trajectory, such as using optical capture systems, like depth cameras, to calibrate upper limb joint points, measuring the motion trajectories of healthy individuals, and then obtaining preset rehabilitation trajectories for the upper limb rehabilitation robot through methods like clustering or spline interpolation [62]. For example, Chakraborty et al. [63] designed a single-degree-of-freedom Stephenson-type six-bar mechanism rehabilitation glove. To measure the motion trajectory of the joints in a normal human hand, they used a depth camera and tracked the motion of the fingers repeatedly in Kinovea 2023 software, using image processing to provide accurate finger trajectories. Zhao Ping and colleagues from Hefei University of Technology [64] extracted the shoulder, elbow, and wrist joint motion trajectories of subjects with different body parameters using a depth camera and then performed clustering regression to generate a rehabilitation task trajectory containing 28 task points. In addition to providing real-time feedback and adjustments, strengthening motor neural pathways, and obtaining target trajectories [65,66], methods also include direct demonstration by rehabilitation therapists and mirror therapy. Hefei University of Technology [67] designed an intelligent upper limb rehabilitation robot that combines flexible cables and an exoskeleton. It employs an optimal adaptive robust control design based on the Udwdia–Kalaba theory and fuzzy set theory. The system uses sensors to detect changes in the tension on the cable, allowing the robot to adjust its position accordingly and achieve drag teaching. National Taiwan University [68], combined with the mirror motion mode, described that the robot can estimate the posture of the upper limb through two IMUs worn by the patient or physician on the healthy side to obtain the upper limb rehabilitation trajectory. Upper limb rehabilitation trajectories refer to the process of helping patients restore upper limb function, improve motor abilities, and enhance quality of life through a series of treatment and training measures. These trajectories are typically developed based on factors such as the patient’s underlying condition, type of injury, rehabilitation goals, and treatment stage. In the passive assistive mode for early-stage rehabilitation patients, simpler rehabilitation trajectories are often selected for use, such as linear trajectories [69], arc-like trajectories, including circles, ellipses, and teardrop shapes [70], spiral trajectories [71], and multi-dimensional composite trajectories [72].
4.1.2. Partial Assistive Mode
Although the passive assistive mode can reduce the patient’s burden and help with muscle strength and neural function recovery, it is only suitable for training during the early stage of rehabilitation when muscle strength is insufficient. To achieve better interaction with the human body, in the middle and later stages of rehabilitation, upper limb rehabilitation robots need to capture the patient’s movement intentions to facilitate interactive rehabilitation training [73]. Therefore, the key technical challenge of partial assistive mode lies in accurately recognizing the patient’s intentions, which can be divided into discrete and continuous variables [74]. Discrete motion control is relatively simple, usually requiring the recognition of the patient’s intent to start or stop movement. The robot then performs a pre-defined action based on discrete signals, with the main difficulty lying in decoding the patient’s intent [75]. Continuous variables typically include the patient’s muscle-generated torque, interaction torque, joint angles, etc. The robot can adjust the speed or assistive torque based on such data [76].
Currently, in the field of rehabilitation robotics, the recognition of human motion intent is based on physical human–machine interaction strategies. According to the type of intent perception, these strategies are mainly divided into those based on human electrical signals and those based on human force signals.
- (a)
- Perception and Control Strategies Based on Human Bioelectric Signals
In 1781, Galvani confirmed that muscle contraction is closely related to electricity, a discovery that marked the beginning of a new era in electrophysiology. Subsequently, numerous discoveries and studies were made regarding “bioelectricity” and the conduction of electromyographic neural impulses. In 1894, German biologist Dubois-Reymond discovered that the electrical activity produced when muscles contract actively could be recorded and introduced the concept of “action potential”. In 1890, Marey recorded the first instance of this “electrical activity,” which we now call an electromyogram (EMG) [77]. Following this, instruments such as the electroencephalogram (EEG), electromyogram (EMG), and electrogastrogram (EGG) were invented based on the characteristic changes in bioelectric signals associated with different physiological states of the human body. These advancements pushed the application and observation of human bioelectric signals into various fields. Among them, surface electromyography (sEMG) signals are closely related to the functional and activity states of muscles and can reflect neuromuscular activity to some extent. Moreover, sEMG has advantages such as non-invasiveness, real-time measurement, simplicity, and the ability to perform multi-target measurements. Therefore, surface EMG technology, which records the tiny electrical changes during muscle movement, is widely used in rehabilitation medicine, sports science, and other fields.
Human voluntary movement intentions are transmitted directly or indirectly through various means, such as speech [78], unaffected-side gestures (mirror therapy) [79], electromyographic (EMG) signals [80], or electroencephalogram (EEG) signals [81].
MAKOWSKI N [82] evaluated the performance of a reaching motion with the assistance of an upper limb rehabilitation robot, demonstrating that even weak EMG signals could provide effective rehabilitation commands for assistive devices. The BORIS team in France [83] developed the EMY wearable robot, which decodes brain activity from patients with limb paralysis using epidural implanted electrodes, thereby assisting patients with movement. However, the surgical difficulty and risks associated with invasive electrodes make them hard to popularize. Moreover, the decoding process of electrophysiological signals is exceptionally complex, and the low success rate and accuracy of decoding remain significant technical challenges [84].
Currently, there are several limitations to robot-assisted rehabilitation training based on bioelectric signals: high cost and technical barriers, susceptibility to signal noise interference, low accuracy in intent recognition, insufficient robustness, and a lack of diverse motion planning strategies, making it difficult to accommodate patients with different physical parameters. Although bioelectric signal-based control methods have not yet been widely adopted in commercial applications, they hold enormous potential for improving human–machine interaction models.
- (b)
- Perception and Control Strategies Based on Human Force Signals
Force feedback signals reflect the contact force between the rehabilitation device and the patient’s upper limb, enabling effective control over the actual contact force at the limb’s end. This is crucial for the progress of the patient’s rehabilitation training.
In the research of force signal perception and control strategies for upper limb rehabilitation robots, most studies focus on impedance control models for robots. Impedance control establishes a relationship between the robot’s end-effector position or velocity and the end-effector contact force. It adjusts the position based on the difference between the actual contact force and the desired force, thereby regulating the end-effector contact force. This approach ensures effective control of the robot in constrained environments and is a simple and efficient method for handling robotic force control [85]. Impedance control was first introduced in 1984 by the HOGAN team at MIT [86], and the MIT-MANUS, designed by their team, was the first rehabilitation robot to use impedance control. This robot provided compliant interaction in the horizontal direction while guiding hand movement, establishing a dynamic relationship between the robot’s motion and external interaction forces, and incorporating a spring-damper-mass model in the controller to enable compliance. Northeastern University [87] proposed a passive compliance control system for upper limb rehabilitation robots based on impedance control. The system consists of trajectory planning and tracking, using the patient’s human–machine interaction force to adjust the initial desired trajectory via an impedance trajectory regulator to achieve compliance control.
However, standalone impedance control is insufficient to meet the complex control requirements of modern rehabilitation devices. When the robot interacts with an uncertain external environment, impedance control fails to maintain accurate trajectory tracking. The performance of the impedance controller is especially impacted when there are external disturbances. Fixed-parameter impedance models are inadequate for uncertain external environments [88]. Therefore, combining impedance control with other control methods that have better performance is needed to improve the system’s adaptability in uncertain environments. The existing robot impedance control models include force-based impedance control models [89], position-based impedance control models [90], adaptive impedance control models [91], and hybrid force-position control models [92], all of which can safely and effectively complete rehabilitation tasks with a certain level of compliance control.
ARMin IV employs feedforward torque compensation and a velocity-based disturbance observer. By installing torque sensors on the patient’s upper limb, it provides more intuitive motor drive torque feedback [93]. Elgeneidy et al. [94] designed a discontinuous control algorithm that enables angle tracking control of the actuator and real-time feedback of both actuator end-angle and contact force. Doulgeri et al. [91] developed an adaptive impedance controller to address the challenges of non-linear systems where accurate mathematical models cannot be established. Xu et al. [95] designed an adaptive impedance scheme, considering system uncertainties and external disturbances, to improve trajectory tracking control for the robot’s desired force signals and enhance system stability. The University of California developed a sensor fusion technique based on Kalman filtering. The team fused information from three sets of torque sensors to obtain the total interactive torque, which was then mapped into the joint space to estimate the external torque on each joint of the EXO-UL8 [96].
While bioelectric signal-based perception and control can help control rehabilitation robots and provide direct feedback on muscle activity in some cases, force signal perception is typically superior. This is because force signals are more closely related to natural human movement, provide better signal stability, and offer more accurate, intuitive, and real-time feedback in practical applications [97]. Force signals can help robotic systems better simulate human movement patterns, enabling more natural and efficient rehabilitation training, particularly in multi-degree-of-freedom movements and complex rehabilitation tasks. Therefore, force signal perception is considered to have greater advantages in the field of rehabilitation robotics.
4.2. Correction Mode
The correction mode is typically used when the patient has a certain level of motor ability but still exhibits movement deviations or lack of coordination. In this mode, robots provide real-time feedback or impose limitations during the patient’s movement, helping to restore the normal movement trajectory. It is used to correct the patient’s poor movement patterns, such as deviation from the target trajectory or improper posture, and provides reliable feedback to help the patient correct movement defects. In correction mode, the robot is mainly used to adjust the patient’s movement trajectory or posture, ensuring that the patient’s movement meets the correct biomechanical requirements. This mode is often applied when the patient exhibits abnormal or deviated movement patterns, such as poor coordination, incorrect joint positioning, or unnatural movement paths.
For example, Jihong Zhu’s team developed an upper limb dressing-assistance robot [98], which uses the elbow angle, a key factor affecting the dressing movement, as a feature. A “virtual tunnel” is defined along a pre-determined dressing path. When the patient’s hand is inside the tunnel, the robot provides partial assistance tangentially. When the patient’s hand moves away from the tunnel, the robot corrects the movement normatively, helping the patient perform the correct motion. A similar “virtual tunnel” strategy is employed in the rope-driven robot CAREX developed by Columbia University [99]. Unlike Jihong Zhu’s upper limb dressing-assistance robot, CAREX features a rope-driven system where each direction (tangential and normal) of the handle’s motion includes a proportional controller. This system acts like a virtual spring for the patient’s arm, adjusting the correction scheme near the virtual path. Clinical experiments show that this type of robot can keep the patient’s upper limb joint posture within the “virtual tunnel” or very close to the desired trajectory, making the correction mode highly effective. Similarly, the ARMEO series upper limb rehabilitation robots developed by the Swiss company Hocoma [100] also use correction mode. In this mode, the ARMEO robot tracks the patient’s upper limb movement trajectory in real time. If the patient’s movement is incorrect or uncoordinated, the robot applies slight external forces to help adjust posture or direction, ensuring proper motion. For example, during arm extension or flexion, the robot guides the movement to prevent the patient from compensating with incorrect actions, such as relying on other muscle groups. The correction mode helps avoid compensatory movements and encourages the use of correct muscle groups. Another example is the portable upper limb rehabilitation robot ReoGo developed by RehabCare [101]. This static arm exoskeleton with a compact wheeled platform can generate wide, repeatable movements in 3D space and correct incorrect movement postures. The MIT-Manus robot also uses correction mode to intervene in the patient’s movement, allowing them to develop muscle memory for precise movements, correcting incorrect trajectories, and improving movement accuracy, thus promoting muscle strength recovery [102].
4.3. Resistance Mode
Resistance mode is suitable for the later stages of rehabilitation, when the patient has acquired a certain level of independent motor ability. By increasing resistance, patients can further challenge their motor skills, promoting muscle strength improvement. In resistance mode, the robot applies specific resistance, requiring the patient to overcome it during active movement. This mode primarily focuses on enhancing muscle strength and endurance by increasing the load on the movement, thereby promoting muscle strengthening and functional recovery. Robots with resistance mode provide real-time feedback or impose limitations during the patient’s movement, helping restore normal movement trajectories and correct poor movement patterns, such as deviation from the target trajectory or improper posture, while providing feedback to help the patient correct movement defects and engage in more challenging training. The MIT-Manus robot can intervene in the patient’s movement through resistance mode, increasing the muscle workload to promote muscle strength recovery. ExoRehab, an exoskeleton rehabilitation robot system, is designed to provide functional movement training to help patients restore motor function. The system offers resistance mode to help enhance muscle strength, requiring patients to resist external resistance applied by the system [103]. The upper limb rehabilitation robot developed by Bournemouth University [104] uses springs to provide resistance. It can offer three stages of rehabilitation within a single structure. In resistance mode, the motor does not provide assistance; the patient overcomes the spring’s pull to achieve training effects with minimal energy consumption. Compared to existing models, this upper limb rehabilitation robot improves the torque-to-weight ratio.
Robots with both correction and resistance modes are still rare, mainly due to factors such as high technical complexity, high costs, variations in clinical needs, individualized patient requirements, comfort and safety concerns, and the lack of unified standards. Although this type of robot theoretically has high application potential, its design and development still face significant challenges. With advancements in technology and changing clinical needs, more rehabilitation robots featuring both functions may enter the market in the future, though it remains a highly specialized field at present.
5. Path Planning for Upper Limb Rehabilitation Robots
Path planning is one of the most important aspects of designing upper limb rehabilitation robots [105]. Rehabilitation robots use path planning methods to assist patients in completing specific rehabilitation tasks, guiding the robot to move the affected limb along a pre-determined path to help restore motor function. Path planning ensures that the robot moves the patient’s limb in a controlled and safe manner, reducing the risk of injury. Additionally, path planning can be used to customize the rehabilitation process according to each patient’s specific needs and abilities, thus providing more personalized and effective treatment. Over the years, path planning methods for rehabilitation robots have mainly focused on improving the efficiency, accuracy, and safety of the robot-assisted treatment.
The development of robot path planning algorithms reflects the ongoing interaction and progress between algorithm theory and practical application needs. From early graph search techniques to today’s intelligent algorithms, path planning technology has undergone significant evolution to adapt to the complexity and diversity of environments [106]. Path planning algorithms began with simple graph search techniques, such as the A* algorithm, which is a new method combining heuristic algorithms like BFS with traditional algorithms like Dijkstra to find the shortest path in known environments. Thanks to its excellent flexibility, the A* algorithm is suitable for various scenarios and has become one of the most popular search methods [107]. The D* algorithm is an improvement of the A* static algorithm and is an incomplete replanning method based on the original data. D* is a fast and efficient optimization method, particularly useful for environments with uncertainty or changing conditions. Unlike A, which searches from the start to the goal, D works in reverse, from the goal to the start [108]. Random sampling methods and biomimetic algorithms provide solutions for path planning in unknown or partially known environments. Currently, deep reinforcement learning algorithms, with their excellent decision-making capabilities, have become a research hotspot for planning paths in unknown and complex dynamic environments. Commonly used path planning algorithms are shown in Figure 8.
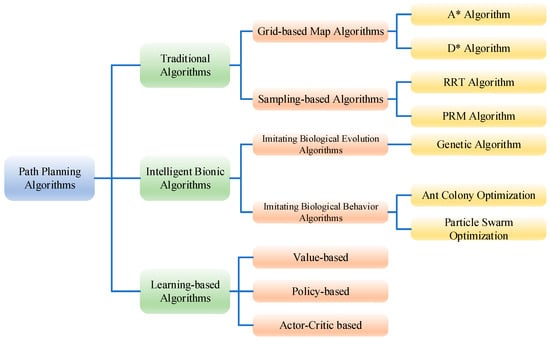
Figure 8.
Commonly used path planning algorithms.
In the early stages of rehabilitation robotics, path planning algorithms relying on predefined trajectories, such as Rapidly-exploring Random Tree (RRT) and Artificial Potential Field (APF) methods, were commonly used [109,110,111]. However, despite the widespread application of the Artificial Potential Field method, especially in situations requiring high real-time performance, its drawbacks—such as the local minima problem, inflexible path planning, and high computational cost—have limited its use in complex environments. To overcome these shortcomings, researchers often combine the Artificial Potential Field method with other path planning techniques (e.g., A* algorithm and Dijkstra’s algorithm) or adopt improved versions of the method (e.g., modified APF or fuzzy logic-based control approaches) to enhance its performance [112]. The Swiss Federal Institute of Technology Zurich [113] proposed a scheme combining adaptive impedance control and trajectory learning, developing a trajectory learning algorithm that provides constant force assistance for repetitive training tasks and applies it to a predefined path coordinate system, allowing the rehabilitation robot to iteratively update based on subsequent interaction forces. Shanghai University, combining the A* path planning method, proposed a path planning approach that guarantees the robot’s motion performance and ensures high conformity to the desired path [114]. The Institute of Automation, Chinese Academy of Sciences [115] proposed a real-time trajectory generation algorithm based on adaptive frequency oscillators to extract high-order motion features of robotic arms and an adaptive correction algorithm for subjects, which was validated on the upper limb rehabilitation robot CASIA-ARM II, showing its ability to provide comfortable and high-quality rehabilitation training for patients. LAURETTI et al. [116] proposed an upper limb exoskeleton motion planning method combining dynamic movement primitives (DMPs) and machine learning, helping patients perform Activities of Daily Living (ADL) tasks in unstructured environments. CORDELLA et al. [116] used DMPs to learn human daily activity trajectories and guide exoskeleton-assisted upper limb rehabilitation.
Currently, rehabilitation robot path planning methods mainly focus on human–robot interaction, adaptive and personalized path planning, multi-objective optimization, and real-time path planning [117,118,119]. These efforts aim to enhance patient acceptance and engagement, ensuring safety and comfort during rehabilitation; adapting to changes in the patient’s physical and cognitive states, and personalizing rehabilitation training based on individual characteristics; considering multiple objectives, such as energy consumption, patient comfort, and rehabilitation outcomes, to find an optimal balance between factors; achieving real-time control of robots with quick, efficient path planning algorithms capable of responding to unexpected changes in the environment or patient condition. Additionally, machine learning and AI technologies are being applied to path planning, enabling personalized rehabilitation that evolves alongside the patient’s progress. In summary, the development of path planning methods for rehabilitation robots is progressing rapidly, holding significant potential for improving the quality of rehabilitation services and enhancing training outcomes.
6. Future Development Directions
With the advancement of technology and the evolving demands of healthcare, upper-limb rehabilitation robots have evolved from early assistive devices to efficient rehabilitation tools, widely applied in the treatment of stroke, spinal cord injury, brain trauma, and other conditions. Despite significant improvements in functionality and accuracy, there remain many technical and application challenges that need to be addressed. In the future, upper limb rehabilitation robots will experience breakthroughs in several critical areas.
- (1)
- Artificial Intelligence and Compliant Control
With the development of artificial intelligence (AI), machine learning (ML), and big data technologies, future upper limb rehabilitation robots will become more intelligent, capable of providing personalized rehabilitation plans tailored to the specific conditions and needs of each patient. Future rehabilitation fields should focus on upgrading decision-making systems for rehabilitation assessments [120]. Using reinforcement learning methods combined with clinical evaluation metrics, patient recovery status will be evaluated based on movement posture features, and individualized reports will be provided. Robots will likely be able to autonomously perform functional assessments, treatment plan formulation, and movement assistance based on large amounts of image, voice, and video data from rehabilitation scenarios, using supervised learning methods to mimic the decisions and operations of rehabilitation physicians [121].
Currently, most robots still rely on preset movement patterns and standardized training plans. In rehabilitation training, different tasks require varying levels of effort from patients, and as rehabilitation progresses, the patients’ muscle strength changes. These factors necessitate the robots adjust their impedance to provide appropriate assistance while continuously monitoring the patient’s physiological responses (e.g., muscle activity, range of motion, and fatigue) to intelligently adjust the intensity, duration, and type of training, thus customizing the rehabilitation program. Additionally, robots will need stronger remote monitoring and cloud collaboration capabilities, allowing real-time data upload to the cloud, enabling doctors and therapists to remotely monitor progress and adjust treatment plans based on data analysis. To address these challenges, impedance coefficients must be set within an appropriate range. The nonlinear dynamics of rehabilitation robots complicate stability analysis, and online adjustments to impedance coefficients increase this complexity. Incorrect impedance settings could destabilize the robot system. Moreover, perceiving and interpreting the patient’s movement intentions to enable intention-driven rehabilitation training will effectively facilitate sensory function recovery [122]. Therefore, developing cost-effective, reliable sensors to accurately estimate the patient’s active intention and primary torque is another challenge. Solving these problems can improve the human–robot interaction experience for patients. As sensor systems advance, more precise feedback information and sensor fusion technologies will drive the application of AI control systems in rehabilitation, achieving more precise control. Moreover, with the development of AI and machine learning, future upper limb rehabilitation robots will aim to replicate the natural movement patterns of the human upper limb more accurately, studying the movement characteristics of the human upper limb in-depth. This will allow rehabilitation equipment to provide mechanical stimuli necessary for remodeling neural circuits and use lightweight, flexible materials to improve ergonomics, enhancing patient comfort and promoting a natural movement experience.
- (2)
- Multisensory Feedback and Interactive Training
Traditional upper limb rehabilitation robots mainly rely on visual and mechanical feedback for training. However, this single feedback method often fails to fully engage patients or stimulate their interest in training. Future robots will integrate more feedback mechanisms, including haptic feedback, auditory feedback, and the use of virtual reality (VR) or augmented reality (AR) technologies, enabling patients to receive richer, more intuitive sensory experiences during training. For example, studies have shown that VR-based therapies using upper limb rehabilitation devices improved quality of life, dynamic stability, and upper limb motor function [123]. VR has been proven more beneficial than traditional therapy in achieving dynamic balance. By integrating VR technology, upper limb rehabilitation robots can provide interactive tasks in virtual environments, simulating everyday actions such as grasping, placing, and picking up objects, which enhances the fun and effectiveness of rehabilitation while increasing patient engagement [119].
- (3)
- Ergonomics and New Drive Technologies
The comfort of wearable exoskeletons requires specific quantification, but there is currently no unified standard for analyzing human upper limb kinematics. Ideally, physiological joints should align well with the exoskeleton. However, physiological joints, which have complex structures, do not operate like traditional mechanical joints, and due to the influence of shoulder rhythms, achieving a perfect fit between the shoulder joint of the human body and the exoskeleton robot is particularly challenging. As a result, the rotational joint axes may shift during rehabilitation training, potentially leading to secondary injuries. Additionally, the connection between the exoskeleton and the human body is not rigid, meaning that sliding may occur between the exoskeleton and the limb during task execution. Researchers can optimize the mechanism based on accurate models to address common issues such as misalignment between the joint axes of the robot and the human body [124]. Some studies have already attempted to overcome joint misalignment by considering complex human biomechanics and developing new flexible compensation mechanisms for connecting robots to the upper limb, improving comfort during movement [125,126,127,128].
- (4)
- Modular and Customizable Design
Despite significant technological breakthroughs in existing upper limb rehabilitation robots, their widespread use is still limited due to factors such as high manufacturing costs and large device size. In the future, upper limb rehabilitation robots will see innovations in manufacturing processes, materials, and production models, lowering costs and enhancing usability. For instance, 3D printing technologies could strike a balance between personalized and mass production, reducing production costs. By simplifying device design or adopting modular structures, both initial purchase costs and the ability to upgrade or adjust robot functions as rehabilitation progresses can be controlled, avoiding unnecessary expenditures. As community- and home-based rehabilitation concepts gain popularity, future upper limb rehabilitation robots will trend toward lightweight and portable designs. Therefore, rehabilitation devices can be lightweight and customizable to accommodate patients with varying physical parameters [129]. While ensuring effective treatment, upper limb rehabilitation robots can reduce high-end features, focusing on basic rehabilitation needs and removing complex operating systems and high-precision sensors, making the overall system more straightforward and easier to maintain, thus lowering costs. With advanced materials and engineering technology, the robot structure will become lighter and more flexible, offering a more comfortable wearing experience. Improvements in lightweight and portability will make rehabilitation training more convenient and sustainable for patients in daily life.
- (5)
- Multimodal Brain Stimulation Technology
With the development of brain–machine interface (BCI) technology, future upper-limb rehabilitation robots may combine with non-invasive neurostimulation technologies to establish multidimensional, collaborative rehabilitation plans. By monitoring and modulating brain neural activity, these technologies can promote the restoration of motor functions. BCI technology allows for direct interaction between electrodes and the brain, enabling more precise control of the robot by the patient and assisting in the retraining of the nervous system. Currently, brain stimulation techniques such as transcranial direct current stimulation, epidural electrical stimulation, focused ultrasound stimulation, transcranial magnetic stimulation, and transcranial photo biomodulation [130,131,132,133,134] have been shown to positively affect neural function remodeling in brain-injured patients. The combination of these brain stimulation methods with rehabilitation robots in collaborative treatment is still in the exploratory stage. Preliminary clinical trials suggest that integrating brain stimulation technologies with rehabilitation robots provides superior outcomes by comparison.
7. Conclusions
Upper limb rehabilitation robots play a crucial role in motion assistance and rehabilitation applications. This paper provides a comprehensive review of the current development of upper limb rehabilitation robots. First, it introduces the physiological structure of the upper limb from an anatomical perspective, explaining the rehabilitation training requirements based on the principle of neural plasticity. Next, it classifies upper limb rehabilitation robots according to their drive mechanisms and interaction modes and discusses methods for evaluating upper limb rehabilitation training in conjunction with the robots’ structural configurations. Various path planning methods are also discussed. Finally, based on the existing limitations, the paper summarizes the future development directions for upper limb rehabilitation robots, including artificial intelligence and compliant control, multisensory feedback and interactive training, ergonomics and new drive technologies, modular and customizable designs, and multimodal brain stimulation techniques. In terms of intelligence and control, upper limb rehabilitation robots can integrate sensor fusion technology to accurately perceive the patient’s movement intentions, enabling precise control. Regarding interactivity, they can incorporate haptic, auditory, and VR/AR technologies to enhance the immersion and engagement of rehabilitation training, thus improving patient participation and therapeutic efficacy. In terms of ergonomics, the exoskeleton structure can be optimized to solve joint alignment issues, lightweight materials can be used to improve wearing comfort, and secondary injuries can be avoided. The final product can reduce costs through 3D printing and modular structures, while exploring collaborative treatment solutions to promote neural function remodeling. This will drive the development of upper limb rehabilitation robots toward intelligence, personalization, and widespread adoption, ultimately improving rehabilitation efficiency and the quality of life for patients.
Upper limb rehabilitation robots have already demonstrated significant value in clinical practice, but their safety, effectiveness, and level of intelligence still need further improvement, including stability analysis, intention perception algorithms, and more. To this end, developers in rehabilitation institutions should closely collaborate with clinical medical experts to establish a more complete technical framework. At the same time, interdisciplinary integration, particularly the combination of sports science, neuroscience, and robotics, will promote further development of rehabilitation robot theory and practice, providing important academic support and application prospects for advancing intelligent rehabilitation devices and elderly care globally.
Author Contributions
Conceptualization, Y.W.; methodology, P.Z. and Y.W.; software, Y.W., X.H.; validation, X.H., Y.W.; formal analysis, Y.W.; investigation, Y.W., X.H., B.X.; resources, Y.W., X.H., B.X.; data curation, Y.W., X.H.; writing—original draft preparation, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Langhorne, P.; Coupar, F.; Pollock, A.J.T.L.N. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Wu, S.; Wu, B.; Liu, M.; Chen, Z.; Wang, W.; Anderson, C.S.; Sandercock, P.; Wang, Y.; Huang, Y.; Cui, L.; et al. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019, 18, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ushiba, J. Brain–machine Interface (BMI)-based neurorehabilitation for post-stroke upper limb paralysis. Keio J. Med. 2022, 71, 82–92. [Google Scholar] [CrossRef]
- Pan, G.-X.; Fu, H.-Q.; Zhang, X.-F.; Ma, F.-L. Research on bionic mechanism of shoulder joint rehabilitation movement. In Wearable Sensors and Robots, Proceedings of International Conference on Wearable Sensors and Robots 2015, Hangzhou, China, 16–18 October 2015; Springer: Berlin/Heidelberg, Germany, 2017; pp. 181–194. [Google Scholar]
- Gull, M.A.; Bai, S.; Bak, T.J.R. A review on design of upper limb exoskeletons. Robotics 2020, 9, 16. [Google Scholar] [CrossRef]
- Ödemiş, E.; Baysal, C.V.; İnci, M. Patient performance assessment methods for upper extremity rehabilitation in assist-as-needed therapy strategies: A comprehensive review. Med. Biol. Eng. Comput. 2025. [Google Scholar] [CrossRef] [PubMed]
- Horgusluoglu, E.; Nudelman, K.; Nho, K.; Saykin, A.J. Adult neurogenesis and neurodegenerative diseases: A systems biology perspective. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 174, 93–112. [Google Scholar] [CrossRef]
- Pollock, A.; Baer, G.; Pomeroy, V.M.; Langhorne, P. Physiotherapy treatment approaches for the recovery of postural control and lower limb function following stroke. Cochrane Database Syst. Rev. 2007. [Google Scholar]
- Bach-y-Rita, P.; Hughes, B. Tactile Vision Substitution: Some Instrumentation and Perceptual Considerations. In Electronic Spatial Sensing for the Blind: Contributions from Perception, Rehabilitation, and Computer Vision; Warren, D.H., Strelow, E.R., Eds.; Springer: Dordrecht, The Netherlands, 1985; pp. 171–186. [Google Scholar]
- Jäger, R.; Sawan, S.A.; Orrú, M.; Tinsley, G.M.; Purpura, M.; Wells, S.D.; Liao, K.; Godavarthi, A. Paraxanthine enhances memory and neuroplasticity more than caffeine in rats. Exp. Brain Res. 2025, 8, 243. [Google Scholar]
- de Almeida, A.; Arida, R. Correspondence to “Exploring the impact of exercise induced BDNF on neuroplasticity in neurodegenerative and neuropsychiatric conditions”. Mol. Biol. Rep. 2025, 52, 398. [Google Scholar] [CrossRef]
- Yan, W.; Lin, Y.; Chen, Y.-F.; Wang, Y.; Wang, J.; Zhang, M. Enhancing Neuroplasticity for Post-Stroke Motor Recovery: Mechanisms, Models, and Neurotechnology. IEEE Trans. Neural Syst. Rehabil. Eng. 2025, 33, 1156–1168. [Google Scholar] [CrossRef]
- Veerbeek, J.M.; van Wegen, E.; van Peppen, R.; Van der Wees, P.J.; Hendriks, E.; Rietberg, M.; Kwakkel, G. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS ONE 2014, 9, e87987. [Google Scholar] [CrossRef]
- Krebs, H.I.; Hogan, N.; Aisen, M.L.; Volpe, B.T. Robot-aided neurorehabilitation. IEEE Trans. Rehabil. Eng. 1998, 6, 75–87. [Google Scholar] [CrossRef]
- Carpinella, I.; Lencioni, T.; Bowman, T.; Bertoni, R.; Turolla, A.; Ferrarin, M.; Jonsdottir, J. Effects of robot therapy on upper body kinematics and arm function in persons post stroke: A pilot randomized controlled trial. J. Neuroeng. Rehabil. 2020, 17, 10. [Google Scholar] [CrossRef]
- Krebs, H.I.; Ferraro, M.; Buerger, S.P.; Newbery, M.J.; Makiyama, A.; Sandmann, M.; Lynch, D.; Volpe, B.T.; Hogan, N. Rehabilitation robotics: Pilot trial of a spatial extension for MIT-Manus. J. Neuroeng. Rehabil. 2004, 1, 5. [Google Scholar] [CrossRef]
- Burgar, C.G.; Lum, P.S.; Shor, P.C.; Van der Loos, H.M. Development of robots for rehabilitation therapy: The Palo Alto VA/Stanford experience. J. Rehabil. Res. Dev. 2000, 37, 663–674. [Google Scholar]
- Perry, J.C.; Zabaleta, H.; Belloso, A.; Keller, T. ARMassist: A low-cost device for telerehabiltation of post-stroke arm deficits. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Munich, Germany, 7–12 September 2009; Volume 25/9, pp. 64–67. [Google Scholar]
- Hesse, S.; Werner, C.; Pohl, M.; Rueckriem, S.; Mehrholz, J.; Lingnau, M.J.S. Computerized arm training improves the motor control of the severely affected arm after stroke: A single-blinded randomized trial in two centers. Stroke 2005, 36, 1960–1966. [Google Scholar] [CrossRef]
- Bovolenta, F.; Goldoni, M.; Clerici, P.; Agosti, M.; Franceschini, M. Robot therapy for functional recovery of the upper limbs: A pilot study on patients after stroke. J. Rehabil. Med. 2009, 41, 971–975. [Google Scholar] [CrossRef]
- Takebayashi, T.; Takahashi, K.; Okita, Y.; Kubo, H.; Hachisuka, K.; Domen, K. Impact of the robotic-assistance level on upper extremity function in stroke patients receiving adjunct robotic rehabilitation: Sub-analysis of a randomized clinical trial. J. Neuroeng. Rehabil. 2022, 19, 25. [Google Scholar] [CrossRef]
- Takebayashi, T.; Takahashi, K.; Amano, S.; Gosho, M.; Sakai, M.; Hashimoto, K.; Hachisuka, K.; Uchiyama, Y.; Domen, K.J.S. Robot-assisted training as self-training for upper-limb hemiplegia in chronic stroke: A randomized controlled trial. Stroke 2022, 53, 2182–2191. [Google Scholar] [CrossRef]
- Yubo, Z.; Zixi, W.; Linhong, J.; Sheng, B. The clinical application of the upper extremity compound movements rehabilitation training robot. In Proceedings of the 9th International Conference on Rehabilitation Robotics, 2005, ICORR 2005, Chicago, IL, USA, 28 June–1 July 2005; pp. 91–94. [Google Scholar]
- Jackson, A.E.; Levesley, M.; Makower, S.; Cozens, J.; Bhakta, B. Development of the iPAM MkII system and description of a randomized control trial with acute stroke patients. In Proceedings of the 2013 IEEE 13th International Conference on Rehabilitation Robotics (ICORR), Seattle, WA, USA, 24–26 June 2013; pp. 1–6. [Google Scholar]
- Zhang, Y.; Zhao, P.; Gong, L.; Deng, X. Trajectory Synthesis and Sensitivity Analysis of Six-Bar Mechanism for Gait Implementation. In Proceedings of the IFToMM World Congress on Mechanism and Machine Science, Tokyo, Japan, 5–10 November 2023; pp. 396–408. [Google Scholar]
- Rosati, G.; Gallina, P.; Masiero, S. Design, implementation and clinical tests of a wire-based robot for neurorehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 560–569. [Google Scholar] [CrossRef]
- Amirabdollahian, F.; Loureiro, R.; Gradwell, E.; Collin, C.; Harwin, W.; Johnson, G. Multivariate analysis of the Fugl-Meyer outcome measures assessing the effectiveness of GENTLE/S robot-mediated stroke therapy. J. Neuroeng. Rehabil. 2007, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Plooij, M.; Apte, S.; Keller, U.; Baines, P.; Sterke, B.; Asboth, L.; Courtine, G.; von Zitzewitz, J.; Vallery, H.J.S.R. Neglected physical human-robot interaction may explain variable outcomes in gait neurorehabilitation research. Sci. Robot. 2021, 6, eabf1888. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; He, M.; Tong, X.; Zhang, M.; Huang, L.J.S. Research on the Motion Control Strategy of a Lower-Limb Exoskeleton Rehabilitation Robot Using the Twin Delayed Deep Deterministic Policy Gradient Algorithm. Sensors 2024, 24, 6014. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.; Lee, H. Development of a low inertia parallel actuated shoulder exoskeleton robot for the characterization of neuromuscular property during static posture and dynamic movement. In Proceedings of the 2019 International Conference on Robotics and Automation (ICRA), Montreal, QC, Canada, 20–24 May 2019; pp. 556–562. [Google Scholar]
- Mihelj, M.; Nef, T.; Riener, R. ARMin II-7 DoF rehabilitation robot: Mechanics and kinematics. In Proceedings of the 2007 IEEE International Conference on Robotics and Automation, Roma, Italy, 10–14 April 2007; pp. 4120–4125. [Google Scholar]
- Lu, L.; Tan, Y.; Klaic, M.; Galea, M.P.; Khan, F.; Oliver, A.; Mareels, I.; Oetomo, D.; Zhao, E. Evaluating rehabilitation progress using motion features identified by machine learning. IEEE Trans. Biomed. Eng. 2020, 68, 1417–1428. [Google Scholar] [CrossRef]
- Rudhe, C.; Albisser, U.; Starkey, M.L.; Curt, A.; Bolliger, M. Reliability of movement workspace measurements in a passive arm orthosis used in spinal cord injury rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 37. [Google Scholar] [CrossRef]
- Sehle, A.; Stuerner, J.; Hassa, T.; Spiteri, S.; Schoenfeld, M.A.; Liepert, J. Behavioral and neurophysiological effects of an intensified robot-assisted therapy in subacute stroke: A case control study. J. Neuroeng. Rehabil. 2021, 18, 6. [Google Scholar] [CrossRef]
- Kim, B.; Deshpande, A.D. An upper-body rehabilitation exoskeleton Harmony with an anatomical shoulder mechanism: Design, modeling, control, and performance evaluation. Int. J. Robot. Res. 2017, 36, 414–435. [Google Scholar] [CrossRef]
- Cheng, Y.; Pan, S. Virtual decomposition control of multi-degree-of-freedom rehabilitation exoskeleton robotic arm. J. Mech. Eng. 2022, 58, 21–30. [Google Scholar]
- Noronha, B.; Ng, C.Y.; Little, K.; Xiloyannis, M.; Kuah, C.W.K.; Wee, S.K.; Kulkarni, S.R.; Masia, L.; Chua, K.S.G.; Accoto, D.; et al. Soft, lightweight wearable robots to support the upper limb in activities of daily living: A feasibility study on chronic stroke patients. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 1401–1411. [Google Scholar] [CrossRef]
- Otten, A.; Voort, C.; Stienen, A.; Aarts, R.; van Asseldonk, E.; van der Kooij, H. LIMPACT: A hydraulically powered self-aligning upper limb exoskeleton. IEEE/ASME Trans. Mechatron. 2015, 20, 2285–2298. [Google Scholar] [CrossRef]
- Pan, M.; Yuan, C.; Liang, X.; Dong, T.; Liu, T.; Zhang, J.; Zou, J.; Yang, H.; Bowen, C. Soft actuators and robotic devices for rehabilitation and assistance. Adv. Intell. Syst. 2022, 4, 2100140. [Google Scholar] [CrossRef]
- Reinkensmeyer, D.J.; Wolbrecht, E.T.; Chan, V.; Chou, C.; Cramer, S.C.; Bobrow, J.E. Rehabilitation. Comparison of three-dimensional, assist-as-needed robotic arm/hand movement training provided with Pneu-WREX to conventional tabletop therapy after chronic stroke. Am. J. Phys. Med. Rehabil. 2012, 91, S232–S241. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.; Wolbrecht, E.; Smith, R.; Liu, J.; Rao, S.; Cramer, S.; Rahman, T.; Bobrow, J.E.; Reinkensmeyer, D.J. A pneumatic robot for re-training arm movement after stroke: Rationale and mechanical design. In Proceedings of the 9th International Conference on Rehabilitation Robotics, 2005, ICORR 2005, Chicago, IL, USA, 28 June–1 July 2005; pp. 500–504. [Google Scholar]
- Klein, J.; Spencer, S.; Allington, J.; Minakata, K.; Wolbrecht, E.; Smith, R.; Bobrow, J.; Reinkensmeyer, D.J. Biomimetic orthosis for the neurorehabilitation of the elbow and shoulder (BONES). In Proceedings of the 2008 2nd IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics, Scottsdale, AZ, USA, 19–22 October 2008; pp. 535–541. [Google Scholar]
- Klein, J.; Spencer, S.; Allington, J.; Bobrow, J.E.; Reinkensmeyer, D.J. Optimization of a parallel shoulder mechanism to achieve a high-force, low-mass, robotic-arm exoskeleton. IEEE Trans. Robot. 2010, 26, 710–715. [Google Scholar] [CrossRef]
- Tu, X.; Han, H.; Huang, J.; Li, J.; Su, C.; Jiang, X.; He, J. Upper Limb Rehabilitation Robot Powered by PAMs Cooperates with FES Arrays to Realize Reach-to-Grasp Trainings. J. Healthc. Eng. 2017, 1, 1282934. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Lien, W.Y.; Chen, C.T.; Wu, Y.C. Implementationof an upper-limb exoskeleton robot driven by pneumatic muscle actuators for rehabilitation. Actuators 2020, 9, 106. [Google Scholar] [CrossRef]
- Al-Fahaam, H.; Davis, S.; Nefti-Meziani, S.J.R.; Systems, A. The design and mathematical modelling of novel extensor bending pneumatic artificial muscles (EBPAMs) for soft exoskeletons. Robot. Auton. Syst. 2018, 99, 63–74. [Google Scholar] [CrossRef]
- Chen, C.; Song, Y.; Chen, D.; Zhu, J.; Ning, H.; Xiao, R. Design and application of pneumatic rehabilitation glove system based on brain-computer interface. Rev. Sci. Instrum. 2024, 95, 095108. [Google Scholar] [CrossRef]
- Das, S.; Kishishita, Y.; Tsuji, T.; Lowell, C.; Ogawa, K.; Kurita, Y. ForceHand glove: A wearable force-feedback glove with pneumatic artificial muscles (PAMs). IEEE Robot. Autom. Lett. 2018, 3, 2416–2423. [Google Scholar] [CrossRef]
- Farag, M.; Azlan, N.Z.; Alsibai, M.H.; Ghafar, A.N.A. Slippage detection for grasping force control of robotic hand using force sensing resistors. In Proceedings of the 2019 5th International Conference on Computer and Technology Applications, Istanbul, Turkey, 16–17 April 2019; pp. 98–102. [Google Scholar]
- Zimmermann, Y.; Forino, A.; Riener, R.; Hutter, M.J.I.R.; Letters, A. ANYexo: A versatile and dynamic upper-limb rehabilitation robot. IEEE Robot. Autom. Lett. 2019, 4, 3649–3656. [Google Scholar] [CrossRef]
- Zimmermann, Y.; Sommerhalder, M.; Wolf, P.; Riener, R.; Hutter, M. ANYexo 2.0: A fully actuated upper-limb exoskeleton for manipulation and joint-oriented training in all stages of rehabilitation. IEEE Trans. Robot. 2023, 39, 2131–2150. [Google Scholar] [CrossRef]
- Crea, S.; Cempini, M.; Moise, M.; Baldoni, A.; Trigili, E.; Marconi, D.; Cortese, M.; Giovacchini, F.; Posteraro, F.; Vitiello, N. A novel shoulder-elbow exoskeleton with series elastic actuators. In Proceedings of the 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob), Singapore, 26–29 June 2016; pp. 1248–1253. [Google Scholar]
- Trigili, E.; Crea, S.; Moisè, M.; Baldoni, A.; Cempini, M.; Ercolini, G.; Marconi, D.; Posteraro, F.; Carrozza, M.C.; Vitiello, N.J.I.A.T.o.M. Design and experimental characterization of a shoulder-elbow exoskeleton with compliant joints for post-stroke rehabilitation. IEEE/ASME Trans. Mechatron. 2019, 24, 1485–1496. [Google Scholar] [CrossRef]
- Pan, J.; Astarita, D.; Baldoni, A.; Dell′Agnello, F.; Crea, S.; Vitiello, N.; Trigili, E.J.I.R.; Letters, A. NESM-γ: An upper-limb exoskeleton with compliant actuators for clinical deployment. IEEE Robot. Autom. Lett. 2022, 7, 7708–7715. [Google Scholar] [CrossRef]
- Qian, W.; Liao, J.; Lu, L.; Ai, L.; Li, M.; Xiao, X. CURER: A lightweight cable-driven compliant upper limb rehabilitation exoskeleton robot. IEEE/ASME Trans. Mechatron. 2022, 28, 1730–1741. [Google Scholar] [CrossRef]
- Richardson, M.C.; Tears, C.; Morris, A.; Alexanders, J. The effects of unilateral versus bilateral motor training on upper limb function in adults with chronic stroke: A systematic review. J. Stroke Cerebrovasc. Dis. 2021, 30, 105617. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Chun, M.H. Combination transcranial direct current stimulation and virtual reality therapy for upper extremity training in patients with subacute stroke. Arch. Phys. Med. Rehabil. 2014, 95, 431–438. [Google Scholar] [CrossRef]
- Ahmed, T.; Islam, M.R.; Brahmi, B.; Rahman, M.H. Robustness and Tracking Performance Evaluation of PID Motion Control of 7 DoF Anthropomorphic Exoskeleton Robot Assisted Upper Limb Rehabilitation. Sensors 2022, 22, 3747. [Google Scholar] [CrossRef]
- Wang, H.; Lu, J. Research on Fractional Order Fuzzy PID Control of the Pneumatic-hydraulic Upper Limb Rehabilitation Training System Based on PSO. Int. J. Control Autom. Syst. 2022, 20, 310–320. [Google Scholar] [CrossRef]
- Hu, B.; Mao, B.; Lu, S.; Yu, H. Design and torque control base on neural network PID of a variable stiffness joint for rehabilitation robot. Front. Neurorobot. 2022, 16, 1007324. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; K-Ouimet, T.; Saad, M.; Kenné, J.P.; Archambault, P.S. Tele-operation of a robotic exoskeleton for rehabilitation and passive arm movement assistance. In Proceedings of the 2011 IEEE International Conference on Robotics and Biomimetics, Karon Beach, Thailand, 7–11 December 2011; pp. 443–448. [Google Scholar]
- Yang, J.; Xie, H.; Shi, J.J.M.; Theory, M. A novel motion-coupling design for a jointless tendon-driven finger exoskeleton for rehabilitation. Mech. Mach. Theory 2016, 99, 83–102. [Google Scholar] [CrossRef]
- Chakraborty, D.; Rathi, A.; Singh, R.; Pathak, V.K.; Srivastava, A.K.; Sharma, A.; Saxena, K.K.; Kumar, G.; Kumar, S.J.R.; Systems, A. External six-bar mechanism rehabilitation device for index finger: Development and shape synthesis. Robot. Auton. Syst. 2023, 161, 104336. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Y.; Guan, H.; Deng, X.; Chen, H. Design of a single-degree-of-freedom immersive rehabilitation device for clustered upper-limb motion. J. Mech. Robot. 2021, 13, 031006. [Google Scholar] [CrossRef]
- Akdoğan, E.; Aktan, M.E.; Koru, A.T.; Arslan, M.S.; Atlıhan, M.; Kuran, B.J.M. Hybrid impedance control of a robot manipulator for wrist and forearm rehabilitation: Performance analysis and clinical results. Mechatronics 2018, 49, 77–91. [Google Scholar] [CrossRef]
- Kumar, S.; Wöhrle, H.; Trampler, M.; Simnofske, M.; Peters, H.; Mallwitz, M.; Kirchner, E.A.; Kirchner, F.J.A.S. Modular design and decentralized control of the recupera exoskeleton for stroke rehabilitation. Appl. Sci. 2019, 9, 626. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, C.; Gu, Z.; Zheng, Y.; Wu, Q. A new design scheme for intelligent upper limb rehabilitation training robot. Int. J. Environ. Res. Public Health 2020, 17, 2948. [Google Scholar] [CrossRef]
- Wang, W.-W.; Fu, L.-C. Mirror therapy with an exoskeleton upper-limb robot based on IMU measurement system. In Proceedings of the 2011 IEEE International Symposium on Medical Measurements and Applications, Bari, Italy, 30–31 May 2011; pp. 370–375. [Google Scholar]
- Wang, X.; Lin, M.; Sang, L.; Wang, H.; Feng, Y.; Niu, J.; Yu, H.; Cheng, B. A Linear Rehabilitative Motion Planning Method with a Multi-Posture Lower-Limb Rehabilitation Robot. Sensors 2024, 24, 7506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, Y.; Zhang, Y.; Wang, Y. Trajectory Synthesis and Linkage Design of Single-Degree-of-Freedom Finger Rehabilitation Device. Actuators 2024, 13, 256. [Google Scholar]
- Mohamaddan, S.; Jamali, A.; Abidin, A.S.Z.; Jamaludin, M.S.; Abd Majid, N.A.; Ashari, M.F.; Hazmi, H.J.P.C.S. Development of upper limb rehabilitation robot device for home setting. Procedia Comput. Sci. 2015, 76, 376–380. [Google Scholar] [CrossRef][Green Version]
- Yan, Y.; Tang, M.; Wang, W.; Zhang, Y.; An, B. Trajectory tracking control of wearable upper limb rehabilitation robot based on Laguerre model predictive control. Robot. Auton. Syst. 2024, 179, 104745. [Google Scholar] [CrossRef]
- Shi, X.; Yang, C.; Lee, P.C.; Xie, D.; Ye, Z.; Li, Z.; Tong, R.K. An interactive soft robotic hand-task training system with wireless task boards and daily objects on post-stroke rehabilitation. Wearable Technol. 2025, 6, e4. [Google Scholar] [CrossRef]
- Jiang, N.; Englehart, K.B.; Parker, P.A. Extracting simultaneous and proportional neural control information for multiple-DOF prostheses from the surface electromyographic signal. IEEE Trans. Biomed. Eng. 2008, 56, 1070–1080. [Google Scholar] [CrossRef]
- Do, A.H.; Wang, P.T.; King, C.E.; Chun, S.N.; Nenadic, Z. Brain-computer interface controlled robotic gait orthosis. J. Neuroeng. Rehabil. 2013, 10, 111. [Google Scholar] [CrossRef]
- Novak, D.; Riener, R.J.R.; Systems, A. A survey of sensor fusion methods in wearable robotics. Robot. Auton. Syst. 2015, 73, 155–170. [Google Scholar] [CrossRef]
- Li, Q. Research on an Exoskeleton Robot Upper Limb Rehabilitation System Based on sEMG Signals. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2009. (In Chinese). [Google Scholar]
- Guo, Y.; Xu, W.; Pradhan, S.; Bravo, C.; Ben-Tzvi, P.J.M. Personalized voice activated grasping system for a robotic exoskeleton glove. Mechatronics 2022, 83, 102745. [Google Scholar] [CrossRef]
- He, B.; Li, M.; He, G. Hand rehabilitation modes combining exoskeleton-assisted training with tactile feedback for hemiplegia patients: A preliminary study. In Proceedings of the International Conference on Intelligent Robotics and Applications, Harbin, China, 1–3 August 2022; pp. 661–669. [Google Scholar]
- Arteaga, M.V.; Castiblanco, J.C.; Mondragon, I.F.; Colorado, J.D.; Alvarado-Rojas, C. EMG-based adaptive trajectory generation for an exoskeleton model during hand rehabilitation exercises. In Proceedings of the 2020 8th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob), New York, NY, USA, 29 November–1 December 2020; pp. 416–421. [Google Scholar]
- Khan, B.A.; Usmani, A.R.; Athar, S.; Hashmi, A.; Farooq, O.; Muzammil, M. EEG-based exoskeleton for rehabilitation therapy. In Ergonomics for Improved Productivity, Proceedings of the HWWE 2017, Aligarh, India, 8–10 December 2017; Springer: Berlin/Heidelberg, Germany, 2021; pp. 645–653. [Google Scholar]
- Makowski, N.S.; Knutson, J.S.; Chae, J.; Crago, P.E. Control of robotic assistance using poststroke residual voluntary effort. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 23, 221–231. [Google Scholar] [CrossRef]
- Benabid, A.L.; Costecalde, T.; Eliseyev, A.; Charvet, G.; Verney, A.; Karakas, S.; Foerster, M.; Lambert, A.; Morinière, B.; Abroug, N. An exoskeleton controlled by an epidural wireless brain–machine interface in a tetraplegic patient: A proof-of-concept demonstration. Lancet Neurol. 2019, 18, 1112–1122. [Google Scholar] [CrossRef]
- Borton, D.; Micera, S.; Millán, J.d.R.; Courtine, G. Personalized neuroprosthetics. Sci. Transl. Med. 2013, 5, 210rv2. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhou, Q.; Xu, J. Research on robotic impedance control. In Proceedings of the 37th Chinese Control Conference (D), Wuhan, China, 25–27 July 2018. (In Chinese). [Google Scholar]
- Hogan, N. Impedance control: An approach to manipulation. In Proceedings of the 1984 American Control Conference, San Diedo, CA, USA, 6–8 June 1984; pp. 304–313. [Google Scholar]
- Zhang, S.; Shan, Q.; Huang, J.; Chen, Y.; Zhou, Y. Passive compliance control of a hybrid arm-wrist upper limb rehabilitation robot. China J. Eng. Mach. 2024, 22, 468–473. (In Chinese) [Google Scholar]
- Ge, S. Research on Tendon-Driven Dexterous Hand Adaptive Control Algorithms. Master’s Thesis, Nanjing University of Posts and Telecommunications, Nanjing, China, 2019. (In Chinese). [Google Scholar]
- Xu, W.; Guo, Y.; Bravo, C.; Ben-Tzvi, P. Design, control, and experimental evaluation of a novel robotic glove system for patients with brachial plexus injuries. IEEE Trans. Robot. 2022, 39, 1637–1652. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Jeong, S.; Lyu, F.; Herrin, K.; Bhatia, S.; Elliott, D.; Kozin, S.; Desai, J.P. FLEXotendon glove-III: Voice-controlled soft robotic hand exoskeleton with novel fabrication method and admittance grasping control. IEEE/ASME Trans. Mechatron. 2022, 27, 3920–3931. [Google Scholar] [CrossRef]
- Bakhtiari, M.; Haghjoo, M.R.; Taghizadeh, M. Model-free adaptive variable impedance control of gait rehabilitation exoskeleton. J. Braz. Soc. Mech. Sci. Eng. 2024, 46, 557. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, S.; Zhang, J.; Luo, S.; Zhang, B. Force/position hybrid control based on the stiffness of a rope-driven parallel robot system. Mech. Transm. 2023, 47, 130–134. (In Chinese) [Google Scholar]
- Just, F.; Özen, Ö.; Bösch, P.; Bobrovsky, H.; Klamroth-Marganska, V.; Riener, R.; Rauter, G. Exoskeleton transparency: Feed-forward compensation vs. disturbance observer. at-Automatisierungstechnik 2018, 66, 1014–1026. [Google Scholar] [CrossRef]
- Elgeneidy, K.; Lohse, N.; Jackson, M. Bending angle prediction and control of soft pneumatic actuators with embedded flex sensors–a data-driven approach. Mechatronics 2018, 50, 234–247. [Google Scholar] [CrossRef]
- Xu, G.; Song, A. Fuzzy variable impedance control for upper-limb rehabilitation robot. In Proceedings of the 2008 Fifth International Conference on Fuzzy Systems and Knowledge Discovery, Jinan, China, 18–20 October 2008; pp. 216–220. [Google Scholar]
- Sun, J.; Shen, Y.; Rosen, J. Sensor reduction, estimation, and control of an upper-limb exoskeleton. IEEE Robot. Autom. Lett. 2021, 6, 1012–1019. [Google Scholar] [CrossRef]
- Kamps, A.; Schüle, K. Cyclic movement training of the lower limb in stroke rehabilitation. Neurol. Rehabil. 2005, 11, S1–S12. [Google Scholar]
- Zhu, J.; Gienger, M.; Franzese, G.; Kober, J. Do you need a hand?—A bimanual robotic dressing assistance scheme. IEEE Trans. Robot. 2024, 40, 1906–1919. [Google Scholar]
- Mao, Y.; Jin, X.; Dutta, G.G.; Scholz, J.P.; Agrawal, S.K. Human movement training with a cable driven arm exoskeleton (CAREX). IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 23, 84–92. [Google Scholar] [CrossRef]
- Atkinson, M.; Tully, A.; Maher, C.A.; Innes-Wong, C.; Russo, R.N.; Osborn, M.P. Safety, Feasibility and Efficacy of Lokomat® and Armeo® Spring Training in Deconditioned Paediatric, Adolescent and Young Adult Cancer Patients. Cancers 2023, 15, 1250. [Google Scholar] [CrossRef]
- Takebayashi, T.; Uchiyama, Y.; Domen, K. Automatic setting optimization for robotic upper-extremity rehabilitation in patients with stroke using ReoGo-J: A cross-sectional clinical trial. Sci. Rep. 2024, 14, 25710. [Google Scholar] [CrossRef]
- Krebs, H.I.; Palazzolo, J.J.; Dipietro, L.; Ferraro, M.; Krol, J.; Rannekleiv, K.; Volpe, B.T.; Hogan, N. Rehabilitation robotics: Performance-based progressive robot-assisted therapy. Auton. Robot. 2003, 15, 7–20. [Google Scholar] [CrossRef]
- Grimaldi, G.; Manto, M. Functional impacts of exoskeleton-based rehabilitation in chronic stroke: Multi-joint versus single-joint robotic training. J. Neuroeng. Rehabil. 2013, 10, 113. [Google Scholar] [CrossRef]
- Manna, S.K.; Dubey, V.N. A portable elbow exoskeleton for three stages of rehabilitation. J. Mech. Robot. 2019, 11, 065002. [Google Scholar] [CrossRef]
- Patle, B.; Pandey, A.; Parhi, D.; Jagadeesh, A. A review: On path planning strategies for navigation of mobile robot. Def. Technol. 2019, 15, 582–606. [Google Scholar] [CrossRef]
- Reda, M.; Onsy, A.; Haikal, A.Y.; Ghanbari, A. Path planning algorithms in the autonomous driving system: A comprehensive review. Robot. Auton. Syst. 2024, 174, 104630. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, C.; Huang, R.; Tong, K.; He, Y.; Xu, L. Path planning for mobile robots in greenhouse orchards based on improved A* and fuzzy DWA algorithms. Comput. Electron. Agric. 2024, 227, 109598. [Google Scholar] [CrossRef]
- Suanpang, P.; Jamjuntr, P. Optimizing Autonomous UAV Navigation with D* Algorithm for Sustainable Development. Sustainability 2024, 16, 7867. [Google Scholar] [CrossRef]
- Islam, M.R.; Assad-Uz-Zaman, M.; Brahmi, B.; Bouteraa, Y.; Wang, I.; Rahman, M.H. Design and development of an upper limb rehabilitative robot with dual functionality. Micromachines 2021, 12, 870. [Google Scholar] [CrossRef]
- Wei, K.; Ren, B. A method on dynamic path planning for robotic manipulator autonomous obstacle avoidance based on an improved RRT algorithm. Sensors 2018, 18, 571. [Google Scholar] [CrossRef]
- Jia, B.; Li, P. Path Planning Based on the Method of Integrating Adjacent Obstacles. In Proceedings of the 2019 Chinese Control and Decision Conference (CCDC), Nanchang, China, 3–5 June 2019; pp. 6197–6202. [Google Scholar]
- Tong, S.; Liu, Q.; Ma, Q.; Qin, J. Integrating deep reinforcement learning and improved artificial potential field method for safe path planning for mobile robots. Robot. Intell. Autom. 2024, 44, 871–886. [Google Scholar] [CrossRef]
- Klamroth-Marganska, V.; Blanco, J.; Campen, K.; Curt, A.; Dietz, V.; Ettlin, T.; Felder, M.; Fellinghauer, B.; Guidali, M.; Kollmar, A. Three-dimensional, task-specific robot therapy of the arm after stroke: A multicentre, parallel-group randomised trial. Lancet Neurol. 2014, 13, 159–166. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, S.; Zhang, L. A path planning method of 6-DOF robot for mirror therapy based on A* algorithm. Technol. Health Care 2022, 30, 105–116. [Google Scholar] [CrossRef]
- Lauretti, C.; Cordella, F.; Ciancio, A.L.; Trigili, E.; Catalan, J.M.; Badesa, F.J.; Crea, S.; Pagliara, S.M.; Sterzi, S.; Vitiello, N. Learning by demonstration for motion planning of upper-limb exoskeletons. Front. Neurorobot. 2018, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Lauretti, C.; Cordella, F.; Guglielmelli, E.; Zollo, L. Learning by demonstration for planning activities of daily living in rehabilitation and assistive robotics. IEEE Robot. Autom. Lett. 2017, 2, 1375–1382. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, H.; Wei, J.; Zhang, F.; Shi, Z.; Li, X. Research on the Safety Design and Trajectory Planning for a New Dual Upper Limb Rehabilitation Robot. Actuators 2024, 13, 364. [Google Scholar]
- Hamaya, M.; Matsubara, T.; Teramae, T.; Noda, T.; Morimoto, J. Design of physical user–robot interactions for model identification of soft actuators on exoskeleton robots. Int. J. Robot. Res. 2021, 40, 397–410. [Google Scholar] [CrossRef]
- Ning, Y.; Sang, L.; Wang, H.; Wang, Q.; Vladareanu, L.; Niu, J. Upper limb exoskeleton rehabilitation robot inverse kinematics modeling and so-lution method based on multi-objective optimization. Sci. Rep. 2024, 14, 25476. [Google Scholar] [CrossRef]
- Meziani, Y.; Morère, Y.; Hadj-Abdelkader, A.; Benmansour, M.; Bourhis, G. Towards adaptive and finer rehabilitation assessment: A learning framework for kinematic evaluation of upper limb rehabilitation on an Armeo Spring exoskeleton. Control. Eng. Pract. 2021, 111, 104804. [Google Scholar] [CrossRef]
- Lee, M.H.; Siewiorek, D.P.; Smailagic, A.; Bernardino, A.; Bermúdez i Badia, S. Enabling AI and Robotic Coaches for Physical Rehabilitation Therapy: Iterative Design and Evaluation with Therapists and Post-stroke Survivors. Int. J. Soc. Robot. 2024, 16, 1–22. [Google Scholar] [CrossRef]
- Carey, L.; Macdonell, R.; Matyas, T.A. SENSe: Study of the Effectiveness of Neurorehabilitation on Sensation: A randomized controlled trial. Neurorehabilit. Neural Repair 2011, 25, 304–313. [Google Scholar] [CrossRef]
- Mani Bharathi, V.; Manimegalai, P.; George, S.T.; Pamela, D.; Mohammed, M.A.; Abdulkareem, K.H.; Jaber, M.M.; Damaševičius, R. A systematic review of techniques and clinical evidence to adopt virtual reality in post-stroke upper limb rehabilitation. Virtual Real. 2024, 28, 172. [Google Scholar] [CrossRef]
- Varghese, R.J.; Mukherjee, G.; King, R.; Keller, S.; Deshpande, A.D. Designing variable stiffness profiles to optimize the physical human robot interface of hand exoskeletons. In Proceedings of the 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob), Enschede, The Netherlands, 26–29 August 2018; pp. 1101–1108. [Google Scholar]
- Christensen, S.; Bai, S. Kinematic analysis and design of a novel shoulder exoskeleton using a double parallelogram linkage. J. Mech. Robot. 2018, 10, 041008. [Google Scholar] [CrossRef]
- Castro, M.N.; Rasmussen, J.; Andersen, M.S.; Bai, S. A compact 3-DOF shoulder mechanism constructed with scissors linkages for exoskeleton applications. Mech. Mach. Theory 2019, 132, 264–278. [Google Scholar] [CrossRef]
- Khan, A.M.; Yun, D.-w.; Ali, M.A.; Zuhaib, K.M.; Yuan, C.; Iqbal, J.; Han, J.; Shin, K.; Han, C. Passivity based adaptive control for upper extremity assist exoskeleton. Int. J. Control. Autom. Syst. 2016, 14, 291–300. [Google Scholar] [CrossRef]
- Yun, Y.; Dancausse, S.; Esmatloo, P.; Serrato, A.; Merring, C.A.; Agarwal, P.; Deshpande, A.D. Maestro: An EMG-driven assistive hand exoskeleton for spinal cord injury patients. In Proceedings of the 2017 IEEE international conference on robotics and automation (ICRA), Singapore, 3 May–3 June 2017; pp. 2904–2910. [Google Scholar]
- Zhang, Y.; Zhao, P.; Li, X.; Zi, B. Design of MMSD Six-Bar Rehab Device Toward the Realization of Multiple Gait Trajectories With One Adjustable Parameter. IEEE/ASME Trans. Mechatron. 2024, 29, 4309–4319. [Google Scholar] [CrossRef]
- Chen, J.-M.; Li, X.-L.; Pan, Q.-H.; Yang, Y.; Xu, S.-M.; Xu, J.-W. Effects of non-invasive brain stimulation on motor function after spinal cord injury: A systematic review and meta-analysis. J. Neuroeng. Rehabil. 2023, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Lorach, H.; Galvez, A.; Spagnolo, V.; Martel, F.; Karakas, S.; Intering, N.; Vat, M.; Faivre, O.; Harte, C.; Komi, S. Walking naturally after spinal cord injury using a brain–spine interface. Nature 2023, 618, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.; Sariev, A.; Lee, S.; Dong, S.-Y.; Royer, S.; Kim, H. Deep cerebellar low-intensity focused ultrasound stimulation restores interhemispheric balance after ischemic stroke in mice. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2073–2079. [Google Scholar] [CrossRef]
- Ambrosini, E.; Zajc, J.; Ferrante, S.; Ferrigno, G.; Dalla Gasperina, S.; Bulgheroni, M.; Baccinelli, W.; Schauer, T.; Wiesener, C.; Russold, M. A hybrid robotic system for arm training of stroke survivors: Concept and first evaluation. IEEE Trans. Biomed. Eng. 2019, 66, 3290–3300. [Google Scholar] [CrossRef]
- Dole, M.; Auboiroux, V.; Langar, L.; Mitrofanis, J. A systematic review of the effects of transcranial photobiomodulation on brain activity in humans. Rev. Neurosci. 2023, 34, 671–693. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).