Dimerized Power: The Antimicrobial and Antiviral Promise of Biflavonoids
Abstract
1. Introduction
2. Biflavonoids
2.1. Chemistry and Natural Occurrence
2.2. Synthesis
- Electrophilic aromatic substitution exploits the reactivity of electron-rich flavonoid rings towards electrophiles. It can enable regioselective bond formation, though controlling product distribution can be difficult [27].
- Oxidative coupling, either enzymatic or chemical, is a versatile route to both C–C and C–O–C linkages [28]. Enzyme-catalyzed approaches, such as peroxidase-mediated coupling, proceed under mild, environmentally benign conditions. In contrast, chemical oxidants (e.g., potassium ferricyanide) provide flexibility but often generate complex product mixtures [23,29].
- Dehydrative coupling involves the condensation of flavonoid hydroxyl groups into ether linkages. This method is conceptually simple but requires careful catalyst choice to avoid degradation of sensitive functional groups.
- Modern cross-coupling reactions, particularly palladium-catalyzed methods such as Suzuki–Miyaura coupling [30,31,32], enable highly selective C–C bond formation. These strategies offer excellent control and efficiency but may be limited by the cost of catalysts and the need for precise reaction conditions.
3. Antimicrobial Activity of Biflavonoids
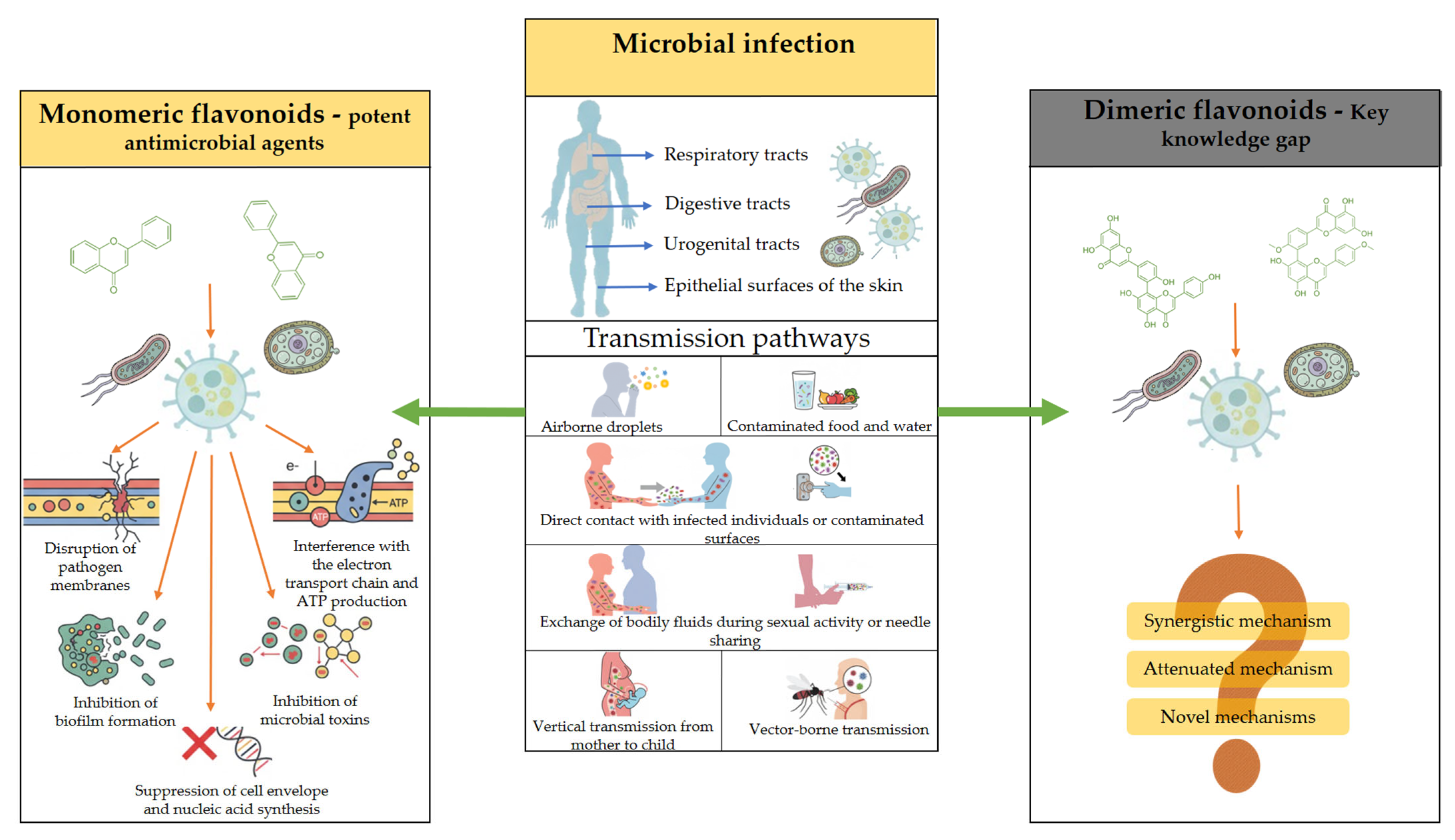
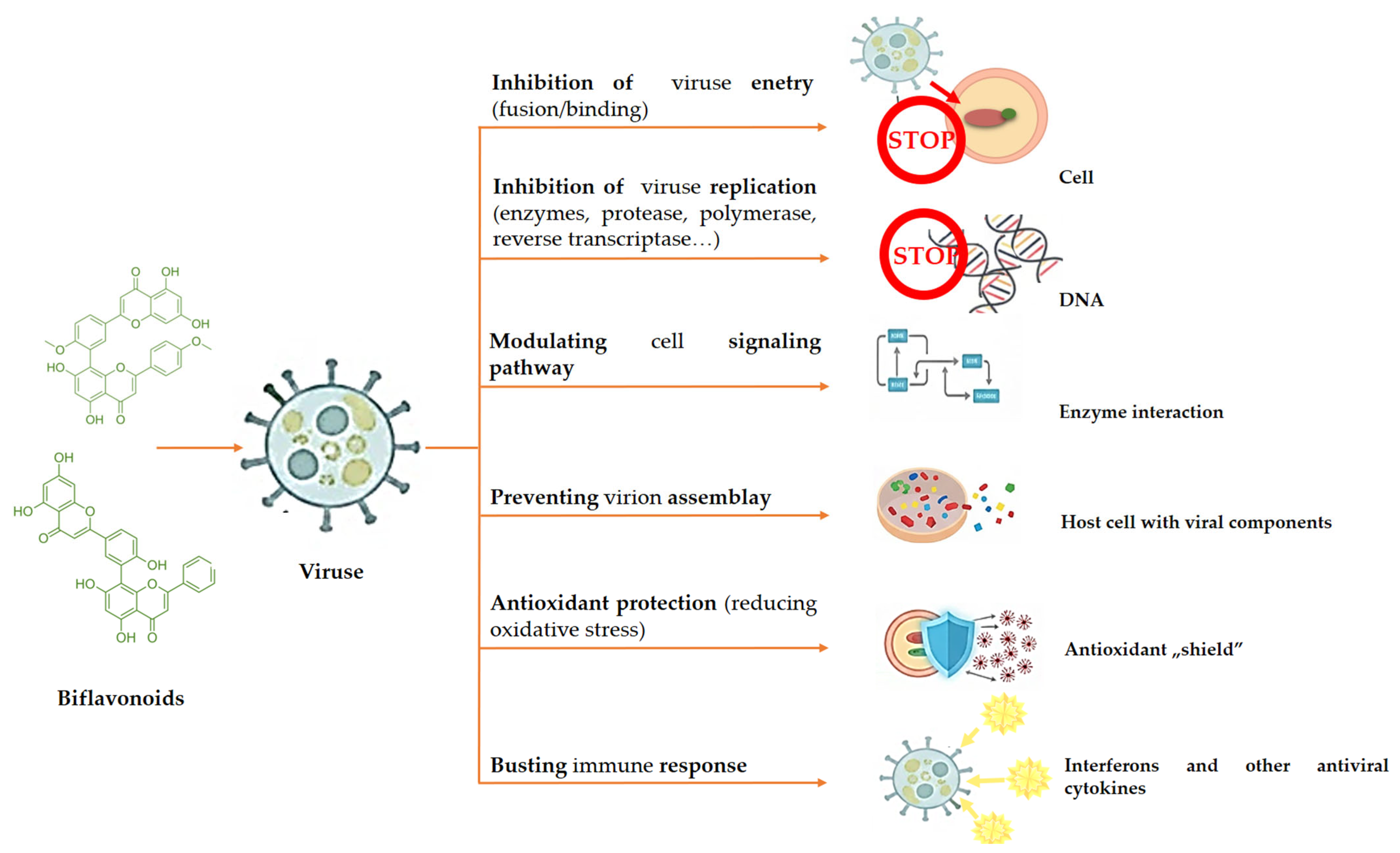
3.1. Antiviral Activity
| Types of Biflavonoids | Source | Pathogen | Outcomes | References |
|---|---|---|---|---|
| Sciadopitysin Ginkgetin Isoginkgetin Amentoflavone Bilobetin | Ginkgo biloba | SARS-CoV-2 | Bioflavones displayed relatively strong SARS-CoV-2 3CLpro inhibition activities (IC50 < 10 μM). | [46] |
| Amentoflavone Bilobetin Ginkgetin Sciadopitysin | Torreya nucifera | SARS-CoV-2 | Amentoflavone exhibited a significant antiviral activity (IC50 = 8.3 ± 1.2 μM). IC50 values of bilobetin, ginkgetin, and sciadopitysin were found as 72.3 ± 4.5 μM, 32.0 ± 1.7 μM, and 38.4 ± 0.2 μM, respectively. | [58] |
| Hinokiflavone Robustaflavone | - | SARS-CoV-2 | The substances exhibited an inhibitory effect against the membrane-fusion-based SARS-CoV-2 spike protein invasion of target cells. | [47] |
| Amentoflavone Agathisflavone Robustaflavone Hinokiflavone Rhusflavanone Succedaneaflavanone | Rhus succedanea | SARS-CoV-2 | Strong interactions are observed between Amentoflavone (~27.04 kcal/mol) and Agathisflavone (~25.87 kcal/mol) and the catalytic residues. All six biflavonoids bind with strong affinity to the same catalytic site of Mpro, as demonstrated by molecular interactions and molecular dynamics. | [48] |
| Amentoflavone Bilobetin Ginkgetin | Torreya nucifera | SARS-CoV-2 | Of the four phytochemicals, only three (amentoflavone, ki = 0.17 mM; bilobetin, ki = 0.21 mM; and ginkgetin, ki = 0.26 mM) exhibited higher binding affinities than lopinavir and N3. | [49] |
| Amentoflavone Bilobetin Ginkgetin Sotetsuflavone | - | SARS-CoV-2 | Significant hydrophilic and hydrophobic bonding interactions were observed between the biflavonoid compounds and either or both catalytic residues (His41 and Cys145) of 3CLpro. | [50] |
| Agathisflavone | Anacardium occidentale | SARS-CoV-2 | Agathisflavone therapy of SARS-CoV-2 replication is effective, with EC50 and CC50 values of 4.23 ± 0.21 and 61.3 ± 0.1 μM, respectively. | [51] |
| Amentoflavone Podovarpusflavone A Isoginkgetin Hinokiflavone | Dacrydium balansae | DV | Biflavonoids were the most potent inhibitors of the DV-NS5 RDRP and DV-NS5, with IC50 values below 3.1 and 5.3 μM. The most potent biflavonoid was hinokiflavone, with an IC50 of 0.26 μM. | [43] |
| Amentoflavone | Selaginella sinensis | RSV | The IC50 value of amentoflavone was 5.5 mg/mL. | [52] |
| Amentoflavone | - | HSV | Amentoflavone exhibited considerable antiviral action against HSV-1 and significantly reduced transcription of viral immediate early genes. | [54] |
| Amentoflavone | - | CVB3 | Amentoflavone and orlistat dramatically decreased CVB3 replication and suppressed virus-induced FAS expression. | [55] |
| Amentoflavone | Selaginella moellendorffii | CVB3 | The total extracts’ IC50 values in the MTT experiment ranged from 19 ± 1.6 to 41 ± 1.2 μg/mL, whereas amentoflavone’s values ranged from 25 ± 1.2 to 52 ± 0.8 μg/mL. | [56] |
| Amentoflavone | - | HCV | It had inhibitory effects on resistant-associated variants of the NS5A inhibitor daclatasvir, as well as on viral entry, replication, and translation of the HCV life cycle. | [57] |
| Agathisflavone | Anacardium occidentale L. | Influenza | The influenza virus was suppressed by agathisflavone, with an EC50 of 1.3 μM. | [53] |
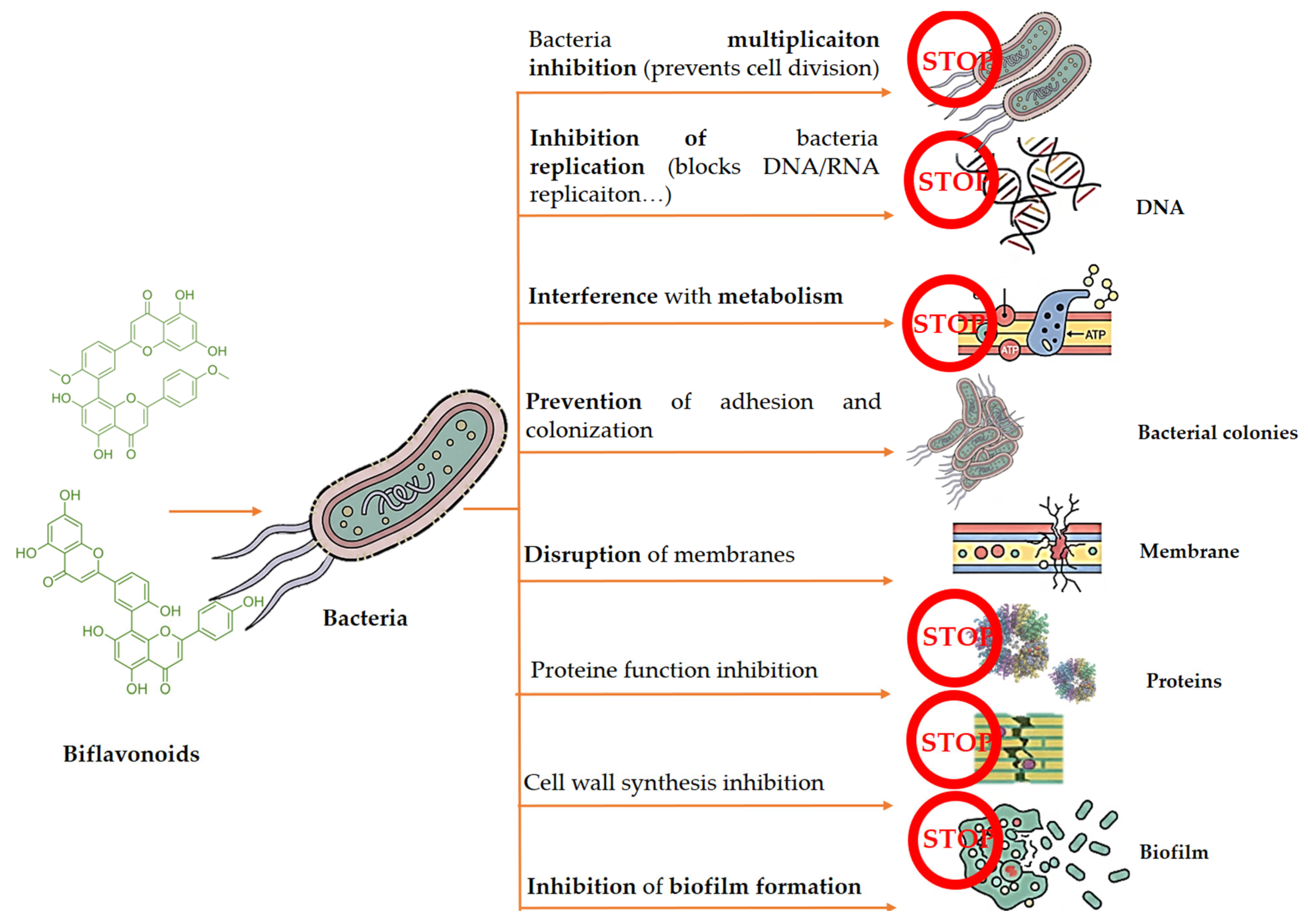
3.2. Antibacterial Activity
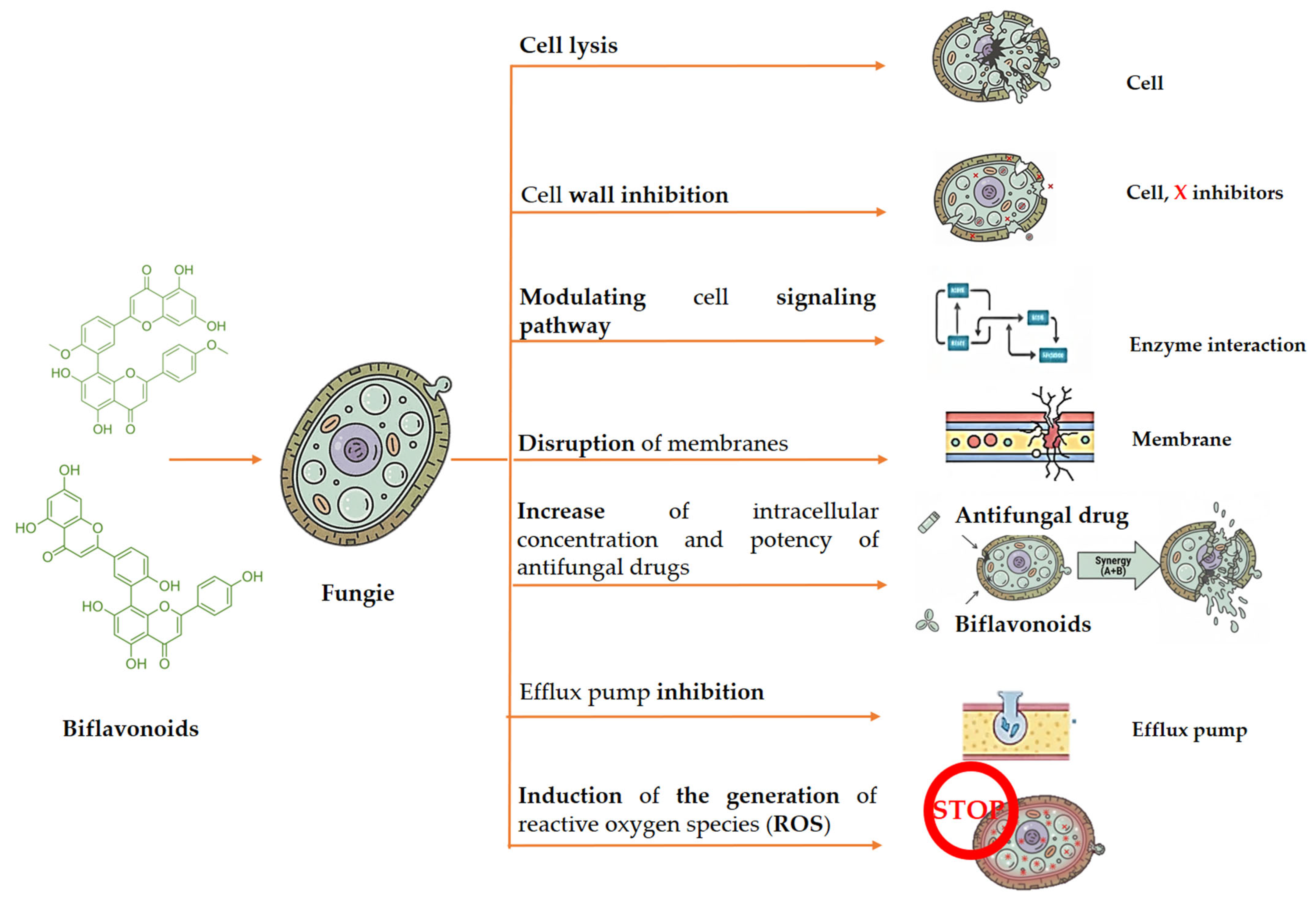
3.3. Antifungal Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Yue, S.-J. Editorial: Flavonoids and Cardiovascular Metabolism. Front. Nutr. 2022, 9, 939798. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Dagur, H.S.; Khan, M.; Malik, N.; Alam, M.; Mushtaque, M. Therapeutic Role of Flavonoids and Flavones in Cancer Prevention: Current Trends and Future Perspectives. Eur. J. Med. Chem. Rep. 2021, 3, 100010. [Google Scholar] [CrossRef]
- Jia, S.; Hou, Y.; Wang, D.; Zhao, X. Flavonoids for Depression and Anxiety: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 8839–8849. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J. The Potential of Flavonoids and Flavonoid Metabolites in the Treatment of Neurodegenerative Pathology in Disorders of Cognitive Decline. Antioxidants 2023, 12, 663. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, W.; Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Chen, D.; Jiao, Z. Identification of Six Flavonoids as Novel Cellular Antioxidants and Their Structure-Activity Relationship. Oxid. Med. Cell. Longev. 2020, 2020, 4150897. [Google Scholar] [CrossRef]
- Santos, E.L.; Maia, B.H.L.N.S.; Ferriani, A.P.; Teixeira, S.D. Flavonoids: Classification, Biosynthesis and Chemical Ecology. In Flavonoids—From Biosynthesis to Human Health; InTech: Rijeka, Croatia, 2017. [Google Scholar]
- Ji, Y.; Li, B.; Qiao, M.; Li, J.; Xu, H.; Zhang, L.; Zhang, X. Advances on the in Vivo and in Vitro Glycosylations of Flavonoids. Appl. Microbiol. Biotechnol. 2020, 104, 6587–6600. [Google Scholar] [CrossRef]
- Boozari, M.; Soltani, S.; Iranshahi, M. Biologically Active Prenylated Flavonoids from the Genus Sophora and Their Structure–Activity Relationship—A Review. Phyther. Res. 2019, 33, 546–560. [Google Scholar] [CrossRef]
- He, X.; Yang, F.; Huang, X. Proceedings of Chemistry, Pharmacology, Pharmacokinetics and Synthesis of Biflavonoids. Molecules 2021, 26, 6088. [Google Scholar] [CrossRef]
- Yue, T.; Sheng, Q.; Luo, Y.; Xiao, Z.; Wang, Y.; Song, W.; Yan, M.; Niu, H.; Zhang, T.; Li, N. Biflavonoids and Oligomeric Flavonoids from Food. In Handbook of Dietary Phytochemicals; Springer: Singapore, 2020; pp. 1–49. [Google Scholar]
- Paquin, A.; Reyes-Moreno, C.; Bérubé, G. Recent Advances in the Use of the Dimerization Strategy as a Means to Increase the Biological Potential of Natural or Synthetic Molecules. Molecules 2021, 26, 2340. [Google Scholar] [CrossRef]
- Šamec, D.; Jurčević Šangut, I.; Karalija, E.; Šarkanj, B.; Zelić, B.; Šalić, A. 3′-8″-Biflavones: A Review of Their Structural Diversity, Natural Occurrence, Role in Plants, Extraction and Identification. Molecules 2024, 29, 4634. [Google Scholar] [CrossRef]
- Kovač Tomas, M.; Jurčević, I.; Šamec, D. Tissue-Specific Profiling of Biflavonoids in Ginkgo (Ginkgo biloba L.). Plants 2022, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Medvedec, B.; Jurčević Šangut, I.; Macanović, A.; Karalija, E.; Šamec, D. Biflavonoid Profiling of Juniperus Species: The Influence of Plant Part and Growing Location. Appl. Sci. 2025, 15, 7082. [Google Scholar] [CrossRef]
- Šamec, D.; Pierz, V.; Srividya, N.; Wüst, M.; Lange, B.M. Assessing Chemical Diversity in Psilotum nudum (L.) Beauv., a Pantropical Whisk Fern That Has Lost Many of Its Fern-like Characters. Front. Plant Sci. 2019, 10, 868. [Google Scholar] [CrossRef]
- Osorio, E.; Londoño, J.; Bastida, J. Low-Density Lipoprotein (LDL)-Antioxidant Biflavonoids from Garcinia madruno. Molecules 2013, 18, 6092–6100. [Google Scholar] [CrossRef]
- Al-Shagdari, A.; Alarcón, A.B.; Cuesta-Rubio, O.; Piccinelli, A.L.; Rastrelli, L. Biflavonoids, Main Constituents from Garcinia Bakeriana Leaves. Nat. Prod. Commun. 2013, 8, 1237–1240. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Dahija, S.; Hassan, S.T.S. Biflavonoids: Important Contributions to the Health Benefits of Ginkgo (Ginkgo biloba L.). Plants 2022, 11, 1381. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Mai, Y.; Li, H.; Wang, Z.; Xu, J.; He, X. Health Benefits of the Flavonoids from Onion: Constituents and Their Pronounced Antioxidant and Anti-Neuroinflammatory Capacities. J. Agric. Food Chem. 2020, 68, 799–807. [Google Scholar] [CrossRef]
- Bagdonaitė, E.; Mártonfi, P.; Repčák, M.; Labokas, J. Variation in Concentrations of Major Bioactive Compounds in Hypericum perforatum L. from Lithuania. Ind. Crops Prod. 2012, 35, 302–308. [Google Scholar] [CrossRef]
- Weng, J.-K.; Noel, J.P. Chemodiversity in Selaginella: A Reference System for Parallel and Convergent Metabolic Evolution in Terrestrial Plants. Front. Plant Sci. 2013, 4, 119. [Google Scholar] [CrossRef]
- Zou, P.; Otero, P.; Mejuto, J.C.; Simal-Gandara, J.; Xiao, J.; Cameselle, C.; Chen, S.; Lin, S.; Cao, H. Exploring the Mechanism of Flavonoids Modification by Dimerization Strategies and Their Potential to Enhance Biological Activity. Food Chem. 2025, 467, 142266. [Google Scholar] [CrossRef]
- Sagrera, G.; Bertucci, A.; Vazquez, A.; Seoane, G. Synthesis and Antifungal Activities of Natural and Synthetic Biflavonoids. Bioorg. Med. Chem. 2011, 19, 3060–3073. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.; de Oliveira, N.C.; Pestana, Y.; Alves, M.A.; da Silva, A.J.M. Regio- and Chemoselective Synthesis of Polyaryl Flavones by Combination of C-O/C-H Activation and Suzuki-Miyaura Cross Coupling Reactions. J. Mol. Struct. 2024, 1299, 137067. [Google Scholar] [CrossRef]
- Pereira, R.; Ribeiro, D.; Silva, V.L.M.; Fernandes, E. Synthetic Flavonoid Dimers: Synthesis Strategies and Biological Activities. Eur. J. Med. Chem. 2025, 291, 117669. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, J.; Szyk, P.; Czarczynska-Goslinska, B.; Goslinski, T. Flavonoids, Chalcones, and Their Fluorinated Derivatives—Recent Advances in Synthesis and Potential Medical Applications. Molecules 2025, 30, 2395. [Google Scholar] [CrossRef]
- Pradayrol, É.; Maciuk, A.; Evanno, L. Oxidative Assemblies of Flavonoids Dimers—Synthesis of Dehydrodicatechin A. ChemistrySelect 2023, 8, e202300779. [Google Scholar] [CrossRef]
- Greule, A.; Stok, J.E.; De Voss, J.J.; Cryle, M.J. Unrivalled Diversity: The Many Roles and Reactions of Bacterial Cytochromes P450 in Secondary Metabolism. Nat. Prod. Rep. 2018, 35, 757–791. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Suzuki, A. Cross-Coupling Reactions Of Organoboranes: An Easy Way To Construct CC Bonds (Nobel Lecture). Angew. Chemie Int. Ed. 2011, 50, 6722–6737. [Google Scholar] [CrossRef]
- Malapit, C.A.; Bour, J.R.; Brigham, C.E.; Sanford, M.S. Base-Free Nickel-Catalysed Decarbonylative Suzuki–Miyaura Coupling of Acid Fluorides. Nature 2018, 563, 100–104. [Google Scholar] [CrossRef]
- Fregoso-López, D.; Miranda, L.D. Visible-Light Mediated Radical Alkylation of Flavones: A Modular Access to Nonsymmetrical 3,3″-Biflavones. Org. Lett. 2022, 24, 8615–8620. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.-H.; Zhu, J.-M.; Wang, G.-Y.; Ren, Y.-H.; Liu, H.-L.; Wang, Y. Gymnosperm-Specific CYP90Js Enable Biflavonoid Biosynthesis and Microbial Production of Amentoflavone. Nat. Commun. 2025, 16, 7792. [Google Scholar] [CrossRef] [PubMed]
- Alfke, J.; Kampermann, U.; Kalinina, S.; Esselen, M. Isolation and Structural Elucidation of Dimeric Epigallocatechin-3-Gallate Autoxidation Products and Their Antioxidant Capacity. Eur. Food Res. Technol. 2021, 247, 2961–2975. [Google Scholar] [CrossRef]
- Choi, J.; Kim, E.-M.; Ko, B.J.; Lee, U.-J.; Seo, J.-H.; Kim, B.-G. Production of Theasinensin A Using Laccase as Antioxidant and Antiaging Agent. Biotechnol. Bioprocess Eng. 2022, 27, 253–261. [Google Scholar] [CrossRef]
- Zhang, L.-B.; Deng, Z.-Q.; Qiu, T.-T.; Yang, W.-W.-J.; Zhu, F.; Ye, X.-Y. Characterisation of a Laccase Isolated from Trametes Hirsuta and Its Application in the Oligomerisation of Phenolic Compounds. Fungal Biol. 2023, 127, 872–880. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial Activity of Flavonoids and Their Structure–Activity Relationship: An Update Review. Phyther. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Gontijo, V.S.; dos Santos, M.H.; Viegas, C., Jr. Biological and Chemical Aspects of Natural Biflavonoids from Plants: A Brief Review. Mini-Rev. Med. Chem. 2017, 17, 834–862. [Google Scholar] [CrossRef]
- Menezes, J.C.J.M.D.S.; Diederich, M.F. Natural Dimers of Coumarin, Chalcones, and Resveratrol and the Link between Structure and Pharmacology. Eur. J. Med. Chem. 2019, 182, 111637. [Google Scholar] [CrossRef]
- Coulerie, P.; Eydoux, C.; Hnawia, E.; Stuhl, L.; Maciuk, A.; Lebouvier, N.; Canard, B.; Figadère, B.; Guillemot, J.-C.; Nour, M. Biflavonoids of Dacrydium Balansae with Potent Inhibitory Activity on Dengue 2 NS5 Polymerase. Planta Med. 2012, 78, 672–677. [Google Scholar] [CrossRef]
- Hossain, R.; Islam, M.T.; Ray, P.; Jain, D.; Saikat, A.S.M.; Nahar, L.; Talukdar, A.D.; Sarker, S.; Ayatollahi, S.A.; Martorell, M.; et al. Amentoflavone, New Hope against SARS-CoV-2: An Outlook through Its Scientific Records and an in Silico Study. Pharmacogn. Res. 2021, 13, 149–157. [Google Scholar] [CrossRef]
- Singh, A.V. Potential of Amentoflavone with Antiviral Properties in COVID-19 Treatment. Asian Biomed. 2021, 15, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhu, G.-H.; Wang, H.-N.; Hu, Q.; Chen, L.-L.; Guan, X.-Q.; Li, H.-L.; Chen, H.-Z.; Tang, H.; Ge, G.-B. Discovery of Naturally Occurring Inhibitors against SARS-CoV-2 3CLpro from Ginkgo Biloba Leaves via Large-Scale Screening. Fitoterapia 2021, 152, 104909. [Google Scholar] [CrossRef]
- Mondal, S.; Karmakar, A.; Mallick, T.; Begum, N.A. Exploring the Efficacy of Naturally Occurring Biflavone Based Antioxidants towards the Inhibition of the SARS-CoV-2 Spike Glycoprotein Mediated Membrane Fusion. Virology 2021, 556, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Lokhande, K.; Nawani, N.; Venkateswara, S.K.; Pawar, S. Biflavonoids from Rhus Succedanea as Probable Natural Inhibitors against SARS-CoV-2: A Molecular Docking and Molecular Dynamics Approach. J. Biomol. Struct. Dyn. 2022, 40, 4376–4388. [Google Scholar] [CrossRef]
- Ghosh, R.; Chakraborty, A.; Biswas, A.; Chowdhuri, S. Computer Aided Identification of Potential SARS-CoV-2 Main Protease Inhibitors from Diterpenoids and Biflavonoids of Torreya Nucifera Leaves. J. Biomol. Struct. Dyn. 2022, 40, 2647–2662. [Google Scholar] [CrossRef]
- Abdizadeh, R.; Hadizadeh, F.; Abdizadeh, T. Evaluation of Apigenin-Based Biflavonoid Derivatives as Potential Therapeutic Agents against Viral Protease (3CLpro) of SARS-CoV-2 via Molecular Docking, Molecular Dynamics and Quantum Mechanics Studies. J. Biomol. Struct. Dyn. 2023, 41, 5915–5945. [Google Scholar] [CrossRef]
- Chaves, O.A.; Lima, C.R.; Fintelman-Rodrigues, N.; Sacramento, C.Q.; de Freitas, C.S.; Vazquez, L.; Temerozo, J.R.; Rocha, M.E.N.; Dias, S.S.G.; Carels, N.; et al. Agathisflavone, a Natural Biflavonoid That Inhibits SARS-CoV-2 Replication by Targeting Its Proteases. Int. J. Biol. Macromol. 2022, 222, 1015–1026. [Google Scholar] [CrossRef]
- Ma, S.-C.; But, P.P.-H.; Ooi, V.E.-C.; He, Y.-H.; Lee, S.H.-S.; Lee, S.-F.; Lin, R.-C. Antiviral Amentoflavone from Selaginella Sinensis. Biol. Pharm. Bull. 2001, 24, 311–312. [Google Scholar] [CrossRef]
- de Freitas, C.S.; Rocha, M.E.N.; Sacramento, C.Q.; Marttorelli, A.; Ferreira, A.C.; Rocha, N.; de Oliveira, A.C.; de Oliveira Gomes, A.M.; dos Santos, P.S.; da Silva, E.O.; et al. Agathisflavone, a Biflavonoid from Anacardium occidentale L., Inhibits Influenza Virus Neuraminidase. Curr. Top. Med. Chem. 2020, 20, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Song, X.; Su, G.; Wang, Y.; Wang, Z.; Jia, J.; Qing, S.; Huang, L.; Wang, Y.; Zheng, K.; et al. Amentoflavone Inhibits HSV-1 and ACV-Resistant Strain Infection by Suppressing Viral Early Infection. Viruses 2019, 11, 466. [Google Scholar] [CrossRef] [PubMed]
- Wilsky, S.; Sobotta, K.; Wiesener, N.; Pilas, J.; Althof, N.; Munder, T.; Wutzler, P.; Henke, A. Inhibition of Fatty Acid Synthase by Amentoflavone Reduces Coxsackievirus B3 Replication. Arch. Virol. 2012, 157, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Li, J.; Lei, X.; Liu, Y.; Yang, Z.; Chen, K. Antiviral Activity of Total Flavonoid Extracts from Selaginella Moellendorffii Hieron against Coxsackie Virus B3 In Vitro and In Vivo. Evid. Based Complement. Altern. Med. 2014, 2014, 950817. [Google Scholar] [CrossRef]
- Lee, W.-P.; Lan, K.-L.; Liao, S.-X.; Huang, Y.-H.; Hou, M.-C.; Lan, K.-H. Inhibitory Effects of Amentoflavone and Orobol on Daclatasvir-Induced Resistance-Associated Variants of Hepatitis C Virus. Am. J. Chin. Med. 2018, 46, 835–852. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Jeong, H.J.; Kim, J.H.; Kim, Y.M.; Park, J.-Y.; Kim, D.; Naguyen, T.T.H.; Park, S.-J.; Chang, J.S.; Park, K.H. Biflavonoids from Torreya Nucifera Displaying SARS-CoV 3CLpro Inhibition. Bioorg. Med. Chem. 2010, 18, 7940–7947. [Google Scholar] [CrossRef]
- Menezes, J.C.J.M.D.S.; Campos, V.R. Natural Biflavonoids as Potential Therapeutic Agents against Microbial Diseases. Sci. Total Environ. 2021, 769, 145168. [Google Scholar] [CrossRef]
- Hwang, J.H. Antibacterial Effect of Amentoflavone and Its Synergistic Effect with Antibiotics. J. Microbiol. Biotechnol. 2013, 23, 953–958. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Park, I.; Lee, J.; Shukla, S.; Nile, S.H.; Chun, H.S.; Khan, I.; Oh, S.Y.; Lee, H.; Huh, Y.S.; et al. Antioxidant and Antimicrobial Efficacy of a Biflavonoid, Amentoflavone from Nandina Domestica in Vitro and in Minced Chicken Meat and Apple Juice Food Models. Food Chem. 2019, 271, 239–247. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, B.; Liu, S.; Wang, L.; Wang, J. Anticytotoxin Effects of Amentoflavone to Pneumolysin. Biol. Pharm. Bull. 2017, 40, 61–67. [Google Scholar] [CrossRef]
- Shen, X.; Niu, X.; Li, G.; Deng, X.; Wang, J. Amentoflavone Ameliorates Streptococcus Suis-Induced Infection in Vitro and in Vivo. Appl. Environ. Microbiol. 2018, 84, e01804-18. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, H.M.; Mabusela, W.T.; Abegaz, B.M.; Martin, O.O. Antimicrobial Activities of a Novel Biflavonoid and Other Constituents from Rhus Natalensis. J. Med. Plants Res. 2013, 7, 619–623. [Google Scholar]
- Xu, H.X.; Mughal, S.; Taiwo, O.; Lee, S.F. Isolation and Characterization of an Antibacterial Biflavonoid from an African Chewing Stick Garcinia Kola Heckel (Clusiaceae). J. Ethnopharmacol. 2013, 147, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Negm, W.A.; El-Aasr, M.; Kamer, A.A.; Elekhnawy, E. Investigation of the Antibacterial Activity and Efflux Pump Inhibitory Effect of Cycas thouarsii R.Br. Extract against Klebsiella pneumoniae Clinical Isolates. Pharmaceuticals 2021, 14, 756. [Google Scholar] [CrossRef]
- Bitchagno, G.T.M.; Tankeo, S.B.; Tsopmo, A.; Simo Mpetga, J.D.; Tchinda, A.T.; Fobofou, S.A.T.; Nkuete, A.H.L.; Wessjohann, L.A.; Kuete, V.; Tane, P. Ericoside, a New Antibacterial Biflavonoid from Erica mannii (Ericaceae). Fitoterapia 2016, 109, 206–211. [Google Scholar] [CrossRef]
- Tang, S.; Bremner, P.; Kortenkamp, A.; Schlage, C.; Gray, A.I.; Gibbons, S.; Heinrich, M. Biflavonoids with Cytotoxic and Antibacterial Activity from Ochna macrocalyx. Planta Med. 2003, 69, 247–253. [Google Scholar] [CrossRef]
- Nandu, T.G.; Subramenium, G.A.; Shiburaj, S.; Viszwapriya, D.; Iyer, P.M.; Balamurugan, K.; Rameshkumar, K.B.; Karutha Pandian, S. Fukugiside, a Biflavonoid from Garcinia travancorica Inhibits Biofilm Formation of Streptococcus pyogenes and Its Associated Virulence Factors. J. Med. Microbiol. 2018, 67, 1391–1401. [Google Scholar] [CrossRef]
- Haas, B.; Grenier, D. Understanding the Virulence of Streptococcus suis: A Veterinary, Medical, and Economic Challenge. Med. Mal. Infect. 2018, 48, 159–166. [Google Scholar] [CrossRef]
- Fittipaldi, N.; Segura, M.; Grenier, D.; Gottschalk, M. Virulence Factors Involved in the Pathogenesis of the Infection Caused by the Swine Pathogen and Zoonotic Agent Streptococcus suis. Future Microbiol. 2012, 7, 259–279. [Google Scholar] [CrossRef]
- Chukwujekwu, J.C.; De Kock, C.A.; Smith, P.J.; Van Heerden, F.R.; Van Staden, J. Antiplasmodial and Antibacterial Activity of Compounds Isolated from Ormocarpum trichocarpum. Planta Med. 2012, 78, 1857–1860. [Google Scholar] [CrossRef]
- Hyun, J.J.; Woo, S.S.; Yeo, S.H.; Hyun, S.K.; Lee, I.S.; Woo, E.R.; Dong, G.L. Antifungal Effect of Amentoflavone Derived from Selaginella tamariscina. Arch. Pharm. Res. 2006, 29, 746–751. [Google Scholar] [CrossRef]
- Jung, H.J.; Park, K.; Lee, I.S.; Kim, H.S.; Yeo, S.H.; Woo, E.R.; Lee, D.G. S-Phase Accumulation of Candida albicans by Anticandidal Effect of Amentoflavone Isolated from Selaginella tamariscina. Biol. Pharm. Bull. 2007, 30, 1969–1971. [Google Scholar] [CrossRef]
- Hwang, I.S.; Lee, J.; Jin, H.G.; Woo, E.R.; Lee, D.G. Amentoflavone Stimulates Mitochondrial Dysfunction and Induces Apoptotic Cell Death in Candida albicans. Mycopathologia 2012, 173, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Krauze-Baranowska, M.; Wiwart, M. Antifungal Activity of Biflavones from Taxus baccata and Ginkgo biloba. Zeitschrift Naturforschung C 2003, 58, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Šangut, I.J.; Šarkanj, B.; Karalija, E.; Šamec, D. A Comparative Analysis of Radical Scavenging, Antifungal and Enzyme Inhibition Activity of 3′-8″-Biflavones and Their Monomeric Subunits. Antioxidants 2023, 12, 1854. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, Y.; Woo, E.R.; Lee, D.G. Isocryptomerin, a Novel Membrane-Active Antifungal Compound from Selaginella tamariscina. Biochem. Biophys. Res. Commun. 2009, 379, 676–680. [Google Scholar] [CrossRef]
- Lee, J.-H. Involvement of T-Cell Immunoregulation by Ochnaflavone in Therapeutic Effect on Fungal Arthritis Due to Candida albicans. Arch. Pharm. Res. 2011, 34, 1209–1217. [Google Scholar] [CrossRef]

| Latin Name | Biflavonoids-Type | Biflavonoids |
|---|---|---|
| Hypericum perforatum L. | 3′,8″ 3,8″ | amentoflavone 3,8″-biapigenin [21] |
| Selaginella sp. | Seven dimeric linking types: 2′-8″, 3-3″,3′-6″,3′-8″,3-O-4‴, 3′-O-4‴ and 4′-O-6′ | 2′,8′-biapigenin, taiwaniaflavone, 7,4′-di-O-methylrobus taflavone, 7″-O-methylrobustaflavone, 4′-O-methylrobustaflavone, robustaflavone, 7,4′-di-O-methyl-2″,3″-dihydrorobustaflavone, 7, 4′,7″-tri-O-methyl-2″,3″-dihydrorobustaflavone, 7,4′,7″,4‴-tetra-O-methylamentoflavone, kayaflavone, heveaflavone, ginkgetin, 7,7″-di-O-methylamentoflavone, 4′,7″-di-O-methylamento flavone, isoginkgetin, bilobetin, podocarpusflavone A, sostetsuflavone, amentoflavone, sumaflavone, 2,3-dihy droamentoflavone, 2″,3″-dihydroamentoflavone, tetrahy droamentoflavone, delicaflavone, ochnaflavone, crypto merin B, pulvinatabiflavone, 7-O-methyl-hinokiflavone, isocryptomerin, hinokiflavone, 2″,3″-dihydroisocryptomerin, 2,3-dihydrohinokiflavone (45), 2″,3″-dihydrohinokiflavone, tetrahydrohinokiflavone [22] |
| Juniperus sp. | 3′,8″, 8,8″, 4′,6″ | amentoflavone, bilobetin, cupressuflavone, hinokiflavone [15] |
| Ginko biloba L. | 3′,8″ | ginkgetin, isoginkgetin, amentoflavone, bilobetin, sciadopitysin, sesquoiaflavone, podocarpusflavone A, and 5′-metoxybilobetin [19] |
| Garcinia madruno L. | 3′,8″ 3″-8″ | amentoflavone, morelloflavone, volkensiflavone, fukugiside, spicataside [17] |
| Types of Biflavonoids | Source | Pathogen | Outcomes | References |
|---|---|---|---|---|
| Amentoflavone | E. faecium S. aureus S. mutans E. coli O-157 P. aeruginosa | Amentoflavone showed remarkable antibacterial activity against Gram-positive and Gram-negative bacteria, with MIC values of 4–32 µg/mL. | [60] | |
| Amentoflavone | Nandina domestica | S. aureus KCTC 1621 E. coli ATCC 43889 | S. aureus and E. coli showed a significant reduction in cell viability at their respective MICs of 62.5 and 125 µg/mL. | [61] |
| Amentoflavone | - | S. pneumoniae | By interacting with the toxin at Ser254, Glu277, and Arg359, amentoflavone efficiently inhibits the oligomerization of wild-type PLY and shields human alveolar epithelial (A549) cells from harm caused by pneumolysin. | [62] |
| Amentoflavone | Chinese herbs | S. suis | Although amentoflavone does not significantly affect the expression of SLY, it was found to be a strong antagonist of SLY-mediated hemolysis. | [63] |
| Rhuschromone | Rhus natalensis | S. aureus E. coli P. aureginosa | Rhuschromone had a comparatively high activity against S. aureus. | [64] |
| GB1 | Garcinia kola Heckel | S. mutans | GB1 showed activity against S. mutans and other oral bacteria with MIC values of 32–64 µg/mL. | [65] |
| 5,7,7″,4‴-tetra-O-methyl-hinokiflavone 2,3-dihydrobilobetin 4′,4‴-O-dimethyl amentoflavone Hinokiflavone | Cycas thouarsii R.Br. | K. pneumoniae | The MICs of the pure compounds ranged from 0.25 to 2 µg/mL against pathogens. | [66] |
| Ericoside | Erica mannii | E. coli AG100 | With a MIC of 64 μg/mL, ericoside showed moderate efficacy against the resistant bacteria. | [67] |
| Types of Biflavonoids | Source | Pathogen | Outcomes | References |
|---|---|---|---|---|
| Amentoflavone | Selaginella tamariscina | C. albicans S. cerevisiae T. beigelli | Amentoflavone exhibited a significant antifungal activity at concentrations ranging from 5–10 µg/mL. | [73] |
| Amentoflavone | Selaginella tamariscina | C. albicans | Cell cycles were markedly inhibited during the S-phase by amentoflavone. | [74] |
| Amentoflavone | Selaginella tamariscina | C. albicans | Amentoflavone causes C. albicans cells to undergo apoptosis and is linked to mitochondrial malfunction. | [75] |
| Amentoflavone Bilobetin Sequoiaflavone Ginkgetin Sciadopitysin 2,3-Dihydrosciadopitysin | Taxus baccata Ginkgo biloba | A. alternata Cladosporium oxysporum F. culmorum | Bilobetin exhibited a significant antifungal activity with values of ED50 14, 11, and 17 μm, respectively. Bilobetin was less effective than ginkgetin and 7-O-methylamentoflavone in their interactions with A. alternata. After being exposed to a 200 μm concentration of ginkgetin, A. alternata showed modest structural alterations in its cell wall. | [76] |
| Amentoflavone Bilobetin Ginkgetin Isoginkgetin Sciadopitysin | - | A. alternata A. flavus A. ochraceus F. graminearum Fusarium verticillioides | The fungus species and measured concentration had a significant impact on the antifungal activity. All the substances showed considerable inhibition of F. graminearum at 0.01 μg/mL, with biflavonoids working better than monomers. | [77] |
| Isocryptomerin | Selaginella tamariscina | C. albicans T. beigelii S. cerevisiae | Isocryptomerin, with an MIC value of 18.11 μM, exhibited against human pathogenic fungi. | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Duman, H.; Karav, S.; Šalić, A.; Šamec, D. Dimerized Power: The Antimicrobial and Antiviral Promise of Biflavonoids. Biomolecules 2026, 16, 24. https://doi.org/10.3390/biom16010024
Duman H, Karav S, Šalić A, Šamec D. Dimerized Power: The Antimicrobial and Antiviral Promise of Biflavonoids. Biomolecules. 2026; 16(1):24. https://doi.org/10.3390/biom16010024
Chicago/Turabian StyleDuman, Hatice, Sercan Karav, Anita Šalić, and Dunja Šamec. 2026. "Dimerized Power: The Antimicrobial and Antiviral Promise of Biflavonoids" Biomolecules 16, no. 1: 24. https://doi.org/10.3390/biom16010024
APA StyleDuman, H., Karav, S., Šalić, A., & Šamec, D. (2026). Dimerized Power: The Antimicrobial and Antiviral Promise of Biflavonoids. Biomolecules, 16(1), 24. https://doi.org/10.3390/biom16010024









