Cold Atmospheric Plasma Enhances TGF-β1, CTGF Protein Expression, and Healing in Full-Thickness Skin Burns: An Animal Study
Abstract
1. Introduction
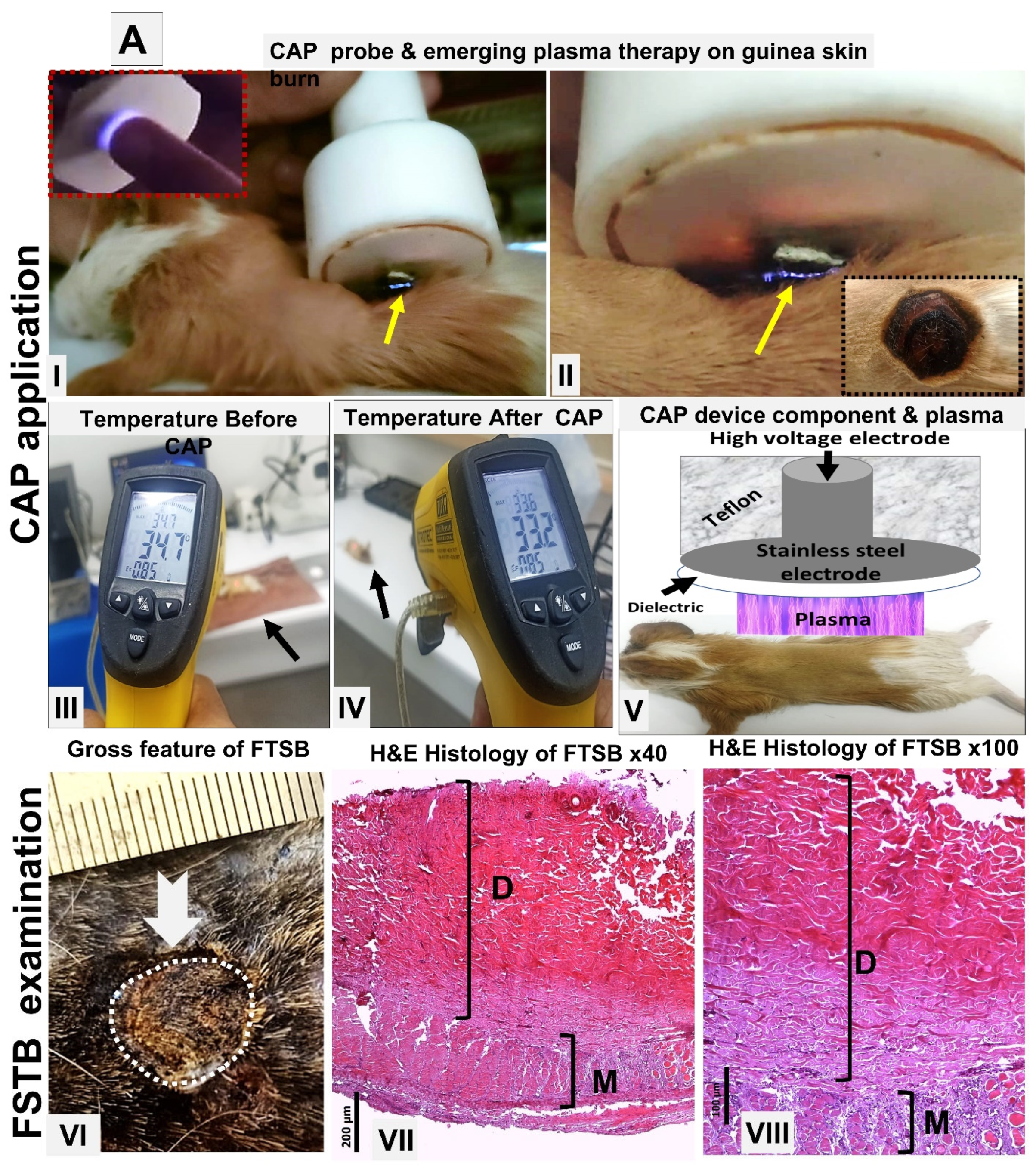
2. Materials and Methods
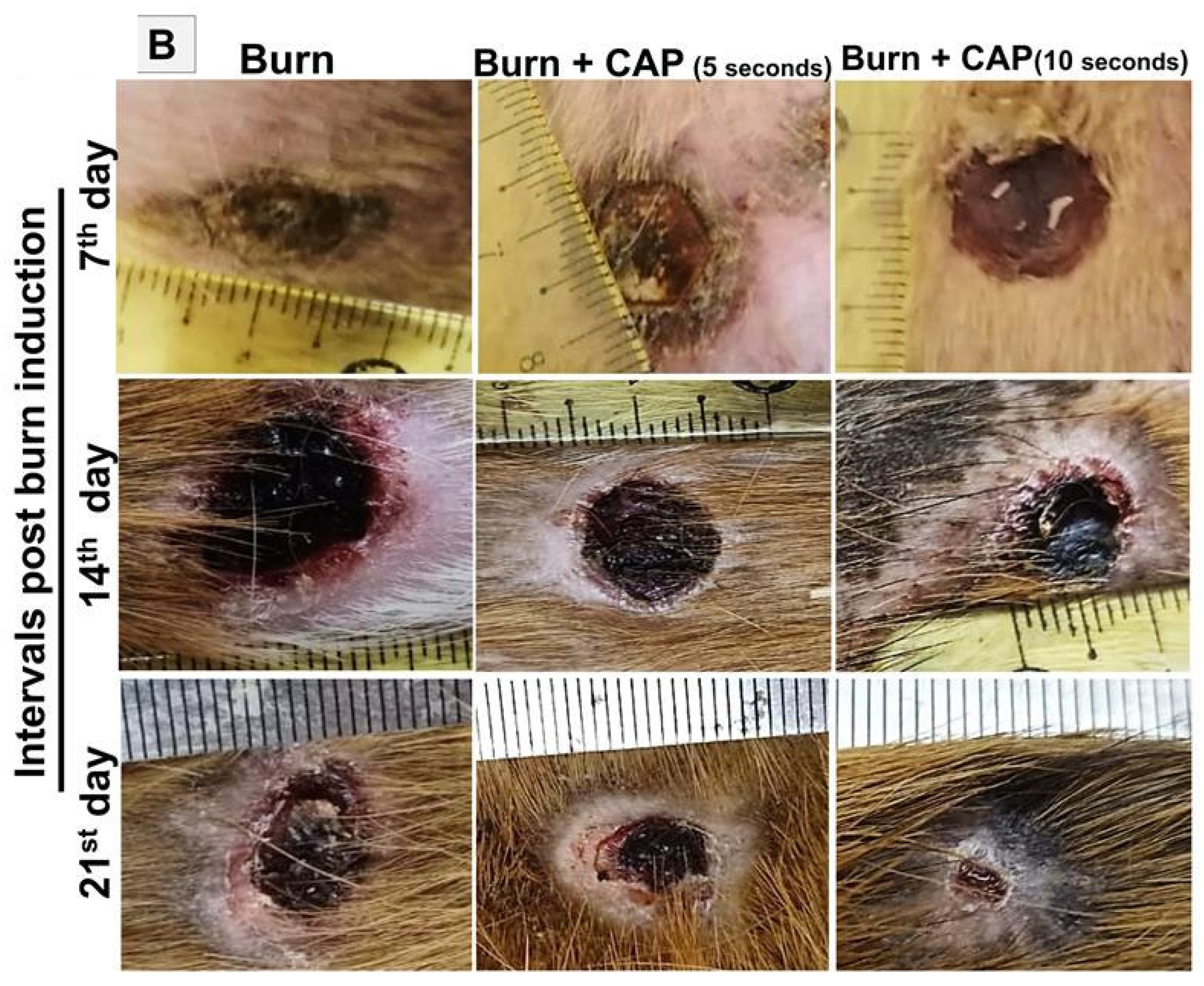
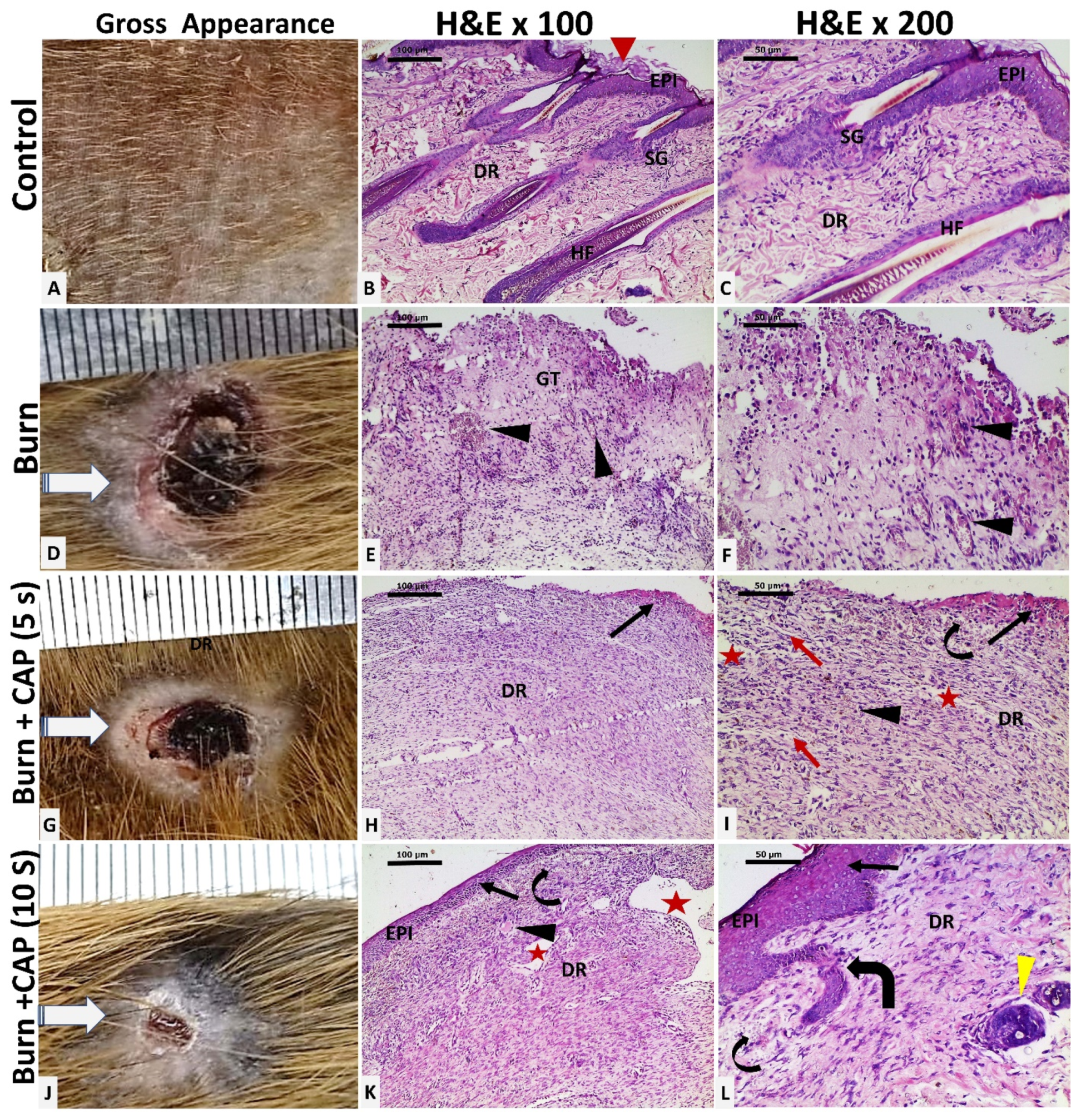
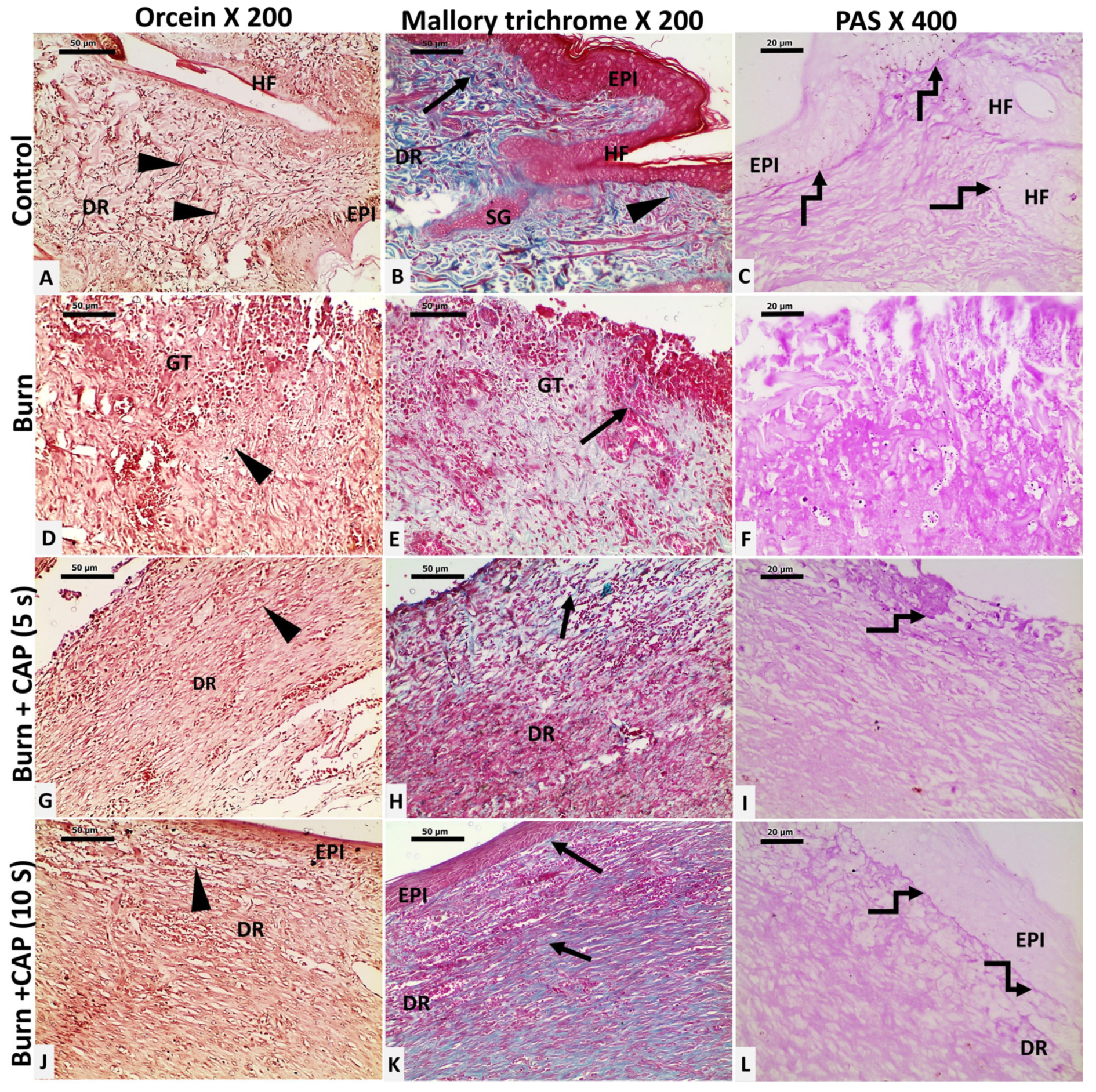
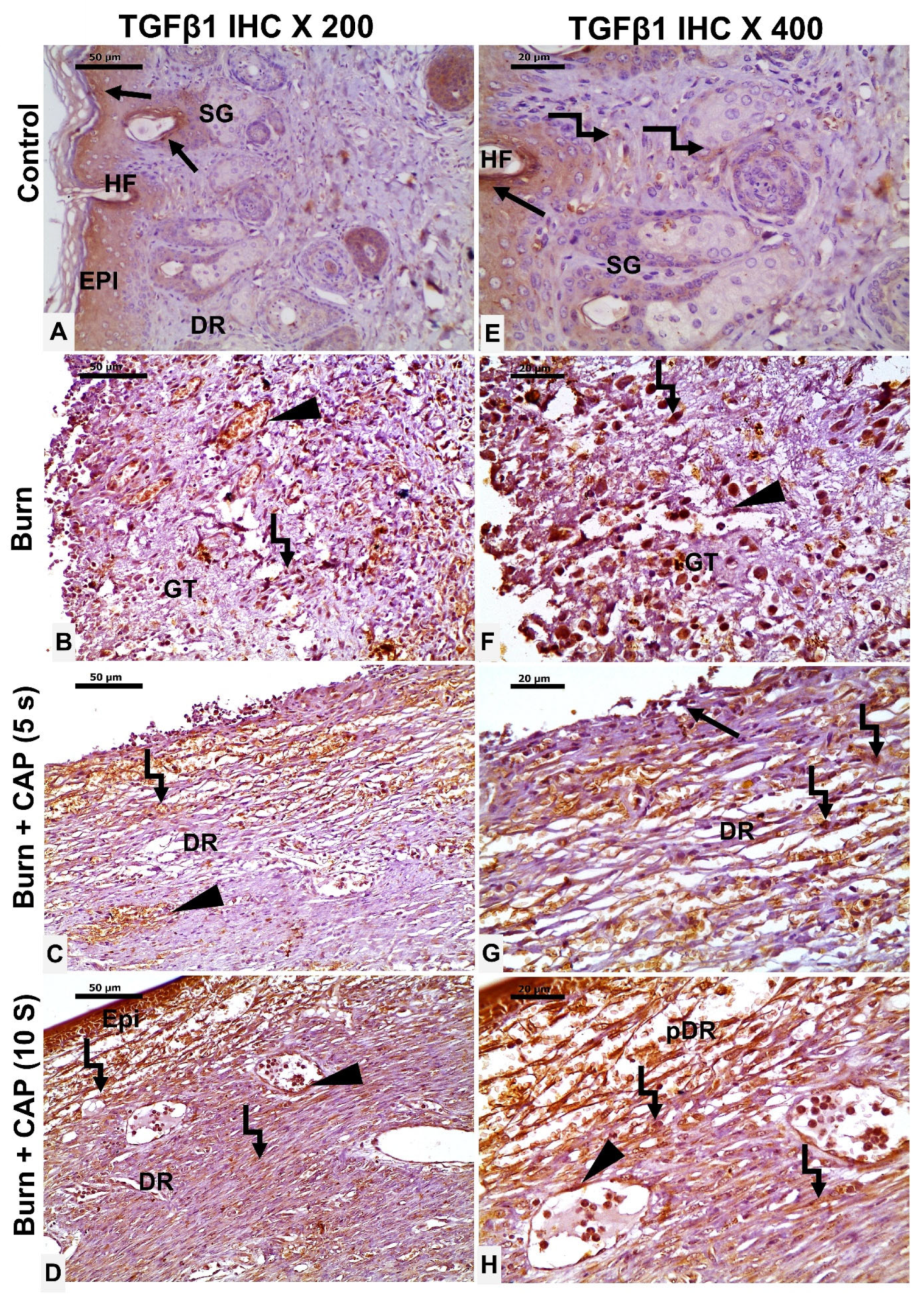
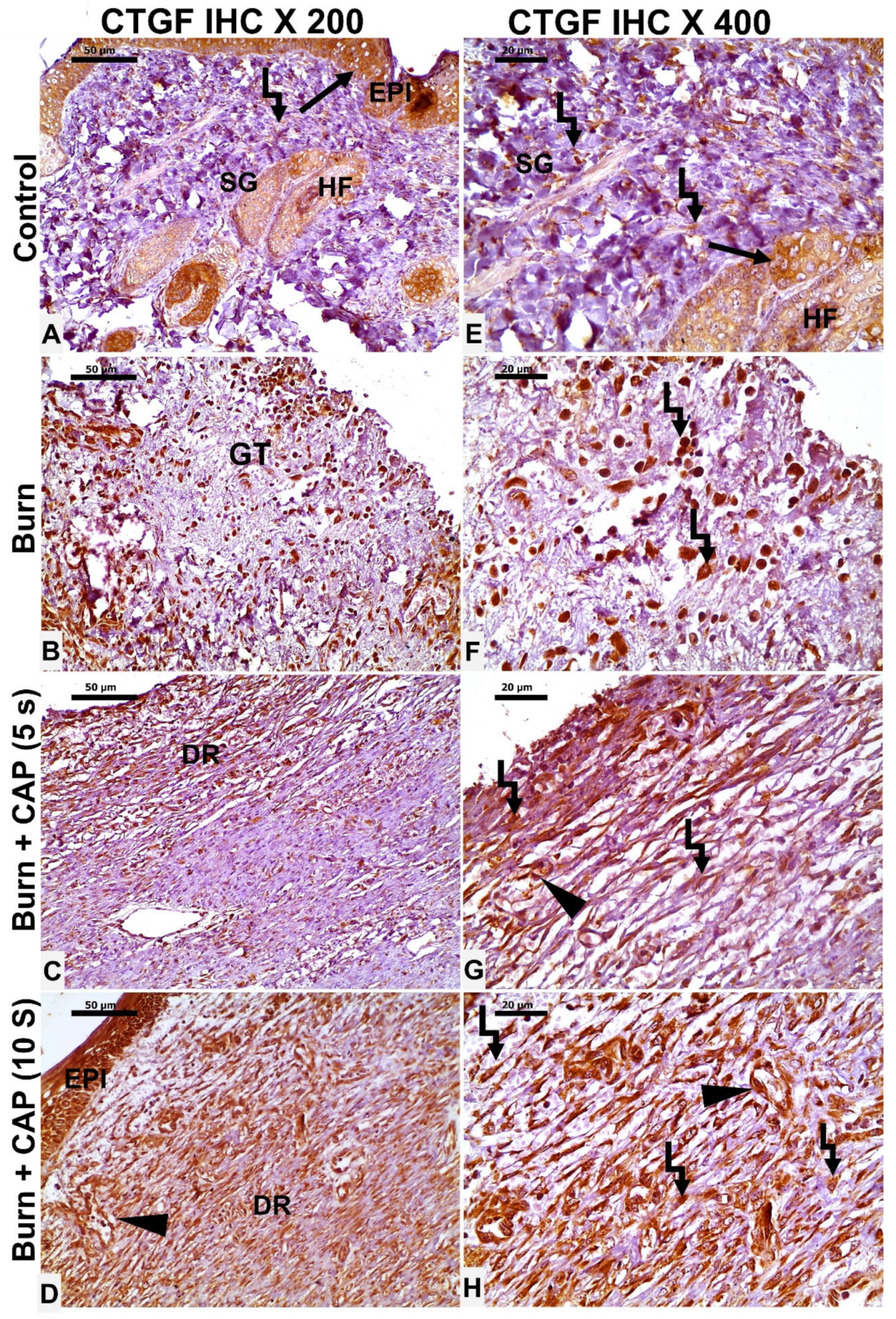
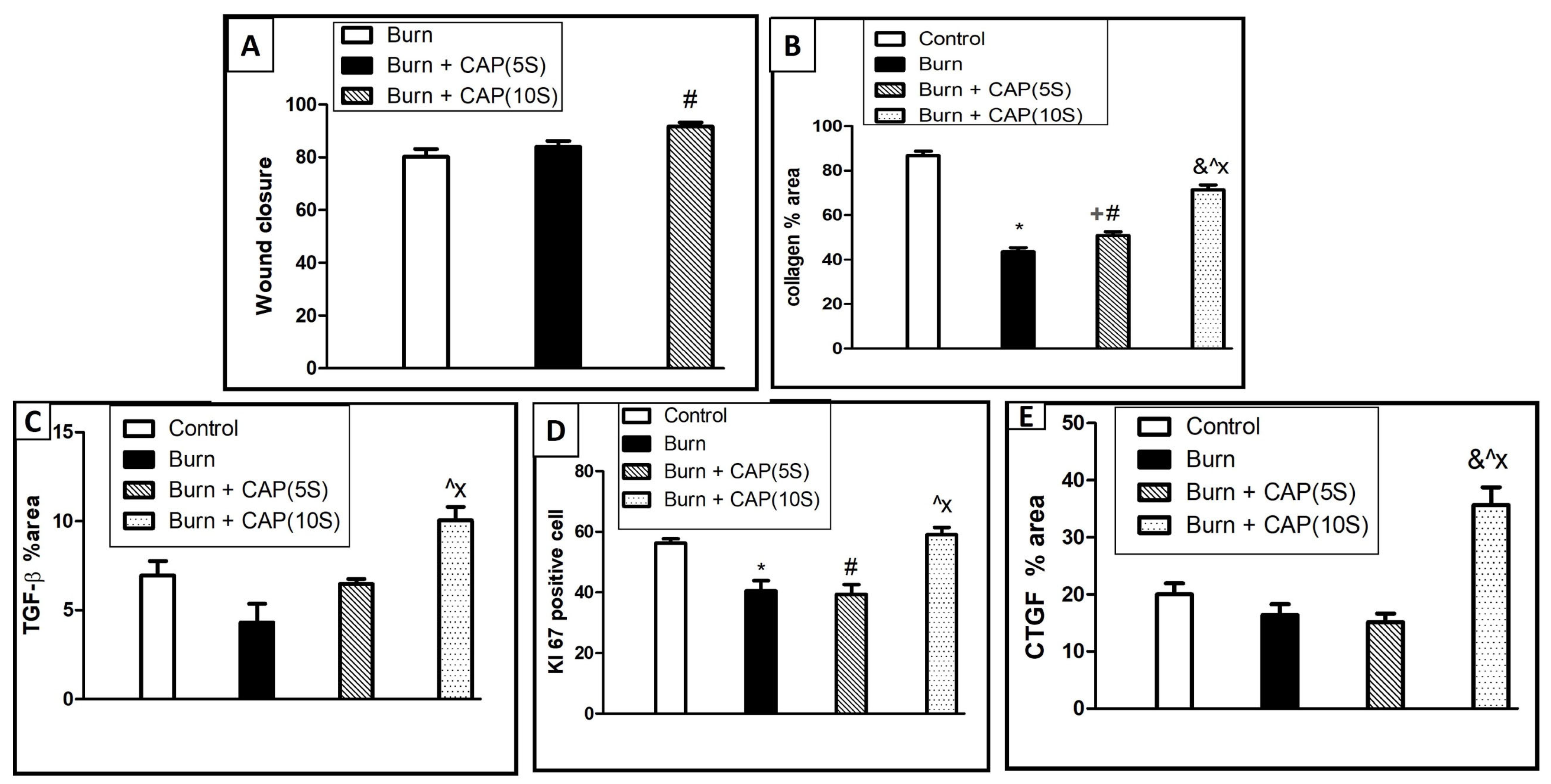
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFTSBs | Acute full-thickness skin burns |
| TGF-β1 | Transforming growth factor-beta1 |
| CTGF | Connective tissue growth factor |
References
- Khan, I.; Rahman, S.U.; Tang, E.; Engel, K.; Hall, B.; Kulkarni, A.B.; Arany, P.R. Accelerated Burn Wound Healing with Photobiomodulation Therapy Involves Activation of Endogenous Latent TGF-βPl1. Sci. Rep. 2021, 11, 13371. [Google Scholar] [CrossRef]
- Ye, H.; De, S. Thermal Injury of Skin and Subcutaneous Tissues: A Review of Experimental Approaches and Numerical Models. Burns 2017, 5, 909–932. [Google Scholar] [CrossRef] [PubMed]
- Bloemsma, G.; Dokter, J.; Boxma, H.; Oen, I. Mortality and Causes of Death in a Burn Centre. Burns 2008, 8, 1103–1107. [Google Scholar] [CrossRef]
- Kelly, E.J.; Oliver, M.A.; Carney, B.C.; Shupp, J.W. Infection and Burn Injury. Eur. Burn. J. 2022, 1, 165–179. [Google Scholar] [CrossRef]
- Warby, R.; Maani, C.V. Burn Classification; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Yuan, N.; Yang, Y.; Tan, C.; Ran, X. Mechanism of Cold Atmospheric Plasma in Treatment of Chronic Skin Ulcer. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2024, 10, 1283–1288. [Google Scholar] [CrossRef]
- Lackmann, J.-W.; Schneider, S.; Edengeiser, E.; Jarzina, F.; Brinckmann, S.; Steinborn, E.; Havenith, M.; Benedikt, J.; Bandow, J.E. Photons and Particles Emitted from Cold Atmospheric-Pressure Plasma Inactivate Bacteria and Biomolecules Independently and Synergistically. J. R. Soc. Interface 2013, 89, 20130591. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.G.; Bernabe, G.; Marchiori, C.; Scarpa, M.; Zuin, M.; Cavazzana, R.; Zaniol, B.; Martines, E. Antibacterial Efficacy and Mechanisms of Action of Low Power Atmospheric Pressure Cold Plasma: Membrane Permeability, Biofilm Penetration and Antimicrobial Sensitization. J. Appl. Microbiol. 2018, 2, 398–408. [Google Scholar] [CrossRef]
- Shi-Wen, X.; Leask, A.; Abraham, D. Regulation and Function of Connective Tissue Growth Factor/Ccn2 in Tissue Repair, Scarring and Fibrosis. Cytokine Growth Factor Rev. 2008, 2, 133–144. [Google Scholar] [CrossRef]
- Kiritsi, D.; Nystrom, A. The Role of Tgfbeta in Wound Healing Pathologies. Mech. Ageing Dev. 2018, 172, 51–58. [Google Scholar] [CrossRef]
- Liu, L.D.; Shi, H.J.; Jiang, L.; Wang, L.C.; Ma, S.H.; Dong, C.H.; Wang, J.J.; Zhao, H.L.; Liao, Y.; Li, Q.H. The Repairing Effect of a Recombinant Human Connective-Tissue Growth Factor in a Burn-Wounded Rhesus-Monkey (Macaca Mulatta) Model. Biotechnol. Appl. Biochem. 2007, 47, 105–112. [Google Scholar] [CrossRef]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.-F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.K.; et al. Cold Atmospheric Plasma (CAP) Changes Gene Expression of Key Molecules of the Wound Healing Machinery and Improves Wound Healing In Vitro and In Vivo. PLoS ONE 2013, 11, e79325. [Google Scholar] [CrossRef]
- Sisco, M.; Kryger, Z.B.; O’Shaughnessy, K.D.; Kim, P.S.; Schultz, G.S.; Ding, X.; Roy, N.K.; Dean, N.M.; Mustoe, T.A. Antisense Inhibition of Connective Tissue Growth Factor (CTGF/CCN2) mRNA Limits Hypertrophic Scarring Without Affecting Wound Healing In Vivo. Wound Repair Regen. 2008, 5, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Seher, A.; Nickel, J.; Mueller, T.D.; Kneitz, S.; Gebhardt, S.; Ter Vehn, T.M.; Schlunck, G.; Sebald, W. Gene Expression Profiling of Connective Tissue Growth Factor (CTGF) Stimulated Primary Human Tenon Fibroblasts Reveals an Inflammatory and Wound Healing Response In Vitro. Mol. Vis. 2011, 17, 53–62. [Google Scholar] [PubMed]
- Robinson, P.M.; Chuang, T.D.; Sriram, S.; Pi, L.; Luo, X.P.; Petersen, B.E.; Schultz, G.S. Microrna Signature in Wound Healing Following Excimer Laser Ablation: Role of Mir-133b on Tgfbeta1, Ctgf, Sma, and Col1a1 Expression Levels in Rabbit Corneal Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2013, 10, 6944–6951. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Gulko, J.; Kim, D.; Sim, K.H.; Gutman, S.; Yang, J.; Cook, J.L.; Lee, C.H. Effect of Dose and Release Rate of CTGF and TGFβ3 on Avascular Meniscus Healing. J. Orthop. Res. 2019, 7, 1555–1562. [Google Scholar] [CrossRef]
- El-Hossary, F.; Badr, G.; Lashein, F.E.-D.M.; Metawa, A.; Khalaf, M.; Gebril, S.M. Improving the Healing Rate of Diabetic Wounds by Applying Dielectric Barrier Discharge: An Applied Study in Male Mice. IEEE Trans. Radiat. Plasma Med Sci. 2025, 1, 3574259. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The Pig as a Model for Human Wound Healing. Wound Repair Regen. 2001, 2, 66–76. [Google Scholar] [CrossRef]
- Dahiya, P. Burns as a Model of Sirs. Front. Biosci. Landmark Ed. 2009, 13, 4962–4967. [Google Scholar] [CrossRef]
- Wenger, S. Anesthesia and Analgesia in Rabbits and Rodents. J. Exot. Pet Med. 2012, 1, 7–16. [Google Scholar] [CrossRef]
- Qu, M.; Nourbakhsh, M. Current Experimental Models of Burns. Discov. Med. 2017, 125, 95–103. [Google Scholar]
- Gebril, S.M.; Lashein, F.E.-D.M.; Khalaf, M.; AbuAmra, E.E.-S.; El-Hossary, F.M. Effect of Cold Atmospheric Plasma on Hyperglycemia and Immunity in the Spleen of STZ Diabetic Mice. IEEE Trans. Radiat. Plasma Med Sci. 2024, 9, 131–140. [Google Scholar] [CrossRef]
- El-Reda, G.A.; Mahmoud, M.A.M.; Khalaf, M.; Saber, A.A.; El-Hossary, F.M. Impact of Cold Atmospheric Plasma Treatment Duration on Distilled Water Physicochemical Characteristics. Sohag J. Sci. 2024, 9, 190–197. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; Wende, K.; Vollmar, B.; von Woedtke, T. A Cold Plasma Jet Accelerates Wound Healing in a Murine Model of Full-Thickness Skin Wounds. Exp. Dermatol. 2017, 2, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.M.; Yoon, H.K.; Jung, C.J.; Jo, S.Y.; Hwang, S.G.; Lee, H.J.; Lee, W.J.; Chang, S.E.; Won, C.H. Cold Plasma Treatment Promotes Full-thickness Healing of Skin Wounds in Murine Models. Int. J. Low. Extremity Wounds 2023, 1, 77–84. [Google Scholar] [CrossRef]
- Singer, A.J.; Zhou, J.W.; Osman, O.B.; Harris, Z.B.; Khani, M.E.; Baer, E.; Zhang, N.; McClain, S.A.; Arbab, M.H. Comparison of Comparable Scald and Contact Burns in a Porcine Model: A Preliminary Report. Wound Repair Regen. 2020, 6, 789–796. [Google Scholar] [CrossRef]
- Brans, T.; Dutrieux, R.; Hoekstra, M.; Kreis, R.; du Pont, J. Histopathological Evaluation of Scalds and Contact Burns in the Pig Model. Burns 1994, 20, S48–S51. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, S.; Clough, E.C.; Thomlinson, A.M.; Chadwick, S.L.; Ferguson, M.W.; Shah, M. Partial Thickness Wound: Does Mechanism of Injury Influence Healing? Burns 2019, 3, 531–542. [Google Scholar] [CrossRef]
- Sheu, S.-Y.; Wang, W.-L.; Fu, Y.-T.; Lin, S.-C.; Lei, Y.-C.; Liao, J.-H.; Tang, N.-Y.; Kuo, T.-F.; Yao, C.-H. The Pig as an Experimental Model for Mid-Dermal Burns Research. Burns 2014, 8, 1679–1688. [Google Scholar] [CrossRef]
- Abdel-Motaleb, A.A.; Zedan, H.; Mostafa, M.M.; Abu-Dief, E.E.; Gebril, S.M.; Hussein, M.R.A. Combined Microneedling with Topical Application of Platelet-Rich Plasma Versus Microneedling Alone in the Treatment of Stria Distensae: Clinicopathological Analysis. J. Dermatol. Treat. 2022, 2, 836–847. [Google Scholar] [CrossRef]
- Fernandez-Flores, A.; Diep, M.; Cassarino, D. Thickening of the Basement Membrane as a Diagnostic Sign of Mycosis Fungoides. J. Cutan. Pathol. 2021, 3, 356–363. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Gebril, S.M.; Ito, Y.; Shibata, M.; Maemura, K.; Abu-Dief, E.E.; Hussein, M.R.A.; Abdelaal, U.M.; Elsayed, H.M.; Otsuki, Y.; Higuchi, K. Indomethacin can Induce Cell Death in Rat Gastric Parietal Cells Through Alteration of Some Apoptosis- and Autophagy-Associated Molecules. Int. J. Exp. Pathol. 2020, 6, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, M.H.; Balgoon, M.J.; El-Sawi, N.M.; Alshubaily, F.A.; Jambi, E.J.; Khojah, S.M.; Baljoon, R.S.; Alkhattabi, N.A.; Baz, L.A.; Alharbi, A.A.; et al. A Biochemical, Theoretical and Immunohistochemical Study Comparing the Therapeutic Efficacy of Curcumin and Taurine on T-2 Toxin Induced Hepatotoxicity in Rats. Front. Mol. Biosci. 2023, 10, 1172403. [Google Scholar] [CrossRef] [PubMed]
- Blaise, O.; Duchesne, C.; Capuzzo, E.; Nahori, M.-A.; Fernandes, J.; Connor, M.G.; Hamon, M.A.; Pizarro-Cerda, J.; Lataillade, J.-J.; McGuckin, C.; et al. Infected Wound Repair Correlates with Collagen I Induction and Nox2 Activation by Cold Atmospheric Plasma. NPJ Regen. Med. 2024, 1, 28. [Google Scholar] [CrossRef]
- Moritz, A.R. Studies of Thermal Injury: III. The Pathology and Pathogenesis of Cutaneous Burns. An Experimental Study. Am. J. Pathol. 1947, 6, 915–941. [Google Scholar]
- Lau, K.; Paus, R.; Tiede, S.; Day, P.; Bayat, A. Exploring the Role of Stem Cells in Cutaneous Wound Healing. Exp. Dermatol. 2009, 11, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Stolz, W.; Shimizu, T.; Monetti, R.; Bunk, W.; Schmidt, H.-U.; Morfill, G.; Klämpfl, T.; Steffes, B.; Thomas, H.; et al. Cold Atmospheric Argon Plasma Treatment May Accelerate Wound Healing in Chronic Wounds: Results of an Open Retrospective Randomized Controlled Study In Vivo. Clin. Plasma Med. 2013, 2, 25–30. [Google Scholar] [CrossRef]
- Dubey, S.K.; Parab, S.; Alexander, A.; Agrawal, M.; Achalla, V.P.K.; Pal, U.N.; Pandey, M.M.; Kesharwani, P. Cold Atmospheric Plasma Therapy in Wound Healing. Process. Biochem. 2022, 112, 112–123. [Google Scholar] [CrossRef]
- Abramo, A.; Viola, J. Heterologous Collagen Matrix Sponge: Histologic and Clinical Response to Its Implantation in Third-Degree Burn Injuries. Br. J. Plast. Surg. 1992, 2, 117–122. [Google Scholar] [CrossRef]
- Zhang, K.; Garner, W.; Cohen, L.; Rodriguez, J.; Phan, S. Increased Types I and III Collagen and Transforming Growth Factor-β1 mRNA and Protein in Hypertrophic Burn Scar. J. Investig. Dermatol. 1995, 5, 750–754. [Google Scholar] [CrossRef]
- Ge, K.; Lu, S.-L.; Qing, C.; Xie, T.; Rong, L.; Niu, Y.-W.; Wang, M.-J.; Liao, Z.-J.; Shi, J.-X. The Influence of L-Arginine on the Angiogenesis in Burn Wounds in Diabetic Rats. Zhonghua Shao Shang Za Zhi 2004, 4, 210–213. [Google Scholar]
- Mansoub, N.H.; Gürdal, M.; Karadadaş, E.; Kabadayi, H.; Vatansever, S.; Ercan, G. The Role of PRP and Adipose Tissue-Derived Keratinocytes on Burn Wound Healing in Diabetic Rats. BioImpacts 2018, 1, 5–12. [Google Scholar] [CrossRef]
- Mai, B.; Jia, M.; Liu, S.; Sheng, Z.; Li, M.; Gao, Y.; Wang, X.; Liu, Q.; Wang, P. Smart Hydrogel-Based Dvdms/Bfgf Nanohybrids for Antibacterial Phototherapy with Multiple Damaging Sites and Accelerated Wound Healing. ACS Appl. Mater. Interfaces 2020, 9, 10156–10169. [Google Scholar] [CrossRef]
- Frescaline, N.; Duchesne, C.; Favier, M.; Onifarasoaniaina, R.; Guilbert, T.; Uzan, G.; Banzet, S.; Rousseau, A.; Lataillade, J. Physical Plasma Therapy Accelerates Wound Re-Epithelialisation and Enhances Extracellular Matrix Formation in Cutaneous Skin Grafts. J. Pathol. 2020, 4, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Pedroza, M.; To, S.; Assassi, S.; Wu, M.; Tweardy, D.; Agarwal, S.K. Role of STAT3 in Skin Fibrosis and Transforming Growth Factor Beta Signalling. Rheumatology 2018, 10, 1838–1850. [Google Scholar] [CrossRef]
- Verrecchia, F.; Mauviel, A. Transforming Growth Factor-Beta and Fibrosis. World J. Gastroenterol. 2007, 22, 3056–3062. [Google Scholar] [CrossRef]
- Durgun, C.; Kirman, G.; Deveci, E. Investigation of the Histopathological Level of Ki-67, Caspase-3 Expressions of the Effects of Hesperidin on Wound Healing in the Rat Esophagus. Acta Cir. Bras. 2023, 38, e381723. [Google Scholar] [CrossRef] [PubMed]
- Omran, S.A.; Ghani, B.A. Effect of Fenugreek Oil on Healing of Experimentally Induced Buccal Mucosal Ulcer by Immunohistochemical Evaluation of Ki-67 Expression. Cell Biochem. Biophys. 2024, 3, 2363–2371. [Google Scholar] [CrossRef]
- Arndt, S.; Landthaler, M.; Zimmermann, J.L.; Unger, P.; Wacker, E.; Shimizu, T.; Li, Y.-F.; Morfill, G.E.; Bosserhoff, A.-K.; Karrer, S.; et al. Effects of Cold Atmospheric Plasma (CAP) on ß-Defensins, Inflammatory Cytokines, and Apoptosis-Related Molecules in Keratinocytes In Vitro and In Vivo. PLoS ONE 2015, 3, e0120041. [Google Scholar] [CrossRef]
- Arndt, S.; Unger, P.; Bosserhoff, A.-K.; Berneburg, M.; Karrer, S. The Anti-Fibrotic Effect of Cold Atmospheric Plasma on Localized Scleroderma In Vitro and In Vivo. Biomedicines 2021, 11, 1545. [Google Scholar] [CrossRef]
- Shome, D.; von Woedtke, T.; Riedel, K.; Masur, K. The HIPPO Transducer YAP and Its Targets CTGF and Cyr61 Drive a Paracrine Signalling in Cold Atmospheric Plasma-Mediated Wound Healing. Oxid. Med. Cell. Longev. 2020, 2020, 4910280. [Google Scholar] [CrossRef]
- Henshaw, F.R.; Boughton, P.; Lo, L.; McLennan, S.V.; Twigg, S.M. Topically Applied Connective Tissue Growth Factor/CCN2 Improves Diabetic Preclinical Cutaneous Wound Healing: Potential Role for CTGF in Human Diabetic Foot Ulcer Healing. J. Diabetes Res. 2015, 2015, 236238. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.J.; Pi, L.; Sriram, S.; Mao, C.; Petersen, B.E.; Scott, E.W.; Leask, A.; Schultz, G.S. Conditional Knockout of CTGF Affects Corneal Wound Healing. Investig. Opthalmol. Vis. Sci. 2014, 4, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cui, L.; Yang, J.M.; Lee, H.S.; Choi, J.S.; Woo, J.M.; Lim, S.K.; Yoon, K.C. The Wound Healing Effects of Adiponectin Eye Drops after Corneal Alkali Burn. Curr. Eye Res. 2016, 41, 1424–1432. [Google Scholar] [CrossRef]
- Hong, Q.; Dong, X.; Jones, J.E.; Hong, L.; Yu, Q.; Sun, H.; Chen, M. A Novel Approach to Expedite Wound Healing with Plasma Brush of Cold Flame. Rev. Sci. Instrum. 2023, 94, 084102. [Google Scholar] [CrossRef]
- Brun, P.; Pathak, S.; Castagliuolo, I.; Palù, G.; Zuin, M.; Cavazzana, R.; Martines, E.; Yousfi, M. Helium Generated Cold Plasma Finely Regulates Activation of Human Fibroblast-Like Primary Cells. PLoS ONE 2014, 8, e104397. [Google Scholar] [CrossRef]
- Rezaeinezhad, A.; Eslami, P.; Mirmiranpour, H.; Ghomi, H. The Effect of Cold Atmospheric Plasma on Diabetes-Induced Enzyme Glycation, Oxidative Stress, and Inflammation; In Vitro and In Vivo. Sci. Rep. 2019, 1, 19958. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, F.; Zafari, P.; Alimohammadi, M.; Golpour, M.; Ghaffari, S.; Rafiei, A. Inhibitory Effects of Cold Atmospheric Plasma on Inflammation and Tumor-Like Feature of Fibroblast-Like Synoviocytes from Patients with Rheumatoid Arthritis. Inflammation 2022, 6, 2433–2448. [Google Scholar] [CrossRef]
- Abdo, A.I.; Kopecki, Z. Comparing Redox and Intracellular Signalling Responses to Cold Plasma in Wound Healing and Cancer. Curr. Issues Mol. Biol. 2024, 5, 4885–4923. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Lin, Z.H.; Cheng, Y.P.; Chiu, H.Y.; Yeh, N.L.; Wu, T.K.; Wu, J.S. Wound Healing in Streptozotocin-Induced Diabetic Rats Using Atmospheric-Pressure Argon Plasma Jet. Sci. Rep. 2018, 1, 12214. [Google Scholar]
- Duchesne, C.; Banzet, S.; Lataillade, J.; Rousseau, A.; Frescaline, N. Cold atmospheric plasma modulates endothelial nitric oxide synthase signalling and enhances burn wound neovascularisation. J. Pathol. 2019, 3, 368–380. [Google Scholar] [CrossRef]
- Hussein, M.R.; Ab-Deif, E.E.; Abdel-Motaleb, A.A.; Zedan, H.; Abdel-Meguid, A.M. Chemical Peeling and Micro-Dermabrasion of the Skin: Comparative Immunohistological and Ultrastructural Studies. J. Dermatol. Sci. 2008, 3, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.R.; Aboulhagag, N.M.; Atta, H.S.; Atta, S.M. Evaluation of the Profile of the Immune Cell Infiltrate in Lichen Planus, Discoid Lupus Erythematosus, and Chronic Dermatitis. Pathology 2008, 7, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, V.A. Transforming Growth Factor-β and Angiotensin in Fibrosis and Burn Injuries. J. Burn. Care Res. 2009, 3, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, X.; He, Y.; Kang, Y.; Jin, F.; Li, Y.; Li, T.; Wei, Z.; Li, S.; Cai, W.; et al. Microrna-411-3p Inhibits Bleomycin-Induced Skin Fibrosis by Regulating Transforming Growth Factor-Beta/Smad Ubiquitin Regulatory Factor-2 Signalling. J. Cell. Mol. Med. 2021, 24, 11290–11299. [Google Scholar] [CrossRef]

| Reagents | Catalog Number | Sources |
|---|---|---|
| Anti-Transforming growth factor-Beta 1(TGF-β1) rabbit pAb | (Cat. No.; A15103) (Dilution of 1:200) | AB clonal, (Wuhan, China) |
| Anti-connective tissue growth factor (CTGF) rabbit pAb | (Cat. No.: E-AB-12339) (Dilution of 1:50) | Elab Science Biotechnology Inc., Houston, TX, United States |
| Anti-Ki-67 rabbit pAb antibody | (Cat. No.: GB111499), (Dilution of 1:300) | Service bio-Technology Co., Ltd., Wuhan, China |
| Normal goat serum | (Cat. No.: 5425) (Dilution of 10%) | Cell signaling technology, Inc., Danvers, MA, United States |
| Pro taqs® 2 step detection goat anti-mouse/rabbit HRP with a peroxidase block and DAB chromogen, Quartet | (Cat. No DSK-211-015) | Schichauweg, (Berlin, Germany) |
| Groups | Design of the Group |
|---|---|
| Group I | Healthy animals, no acute FTSBs (n = 6) |
| Group II | Animals suffering from acute FTSBs receiving no treatment (n = 6) |
| Group III | Animals suffering from acute FTSBs receiving topical CAP treatment for 5 s per day for 21 days (n = 6) |
| Group IV | Animals suffering from acute FTSBs receiving topical CAP treatment for 10 s per day for 21 days (n = 6) |
| Groups | Size of the Wound Closure | Collagen Percentage Area |
|---|---|---|
| Group I | Non wounded | 86.12% ± 6.152 |
| Group II | 81.36 ± 10.17 | 43.56% ± 5.784 |
| Group III (5 s) | 84.7 ± 7.11 | 50.83% ± 5.442 |
| Group IV (10 s) | 91.67 ± 6.087 | 71.36% ± 7.153 |
| Groups | Size of the Wound Closure (7 Days) | Size of the Wound Closure (14 Days) | Size of the Wound Closure (21 Days) |
|---|---|---|---|
| Group I | Non wounded | Non wounded | Non wounded |
| Group II | 33.1 ± 6.91 | 44.84 ± 4.037 | 81.36 ± 10.17 |
|
Group III
(5 s) | 32.93 ± 6.234 | 58.3 ± 3.8 | 84.7 ± 7.11 |
|
Group IV
(10 s) | 34.01 ± 6 | 71.48 ± 4.544 | 91.67 ± 6.087 |
| Groups | Size of the Wound Closure | Collagen Percentage Area | TGF-β1 %Area | CTGF Positive Cell | Ki-67 Positive Cell |
|---|---|---|---|---|---|
| Groups | Size of the wound closure | Collagen percentage area | TGF-β1 | CTGF | Ki-67 |
| Group I | Non wounded | 86.12% ± 6.152 | 6.947 ± 2.438 | 20 ± 5.451 | 56.25 ± 4.132 |
| Group II | 81.36 ± 10.17 | 43.56% ± 5.784 (* p < 0.05) | 4.301 ± 3.797 | 16.38 ± 5.397 | 40.5 ± 9.577 (* p < 0.05) |
| Group III (5 s) | 84.7 ± 7.11 | 50.83% ± 5.442 (# + p < 0.05) | 6.471 ± 1.047 (p < 0.05) | 15.13 ± 4.357 | 39.38 ± 8.975 (# p < 0.05) |
| Group IV (10 s) | 91.67 ± 6.087 (@ p < 0.05) | 71.36 % ± 7.153 (&@X p < 0.05) | 10.04 ± 2.632 (@X p < 0.05) | 35.63 ± 8.911 (&@X p < 0.05) | 59 ± 6.928 (@X p < 0.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gebril, S.M.; El din M. Lasheen, F.; Khalaf, M.; Abdelhamed, A.; Bahkali, M.I.; El Hossary, F.; Hussein, M.R.A. Cold Atmospheric Plasma Enhances TGF-β1, CTGF Protein Expression, and Healing in Full-Thickness Skin Burns: An Animal Study. Biomolecules 2025, 15, 924. https://doi.org/10.3390/biom15070924
Gebril SM, El din M. Lasheen F, Khalaf M, Abdelhamed A, Bahkali MI, El Hossary F, Hussein MRA. Cold Atmospheric Plasma Enhances TGF-β1, CTGF Protein Expression, and Healing in Full-Thickness Skin Burns: An Animal Study. Biomolecules. 2025; 15(7):924. https://doi.org/10.3390/biom15070924
Chicago/Turabian StyleGebril, Sahar M., Fakhr El din M. Lasheen, Mohamed Khalaf, Amr Abdelhamed, Manal I. Bahkali, Fayez El Hossary, and Mahmoud Rezk Abdelwahed Hussein. 2025. "Cold Atmospheric Plasma Enhances TGF-β1, CTGF Protein Expression, and Healing in Full-Thickness Skin Burns: An Animal Study" Biomolecules 15, no. 7: 924. https://doi.org/10.3390/biom15070924
APA StyleGebril, S. M., El din M. Lasheen, F., Khalaf, M., Abdelhamed, A., Bahkali, M. I., El Hossary, F., & Hussein, M. R. A. (2025). Cold Atmospheric Plasma Enhances TGF-β1, CTGF Protein Expression, and Healing in Full-Thickness Skin Burns: An Animal Study. Biomolecules, 15(7), 924. https://doi.org/10.3390/biom15070924








