Humanized Bone Model Identifies BMP6 as a Multifunctional Regulator in Myeloma Bone Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture, Osteogenic Differentiation, and Coculture
2.2. Bone Evaluation in Human BM-like Scaffold (huBMsc) Mice
2.3. Gene Expression Analysis by the Affymetrix GeneChip System or qRT-PCR
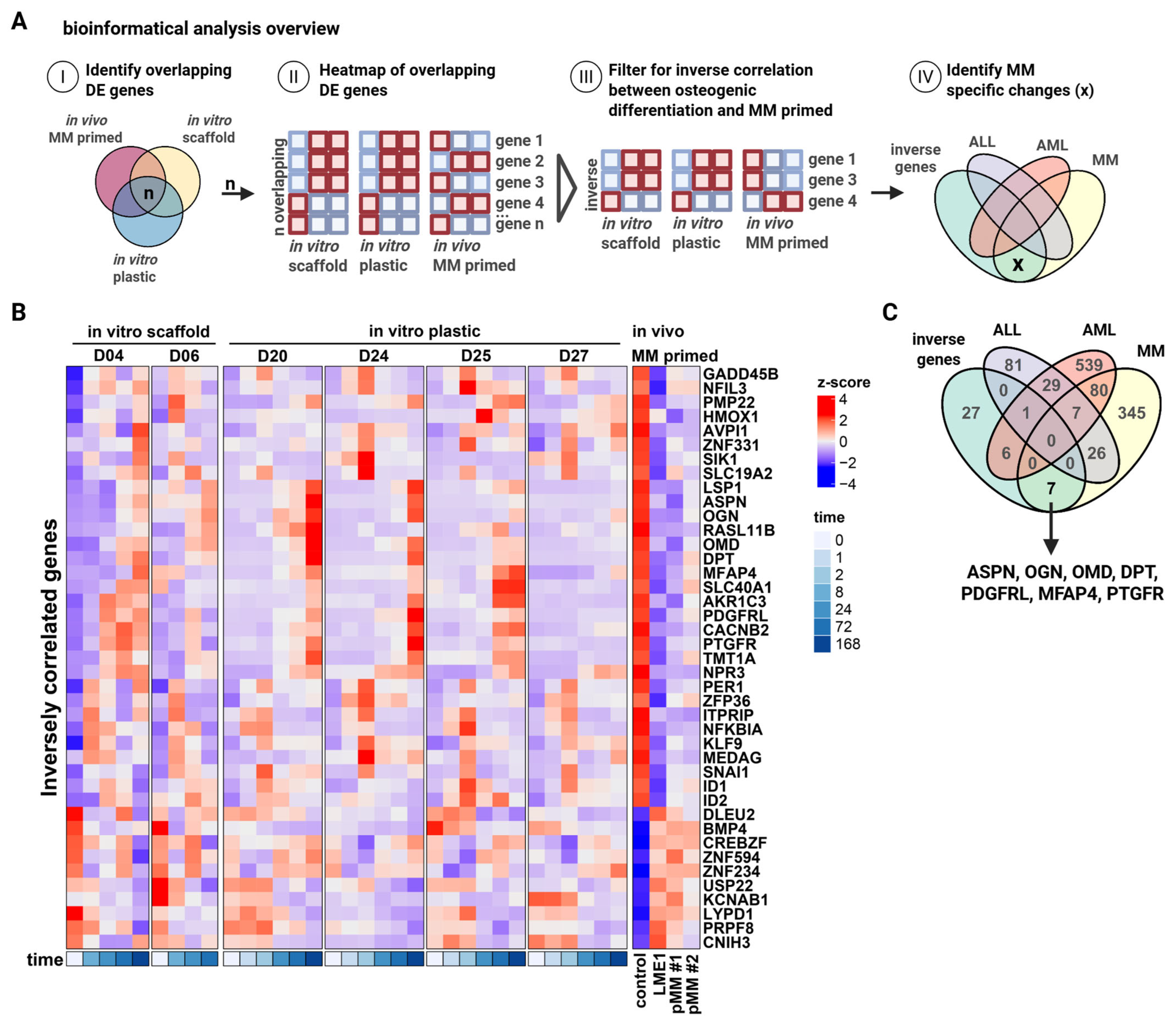
2.4. Bioinformatics Analysis
2.5. Alkaline Phosphatase Staining
2.6. Osteoclast Differentiation
2.7. Statistical Analysis
3. Results
3.1. Evaluation of In Vivo Bone Formation by MM-Derived MSCs and Direct Inhibition by MM Cells Using the huBMsc Model
3.2. Identification of Small Leucine-Rich Proteoglycans ASPN, OGN, and OMD as Intermediates of Bone Formation Inhibited in MM
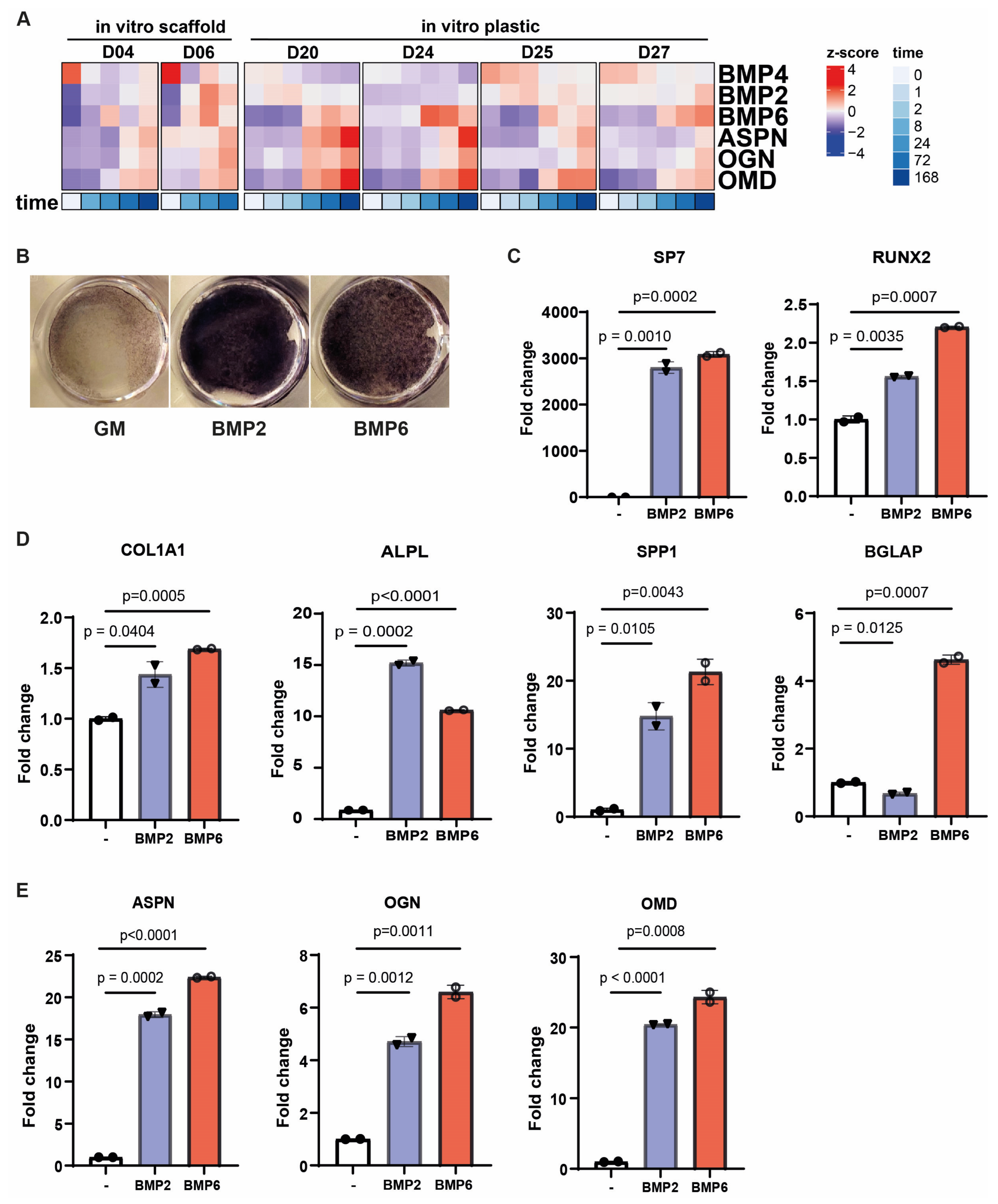
3.3. Bone Morphogenetic Proteins Regulate Small Leucine-Rich Proteoglycans During Osteogenic Differentiation
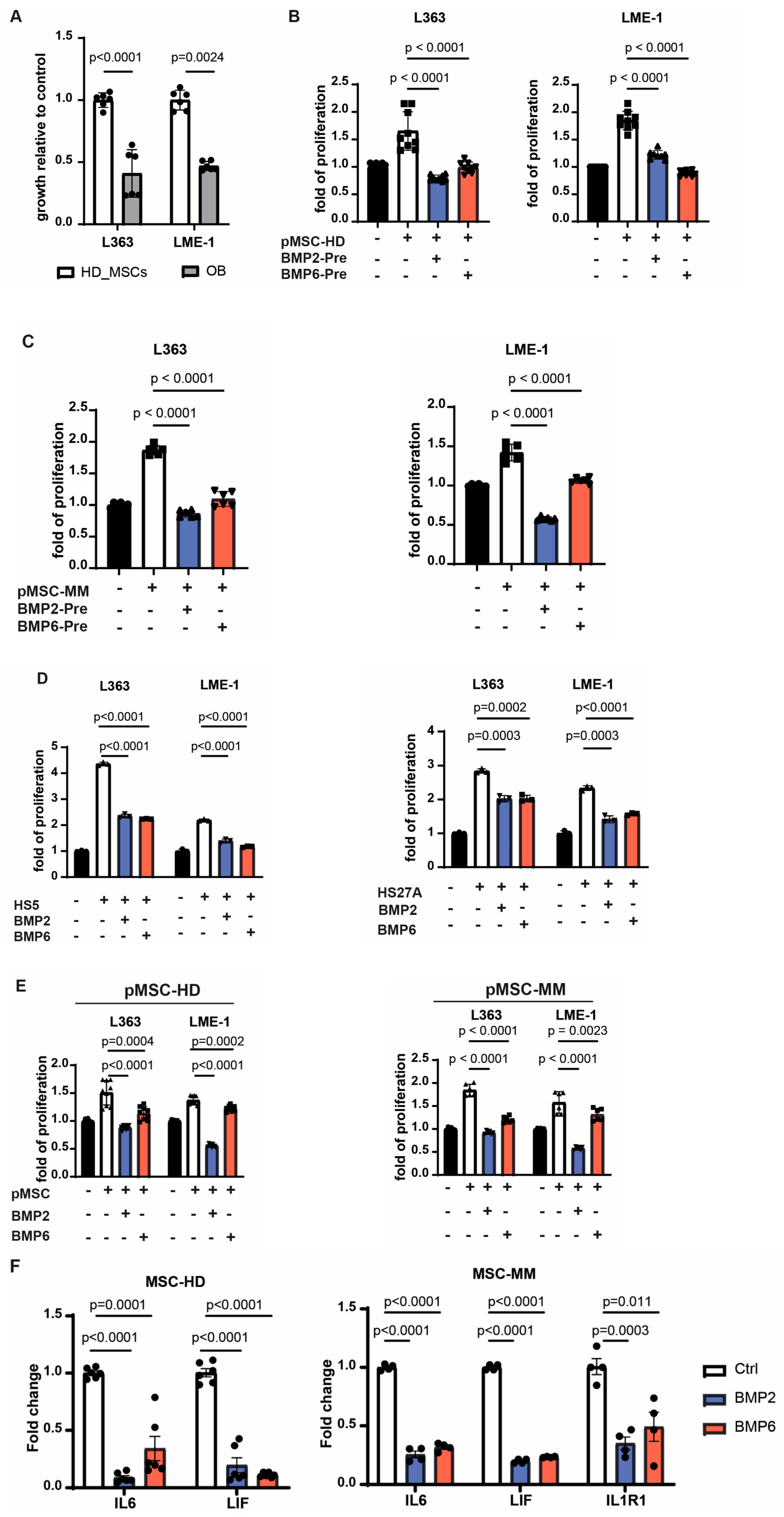
3.4. BMP2 and BMP6 Abolish the Supportive Role of MSCs in the MM Microenvironment
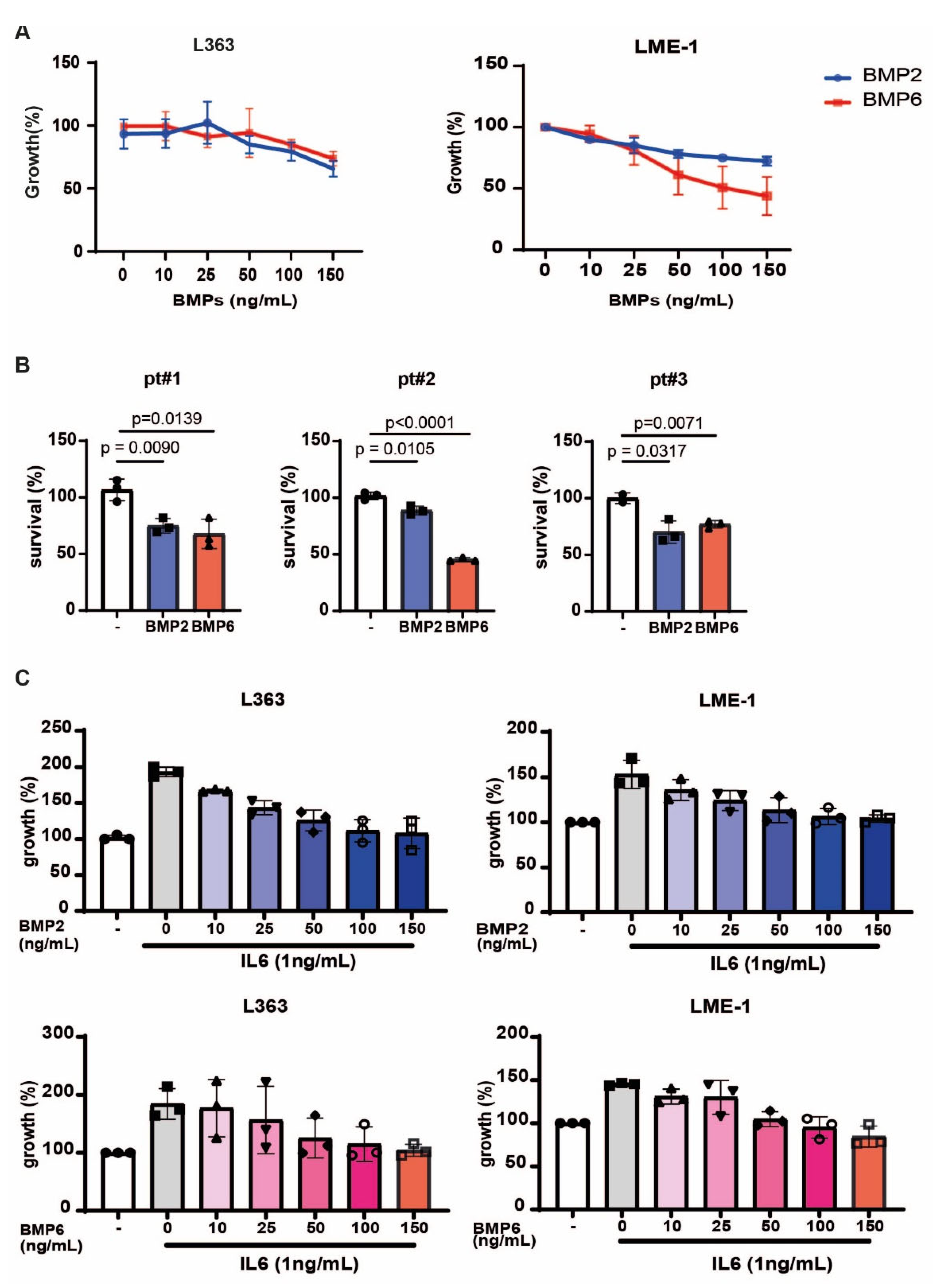
3.5. BMP2 and BMP6 Inhibits MM Growth, as Well as IL6-Induced Proliferation
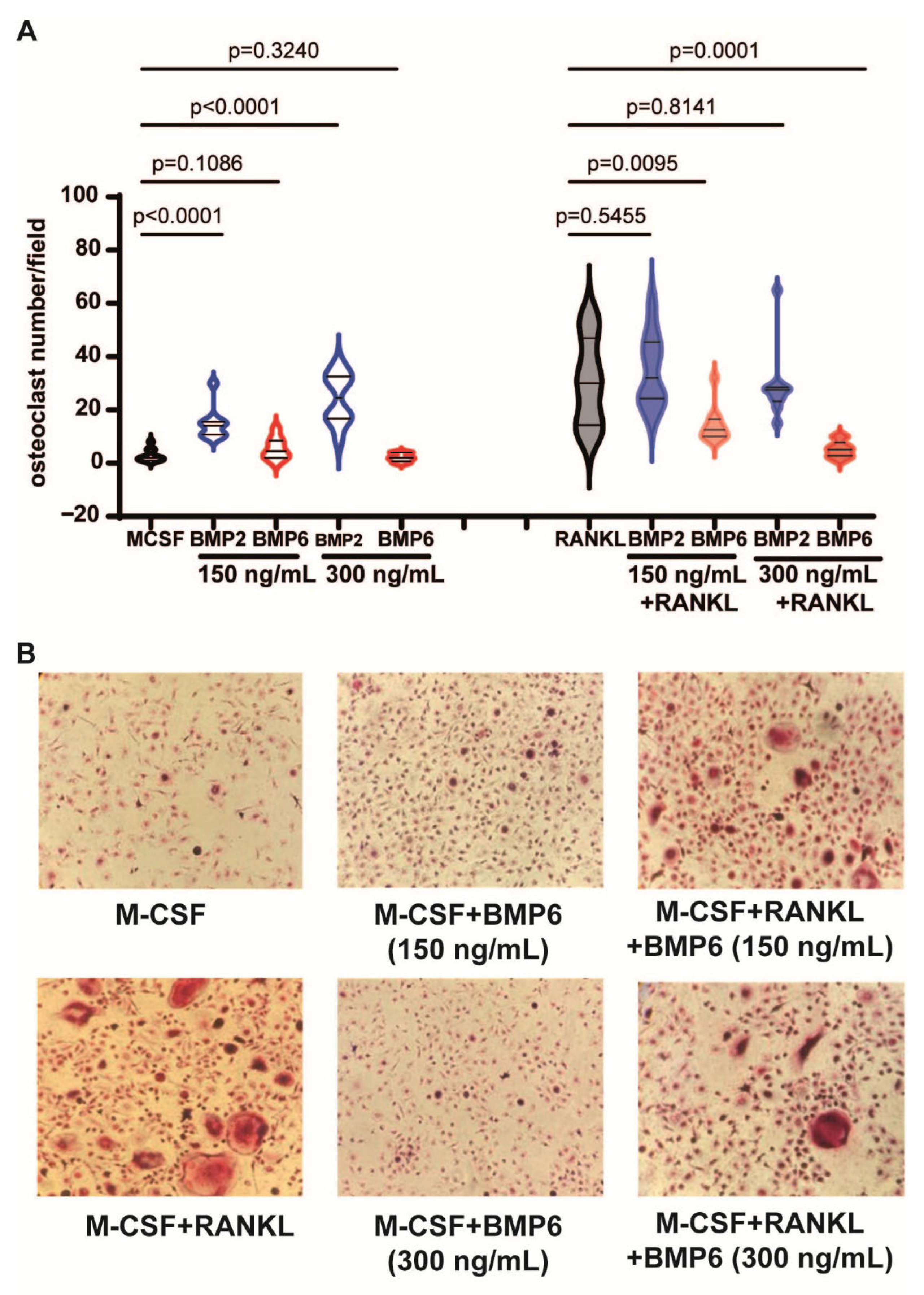
3.6. BMP6, but Not BMP2, Inhibits the RANKL-Dependent Osteoclastogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roodman, G.D. Pathogenesis of myeloma bone disease. Leukemia 2009, 23, 435–441. [Google Scholar] [CrossRef]
- Palumbo, A.; Anderson, K. Multiple Myeloma. New Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Barillé-Nion, S.; Bataille, R. New insights in myeloma-induced osteolysis. Leuk Lymphoma 2003, 44, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.M.; Zhuang, J.; Mundy, G.R. The pathogenesis of the bone disease of multiple myeloma. Bone 2008, 42, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Yee, G.C.; Somerfield, M.R.; Flynn, P.J.; Halabi, S.; Jagannath, S.; Orlowski, R.Z.; Roodman, D.G.; Twilde, P.; Anderson, K. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J. Clin. Oncol. 2007, 25, 2464–2472. [Google Scholar] [CrossRef]
- Pozzi, S.; Raje, N. The role of bisphosphonates in multiple myeloma: Mechanisms, side effects, and the future. Oncologist 2011, 16, 651–662. [Google Scholar] [CrossRef]
- Croucher, P.I.; De Hendrik, R.; Perry, M.J.; Hijzen, A.; Shipman, C.M.; Lippitt, J.; Green, J.; Van Marck, E.; Van Camp, B.; Vanderkerken, K. Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: Evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J. Bone Miner. Res. 2003, 18, 482–492. [Google Scholar] [CrossRef]
- Ring, E.S.; Lawson, M.A.; Snowden, J.A.; Jolley, I.; Chantry, A.D. New agents in the Treatment of Myeloma Bone Disease. Calcif. Tissue Int. 2018, 102, 196–209. [Google Scholar] [CrossRef]
- Jensen, P.R.; Andersen, T.L.; Chavassieux, P.; Roux, J.P.; Delaisse, J.M. Bisphosphonates impair the onset of bone formation at remodeling sites. Bone 2021, 145, 115850. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Xu, S.; De Veirman, K.; De Becker, A.; Vanderkerken, K.; Van Riet, I. Mesenchymal stem cells in multiple myeloma: A therapeutical tool or target? Leukemia 2018, 32, 1500–1514. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.M.E.; Kellermayer, Z.; Papazian, N.; Tahri, S.; Hofste op Bruinink, D.; Hoogenboezem, R.; Sanders, M.A.; van de Woestijne, P.C.; Bos, P.K.; Khandanpour, C.; et al. The multiple myeloma microenvironment is defined by an inflammatory stromal cell landscape. Nat. Immunol. 2021, 22, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Arnulf, B.; Lecourt, S.; Soulier, J.; Ternaux, B.; Lacassagne, M.N.; Crinquette, A.; Dessoly, J.; Sciaini, A.K.; Benbunan, M.; Chomienne, C.; et al. Phenotypic and functional characterization of bone marrow mesenchymal stem cells derived from patients with multiple myeloma. Leukemia 2007, 21, 158–163. [Google Scholar] [CrossRef]
- Corre, J.; Mahtouk, K.; Attal, M.; Gadelorge, M.; Huynh, A.; Fleury-Cappellesso, S.; Danho, C.; Laharrague, P.; Klein, B.; Rème, T.; et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia 2007, 21, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Garderet, L.; Mazurier, C.; Chapel, A.; Ernou, I.; Boutin, L.; Holy, X.; Gorin, N.C.; Lopez, M.; Doucet, C.; Lataillade, J.J. Mesenchymal stem cell abnormalities in patients with multiple myeloma. Leuk Lymphoma 2007, 48, 2032–2041. [Google Scholar] [CrossRef]
- McNee, G.; Eales, K.L.; Wei, W.; Williams, D.S.; Barkhuizen, A.; Bartlett, D.B.; Essex, S.; Anandram, S.; Filer, A.; Moss, P.A.H.; et al. Citrullination of histone H3 drives IL-6 production by bone marrow mesenchymal stem cells in MGUS and multiple myeloma. Leukemia 2017, 31, 373–381. [Google Scholar] [CrossRef]
- Lemaitre, L.; Do Souto Ferreira, L.; Joubert, M.-V.; Avet-Loiseau, H.; Martinet, L.; Corre, J.; Couderc, B. Imprinting of Mesenchymal Stromal Cell Transcriptome Persists even after Treatment in Patients with Multiple Myeloma. Int. J. Mol. Sci. 2020, 21, 3854. [Google Scholar] [CrossRef]
- André, T.; Meuleman, N.; Stamatopoulos, B.; De Bruyn, C.; Pieters, K.; Bron, D.; Lagneaux, L. Evidences of Early Senescence in Multiple Myeloma Bone Marrow Mesenchymal Stromal Cells. PLoS ONE 2013, 8, e59756. [Google Scholar] [CrossRef]
- Fairfield, H.; Costa, S.; Falank, C.; Farrell, M.; Murphy, C.S.; D’Amico, A.; Driscoll, H.; Reagan, M.R. Multiple myeloma cells alter adipogenesis, increase senescence-related and inflammatory gene transcript expression, and alter metabolism in preadipocytes. Front. Oncol. 2021, 10, 584683. [Google Scholar] [CrossRef]
- Plakhova, N.; Panagopoulos, V.; Cantley, M.D.; Trainor, L.J.; Hewett, D.R.; Clark, K.C.; Gardiner, J.; Yong, A.; Lee, C.; Horvath, N.; et al. Age-related mesenchymal stromal cell senescence is associated with progression from MGUS to multiple myeloma. Leukemia 2025, 39, 1464–1475. [Google Scholar] [CrossRef]
- Ghamlouch, H.; Gagler, D.C.; Blaney, P.; Boyle, E.M.; Wang, Y.; Avigan, J.; Choi, J.; Landgren, O.; Tsirigos, A.; Maura, F.; et al. A proinflammatory response and polarized differentiation of stromal elements characterizes the murine myeloma bone marrow niche. Exp. Hematol. Oncol. 2025, 14, 22. [Google Scholar] [CrossRef]
- Xu, S.; Evans, H.; Buckle, C.; De Veirman, K.; Hu, J.; Xu, D.; Menu, E.; De Becker, A.; Vande Broek, I.; Leleu, X.; et al. Impaired osteogenic differentiation of mesenchymal stem cells derived from multiple myeloma patients is associated with a blockade in the deactivation of the Notch signaling pathway. Leukemia 2012, 26, 2546–2549. [Google Scholar] [CrossRef]
- Kassen, D.; Moore, S.; Percy, L.; Herledan, G.; Bounds, D.; Rodriguez-Justo, M.; Croucher, P.; Yong, K. The bone marrow stromal compartment in multiple myeloma patients retains capability for osteogenic differentiation in vitro: Defining the stromal defect in myeloma. Br. J. Haematol. 2014, 167, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Noll, J.E.; Williams, S.A.; Tong, C.M.; Wang, H.; Quach, J.M.; Purton, L.E.; Pilkington, K.; To, L.B.; Evdokiou, A.; Gronthos, S.; et al. Myeloma plasma cells alter the bone marrow microenvironment by stimulating the proliferation of mesenchymal stromal cells. Haematologica 2014, 99, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.; Scutt, A.M.; Croucher, P.I. Human myeloma cells promote the recruitment of osteoblast precursors: Mediation by interleukin-6 and soluble interleukin-6 receptor. J. Bone Miner. Res. 2000, 15, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Prins, H.J.; Rozemuller, H.; Vonk-Griffioen, S.; Verweij, V.G.; Dhert, W.J.; Slaper-Cortenbach, I.C.; Martens, A.C. Bone-forming capacity of mesenchymal stromal cells when cultured in the presence of human platelet lysate as substitute for fetal bovine serum. Tissue Eng. Part A 2009, 15, 3741–3751. [Google Scholar] [CrossRef]
- Astori, G.; Amati, E.; Bambi, F.; Bernardi, M.; Chieregato, K.; Schäfer, R.; Sella, S.; Rodeghiero, F. Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: Present and future. Stem Cell Res. Ther. 2016, 7, 93. [Google Scholar] [CrossRef]
- Das, R.; Strowig, T.; Verma, R.; Koduru, S.; Hafemann, A.; Hopf, S.; Kocoglu, M.H.; Borsotti, C.; Zhang, L.; Branagan, A.; et al. Microenvironment-dependent growth of preneoplastic and malignant plasma cells in humanized mice. Nat. Med. 2016, 22, 1351–1357. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Claiborne, D.T.; Maldini, C.R.; Phelps, M.; Vrbanac, V.; Karpel, M.E.; Krupp, K.L.; Power, K.A.; Boutwell, C.L.; Balazs, A.B.; et al. Innate Immune Reconstitution in Humanized Bone Marrow-Liver-Thymus (HuBLT) Mice Governs Adaptive Cellular Immune Function and Responses to HIV-1 Infection. Front. Immunol. 2021, 12, 667393. [Google Scholar] [CrossRef]
- Hasanali, Z.S.; Garfall, A.L.; Burzenski, L.; Shultz, L.D.; Tang, Y.; Kadu, S.; Sheppard, N.C.; Liu, W.; Dopkin, D.; Vogl, D.T.; et al. Human IL-6 fosters long-term engraftment of patient-derived disease-driving myeloma cells in immunodeficient mice. JCI Insight 2024, 9, e177300. [Google Scholar] [CrossRef]
- Groen, R.W.J.; Noort, W.A.; Raymakers, R.A.; Prins, H.-J.; Aalders, L.; Hofhuis, F.M.; Moerer, P.; van Velzen, J.F.; Bloem, A.C.; van Kessel, B.; et al. Reconstructing the human hematopoietic niche in immunodeficient mice: Opportunities for studying primary multiple myeloma. Blood 2012, 120, e9–e16. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Lutsik, P.; Baude, A.; Mancarella, D.; Öz, S.; Kühn, A.; Toth, R.; Hey, J.; Toprak, U.H.; Lim, J.; Nguyen, V.H.; et al. Globally altered epigenetic landscape and delayed osteogenic differentiation in H3.3-G34W-mutant giant cell tumor of bone. Nat. Commun. 2020, 11, 5414. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- Boyd, A.L.; Reid, J.C.; Salci, K.R.; Aslostovar, L.; Benoit, Y.D.; Shapovalova, Z.; Nakanishi, M.; Porras, D.P.; Almakadi, M.; Campbell, C.J.V.; et al. Acute myeloid leukaemia disrupts endogenous myelo-erythropoiesis by compromising the adipocyte bone marrow niche. Nat. Cell Biol. 2017, 19, 1336–1347. [Google Scholar] [CrossRef]
- Smeets, M.W.E.; Steeghs, E.M.P.; Orsel, J.; Stalpers, F.; Vermeeren, M.M.P.; Veltman, C.H.J.; Slenders, L.; Nierkens, S.; Van de Ven, C.; Den Boer, M.L. B-cell precursor acute lymphoblastic leukemia elicits an interferon-α/β response in bone marrow-derived mesenchymal stroma. Haematologica 2024, 109, 2073–2084. [Google Scholar] [CrossRef]
- Boissy, P.; Andersen, T.L.; Abdallah, B.M.; Kassem, M.; Plesner, T.; Delaissé, J.M. Resveratrol inhibits myeloma cell growth, prevents osteoclast formation, and promotes osteoblast differentiation. Cancer Res. 2005, 65, 9943–9952. [Google Scholar] [CrossRef]
- Zhu, F.; Friedman, M.S.; Luo, W.; Woolf, P.; Hankenson, K.D. The transcription factor osterix (SP7) regulates BMP6-induced human osteoblast differentiation. J. Cell Physiol. 2012, 227, 2677–2685. [Google Scholar] [CrossRef]
- Asurappulige, H.S.H.; Ladomery, M.R.; Ruth Morse, H. IL-6 knockdown in a model of the human bone marrow, abrogates DNA damage induction in bystander cells post-chemotherapy induced cytokine release syndrome. Transl. Oncol. 2024, 46, 102030. [Google Scholar] [CrossRef]
- Pillai, M.M.; Yang, X.; Balakrishnan, I.; Bemis, L.; Torok-Storb, B. MiR-886-3p down regulates CXCL12 (SDF1) expression in human marrow stromal cells. PLoS ONE 2010, 5, e14304. [Google Scholar] [CrossRef]
- Westhrin, M.; Holien, T.; Zahoor, M.; Moen, S.H.; Buene, G.; Størdal, B.; Hella, H.; Yuan, H.; de Bruijn, J.D.; Martens, A.; et al. Bone Morphogenetic Protein 4 Gene Therapy in Mice Inhibits Myeloma Tumor Growth, But Has a Negative Impact on Bone. JBMR Plus 2020, 4, e10247. [Google Scholar] [CrossRef] [PubMed]
- Seckinger, A.; Meissner, T.; Moreaux, J.; Goldschmidt, H.; Fuhler, G.M.; Benner, A.; Hundemer, M.; Rème, T.; Shaughnessy, J.D., Jr.; Barlogie, B.; et al. Bone morphogenic protein 6: A member of a novel class of prognostic factors expressed by normal and malignant plasma cells inhibiting proliferation and angiogenesis. Oncogene 2009, 28, 3866–3879. [Google Scholar] [CrossRef] [PubMed]
- Lagler, C.; El-Mesery, M.; Kübler, A.C.; Müller-Richter, U.D.A.; Stühmer, T.; Nickel, J.; Müller, T.D.; Wajant, H.; Seher, A. The anti-myeloma activity of bone morphogenetic protein 2 predominantly relies on the induction of growth arrest and is apoptosis-independent. PLoS ONE 2017, 12, e0185720. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, C.; Kizaki, M.; Ikeda, Y. Bone morphogenetic protein (BMP)-2 induces apoptosis in human myeloma cells. Leuk Lymphoma 2002, 43, 635–639. [Google Scholar] [CrossRef]
- Grab, A.L.; Seckinger, A.; Horn, P.; Hose, D.; Cavalcanti-Adam, E.A. Hyaluronan hydrogels delivering BMP-6 for local targeting of malignant plasma cells and osteogenic differentiation of mesenchymal stromal cells. Acta Biomater. 2019, 96, 258–270. [Google Scholar] [CrossRef]
- Urashima, M.; Ogata, A.; Chauhan, D.; Vidriales, M.B.; Teoh, G.; Hoshi, Y.; Schlossman, R.L.; DeCaprio, J.A.; Anderson, K.C. Interleukin-6 promotes multiple myeloma cell growth via phosphorylation of retinoblastoma protein. Blood 1996, 88, 2219–2227. [Google Scholar] [CrossRef]
- Shain, K.H.; Yarde, D.N.; Meads, M.B.; Huang, M.; Jove, R.; Hazlehurst, L.A.; Dalton, W.S. β1 integrin adhesion enhances IL-6–mediated STAT3 signaling in myeloma cells: Implications for microenvironment influence on tumor survival and proliferation. Cancer Res. 2009, 69, 1009–1015. [Google Scholar] [CrossRef]
- Regelink, J.C.; Raijmakers, P.G.; Bravenboer, N.; Milek, R.; Hoetjes, N.J.; de Kreuk, A.M.; van Duin, M.; Wondergem, M.J.; Lips, P.; Sonneveld, P.; et al. (18)F-fluoride-PET for dynamic in vivo monitoring of bone formation in multiple myeloma. EJNMMI Res. 2016, 6, 46. [Google Scholar] [CrossRef]
- Kalamajski, S.; Aspberg, A.; Lindblom, K.; Heinegård, D.; Oldberg, Å. Asporin competes with decorin for collagen binding, binds calcium and promotes osteoblast collagen mineralization. Biochem. J. 2009, 423, 53–59. [Google Scholar] [CrossRef]
- Tashima, T.; Nagatoishi, S.; Sagara, H.; Ohnuma, S.; Tsumoto, K. Osteomodulin regulates diameter and alters shape of collagen fibrils. Biochem. Biophys. Res. Commun. 2015, 463, 292–296. [Google Scholar] [CrossRef]
- Chen, S.; Birk, D.E. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J. 2013, 280, 2120–2137. [Google Scholar] [CrossRef] [PubMed]
- Sworder, B.J.; Yoshizawa, S.; Mishra, P.J.; Cherman, N.; Kuznetsov, S.A.; Merlino, G.; Balakumaran, A.; Robey, P.G. Molecular profile of clonal strains of human skeletal stem/progenitor cells with different potencies. Stem Cell Res. 2015, 14, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.D.; Pham, L.; Billington, C.J., Jr.; Espe, K.; Carlson, A.E.; Westendorf, J.J.; Petryk, A.; Gopalakrishnan, R.; Mansky, K. Bone morphogenic protein 2 directly enhances differentiation of murine osteoclast precursors. J. Cell Biochem. 2010, 109, 672–682. [Google Scholar] [CrossRef]
- Bordukalo-Nikšić, T.; Kufner, V.; Vukičević, S. The Role Of BMPs in the Regulation of Osteoclasts Resorption and Bone Remodeling: From Experimental Models to Clinical Applications. Front. Immunol. 2022, 13, 869422. [Google Scholar] [CrossRef] [PubMed]
- McClellan, J.W.; Mulconrey, D.S.; Forbes, R.J.; Fullmer, N. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2). J. Spinal Disord. Tech. 2006, 19, 483–486. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Chiari, C.; Grgurevic, L.; Bordukalo-Niksic, T.; Oppermann, H.; Valentinitsch, A.; Nemecek, E.; Staats, K.; Schreiner, M.; Trost, C.; Kolb, A.; et al. Recombinant Human BMP6 Applied Within Autologous Blood Coagulum Accelerates Bone Healing: Randomized Controlled Trial in High Tibial Osteotomy Patients. J. Bone Miner. Res. 2020, 35, 1893–1903. [Google Scholar] [CrossRef]
- Durdevic, D.; Vlahovic, T.; Pehar, S.; Miklic, D.; Oppermann, H.; Bordukalo-Niksic, T.; Gavrankapetanovic, I.; Jamakosmanovic, M.; Milosevic, M.; Martinovic, S.; et al. A novel autologous bone graft substitute comprised of rhBMP6 blood coagulum as carrier tested in a randomized and controlled Phase I trial in patients with distal radial fractures. Bone 2020, 140, 115551. [Google Scholar] [CrossRef]
- Lavery, K.; Swain, P.; Falb, D.; Alaoui-Ismaili, M.H. BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J. Biol. Chem. 2008, 283, 20948–20958. [Google Scholar] [CrossRef]
- Yadin, D.; Knaus, P.; Mueller, T.D. Structural insights into BMP receptors: Specificity, activation and inhibition. Cytokine Growth Factor Rev. 2016, 27, 13–34. [Google Scholar] [CrossRef]
- Sanchez-Duffhues, G.; Williams, E.; Goumans, M.J.; Heldin, C.H.; Ten Dijke, P. Bone morphogenetic protein receptors: Structure, function and targeting by selective small molecule kinase inhibitors. Bone 2020, 138, 115472. [Google Scholar] [CrossRef]
- Gooding, S.; Olechnowicz, S.W.Z.; Morris, E.V.; Armitage, A.E.; Arezes, J.; Frost, J.; Repapi, E.; Edwards, J.R.; Ashley, N.; Waugh, C.; et al. Transcriptomic profiling of the myeloma bone-lining niche reveals BMP signalling inhibition to improve bone disease. Nat. Commun. 2019, 10, 4533. [Google Scholar] [CrossRef]
- Baade Ro, T.; Utne Holt, R.; Brenne, A.-T.; Hjorth-Hansen, H.; Waage, A.; Hjertner, O.; Sundan, A.; Borset, M. Bone morphogenetic protein-5, -6 and -7 inhibit growth and induce apoptosis in human myeloma cells. Oncogene 2004, 23, 3024–3032. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Baardemans, T.; de Matos Simoes, R.; Noort, W.; Ruiter, R.W.J.; Prins, H.-J.; van Hal-van Veen, S.E.; Yuan, H.; de Bruijn, J.D.; Martens, A.C.M.; et al. Humanized Bone Model Identifies BMP6 as a Multifunctional Regulator in Myeloma Bone Disease. Biomolecules 2025, 15, 1747. https://doi.org/10.3390/biom15121747
Wang J, Baardemans T, de Matos Simoes R, Noort W, Ruiter RWJ, Prins H-J, van Hal-van Veen SE, Yuan H, de Bruijn JD, Martens ACM, et al. Humanized Bone Model Identifies BMP6 as a Multifunctional Regulator in Myeloma Bone Disease. Biomolecules. 2025; 15(12):1747. https://doi.org/10.3390/biom15121747
Chicago/Turabian StyleWang, Jiaxian, Thomas Baardemans, Ricardo de Matos Simoes, Willy Noort, Ruud W. J. Ruiter, Henk-Jan Prins, Susan E. van Hal-van Veen, Huipin Yuan, Joost D. de Bruijn, Anton C. M. Martens, and et al. 2025. "Humanized Bone Model Identifies BMP6 as a Multifunctional Regulator in Myeloma Bone Disease" Biomolecules 15, no. 12: 1747. https://doi.org/10.3390/biom15121747
APA StyleWang, J., Baardemans, T., de Matos Simoes, R., Noort, W., Ruiter, R. W. J., Prins, H.-J., van Hal-van Veen, S. E., Yuan, H., de Bruijn, J. D., Martens, A. C. M., Mitsiades, C. S., Zweegman, S., Themeli, M., & Groen, R. W. J. (2025). Humanized Bone Model Identifies BMP6 as a Multifunctional Regulator in Myeloma Bone Disease. Biomolecules, 15(12), 1747. https://doi.org/10.3390/biom15121747




