Inflammatory Biomarkers for Thrombotic Risk Assessment in Multiple Myeloma Patients on IMiD/aCD38-Based Regimens: Insights from a Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Data Collection
2.3. VTE Risk Assessment
2.4. Multiplex Analysis of Plasma Cytokines and Chemokines
2.5. Statistical Analysis
3. Results
3.1. Patient’ Characteristics
3.2. Thrombosis Risk Score Evaluation
3.3. Incidence and Timing of Thrombotic Events
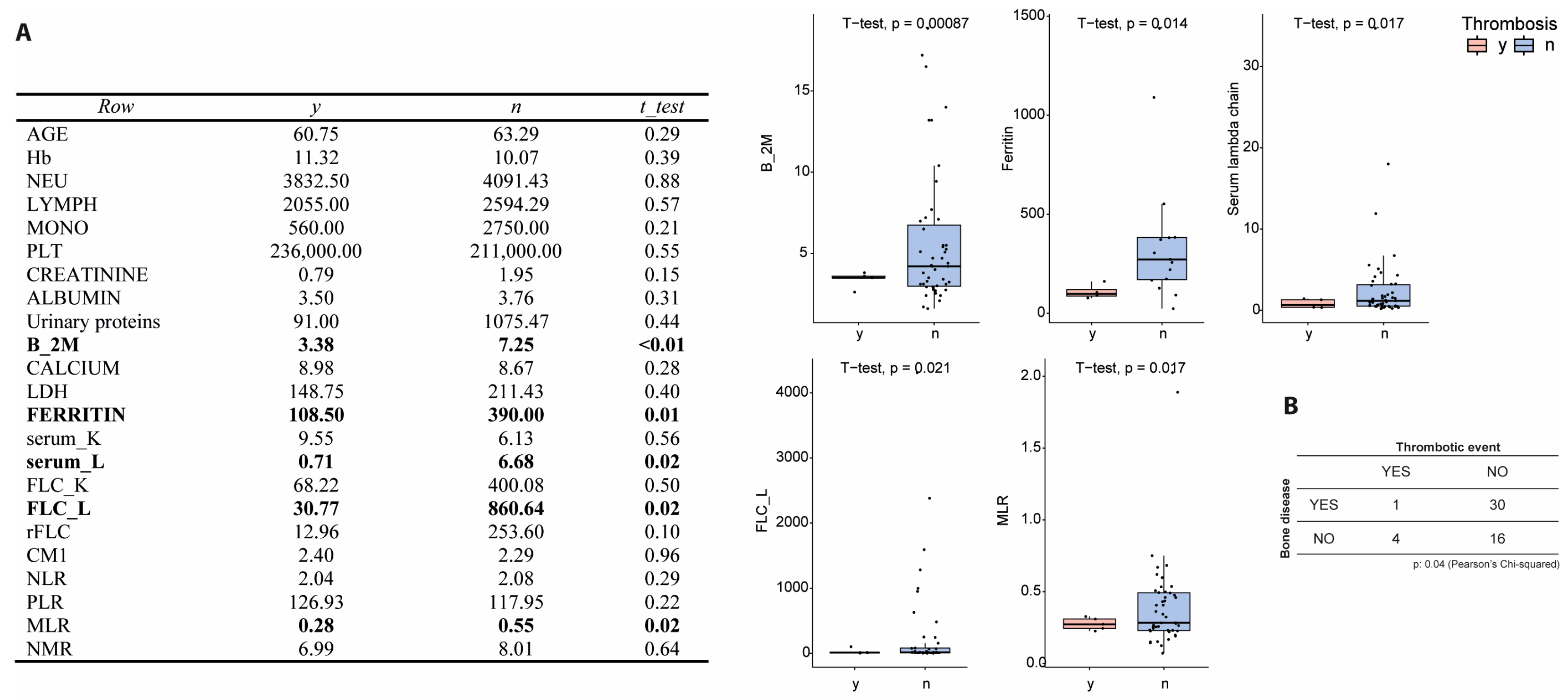
3.4. Association with Hematological and Inflammatory Markers
3.5. Cytokine Profile and Immune Response in Thrombosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MM | Multiple myeloma |
| IMiDs | Immunomodulatory drugs |
| TE | Thrombotic events |
| LMWH | Low molecular weight heparin |
| Thal | Thalidomide |
| Len | Lenalidomide |
| Pom | Pomalidomide |
| VTE | Venous thromboembolism |
| β2M | Beta-2 microglobulin |
| IMWG | International Myeloma Working Group |
| SA-PE | Streptavidin-phycoerythrin |
| ASA | Aspirin |
| MLR | Monocyte-to-lymphocyte ratio |
| DOAC | Direct oral anticoagulants |
References
- Dimopoulos, M.A.; Terpos, E.; Boccadoro, M.; Moreau, P.; Mateos, M.-V.; Zweegman, S.; Cook, G.; Engelhardt, M.; Delforge, M.; Hajek, R.; et al. EHA-EMN Evidence-Based Guidelines for Diagnosis, Treatment and Follow-up of Patients with Multiple Myeloma. Nat. Rev. Clin. Oncol. 2025, 22, 680–700. [Google Scholar] [CrossRef]
- De Stefano, V.; Larocca, A.; Carpenedo, M.; Cavo, M.; Di Raimondo, F.; Falanga, A.; Offidani, M.; Petrucci, M.T.; Ruggeri, M.; Santi, R.M.; et al. Thrombosis in Multiple Myeloma: Risk Stratification, Antithrombotic Prophylaxis, and Management of Acute Events. A Consensus-Based Position Paper from an Ad Hoc Expert Panel. Haematologica 2022, 107, 2536–2547. [Google Scholar] [CrossRef]
- Singh, A.; Gajra, A. Thromboembolism with Immunomodulatory Agents in the Treatment of Multiple Myeloma. Cardiovasc. Hematol. Agents Med. Chem. 2011, 9, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Charalampous, C.; Goel, U.; Kapoor, P.; Binder, M.; Buadi, F.K.; Dingli, D.; Dispenzieri, A.; Fonder, A.L.; Gertz, M.A.; Gonsalves, W.; et al. Thrombosis in Multiple Myeloma: Risk Estimation by Induction Regimen and Association with Overall Survival. Am. J. Hematol. 2023, 98, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Fukatsu, M.; Ikezoe, T. Cancer-Associated Thrombosis in Hematologic Malignancies. Int. J. Hematol. 2024, 119, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Verso, M.; Moik, F.; Graziani, M.; Cohen, A.T. Targeted Anti-Cancer Agents and Risk of Venous Thromboembolism. Haematologica 2024, 109, 3868–3878. [Google Scholar] [CrossRef]
- Sborov, D.W.; Baljevic, M.; Reeves, B.; Laubach, J.; Efebera, Y.A.; Rodriguez, C.; Costa, L.J.; Chari, A.; Silbermann, R.; Holstein, S.A.; et al. Daratumumab plus Lenalidomide, Bortezomib and Dexamethasone in Newly Diagnosed Multiple Myeloma: Analysis of Vascular Thrombotic Events in the GRIFFIN Study. Br. J. Haematol. 2022, 199, 355–365. [Google Scholar] [CrossRef]
- Botta, C.; Gigliotta, E.; Paiva, B.; Anselmo, R.; Santoro, M.; Otero, P.R.; Carlisi, M.; Conticello, C.; Romano, A.; Solimando, A.G.; et al. Network Meta-Analysis of Randomized Trials in Multiple Myeloma: Efficacy and Safety in Frontline Therapy for Patients Not Eligible for Transplant. Hematol. Oncol. 2022, 40, 987–998. [Google Scholar] [CrossRef]
- Fotiou, D.; Gavriatopoulou, M.; Terpos, E. Multiple Myeloma and Thrombosis: Prophylaxis and Risk Prediction Tools. Cancers 2020, 12, 191. [Google Scholar] [CrossRef]
- Stefano, V.; Za, T.; Rossi, E. Venous Thromboembolism in Multiple Myeloma. Semin. Thromb. Hemost. 2014, 40, 338–347. [Google Scholar] [CrossRef]
- Falanga, A.; Marchetti, M. Venous Thromboembolism in the Hematologic Malignancies. J. Clin. Oncol. 2009, 27, 4848–4857. [Google Scholar] [CrossRef]
- Carrier, M.; Le Gal, G.; Tay, J.; Wu, C.; Lee, A.Y. Rates of Venous Thromboembolism in Multiple Myeloma Patients Undergoing Immunomodulatory Therapy with Thalidomide or Lenalidomide: A Systematic Review and Meta-analysis. J. Thromb. Haemost. 2011, 9, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Annibali, O.; Napolitano, M.; Avvisati, G.; Siragusa, S. Incidence of Venous Thromboembolism and Use of Anticoagulation in Hematological Malignancies: Critical Review of the Literature. Crit. Rev. Oncol./Hematol. 2018, 124, 41–50. [Google Scholar] [CrossRef]
- Wang, J.; Kim, Y. Risk of Thromboembolism in Patients with Multiple Myeloma Treated with Daratumumab: A Systemic Review and Meta-Analysis. Int. J. Hematol. 2020, 112, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Comerford, C.; Glavey, S.; O’Sullivan, J.M.; Quinn, J. Potential Mechanisms of Resistance to Current Anti-Thrombotic Strategies in Multiple Myeloma. Cancer Drug Resist. 2022, 5, 214. [Google Scholar] [CrossRef] [PubMed]
- Plano, F.; Gigliotta, E.; Corsale, A.M.; Azgomi, M.S.; Santonocito, C.; Ingrascì, M.; Di Carlo, L.; Augello, A.E.; Speciale, M.; Vullo, C.; et al. Ferritin Metabolism Reflects Multiple Myeloma Microenvironment and Predicts Patient Outcome. Int. J. Mol. Sci. 2023, 24, 8852. [Google Scholar] [CrossRef]
- Botta, C.; Di Martino, M.T.; Ciliberto, D.; Cucè, M.; Correale, P.; Rossi, M.; Tagliaferri, P.; Tassone, P. A Gene Expression Inflammatory Signature Specifically Predicts Multiple Myeloma Evolution and Patients Survival. Blood Cancer J. 2016, 6, e511. [Google Scholar] [CrossRef]
- Susca, N.; Borrelli, P.; Botta, C.; Solimando, A.G. Prognostic Impact of the Immune Microenvironment in Multiple Myeloma: A Meta-Analysis of Current Studies. Haematologica 2025, 999. [Google Scholar] [CrossRef]
- Palomo, M.; Moreno-Castaño, A.B.; Salas, M.Q.; Escribano-Serrat, S.; Rovira, M.; Guillen-Olmos, E.; Fernandez, S.; Ventosa-Capell, H.; Youssef, L.; Crispi, F.; et al. Endothelial Activation and Damage as a Common Pathological Substrate in Different Pathologies and Cell Therapy Complications. Front. Med. 2023, 10, 1285898. [Google Scholar] [CrossRef]
- Gidaro, A.; Manetti, R.; Delitala, A.P.; Soloski, M.J.; Lambertenghi Deliliers, G.; Castro, D.; Soldini, D.; Castelli, R. Incidence of Venous Thromboembolism in Multiple Myeloma Patients across Different Regimens: Role of Procoagulant Microparticles and Cytokine Release. J. Clin. Med. 2022, 11, 2720. [Google Scholar] [CrossRef]
- Shen, F.; Shen, W. Isatuximab in the Treatment of Multiple Myeloma: A Review and Comparison With Daratumumab. Technol. Cancer Res. Treat. 2022, 21, 15330338221106563. [Google Scholar] [CrossRef]
- Nooka, A.K.; Kaufman, J.L.; Hofmeister, C.C.; Joseph, N.S.; Heffner, T.L.; Gupta, V.A.; Sullivan, H.C.; Neish, A.S.; Dhodapkar, M.V.; Lonial, S. Daratumumab in Multiple Myeloma. Cancer 2019, 125, 2364–2382. [Google Scholar] [CrossRef]
- Palumbo, A.; Rajkumar, S.V.; San Miguel, J.F.; Larocca, A.; Niesvizky, R.; Morgan, G.; Landgren, O.; Hajek, R.; Einsele, H.; Anderson, K.C.; et al. International Myeloma Working Group Consensus Statement for the Management, Treatment, and Supportive Care of Patients With Myeloma Not Eligible for Standard Autologous Stem-Cell Transplantation. J. Clin. Oncol. 2014, 32, 587–600. [Google Scholar] [CrossRef]
- Sanfilippo, K.M.; Luo, S.; Wang, T.-F.; Wildes, T.; Mikhael, J.; Keller, J.W.; Thomas, T.S.; Carson, K.R.; Gage, B.F. Predicting Risk of Venous Thromboembolism in Multiple Myeloma: The Impede VTE Score. Blood 2018, 132, 141. [Google Scholar] [CrossRef]
- Li, A.; Wu, Q.; Luo, S.; Warnick, G.S.; Zakai, N.A.; Libby, E.N.; Gage, B.F.; Garcia, D.A.; Lyman, G.H.; Sanfilippo, K.M. Derivation and Validation of a Risk Assessment Model for Immunomodulatory Drug–Associated Thrombosis Among Patients With Multiple Myeloma. J. Natl. Compr. Cancer Netw. 2019, 17, 840–847. [Google Scholar] [CrossRef]
- Chakraborty, R.; Rybicki, L.; Wei, W.; Valent, J.; Faiman, B.M.; Samaras, C.J.; Anwer, F.; Khorana, A.A. Abnormal Metaphase Cytogenetics Predicts Venous Thromboembolism in Myeloma: Derivation and Validation of the PRISM Score. Blood 2022, 140, 2443–2450. [Google Scholar] [CrossRef]
- Abdi, J.; Nasr, P. Abnormalities of Primary and Secondary Hemostasis in Multiple Myeloma: Insights from Studies on Thrombopoiesis, the Coagulation System, and the Bone Marrow Microenvironment. Front. Hematol. 2025, 4, 1435193. [Google Scholar] [CrossRef]
- Isozumi, Y.; Arai, R.; Fujimoto, K.; Koyama, T. Activation of Coagulation by Lenalidomide-Based Regimens for the Treatment of Multiple Myeloma. PLoS ONE 2013, 8, e64369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ghansah, H.; Debreceni, I.B.; Váróczy, L.; Rejtő, L.; Lóczi, L.; Bagoly, Z.; Kappelmayer, J. Patients with Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance Have Variably Increased Thrombin Generation and Different Sensitivity to the Anticoagulant Effect of Activated Protein C. Thromb. Res. 2023, 223, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Fotiou, D.; Dimopoulos, M.A.; Kastritis, E. Approach to Contemporary Risk Assessment, Prevention and Management of Thrombotic Complications in Multiple Myeloma. Cancers 2022, 14, 6216. [Google Scholar] [CrossRef]
- Esmon, C.T. Basic Mechanisms and Pathogenesis of Venous Thrombosis. Blood Rev. 2009, 23, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Multiple Myeloma: 2022 Update on Diagnosis, Risk Stratification, and Management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef] [PubMed]
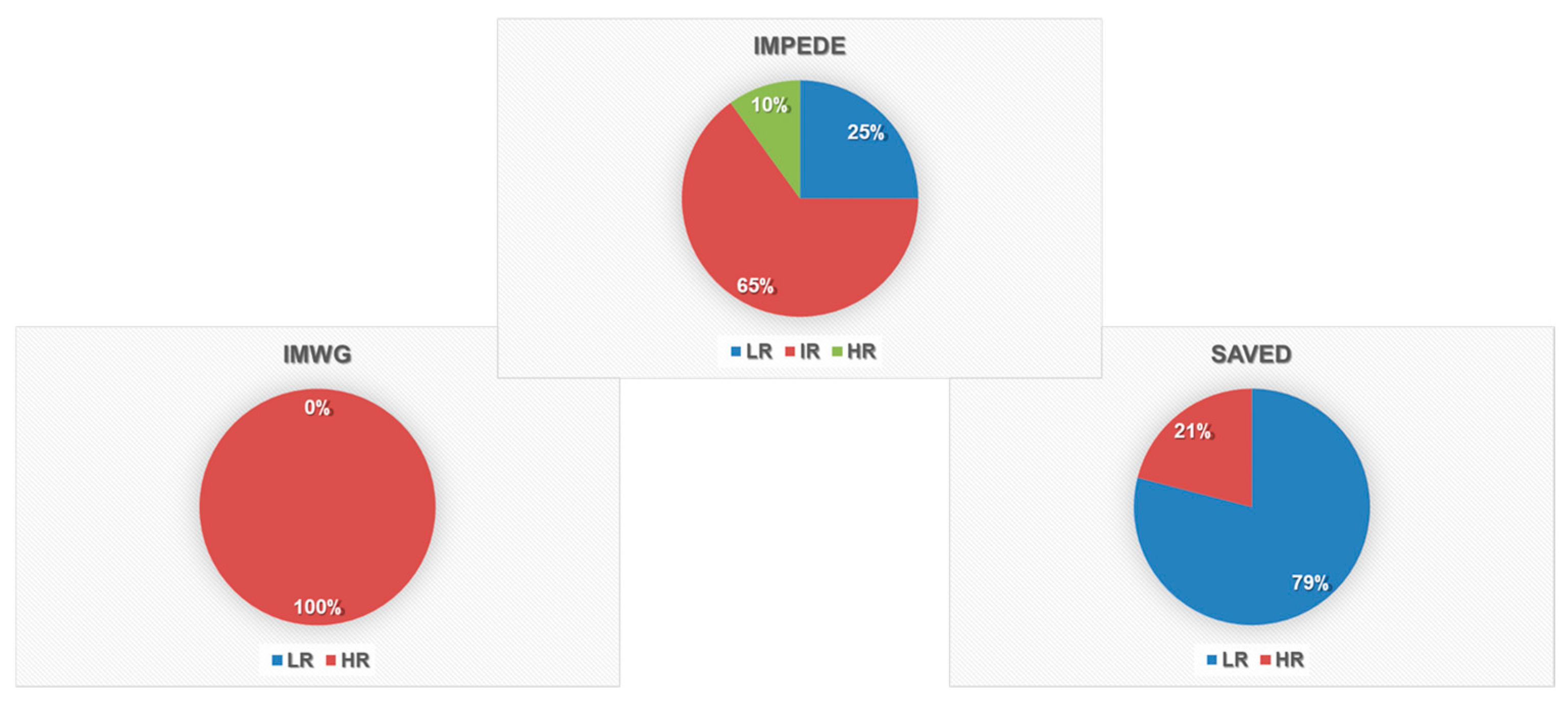

| Characteristic | Patients (n.53) |
|---|---|
| Sex (n) | M: 27 (51%) F: 26 (49%) |
| Age (median) | 72y (47–86y) |
| Isotype | IgG: 37 (70%) IgA: 12 (23%) LC: 4 (7%) K: 35 (66%) L: 18 (34) |
| ISS (median) | ISS 2 (1–3) |
| Lytic lesions (n) | Y: 31 (58%) N:20 (38%)NA: 2 (4%) |
| Previous ASA (n) | Y: 38 (72%) N: 15 (28%) |
| Treatment (n) | Dara-VTD: 17 (32%) Dara-Rd: 34 (64%) Isa-Pd: 2 (4%) |
| Comorbidities | Cardiovascular: 15 (28%) Diabetes: 8 (15%) Chronic renal disease: 5 (9%) |
| ECOG | 0: 19 (36%) 1: 25 (47%) 2: 6 (11%) N/A: 3 (6%) |
| Cytogenetic alterations | Failed or N/A: 42 (79%) SR: 5 (9%) HR: 6 (11%) (n. 3 t(4;14) and n. 3 amp 1q21) |
| VTE Pt 1 | VTE Pt 2 | VTE Pt 3 | VTE Pt 4 | VTE Pt 5 | |
|---|---|---|---|---|---|
| BMI > 25 | Yes | Yes | Yes | No | No |
| Concomitant steroids * | Yes | Yes | Yes | Yes | Yes |
| Main comorbidities # | No | No | No | No | C/K/D |
| Previous TVE History | Yes | No | No | No | No |
| IMIDs | Len | Thal | Len | Len | Len |
| ASA prophylaxis | Yes | Yes | Yes | Yes | Yes |
| Type of TVE | SVT | DVT | SVT | DVT | SVT |
| Site of TVE | Saphenous collateral vein | Sural vein | Great saphenous vein | Sural vein | Saphenous collateral vein |
| Cycle at event onset | 1 | 2 | 6 | 1 | 10 |
| Anticoagulation Therapy | LMWH | LMWH | LMWH | LMWH | LMWH |
| Duration | 3 m | 4 m | 17 m | 5 m | 2 m |
| Time to resolution (d) | 35 | 29 | 63 | 28 | 29 |
| VTE prophylaxis thereafter | Apixaban | Edoxaban | LMWH/Apixaban | Edoxaban | Apixaban |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botta, C.; Corsale, A.M.; Cammarata, C.; Di Fazio, F.; Gigliotta, E.; Rizzuto, A.; Ingrascì, M.; Speciale, M.; Aquilina, C.; Biondo, M.; et al. Inflammatory Biomarkers for Thrombotic Risk Assessment in Multiple Myeloma Patients on IMiD/aCD38-Based Regimens: Insights from a Prospective Observational Study. Biomolecules 2025, 15, 1533. https://doi.org/10.3390/biom15111533
Botta C, Corsale AM, Cammarata C, Di Fazio F, Gigliotta E, Rizzuto A, Ingrascì M, Speciale M, Aquilina C, Biondo M, et al. Inflammatory Biomarkers for Thrombotic Risk Assessment in Multiple Myeloma Patients on IMiD/aCD38-Based Regimens: Insights from a Prospective Observational Study. Biomolecules. 2025; 15(11):1533. https://doi.org/10.3390/biom15111533
Chicago/Turabian StyleBotta, Cirino, Anna Maria Corsale, Claudia Cammarata, Fabiana Di Fazio, Emilia Gigliotta, Andrea Rizzuto, Manuela Ingrascì, Maria Speciale, Cristina Aquilina, Marta Biondo, and et al. 2025. "Inflammatory Biomarkers for Thrombotic Risk Assessment in Multiple Myeloma Patients on IMiD/aCD38-Based Regimens: Insights from a Prospective Observational Study" Biomolecules 15, no. 11: 1533. https://doi.org/10.3390/biom15111533
APA StyleBotta, C., Corsale, A. M., Cammarata, C., Di Fazio, F., Gigliotta, E., Rizzuto, A., Ingrascì, M., Speciale, M., Aquilina, C., Biondo, M., Romano, A., Napolitano, M., Mattana, M., & Siragusa, S. (2025). Inflammatory Biomarkers for Thrombotic Risk Assessment in Multiple Myeloma Patients on IMiD/aCD38-Based Regimens: Insights from a Prospective Observational Study. Biomolecules, 15(11), 1533. https://doi.org/10.3390/biom15111533







