Insulin Resistance at the Crossroads of Metabolic Inflammation, Cardiovascular Disease, Organ Failure, and Cancer
Abstract
1. Introduction
2. Research Strategy
3. Etiology and Assessment of Insulin Resistance
3.1. Causes of Insulin Resistance
3.2. Assessment of Insulin Resistance in Clinics and in Epidemiological Studies
3.3. Assessment of Insulin Resistance in the Diabetes Research Setting
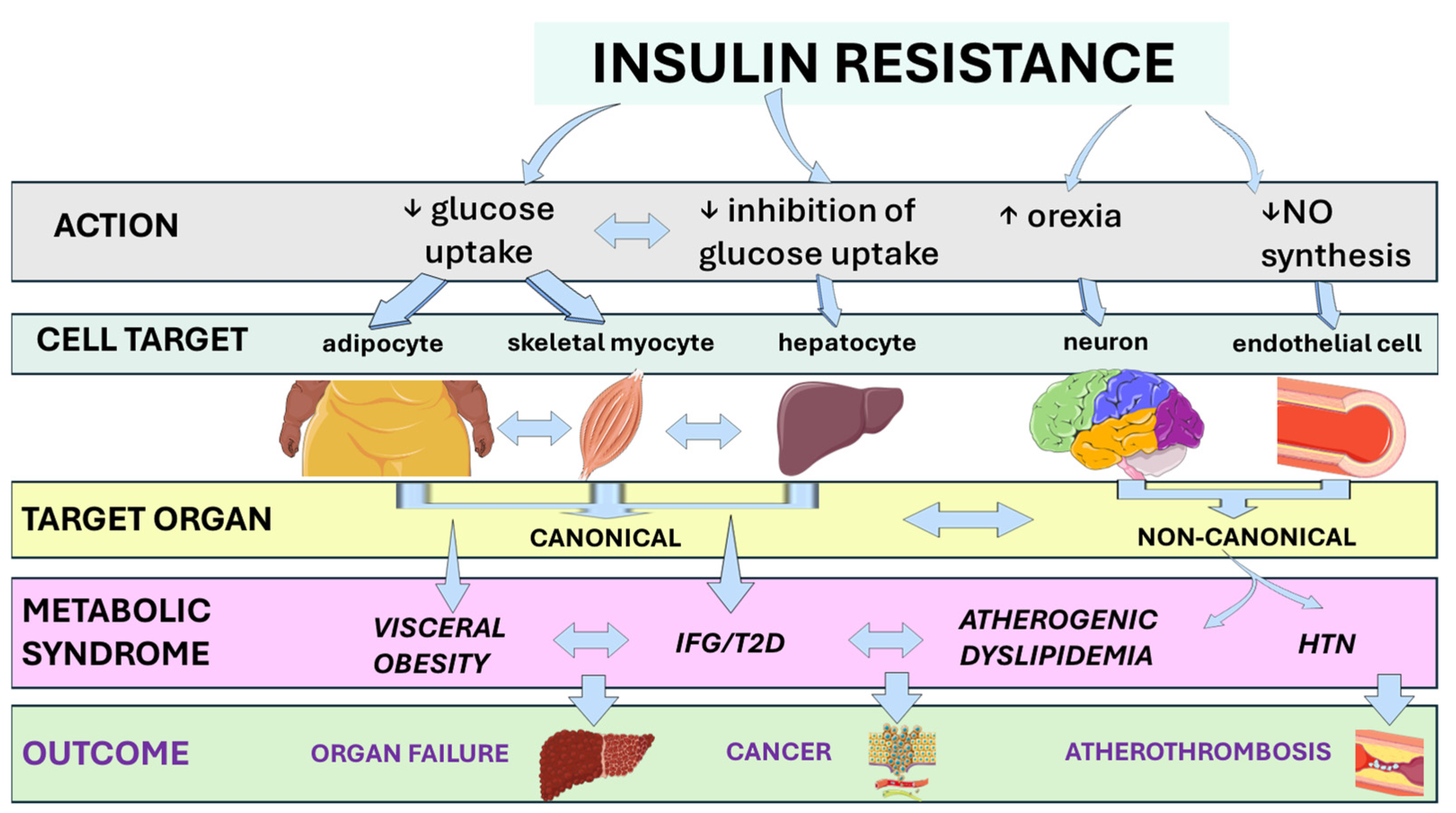
4. Molecular Physiology of Insulin Signaling and Mechanisms of Insulin Resistance
4.1. Inter-Organ Crosstalk: Hepatokines, Myokines, and Adipokines
4.2. Lipotoxicity and the Self-Expanding Network of the Metabolic Syndrome
4.3. Drug-Induced Derailment of Insulin Signaling
5. Pathobiology and Clinical Science Associating Ethnicity, Insulin Resistance, Sex and Aging
5.1. Pathobiology Associating Insulin Resistance, Ethnicity Sex and Aging
5.2. Clinical Science Associating Insulin Resistance, Ethnicity, Sex, and Aging
6. Pathobiology and Clinical Science Associating Insulin Resistance and Metabolic Inflammation
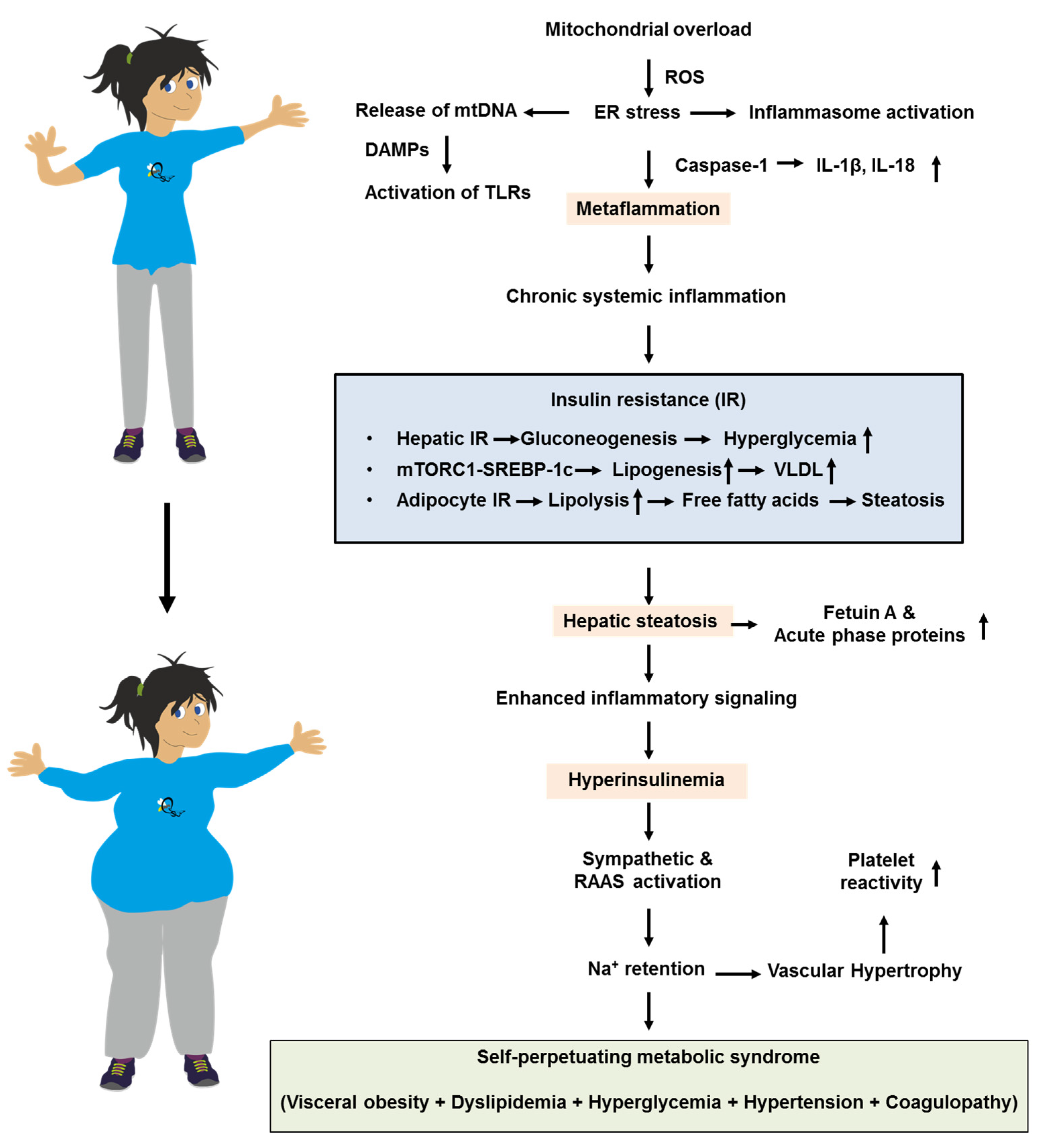
6.1. Pathobiology Associating Insulin Resistance and Metabolic Inflammation
6.2. Clinical Science Associating Insulin Resistance and Metabolic Inflammation
7. Pathobiology and Clinical Science Associating Insulin Resistance and Cardiovascular Disease
7.1. Pathobiology Associating Insulin Resistance and Cardiovascular Disease
7.2. Clinical Science Associating Insulin Resistance and Cardiovascular Disease
8. Pathobiology and Clinical Science Associating Insulin Resistance and Organ Failure
8.1. Pathobiology Associating Insulin Resistance and Organ Failure
8.2. Clinical Science Associating Insulin Resistance and Organ Failure
9. Pathobiology and Clinical Science Associating Insulin Resistance and Cancer
9.1. Pathobiology Associating Insulin Resistance and Cancer
9.2. Clinical Science Associating Insulin Resistance and Cancer
10. Principles of Treatment of Insulin Resistance
11. Conclusions and Research Agenda
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CKD | chronic kidney disease |
| CI | confidence interval |
| CPT1 | carnitine O-palmitoiltransferase 1 |
| CVD | cardiovascular disease |
| DAMP(s) | damage-associated molecular pattern(s) |
| GLP-1RA | glucagon-like peptide receptor 1 agonist(s) |
| HOMA-IR | homeostasis model of insulin resistance |
| IL-6 | interleukin-6 |
| IR | insulin resistance |
| IRR | incidence rate ratio |
| ISR1/2 | insulin receptor substrate 1/2) |
| IST | insulin tolerance test |
| MACE | major adverse cardiovascular events |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| MASH | metabolic dysfunction-associated steatohepatitis |
| mtDNA | mitochondrial DNA |
| OR | odds ratio |
| PCOS | polycystic ovary syndrome |
| QUICKI | quantitative insulin sensitivity check index |
| ROS | reactive oxygen species |
| SGLT2 | sodium-glucose transporter 2 |
| SHBG | sex hormone binding globulin |
| SRRI | selective serotonin reuptake inhibitors |
| THR-β | thyroid hormone receptor-β |
| T2D | type 2 diabetes |
| TLR4 | toll-like receptor 4 |
| TyG | triglyceride-glucose index |
References
- Yalow, R.S.; Berson, S.A. Immunoassay of endogenous plasma insulin in man. J. Clin. Investig. 1960, 39, 1157–1175. [Google Scholar] [CrossRef]
- Yalow, R.S.; Berson, S.A. Plasma insulin concentrations in nondiabetic and early diabetic subjects. Determinations by a new sensitive immuno-assay technic. Diabetes 1960, 9, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Aronis, K.N.; Mantzoros, C.S. A brief history of insulin resistance: From the first insulin radioimmunoassay to selectively targeting protein kinase C pathways. Metabolism 2012, 61, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.R.; Neville, D.M., Jr.; Roth, J. Insulin-receptor interaction in the obese-hyperglycemic mouse. A model of insulin resistance. J. Biol. Chem. 1973, 248, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Goldfine, I.D.; Kahn, C.R.; Neville DMJr Roth, J.; Garrison, M.M.; Bates, R.W. Decreased binding of insulin to its receptors in rats with hormone induced insulin resistance. Biochem. Biophys. Res. Commun. 1973, 53, 852–857. [Google Scholar] [CrossRef]
- Kahn, C.R.; Flier, J.S.; Bar, R.S.; Archer, J.A.; Gorden, P.; Martin, M.M.; Roth, J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N. Engl. J. Med. 1976, 294, 739–745. [Google Scholar] [CrossRef]
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef]
- Muniyappa, R.; Chen, H.; Montagnani, M.; Sherman, A.; Quon, M.J. Endothelial dysfunction due to selective insulin resistance in vascular endothelium: Insights from mechanistic modeling. Am. J. Physiol. Metab. 2020, 319, E629–E646. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andreadi, A.; Bellia, A.; Di Daniele, N.; Meloni, M.; Lauro, R.; Della-Morte, D.; Lauro, D. The molecular link between oxidative stress, insulin resistance, and type 2 diabetes: A target for new therapies against cardiovascular diseases. Curr. Opin. Pharmacol. 2022, 62, 85–96. [Google Scholar] [CrossRef]
- Giangregorio, F.; Mosconi, E.; Debellis, M.G.; Provini, S.; Esposito, C.; Garolfi, M.; Oraka, S.; Kaloudi, O.; Mustafazade, G.; Marín-Baselga, R.; et al. A Systematic Review of Metabolic Syndrome: Key Correlated Pathologies and Non-Invasive Diagnostic Approaches. J. Clin. Med. 2024, 13, 5880. [Google Scholar] [CrossRef]
- Zhan, Z.Q.; Chen, Y.Z.; Huang, Z.M.; Luo, Y.H.; Zeng, J.J.; Wang, Y.; Tan, J.; Chen, Y.X.; Fang, J.Y. Metabolic syndrome, its components, and gastrointestinal cancer risk: A meta-analysis of 31 prospective cohorts and Mendelian randomization study. J. Gastroenterol. Hepatol. 2024, 39, 630–641. [Google Scholar] [CrossRef]
- Hamooya, B.M.; Siame, L.; Muchaili, L.; Masenga, S.K.; Kirabo, A. Metabolic syndrome: Epidemiology, mechanisms, and current therapeutic approaches. Front. Nutr. 2025, 12, 1661603. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Ballestri, S.; Marchesini, G.; Angulo, P.; Loria, P. Nonalcoholic fatty liver disease: A precursor of the metabolic syndrome. Dig. Liver Dis. 2015, 47, 181–190. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Bousvarou, M.D.; Kostara, C.E.; Papakonstantinou, E.J.; Salamou, E.; Guzman, E. Insulin resistance and cardiovascular disease. J. Int. Med. Res. 2023, 51, 3000605231164548. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.K.; Bhimineni, C.; Modi, S.; Jahanshahi, A.; Quiza, K.; Bitetto, D. The Role of Insulin Resistance in Cancer. Curr. Oncol. 2025, 32, 477. [Google Scholar] [CrossRef] [PubMed]
- Nzobokela, J.; Muchaili, L.; Mwambungu, A.; Masenga, S.K.; Kirabo, A. Pathophysiology and emerging biomarkers of cardiovascular-renal-hepato-metabolic syndrome. Front. Cardiovasc. Med. 2025, 12, 1661563. [Google Scholar] [CrossRef]
- Lonardo, A. The heterogeneity of metabolic syndrome presentation and challenges this causes in its pharmacological management: A narrative review focusing on principal risk modifiers. Expert Rev. Clin. Pharmacol. 2023, 16, 891–911. [Google Scholar] [CrossRef]
- Abdullah, M.Y.; Alruwaili, Y.M.; Alsairra, M.N.; Almnaa, S.A.; Mosly, A.F.; Alsuwaidan, D.T.; Al Asmari, R.A.; Alsaffar, H.F.; Alhudaib, R.S.; Althobaiti, A.M.; et al. The Economic and Social Burden of Insulin Resistance in Obesity. J. Healthc. Sci. 2024, 4, JOHS2024000873. [Google Scholar] [CrossRef]
- Sandri, E.; Piredda, M.; Sguanci, M.; Mancin, S. What Factors Influence Obesity in Spain? A Multivariate Analysis of Sociodemographic, Nutritional, and Lifestyle Factors Affecting Body Mass Index in the Spanish Population. Healthcare 2025, 13, 386. [Google Scholar] [CrossRef]
- Freeman, A.M.; Acevedo, L.A.; Pennings, N. Insulin Resistance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507839/ (accessed on 17 August 2023).
- Bak, A.M.; Vendelbo, M.H.; Christensen, B.; Viggers, R.; Bibby, B.M.; Rungby, J.; Jørgensen, J.O.L.; Møller, N.; Jessen, N. Prolonged fasting-induced metabolic signatures in human skeletal muscle of lean and obese men. PLoS ONE 2018, 13, e0200817. [Google Scholar] [CrossRef]
- Flockhart, M.; Nilsson, L.C.; Tais, S.; Ekblom, B.; Apró, W.; Larsen, F.J. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab. 2021, 33, 957–970.e6. [Google Scholar] [CrossRef]
- Hannon, T.S.; Janosky, J.; Arslanian, S.A. Longitudinal Study of Physiologic Insulin Resistance and Metabolic Changes of Puberty. Pediatr. Res. 2006, 60, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Barbour, L.A.; McCurdy, C.E.; Hernandez, T.L.; Kirwan, J.P.; Catalano, P.M.; Friedman, J.E. Cellular Mechanisms for Insulin Resistance in Normal Pregnancy and Gestational Diabetes. Diabetes Care 2007, 30, S112–S119. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-Y.; Liu, C.-H.; Chen, F.-Y.; Kuo, C.-H.; Pitrone, P.; Liu, J.-S. Aging Affects Insulin Resistance, Insulin Secretion, and Glucose Effectiveness in Subjects with Normal Blood Glucose and Body Weight. Diagnostics 2023, 13, 2158. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Maleki, M.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Pathophysiology of Physical Inactivity-Dependent Insulin Resistance: A Theoretical Mechanistic Review Emphasizing Clinical Evidence. J. Diabetes Res. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Pinheiro, M.C.; Costa, H.E.; Mariana, M.; Cairrao, E. Sleep Deprivation and Its Impact on Insulin Resistance. Endocrines 2025, 6, 49. [Google Scholar] [CrossRef]
- Bergman, B.C.; Perreault, L.; Hunerdosse, D.; Kerege, A.; Playdon, M.; Samek, A.M.; Eckel, R.H. Novel and Reversible Mechanisms of Smoking-Induced Insulin Resistance in Humans. Diabetes 2012, 61, 3156–3166. [Google Scholar] [CrossRef]
- Al-Hassani, I.; Khan, N.A.; Elmenyar, E.; Al-Hassani, A.; Rizoli, S.; Al-Thani, H.; El-Menyar, A. The Interaction and Implication of Stress-Induced Hyperglycemia and Cytokine Release Following Traumatic Injury: A Structured Scoping Review. Diagnostics 2024, 14, 2649. [Google Scholar] [CrossRef]
- Gjessing, P.F.; Constantin-Teodosiu, D.; Hagve, M.; Lobo, D.N.; Revhaug, A.; Irtun, Ø. Preoperative carbohydrate supplementation attenuates post-surgery insulin resistance via reduced inflammatory inhibition of the insulin-mediated restraint on muscle pyruvate dehydrogenase kinase 4 expression. Clin. Nutr. 2015, 34, 1177–1183. [Google Scholar] [CrossRef]
- Kitabchi, A.E.; Umpierrez, G.E.; Miles, J.M.; Fisher, J.N. Hyperglycemic Crises in Adult Patients With Diabetes. Diabetes Care 2009, 32, 1335–1343. [Google Scholar] [CrossRef]
- Wu, Q.; Burley, G.; Li, L.; Lin, S.; Shi, Y. The role of dietary salt in metabolism and energy balance: Insights beyond cardiovascular disease. Diabetes, Obes. Metab. 2023, 25, 1147–1161. [Google Scholar] [CrossRef]
- Thomas, S.S.; Zhang, L.; Mitch, W.E. Molecular mechanisms of insulin resistance in chronic kidney disease. Kidney Int. 2015, 88, 1233–1239. [Google Scholar] [CrossRef]
- Clarembeau, F.; Bale, G.; Lanthier, N. Cirrhosis and insulin resistance: Current knowledge, pathophysiological mechanisms, complications and potential treatments. Clin. Sci. 2020, 134, 2117–2135. [Google Scholar] [CrossRef]
- Rivas, A.M.; Nugent, K. Hyperglycemia, Insulin, and Insulin Resistance in Sepsis. Am. J. Med Sci. 2021, 361, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.T.; Tassiopoulos, K.; Bosch, R.J.; Shikuma, C.; McComsey, G.A. Association Between Systemic Inflammation and Incident Diabetes in HIV-Infected Patients After Initiation of Antiretroviral Therapy. Diabetes Care 2010, 33, 2244–2249. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Papavassiliou, A.G. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol. Med. 2006, 12, 324–332. [Google Scholar] [CrossRef]
- Pivonello, R.; De Leo, M.; Vitale, P.; Cozzolino, A.; Simeoli, C.; De Martino, M.C.; Lombardi, G.; Colao, A. Pathophysiology of Diabetes Mellitus in Cushing’s Syndrome. Neuroendocrinology 2010, 92, 77–81. [Google Scholar] [CrossRef]
- Sharma, R.; Kopchick, J.J.; Puri, V.; Sharma, V.M. Effect of growth hormone on insulin signaling. Mol. Cell. Endocrinol. 2020, 518, 111038. [Google Scholar] [CrossRef] [PubMed]
- Vila, G.; Jørgensen, J.O.L.; Luger, A.; Stalla, G.K. Insulin Resistance in Patients With Acromegaly. Front. Endocrinol. 2019, 10, 509. [Google Scholar] [CrossRef]
- Beaupere, C.; Liboz, A.; Fève, B.; Blondeau, B.; Guillemain, G. Molecular Mechanisms of Glucocorticoid-Induced Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 623. [Google Scholar] [CrossRef]
- Fuhrmann, A.; Lopes, P.; Sereno, J.; Pedro, J.; Espinoza, D.; Pereira, M.; Reis, F.; Eriksson, J.; Carvalho, E. Molecular mechanisms underlying the effects of cyclosporin A and sirolimus on glucose and lipid metabolism in liver, skeletal muscle and adipose tissue in an in vivo rat model. Biochem. Pharmacol. 2014, 88, 216–228. [Google Scholar] [CrossRef]
- Kang, I.; Kim, S.-W.; Youn, J.H. Effects of Nicotinic Acid on Gene Expression: Potential Mechanisms and Implications for Wanted and Unwanted Effects of the Lipid-Lowering Drug. J. Clin. Endocrinol. Metab. 2011, 96, 3048–3055. [Google Scholar] [CrossRef]
- Yang, H.; Yang, L. Targeting cAMP/PKA pathway for glycemic control and type 2 diabetes therapy. J. Mol. Endocrinol. 2016, 57, R93–R108. [Google Scholar] [CrossRef]
- Rudich, A.; Ben-Romano, R.; Etzion, S.; Bashan, N. Cellular mechanisms of insulin resistance, lipodystrophy and atherosclerosis induced by HIV protease inhibitors. Acta Physiol. Scand. 2005, 183, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Isaac, R.; Boura-Halfon, S.; Gurevitch, D.; Shainskaya, A.; Levkovitz, Y.; Zick, Y. Selective serotonin reuptake inhibitors (SSRIs) inhibit insulin secretion and action in pancreatic β cells. J. Biol. Chem. 2018, 293, 4577–4578. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.-F.; Shao, R.; Chen, C.; Deng, C. Molecular Mechanisms of Antipsychotic Drug-Induced Diabetes. Front. Neurosci. 2017, 11, 643. [Google Scholar] [CrossRef]
- Burghardt, K.J.; Seyoum, B.; Mallisho, A.; Burghardt, P.R.; Kowluru, R.A.; Yi, Z. Atypical antipsychotics, insulin resistance and weight; a meta-analysis of healthy volunteer studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 83, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.-C.; Lin, H.-Y.; Sparks, J.D.; Yeh, S.; Chang, C. Androgen Receptor Roles in Insulin Resistance and Obesity in Males: The Linkage of Androgen-Deprivation Therapy to Metabolic Syndrome. Diabetes 2014, 63, 3180–3188. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, P.-H.; Freedland, S.J.; Chi, J.-T. Metabolic Response to Androgen Deprivation Therapy of Prostate Cancer. Cancers 2024, 16, 1991. [Google Scholar] [CrossRef]
- Laakso, M.; Silva, L.F. Statins and risk of type 2 diabetes: Mechanism and clinical implications. Front. Endocrinol. 2023, 14, 1239335. [Google Scholar] [CrossRef]
- Han, X.; Liu, J.; Gu, Y.; Li, Y.; Zhang, W.; Lv, N.; Dang, A. Diabetes Risks of Statin Therapy—Coenzyme Q10 May Help. Rev. Cardiovasc. Med. 2025, 26, 26437. [Google Scholar] [CrossRef]
- Rajamohan, H.H.; Boyer, P.-N.; Davis, T.; Lane, M.; Love, A. Exogenous Insulin Antibody Syndrome: A Rare Cause of Extreme Insulin Resistance Treated With High-dose Corticosteroids. Jcem Case Rep. 2025, 3. [Google Scholar] [CrossRef]
- Swarup, S.; Ahmed, I.; Grigorova, Y.; Zeltser, R. Metabolic Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459248/ (accessed on 7 March 2024).
- Amisi, C.A. Markers of insulin resistance in Polycystic ovary syndrome women: An update. World, J. Diabetes 2022, 13, 129–149. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Rasquin, L.I.; Anastasopoulou, C. Polycystic Ovarian Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459251/ (accessed on 7 July 2025).
- Madan, R.; Varghese, R.T.; Ranganath. Assessing Insulin Sensitivity and Resistance in Humans. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278954/ (accessed on 16 October 2024).
- Vaidya, R.A.; Desai, S.; Moitra, P.; Salis, S.; Agashe, S.; Battalwar, R.; Mehta, A.; Madan, J.; Kalita, S.; Udipi, S.A.; et al. Hyperinsulinemia: An early biomarker of metabolic dysfunction. Front. Clin. Diabetes Health 2023, 4, 1159664. [Google Scholar] [CrossRef]
- Jarvis, P.R.E.; Cardin, J.L.; Nisevich-Bede, P.M.; McCarter, J.P. Continuous glucose monitoring in a healthy population: Understanding the post-prandial glycemic response in individuals without diabetes mellitus. Metabolism 2023, 146, 155640. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Launico, M.V. Glucose Tolerance Test. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532915/ (accessed on 15 September 2025).
- Sun, Y.; Ji, H.; Sun, W.; An, X.; Lian, F. Triglyceride glucose (TyG) index: A promising biomarker for diagnosis and treatment of different diseases. Eur. J. Intern. Med. 2024, 131, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Budak, G.G.; Vatan, A.; Güçlü, E.; Karabay, O. Concordance between homeostatic model assessment and triglyceride glucose index in assessing insulin resistance among HIV-infected patients. Saudi Med. J. 2025, 46, 157–162. [Google Scholar] [CrossRef]
- Avagimyan, A.; Pogosova, N.; Fogacci, F.; Aghajanova, E.; Djndoyan, Z.; Patoulias, D.; Sasso, L.L.; Bernardi, M.; Faggiano, A.; Mohammadifard, N.; et al. Triglyceride-glucose index (TyG) as a novel biomarker in the era of cardiometabolic medicine. Int. J. Cardiol. 2025, 418, 132663, Erratum in Int. J. Cardiol. 2025, 421, 132907. https://doi.org/10.1016/j.ijcard.2024.132907. [Google Scholar] [CrossRef]
- Salazar, J.; Bermúdez, V.; Calvo, M.; Olivar, L.C.; Luzardo, E.; Navarro, C.; Mencia, H.; Cerda, M.; Graterol, M.; Graterol, R.; et al. Optimal cutoff for the evaluation of insulin resistance through triglyceride-glucose index: A cross-sectional study in a Venezuelan population. F1000Research 2017, 6, 1337. [Google Scholar] [CrossRef] [PubMed]
- de Cassia da Silva, C.; Zambon, M.P.; Vasques, A.C.J.; Camilo, D.F.; de Góes Monteiro Antonio, M.Â.R.; Geloneze, B. The threshold value for identifying insulin resistance (HOMA-IR) in an admixed adolescent population: A hyperglycemic clamp validated study. Arch. Endocrinol. Metab. 2023, 67, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Park, J.H.; Jang, E.-J.; Park, Y.-K.; Yu, J.M.; Park, J.-S.; Ahn, Y.; Choi, S.-H.; Yoo, H.J. The Cut-off Values of Surrogate Measures for Insulin Sensitivity in a Healthy Population in Korea according to the Korean National Health and Nutrition Examination Survey (KNHANES) 2007–2010. J. Korean Med. Sci. 2018, 33, e197. [Google Scholar] [CrossRef]
- Beaudry, M.; Bissonnette, S.; Lamantia, V.; Devaux, M.; Faraj, M. Sex-Specific Models to Predict Insulin Secretion and Sensitivity in Subjects with Overweight and Obesity. Int. J. Mol. Sci. 2023, 24, 6130. [Google Scholar] [CrossRef]
- Tam, C.S.; Xie, W.; Johnson, W.D.; Cefalu, W.T.; Redman, L.M.; Ravussin, E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care 2012, 35, 1605–1610. [Google Scholar] [CrossRef]
- Mari, A.; Pacini, G.; Brazzale, A.R.; Ahrén, B. Comparative evaluation of simple insulin sensitivity methods based on the oral glucose tolerance test. Diabetologia 2005, 48, 748–751. [Google Scholar] [CrossRef]
- Bo, T.; Gao, L.; Yao, Z.; Shao, S.; Wang, X.; Proud, C.G.; Zhao, J. Hepatic selective insulin resistance at the intersection of insulin signaling and metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2024, 36, 947–968. [Google Scholar] [CrossRef]
- Iglesias, P. The endocrine role of hepatokines: Implications for human health and disease. Front. Endocrinol. 2025, 16, 1663353. [Google Scholar] [CrossRef]
- Calcaterra, V.; Magenes, V.C.; Bianchi, A.; Rossi, V.; Gatti, A.; Marin, L.; Vandoni, M.; Zuccotti, G. How Can Promoting Skeletal Muscle Health and Exercise in Children and Adolescents Prevent Insulin Resistance and Type 2 Diabetes? Life 2024, 14, 1198. [Google Scholar] [CrossRef] [PubMed]
- Consitt, L.A.; Clark, B.C. The Vicious Cycle of Myostatin Signaling in Sarcopenic Obesity: Myostatin Role in Skeletal Muscle Growth, Insulin Signaling and Implications for Clinical Trials. J. Frailty Aging 2018, 7, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Schon, H.T.; Weiskirchen, R. Exercise-Induced Release of Pharmacologically Active Substances and Their Relevance for Therapy of Hepatic Injury. Front. Pharmacol. 2016, 7, 283. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Choi, W.; Woo, G.H.; Kwon, T.H.; Jeon, J.H. Obesity-Driven Metabolic Disorders: The Interplay of Inflammation and Mitochondrial Dysfunction. Int. J. Mol. Sci. 2025, 26, 9715. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The Role of SCAP/SREBP as Central Regulators of Lipid Metabolism in Hepatic Steatosis. Int. J. Mol. Sci. 2024, 25, 1109. [Google Scholar] [CrossRef] [PubMed]
- Masson, W.; Lobo, M.; Siniawski, D.; Huerín, M.; Molinero, G.; Valéro, R.; Nogueira, J.P. Therapy with cholesteryl ester transfer protein (CETP) inhibitors and diabetes risk. Diabetes Metab. 2018, 44, 508–513. [Google Scholar] [CrossRef]
- da Silva, A.A.; do Carmo, J.M.; Li, X.; Wang, Z.; Mouton, A.J.; Hall, J.E. Role of Hyperinsulinemia and Insulin Resistance in Hypertension: Metabolic Syndrome Revisited. Can. J. Cardiol. 2020, 36, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; McQueen, A.; Chen, T.C.; Wang, J.C. Regulation of Glucose Homeostasis by Glucocorticoids. Adv. Exp. Med. Biol. 2015, 872, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yao, H.; Sun, Z.; Wang, Y.; Zhao, Y.; Wang, Z.; Li, L. Role of NFAT in the Progression of Diabetic Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 635172. [Google Scholar] [CrossRef]
- Chakkera, H.A.; Mandarino, L.J. Calcineurin inhibition and new-onset diabetes mellitus after transplantation. Transplantation 2013, 95, 647–652. [Google Scholar] [CrossRef]
- Murata, H.; Hruz, P.W.; Mueckler, M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J. Biol. Chem. 2000, 275, 20251–20254. [Google Scholar] [CrossRef]
- Hertel, J.; Struthers, H.; Horj, C.B.; Hruz, P.W. A structural basis for the acute effects of HIV protease inhibitors on GLUT4 intrinsic activity. J. Biol. Chem. 2004, 279, 55147–55152. [Google Scholar] [CrossRef]
- Xu, H.; Zhuang, X. Atypical antipsychotics-induced metabolic syndrome and nonalcoholic fatty liver disease: A critical review. Neuropsychiatr. Dis. Treat. 2019, 15, 2087–2099. [Google Scholar] [CrossRef]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Rizi, E.P.; Teo, Y.; Leow, M.K.-S.; Khoo, E.Y.H.; Yeo, C.R.; Chan, E.; Song, T.; Sadananthan, S.A.; Velan, S.S.; Gluckman, P.D.; et al. Ethnic Differences in the Role of Adipocytokines Linking Abdominal Adiposity and Insulin Sensitivity Among Asians. J. Clin. Endocrinol. Metab. 2015, 100, 4249–4256, Erratum in J. Clin. Endocrinol. Metab. 2016, 101, 1887. https://doi.org/10.1210/jc.2016-1453. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chávez-Guevara, I.A.; Amaro-Gahete, F.J.; Osuna-Prieto, F.J.; Labayen, I.; Aguilera, C.M.; Ruiz, J.R. The role of sex in the relationship between fasting adipokines levels, maximal fat oxidation during exercise, and insulin resistance in young adults with excess adiposity. Biochem. Pharmacol. 2023, 216, 115757. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Lonardo, A. Sex Hormones and Metabolic Dysfunction-Associated Steatotic Liver Disease. Int. J. Mol. Sci. 2025, 26, 9594. [Google Scholar] [CrossRef]
- Alizadeh Pahlavani, H.; Laher, I.; Knechtle, B.; Zouhal, H. Exercise and mitochondrial mechanisms in patients with sarcopenia. Front. Physiol. 2022, 13, 1040381. [Google Scholar] [CrossRef]
- Raygor, V.; Abbasi, F.; Lazzeroni, L.C.; Kim, S.; Ingelsson, E.; Reaven, G.M.; Knowles, J.W. Impact of race/ethnicity on insulin resistance and hypertriglyceridaemia. Diab Vasc. Dis. Res. 2019, 16, 153–159. [Google Scholar] [CrossRef]
- Vasishta, S.; Ganesh, K.; Umakanth, S.; Joshi, M.B. Ethnic disparities attributed to the manifestation in and response to type 2 diabetes: Insights from metabolomics. Metabolomics 2022, 18, 45. [Google Scholar] [CrossRef]
- Palmer, A.K.; Jensen, M.D. Metabolic changes in aging humans: Current evidence and therapeutic strategies. J. Clin. Investig. 2022, 132, e158451. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Chen, P.J.; Xiao, W.H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol. Metab. Syndr. 2020, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Ciarambino, T.; Crispino, P.; Guarisco, G.; Giordano, M. Gender Differences in Insulin Resistance: New Knowledge and Perspectives. Curr. Issues Mol. Biol. 2023, 45, 7845–7861. [Google Scholar] [CrossRef]
- Lonardo, A.; Jamalinia, M.; Weiskirchen, R. Sex differences in MASLD. Scierxiv 2025, 2025110738. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, H.; Wang, T.; Yu, B.; Mao, S.; Wang, X.; Zhang, W.; Wang, L.; Zhang, Y.; Ren, Z.; et al. TyG index is positively associated with risk of CHD and coronary atherosclerosis severity among NAFLD patients. Cardiovasc. Diabetol. 2022, 21, 123. [Google Scholar] [CrossRef]
- El Assar, M.; Angulo, J.; Carnicero, J.A.; Molina-Baena, B.; García-García, F.J.; Sosa, P.; Rodríguez-Mañas, L. Gender-specific capacity of insulin resistance proxies to predict functional decline in older adults. J. Nutr. Health Aging 2024, 28, 100376. [Google Scholar] [CrossRef]
- Yao, J.; Wu, D.; Qiu, Y. Adipose tissue macrophage in obesity-associated metabolic diseases. Front. Immunol. 2022, 13, 977485. [Google Scholar] [CrossRef]
- Li, B.; Leung, J.C.K.; Chan, L.Y.Y.; Yiu, W.H.; Tang, S.C.W. A global perspective on the crosstalk between saturated fatty acids and Toll-like receptor 4 in the etiology of inflammation and insulin resistance. Prog. Lipid Res. 2020, 77, 101020. [Google Scholar] [CrossRef]
- Misceo, D.; Mocciaro, G.; D’Amore, S.; Vacca, M. Diverting hepatic lipid fluxes with lifestyles revision and pharmacological interventions as a strategy to tackle steatotic liver disease (SLD) and hepatocellular carcinoma (HCC). Nutr. Metab. 2024, 21, 112. [Google Scholar] [CrossRef]
- Pal, D.; Dasgupta, S.; Kundu, R.; Maitra, S.; Das, G.; Mukhopadhyay, S.; Ray, S.; Majumdar, S.S.; Bhattacharya, S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 2012, 18, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, R.; Thurmond, D.C. Mechanisms by Which Skeletal Muscle Myokines Ameliorate Insulin Resistance. Int. J. Mol. Sci. 2022, 23, 4636. [Google Scholar] [CrossRef]
- Crasan, I.-M.; Tanase, M.; Delia, C.E.; Gradisteanu-Pircalabioru, G.; Cimpean, A.; Ionica, E. Metaflammation’s Role in Systemic Dysfunction in Obesity: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 10445. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Kirichenko, T.V.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Varaeva, Y.R.; Starodubova, A.V. The Role of Adipokines in Inflammatory Mechanisms of Obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef]
- Janssen, J.A.M.J.L. The Pivotal Role of the Western Diet, Hyperinsulinemia, Ectopic Fat, and Diacylglycerol-Mediated Insulin Resistance in Type 2 Diabetes. Int. J. Mol. Sci. 2025, 26, 9191. [Google Scholar] [CrossRef]
- Hossain, I.A.; Akter, S.; Bhuiyan, F.R.; Shah, M.R.; Rahman, M.K.; Ali, L. Subclinical inflammation in relation to insulin resistance in prediabetic subjects with nonalcoholic fatty liver disease. BMC Res. Notes 2016, 9, 266. [Google Scholar] [CrossRef]
- Yan, Y.; Li, S.; Liu, Y.; Bazzano, L.; He, J.; Mi, J.; Chen, W. Temporal relationship between inflammation and insulin resistance and their joint effect on hyperglycemia: The Bogalusa Heart Study. Cardiovasc. Diabetol. 2019, 18, 109. [Google Scholar] [CrossRef]
- Symons, J.D.; McMillin, S.L.; Riehle, C.; Tanner, J.; Palionyte, M.; Hillas, E.; Jones, D.; Cooksey, R.C.; Birnbaum, M.J.; McClain, D.A.; et al. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ. Res. 2009, 104, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- De Pascali, F.; Hemann, C.; Samons, K.; Chen, C.A.; Zweier, J.L. Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and S-glutathionylation. Biochemistry 2014, 53, 3679–3688. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Lucke-Wold, B.P.; Li, X.; Logsdon, A.F.; Xu, L.-C.; Xu, S.; LaPenna, K.B.; Wang, H.; Talukder, M.A.H.; Siedlecki, C.A.; et al. Reduction of Endothelial Nitric Oxide Increases the Adhesiveness of Constitutive Endothelial Membrane ICAM-1 through Src-Mediated Phosphorylation. Front. Physiol. 2018, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; RKashyap, S.; AMajor, J.; Silverstein, R.L. Insulin promotes macrophage foam cell formation: Potential implications in diabetes-related atherosclerosis. Lab. Investig. 2012, 92, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Cerf, M.E. Cardiac Glucolipotoxicity and Cardiovascular Outcomes. Medicina 2018, 54, 70. [Google Scholar] [CrossRef] [PubMed]
- Sorop, O.; van de Wouw, J.; Chandler, S.; Ohanyan, V.; Tune, J.D.; Chilian, W.M.; Merkus, D.; Bender, S.B.; Duncker, D.J. Experimental animal models of coronary microvascular dysfunction. Cardiovasc. Res. 2020, 116, 756–770. [Google Scholar] [CrossRef]
- D’aMario, D.; Migliaro, S.; Borovac, J.A.; Restivo, A.; Vergallo, R.; Galli, M.; Leone, A.M.; Montone, R.A.; Niccoli, G.; Aspromonte, N.; et al. Microvascular Dysfunction in Heart Failure With Preserved Ejection Fraction. Front. Physiol. 2019, 10, 1347. [Google Scholar] [CrossRef]
- Thorp, A.A.; Schlaich, M.P. Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J. Diabetes Res. 2015, 2015, 341583. [Google Scholar] [CrossRef]
- Shan, P.F.; Li, Q.; Khamaisi, M.; Qiang, G.F. Type 2 Diabetes Mellitus and Macrovascular Complications. Int. J. Endocrinol. 2017, 2017, 4301461. [Google Scholar] [CrossRef]
- Di Pino, A.; DeFronzo, R.A. Insulin Resistance and Atherosclerosis: Implications for Insulin-Sensitizing Agents. Endocr. Rev. 2019, 40, 1447–1467. [Google Scholar] [CrossRef]
- Salvia, M.G. The Look AHEAD Trial: Translating Lessons Learned Into Clinical Practice and Further Study. Diabetes Spectr. 2017, 30, 166–170. [Google Scholar] [CrossRef]
- Balducci, S.; Sacchetti, M.; Haxhi, J.; Orlando, G.; Zanuso, S.; Cardelli, P.; Cavallo, S.; D’errico, V.; Ribaudo, M.C.; Di Biase, N.; et al. The Italian Diabetes and Exercise Study 2 (IDES-2): A long-term behavioral intervention for adoption and maintenance of a physically active lifestyle. Trials 2015, 16, 569. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Marx, N.; Marx-Schütt, K. Cardiovascular Risk Reduction in Patients with Type 2 Diabetes: What Does the Cardiologist Need to Know? Eur. Cardiol. 2025, 20, e09. [Google Scholar] [CrossRef]
- English, W.J.; Spann, M.D.; Aher, C.V.; Williams, D.B. Cardiovascular risk reduction following metabolic and bariatric surgery. Ann. Transl. Med. 2020, 8, S12. [Google Scholar] [CrossRef]
- Joseph, J.J.; Deedwania, P.; Acharya, T.; Aguilar, D.; Bhatt, D.L.; Chyun, D.A.; Di Palo, K.E.; Golden, S.H.; Sperling, L.S. Comprehensive Management of Cardiovascular Risk Factors for Adults With Type 2 Diabetes: A Scientific Statement From the American Heart Association. Circulation 2022, 145, e722–e759. [Google Scholar] [CrossRef] [PubMed]
- Prentki, M.; Peyot, M.L.; Masiello, P.; Madiraju, S.R.M. Nutrient-Induced Metabolic Stress, Adaptation, Detoxification, and Toxicity in the Pancreatic β-Cell. Diabetes 2020, 69, 279–290. [Google Scholar] [CrossRef]
- Yu, T.; Robotham, J.L.; Yoon, Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. USA 2006, 103, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 diabetes mellitus in adults: Pathogenesis, prevention and therapy. Signal Transduct. Target. Ther. 2024, 9, 262. [Google Scholar] [CrossRef]
- Tang, S.P.; Mao, X.L.; Chen, Y.H.; Yan, L.L.; Ye, L.P.; Li, S.W. Reactive Oxygen Species Induce Fatty Liver and Ischemia-Reperfusion Injury by Promoting Inflammation and Cell Death. Front. Immunol. 2022, 13, 870239. [Google Scholar] [CrossRef]
- Menendez-Montes, I.; Abdisalaam, S.; Xiao, F.; Lam, N.T.; Mukherjee, S.; Szweda, L.I.; Asaithamby, A.; Sadek, H.A. Mitochondrial fatty acid utilization increases chromatin oxidative stress in cardiomyocytes. Proc. Natl. Acad. Sci. USA 2021, 118, e2101674118. [Google Scholar] [CrossRef]
- Mackesy, D.Z.; Goalstone, M.L. Insulin augments tumor necrosis factor-alpha stimulated expression of vascular cell adhesion molecule-1 in vascular endothelial cells. J. Inflamm. 2011, 8, 34. [Google Scholar] [CrossRef]
- Kasuga, M. Insulin resistance and pancreatic beta cell failure. J. Clin. Investig. 2006, 116, 1756–1760. [Google Scholar] [CrossRef]
- Tanday, N.; Tarasov, A.I.; Moffett, R.C.; Flatt, P.R.; Irwin, N. Pancreatic islet cell plasticity: Pathogenic or therapeutically exploitable? Diabetes Obes. Metab. 2024, 26, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Gloyn, A.L.; Ibberson, M.; Marchetti, P.; Powers, A.C.; Rorsman, P.; Sander, M.; Solimena, M. Every islet matters: Improving the impact of human islet research. Nat. Metab. 2022, 4, 970–977, Erratum in Nat. Metab. 2024, 6, 1415. https://doi.org/10.1038/s42255-024-01091-y. [Google Scholar] [CrossRef]
- Prentki, M.; Nolan, C.J. Islet beta cell failure in type 2 diabetes. J. Clin. Investig. 2006, 116, 1802–1812. [Google Scholar] [CrossRef]
- Alejandro, E.U.; Gregg, B.; Blandino-Rosano, M.; Cras-Méneur, C.; Bernal-Mizrachi, E. Natural history of β-cell adaptation and failure in type 2 diabetes. Mol. Asp. Med. 2015, 42, 19–41. [Google Scholar] [CrossRef]
- Lonardo, A.; Weiskirchen, R. Liver and obesity: A narrative review. Explor. Med. 2025, 6, 1001334. [Google Scholar] [CrossRef]
- Lonardo, A.; Weiskirchen, R. PPARs in molecular pathogenesis and drug treatment of type 2 diabetes-related MASLD. Explor. Dig. Dis. 2025, 4, 100590. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Weiss, N.S.; Kowdley, K.V.; Dominitz, J.A. Is obesity a risk factor for cirrhosis-related death or hospitalization? A population-based cohort study. Gastroenterology 2003, 125, 1053–1059. [Google Scholar] [CrossRef]
- Ahn, S.B.; Powell, E.E.; Russell, A.; Hartel, G.; Irvine, K.M.; Moser, C.; Valery, P.C. Type 2 Diabetes: A Risk Factor for Hospital Readmissions and Mortality in Australian Patients With Cirrhosis. Hepatol. Commun. 2020, 4, 1279–1292. [Google Scholar] [CrossRef]
- Lugari, S.; Baldelli, E.; Lonardo, A. Metabolic primary liver cancer in adults: Risk factors and pathogenic mechanisms. Metab. Target Organ Damage 2023, 3, 5. [Google Scholar] [CrossRef]
- Pinto, K.R.D.; Feckinghaus, C.M.; Hirakata, V.N. Obesity as a predictive factor for chronic kidney disease in adults: Systematic review and meta-analysis. Braz. J. Med. Biol. Res. 2021, 54, e10022. [Google Scholar] [CrossRef] [PubMed]
- Fenta, E.T.; Eshetu, H.B.; Kebede, N.; Bogale, E.K.; Zewdie, A.; Kassie, T.D.; Anagaw, T.F.; Mazengia, E.M.; Gelaw, S.S. Prevalence and predictors of chronic kidney disease among type 2 diabetic patients worldwide, systematic review and meta-analysis. Diabetol. Metab. Syndr. 2023, 15, 245. [Google Scholar] [CrossRef]
- Lonardo, A.; Mantovani, A.; Targher, G.; Baffy, G. Nonalcoholic Fatty Liver Disease and Chronic Kidney Disease: Epidemiology, Pathogenesis, and Clinical and Research Implications. Int. J. Mol. Sci. 2022, 23, 13320. [Google Scholar] [CrossRef]
- Lonardo, A. Association of NAFLD/NASH, and MAFLD/MASLD with chronic kidney disease: An updated narrative review. Metab. Target Organ Damage 2024, 4, 16. [Google Scholar] [CrossRef]
- Yoon, J.H.; Hwang, J.; Son, S.U.; Choi, J.; You, S.W.; Park, H.; Cha, S.Y.; Maeng, S. How Can. Insulin Resistance Cause Alzheimer’s Disease? Int. J. Mol. Sci. 2023, 24, 3506. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Huang, W.; Wang, Z.; Zhang, D.; Qiao, H.; Chen, N.; Ni, X.; Cheng, J.; Ni, W. Insulin resistance surrogates predict major adverse cardiovascular events in patients with heart failure with preserved ejection fraction and chronic kidney disease: A retrospective cohort study. Lipids Health Dis. 2025, 24, 349. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Jiang, R.; Xu, D.; Zhu, J.; Chen, J.; Lin, Y.; Zhou, H. Association between insulin resistance indices and outcomes in patients with heart failure with preserved ejection fraction. Cardiovasc. Diabetol. 2025, 24, 32. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016, 65, 589–600. [Google Scholar] [CrossRef]
- Jamalinia, M.; Zare, F.; Lonardo, A. Liver Fibrosis and Risk of Incident Dementia in the General Population: Systematic Review With Meta-Analysis. Health Sci. Rep. 2025, 8, e71530. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fazio, S.; Bellavite, P.; Affuso, F. Chronically Increased Levels of Circulating Insulin Secondary to Insulin Resistance: A Silent Killer. Biomedicines 2024, 12, 2416. [Google Scholar] [CrossRef]
- Stanciu, S.M.; Jinga, M.; Miricescu, D.; Stefani, C.; Nica, R.I.; Stanescu-Spinu, I.I.; Vacaroiu, I.A.; Greabu, M.; Nica, S. mTOR Dysregulation, Insulin Resistance, and Hypertension. Biomedicines 2024, 12, 1802. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Nestler, J.E.; Powers, L.P.; Matt, D.W.; Steingold, K.A.; Plymate, S.R.; Rittmaster, R.S.; Clore, J.N.; Blackard, W.G. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1991, 72, 83–89. [Google Scholar] [CrossRef]
- Schrijnders, D.; Hendriks, S.H.; Kleefstra, N.; Vissers, P.A.J.; Johnson, J.A.; de Bock, G.H.; Bilo, H.J.G.; Landman, G.W.D. Sex differences in obesity related cancer incidence in relation to type 2 diabetes diagnosis (ZODIAC-49). PLoS ONE 2018, 13, e0190870. [Google Scholar] [CrossRef]
- Nigam, M.; Punia, B.; Dimri, D.B.; Mishra, A.P.; Radu, A.F.; Bungau, G. Reactive Oxygen Species: A Double-Edged Sword in the Modulation of Cancer Signaling Pathway Dynamics. Cells 2025, 14, 1207. [Google Scholar] [CrossRef]
- Milani, I.; Codini, M.; Guarisco, G.; Chinucci, M.; Gaita, C.; Leonetti, F.; Capoccia, D. Hepatokines and MASLD: The GLP1-Ras-FGF21-Fetuin-A Crosstalk as a Therapeutic Target. Int. J. Mol. Sci. 2024, 25, 10795. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Pottanat, T.G.; Siegel, R.W.; Ehsani, M.; Qian, Y.-W.; Zhen, E.Y.; Regmi, A.; Roell, W.C.; Guo, H.; Luo, M.J.; et al. Angiopoietin-like protein 8 differentially regulates ANGPTL3 and ANGPTL4 during postprandial partitioning of fatty acids. J. Lipid Res. 2020, 61, 1203–1220. [Google Scholar] [CrossRef]
- Garstka, M.A.; Kedzierski, L.; Maj, T. Diabetes can impact cellular immunity in solid tumors. Trends Immunol. 2025, 46, 295–309. [Google Scholar] [CrossRef]
- Lonardo, F. Cancer in individuals with obesity and metabolic disorders. A preventable epidemic? Metab. Target Organ Damage 2022, 2, 20. [Google Scholar] [CrossRef]
- Pan, S.Y.; Johnson, K.C.; Ugnat, A.M.; Wen, S.W.; Mao, Y.; Canadian Cancer Registries Epidemiology Research Group. Association of obesity and cancer risk in Canada. Am. J. Epidemiol. 2004, 159, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Abonyi-Tóth, Z.; Rokszin, G.; Sütő, G.; Fábián, I.; Kiss, Z.; Jermendy, G.; Kempler, P.; Lengyel, C.; Wittmann, I.; Molnár, G.A. Incident Cancer Risk of Patients with Prevalent Type 2 Diabetes Mellitus in Hungary (Part 2). Cancers 2024, 16, 2414. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A. Is liver fibrosis a risk factor for gynecological cancers? Metab. Target Organ Damage 2024, 4, 7. [Google Scholar] [CrossRef]
- Mantovani, A.; Lonardo, A.; Stefan, N.; Targher, G. Metabolic dysfunction-associated steatotic liver disease and extrahepatic gastrointestinal cancers. Metabolism 2024, 160, 156014. [Google Scholar] [CrossRef]
- Lonardo, A.; Stefan, N.; Mantovani, A. Widening research horizons on metabolic dysfunction-associated steatotic liver disease and cancer. Trends Endocrinol. Metab. 2025, 36, 610–613. [Google Scholar] [CrossRef]
- Wu, C.; Targher, G.; Byrne, C.D.; Mao, Y.; Cheung, T.T.; Yilmaz, Y.; Valenti, L.; Méndez-Sánchez, N.; Sookoian, S.; Chan, W.-K.; et al. Global, Regional, and National Burden of Primary Liver Cancer Attributable to Metabolic Risks: An Analysis of the Global Burden of Disease Study 1990–2021. Am. J. Gastroenterol. 2025, 120, 2280–2290. [Google Scholar] [CrossRef]
- Bril, F.; Elbert, A. Metabolic dysfunction-associated steatotic liver disease and urinary system cancers: Mere coincidence or reason for concern? Metabolism 2025, 162, 156066. [Google Scholar] [CrossRef]
- Schenk, S.; Harber, M.P.; Shrivastava, C.R.; Burant, C.F.; Horowitz, J.F. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. J. Physiol. 2009, 587 Pt 20, 4949–4961. [Google Scholar] [CrossRef]
- Flynn, C.R.; Tamboli, R.A.; Antoun, J.; Sidani, R.M.; Williams, B.; Spann, M.D.; English, W.J.; Welch, E.B.; Sundaresan, S.; Abumrad, N.N. Caloric Restriction and Weight Loss Are Primary Factors in the Early Tissue-Specific Metabolic Changes After Bariatric Surgery. Diabetes Care 2022, 45, 1914–1916. [Google Scholar] [CrossRef] [PubMed]
- Pareek, M.; Schauer, P.R.; Kaplan, L.M.; Leiter, L.A.; Rubino, F.; Bhatt, D.L. Metabolic Surgery: Weight Loss, Diabetes, and Beyond. J. Am. Coll. Cardiol. 2018, 71, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, S.J.; Gershuni, V.M.; Hazbun, T.L.; Athinarayanan, S.J. Reversing Type 2 Diabetes: A Narrative Review of the Evidence. Nutrients 2019, 11, 766. [Google Scholar] [CrossRef] [PubMed]
- Ram Sohan, P.; Mahakalkar, C.; Kshirsagar, S.; Bikkumalla, S.; Reddy, S.; Hatewar, A.; Dixit, S. Long-Term Effectiveness and Outcomes of Bariatric Surgery: A Comprehensive Review of Current Evidence and Emerging Trends. Cureus 2024, 16, e66500. [Google Scholar] [CrossRef]
- Herman, R.; Kravos, N.A.; Jensterle, M.; Janež, A.; Dolžan, V. Metformin and Insulin Resistance: A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. Int. J. Mol. Sci. 2022, 23, 1264. [Google Scholar] [CrossRef]
- Mesquita, L.A.; Spiazzi, B.F.; Piccoli, G.F.; Nogara, D.A.; da Natividade, G.R.; Garbin, H.I.; Wayerbacher, L.F.; Wiercinski, V.M.; Baggio, V.A.; Zingano, C.P.; et al. Does metformin reduce the risk of cancer in obesity and diabetes? A systematic review and meta-analysis. Diabetes Obes. Metab. 2024, 26, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Said, A.; Akhter, A. Meta-Analysis of Randomized Controlled Trials of Pharmacologic Agents in Non-alcoholic Steatohepatitis. Ann. Hepatol. 2017, 16, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Eggleton, J.S.; Jialal, I. Thiazolidinediones. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551656/ (accessed on 20 February 2023).
- Weiskirchen, R.; Lonardo, A. How ‘miracle’ weight-loss semaglutide promises to change medicine but can we afford the expense? Br. J. Pharmacol. 2025, 182, 1651–1670. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Lonardo, A. Semaglutide from Bench to Bedside: The Experimental Journey Towards a Transformative Therapy for Diabetes, Obesity and Metabolic Liver Disorders. Med. Sci. 2025, 13, 265. [Google Scholar] [CrossRef]
- Karim, G.; Bansal, M.B. Resmetirom: An Orally Administered, Smallmolecule, Liver-directed, β-selective THR Agonist for the Treatment of Non-alcoholic Fatty Liver Disease and Non-alcoholic Steatohepatitis. touchREV. Endocrinol. 2023, 19, 60–70. [Google Scholar] [CrossRef]
- Tiwari, A.; Sharma, A.; Kumar, H.; Gupta, V.; Deshpande, V.; Mupparaju, J.S.; Mishra, T.; Bharadwaj, H.R.; Dahiya, D.S.; Jain, V. Resmetirom for MASH: A Comprehensive Review of a Novel Therapeutic Frontier. Biomedicines 2025, 13, 2079. [Google Scholar] [CrossRef]
- Shakeel, L.; Shaukat, A.; Akilimali, A. Resmetirom: A Breakthrough in the Treatment of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Health Sci. Rep. 2025, 8, e70920. [Google Scholar] [CrossRef]
- Lonardo, A.; Weiskirchen, R. Tirzepatide in metabolic dysfunction-associated steatotic liver disease and steatohepatitis: A novel star on the horizon? Explor. Drug Sci. 2025, 3, 1008128. [Google Scholar] [CrossRef]
- Lonardo, A.; Zheng, M.H.; Weiskirchen, R. Food insecurity is an emerging risk factor for liver disease: A scoping review. Expert Rev. Gastroenterol. Hepatol. 2025, 19, 1033–1049. [Google Scholar] [CrossRef]
- Hill-Briggs, F.; Adler, N.E.; Berkowitz, S.A.; Chin, M.H.; Gary-Webb, T.L.; Navas-Acien, A.; Thornton, P.L.; Haire-Joshu, D. Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care 2020, 44, 258–279. [Google Scholar] [CrossRef]
- Lonardo, A.; Bellentani, S.; Ratziu, V.; Loria, P. Insulin resistance in nonalcoholic steatohepatitis: Necessary but not sufficient—Death of a dogma from analysis of therapeutic studies? Expert Rev. Gastroenterol. Hepatol. 2011, 5, 279–289. [Google Scholar] [CrossRef] [PubMed]


| Model | Mechanisms | Metabolic Effects | Ref. | Comment | |
|---|---|---|---|---|---|
| Physiological | |||||
| Prolonged fasting | Lean and obese men. | Markedly increased levels of β-hydroxybutyrate and β- hydroxybutyrylcarnitine in skeletal muscle. | Substrate competition due to increased metabolism of ketone bodies rather than oxidation of long-chain fatty acids. | [22] | Prolonged fasting should be avoided to prevent IR. |
| Strenuous exercise | Healthy volunteers. | Mitochondrial functional impairment | Impaired glucose tolerance | [23] | Excessive exercise training should be avoided to prevent IR. |
| Puberty | Healthy, normal-weight, prepubertal children. | Adiponectin levels were halved, partially mediated by increased GH secretion. | Increased total body lipolysis and decreased glucose oxidation. | [24] | Balanced diet and non-strenuous physical activity may be a valuable approach for preventing puberty-related IR. |
| Pregnancy | Pregnant and GDM women during the third trimester. | hPGH increases the expression of the p85α subunit of PI3K in skeletal muscle, which, in turn, acts as a dominant-negative competitor to forming a PI3K heterodimer with the p110 subunit, thus inhibiting the PI3K activity and attenuating the downstream insulin signaling. | Increased risk of GDM | [25] | Balanced diet and moderate physical activity should be suggested for preventing IR in pregnancy. |
| Aging | Individuals with normal FPG and BMI values. | Age is negatively related to IR and GE in both sexes. | The risk of T2D increases with age. | [26] | Normal weight should be maintained during ageing with appropriate lifestyle habits to prevent age-related IR. |
| Pathological | |||||
| Obesity | Humans | Ectopic accumulation of lipotoxic intermediates such as DAGs, Ceramides, and LPA. | Obesity is a major risk factor for incident T2D. | [27] | Obesity should be treated with appropriate lifestyle habits, drug treatment and bariatric surgery whenever indicated. |
| Physical inactivity | Humans | Impaired muscle glucose uptake, altered lipid metabolism, oxidative stress, inflammation, and endothelial dysfunction | Sedentary behavior is a major risk factor for incident T2D. | [28] | Sedentary behavior should be discouraged at the general population level through appropriate public health policies. |
| Altered sleep–wake cycle | Healthy non-obese subjects | Reduced β-cell secretory response and increased NEFAs. | Sleep deprivation is a risk factor for an increase incidence of T2D. | [29] | Healthy sleep habits should be promoted as part of public health strategies for preventing T2D. |
| Smoking | Muscle cell culture. | Nicotine induces IR in skeletal muscle by activating mTOR. | Tobacco smoking increases the risk of T2D. | [30] | Public health campaigns and specific tax policies should be implemented to discourage smoking. |
| Stressful conditions | |||||
| Trauma | SIH individuals compared to NDN, DN, and DH patients. | SIH patients had elevated IL-6 concentrations relative to NDN, DN, and DH patients. | SIH is linked to higher mortality in trauma. | [31] | Prompt treatment of SIH is a rational approach to reduce mortality in the context of major trauma. |
| Surgery | Postoperative IR results from muscle inflammation and reduced suppression of FOXO1-driven PDH kinase-4 mRNA and protein expression after surgery. | Reduced oxidation of glucose, resulting in impaired glucose uptake in muscle. | [32] | Preoperative carbohydrate supplementation may limit muscle inflammation while improving the inhibition of PDH kinase-4 activity mediated by insulin. | |
| DKA | Adult patients with diabetes. | Absolute insulin deficiency, increased counter-regulatory hormones, and a surge in pro-inflammatory cytokines. | Increased lipolysis determines the release of FFAs from the adipose tissue into the bloodstream and unrestrained hepatic fatty acid oxidation in the liver leads to ketone bodies | [33] | Key steps to prevent DKA include consistently monitoring glycemic levels, adhering to prescribed antidiabetic medications, assessing for ketones (if blood glucose exceeds 250 mg/dL or during illness) and maintenance of adequate hydration status. |
| High-sodium diets | Humans | Overproduction of fructose and ghrelin, leptin resistance and IR, reduced circulating levels of adiponectin and GLP-1. These may eventually facilitate obesity development via increased food intake and expanded WAT. | High-salt intake is closely linked to CVD, especially HTN and, as suggested by emerging evidence, also to metabolic disorders. | [34] | Limiting salt intake is an important recommendation to promote cardiovascular health. |
| Organ failure | |||||
| Uremia | Humans with CKD of variable severity. | Uremic toxins disrupt insulin signaling, reducing glucose uptake even with normal insulin, due to inflammation, oxidative stress, and inhibitory molecules that degrade proteins like IRS-1, ultimately impairing glucose metabolism. | IR, commonly observed among individuals with ESRD, also occurs in CKD patients with minimally increased creatinine serum levels. | [35] | Treating IR in CKD is critical to slowing the progression to ESRD, reducing the odds of CVD, and decreasing the risks of fluid/electrolyte imbalances and infections. To achieve this goal, it is recommended to manage underlying mineral bone disorders, enhance dialysis adequacy, implement appropriate lifestyle modifications, and consider the use of medications such as ACE inhibitors. |
| Liver cirrhosis | Humans | Hyperinsulinemia results from impaired muscular glucose uptake and liver dysfunction eventually causing defective glucose storage and defective signaling in peripheral tissues. | The primary contributing factors are inflammation, lipotoxins, sarcopenia, intestinal dysbiosis, and chronic hyperinsulinemia. | [36] | Correcting IR in cirrhosis is crucial to prevent complications such as HCC, infections, CKD, and decrease mortality. The therapeutic strategy involves diet and exercise, alongside medications such as (cautiously) Metformin, TZDs, DPP-4i, and GLP-1RA, with liver transplantation often restoring NGT. |
| Infections | |||||
| Sepsis | Humans | Inhibition of tyrosine kinase activity in the beta subunit, enhanced proteolytic activity leading to receptor loss from the plasma membrane, and the potential translocation of insulin receptors into the nucleus, where they may associate with gene promoters. | In sepsis, hyperglycemia is caused by impaired insulin receptor function, decreased GLUT4 translocation, activation of inflammatory JNK1 signaling, increased lipolysis, reduced tissue glucose uptake, and elevated hepatic glucose production. | [37] | Correcting hyperglycemia in sepsis is crucial to prevent impaired immune responses, reduced WBC functionality, organ dysfunction, and the increased risk of mortality due to metabolic stress and systemic inflammation. |
| HIV | Humans | Macrophages present within adipose tissue secrete inflammatory cytokines such as TNF-alpha and IL-6 that are likely mediators of IR in HIV infection. | HIV individuals are exposed to the risk of T2D | [38] | The management of IR in HIV-infected individuals involves dietary modifications, regular physical activity, and pharmacological interventions. Available drugs include Metformin (which requires careful monitoring with some ART agents), medications to reduce visceral fat, and possibly TZDs, DPP-4i, or angiotensin receptor blockers. Specific ARTs that may cause IR should also be addressed. |
| Endocrinological | |||||
| PCOS | Humans | IR in PCOS arises due to a shift from normal tyrosine phosphorylation to impaired serine phosphorylation of the insulin receptor and IRS proteins, thereby disrupting the PI3K/Akt pathway essential for glucose uptake. | IR in PCOS disrupts glucose and lipid metabolism, resulting in hyperinsulinemia. This further stimulates increased androgen production contributing to menstrual irregularities and infertility, while also significantly elevating the risk for T2D, MetS, MASLD, and CVD. | [39] | Dietary modifications, physical activity, weight reduction, and inositol supplementation—with or without insulin-sensitizing medications—are implemented to improve insulin sensitivity, decrease hyperandrogenism, restore ovulatory function, and address metabolic dysfunction. |
| Cushing’s syndrome | Humans | IR owing to hyper-cortisolemia results from blocked GLUT4 translocation, inhibited glycogen synthase, increased hepatic gluconeogenesis, and stimulated lipolysis that further impairs insulin action through high FFAs levels. | Cushing’s syndrome causes a wide spectrum of metabolic derangements due to excess cortisol, primarily manifesting as MetS. | [40] | Managing IR in Cushing’s syndrome involves addressing the root cause, hyper-cortisolemia, with medications like metyrapone or mifepristone, alongside lifestyle changes such as diet, exercise, and weight loss. Specific diabetes drugs should be used to lower glycemia, with lifestyle adjustments being crucial for long-term prevention and management. |
| Acromegaly | Humans | An excess of GH antagonizes insulin action at the level of liver, skeletal muscle, and adipose tissue via multiple pathways, most prominently by enhancing lipolysis and disrupting insulin signaling at the post-receptor level. | Acromegaly leads to a variety of cardiometabolic disorders, including HTN, IR, T2D atherogenic dyslipidemia OSA and accelerated CVD. Driven by GH and IGF-1, these conditions raise the risk of heart failure, arrhythmias and early cardiovascular death. | [41] | Treating IR in acromegaly involves targeting the underlying GH excess through surgery or medications to normalize GH/IGF-1 levels, which significantly improves glucose metabolism. Additionally, standard diabetes management is crucial, with pegvisomant offering unique benefits in improving IR irrespective of weight loss [42]. |
| Drug-induced | |||||
| Long-term glucocorticoids | Humans, animal models and cell culture studies | Enhanced gluconeogenesis and endogenous glucose production. | Glucocorticoids induce hyperglycemia, glucose intolerance and steroid-induced diabetes, weight gain with deleterious fat redistribution, and increased levels of circulating FFAs, sarcopenia, and osteoporosis | [43] | The prevention and management of glucocorticoid-induced IR primarily focus on dietary modifications, regular physical activity, and insulin-sensitizing drugs. |
| Cyclosporine A, Syrolimus | In vivo rat model. | Reduced expression of genes (IRS-1, Glut4, and Glut1), which are associated with insulin action and glucose uptake and upregulation of genes and/or proteins involved in hepatic lipogenesis and gluconeogenesis, accompanied by a decrease in these factors in adipose tissue. | Cyclosporin A and sirolimus are immunosuppressive agents that have been linked to the development of dyslipidemia, IR, and new-onset diabetes following transplantation. | [44] | Maintaining a healthy lifestyle, avoiding high-risk medications, and controlling glucose early help preserve insulin sensitivity in individuals undergoing immunosuppressive pharmacotherapy. |
| Niacin (nicotinic acid; vitamin B3) | Rats | Niacin primarily determines IR through activation of its receptor GPR109A, which reduces insulin signaling in adipose tissue by downregulating IRS-1, PDE3B, and β-adrenergic receptors, thereby decreasing lipolysis. In pancreatic beta-cells, GPR109A activation increases ROS, raising UCP2 and PPARγ, and impairs glucose-stimulated insulin secretion. | Niacin, while vital for energy metabolism, DNA repair and improving atherogenic dysplidemia, also increases glycemic levels, causes flushing, and promotes inflammation through its breakdown products, making its therapeutic use controversial when taken in excess. | [45] | The combination of niacin with regular exercise may potentially improve glycemic control, lower FFAs and lessen negative effects on insulin sensitivity. |
| Beta-adrenergic blocking agents | Mammalian cells | Prolonged activation of the cAMP/PKA pathway can disrupt insulin signaling due to negative crosstalk mechanisms, including PKA-mediated serine phosphorylation of both the IRS and Akt. This process inhibits PI3K/Akt-dependent glucose uptake by preventing GLUT4 translocation. | Beta-blockers induce a diverse metabolic spectrum, primarily by slowing metabolism, impairing glucose/lipid mobilization, potentially causing weight gain/body fat increase, and blunting hypoglycemia symptoms, though carvedilol may offer benefits like reduced IR and antioxidant effects. | [46] | Use vasodilating beta-blockers like carvedilol, monitor glucose and lipids, adjust diet or medications as needed, educate about hypoglycemia, and consider SGLT-2i or GLP-1RAs for high-risk patients. Avoid stopping treatment abruptly. |
| Protease inhibitors | Humans | Acute inhibition of GLUT4-mediated glucose transport, and defective insulin signaling account for IR whereas interference with adipogenesis and adipocyte apoptosis and activation of lipolysis are potential mechanisms of drug-induced lipodystrophy among individuals exposed to Protease inhibitors. | HIV Protease inhibitors are associated with the pathogenesis of IR, dyslipidaemia, lipodystrophy and atherosclerosis, described with highly active ART therapy. | [47] | Preventing and managing HIV protease inhibitors involves maintaining healthy diet, regular physical activity, weight reduction when necessary, carefully selecting ART therapy, and consistently monitoring glycemic values. |
| SRRI | Mouse model. | SSRIs can cause IR by disrupting insulin signaling, mainly through kinase activation that phosphorylates IRS proteins. This impairs insulin function, reduces glucose-stimulated insulin secretion from pancreatic beta cells, and may trigger ER stress and beta-cell apoptosis, contributing to T2D. | SSRI use may cause metabolic changes, mainly due to weight gain from increased appetite, which can contribute to MetS, though clinical impact varies among patients. | [48] | To prevent and manage SSRI-related metabolic dysfunction, monitoring body weight, lipids, and glucose s is important. It is also recommended to follow a healthy diet and exercise regimen, consider SSRIs with lower metabolic risks, and adjust treatment as needed under medical supervision. |
| Atypical antipsychotics | Humans | Atypical antipsychotics can cause IR by directly interfering with insulin signaling in myocytes, adipocytes, and hepatocytes. | Atypical antipsychotics promote weight gain that harms pancreatic beta-cells and disrupt neurotransmitter systems involved in glucose and appetite regulation, which may lead to hyperglycemia and MetS. | [49] | The longer the duration of treatment, the higher the risk of developing IR [50]. |
| Androgen-deprivation therapy | Mouse models | Global deletion of androgen receptor in male mice results in the characteristics of MetS featuring IR owing to reduced ability of insulin to stimulate activation of downstream PI3K in skeletal muscle and liver. Leptin resistance was also demonstrated with deregulated food intake and increased body weight. | Anti-androgenic therapy leads to sarcopenic obesity, which increases the risk of IR, hyperglycemia, T2D, atherogenic dyslipidemia, CVD, and osteoporosis-related fractures. | [51,52] | To prevent and treat IR from anti-androgen therapy, diet, exercise, and weight loss should be prioritized while considering insulin-sensitizing medications in combination with anti-androgens for high-risk individuals to mitigate metabolic risks linked to hypotestosteronemia. |
| Statins | Mouse model | Statin-induced IR results from disruption of the mevalonate pathway, impairing insulin signaling in liver, muscle, and pancreatic cells; it can also affect lipid metabolism, mitochondrial function, gluconeogenesis, and inflammation. | Statin therapy can cause metabolic dysfunction, including IR, new-onset diabetes, muscle complaints, often linked to lipophilic statins and high doses, affecting CoQ10 levels and gut microbiota, though benefits usually outweigh risks for cardiovascular health | [53] | CoQ10 supplementation in individuals taking statins is associated with a reduced risk of NOD, independent of the CoQ10 dose [54]. |
| Insulin therapy | Humans | Exogenous insulin antibody syndrome is a rare cause of extreme IR. | Exogenous insulin therapy, while vital, introduces its own metabolic risks, comprising hypoglycemia, weight gain, fluid retention, and exacerbating underlying IR. A spectrum of dysmetabolic features will potentially ensue (e.g., dyslipidemia, inflammation, HTN, localized lipodystrophy, and increased CVD risk). | [55] | Treatment with high-dose methylprednisolone and mycophenolate mofetil, followed by a tapering regimen of prednisone, resulted in a significant improvement in a reported case of extreme IR [55]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lonardo, A.; Weiskirchen, R. Insulin Resistance at the Crossroads of Metabolic Inflammation, Cardiovascular Disease, Organ Failure, and Cancer. Biomolecules 2025, 15, 1745. https://doi.org/10.3390/biom15121745
Lonardo A, Weiskirchen R. Insulin Resistance at the Crossroads of Metabolic Inflammation, Cardiovascular Disease, Organ Failure, and Cancer. Biomolecules. 2025; 15(12):1745. https://doi.org/10.3390/biom15121745
Chicago/Turabian StyleLonardo, Amedeo, and Ralf Weiskirchen. 2025. "Insulin Resistance at the Crossroads of Metabolic Inflammation, Cardiovascular Disease, Organ Failure, and Cancer" Biomolecules 15, no. 12: 1745. https://doi.org/10.3390/biom15121745
APA StyleLonardo, A., & Weiskirchen, R. (2025). Insulin Resistance at the Crossroads of Metabolic Inflammation, Cardiovascular Disease, Organ Failure, and Cancer. Biomolecules, 15(12), 1745. https://doi.org/10.3390/biom15121745








