Influence of Stress-Induced Senescence on the Secretome of Primary Mesenchymal Stromal Cells
Abstract
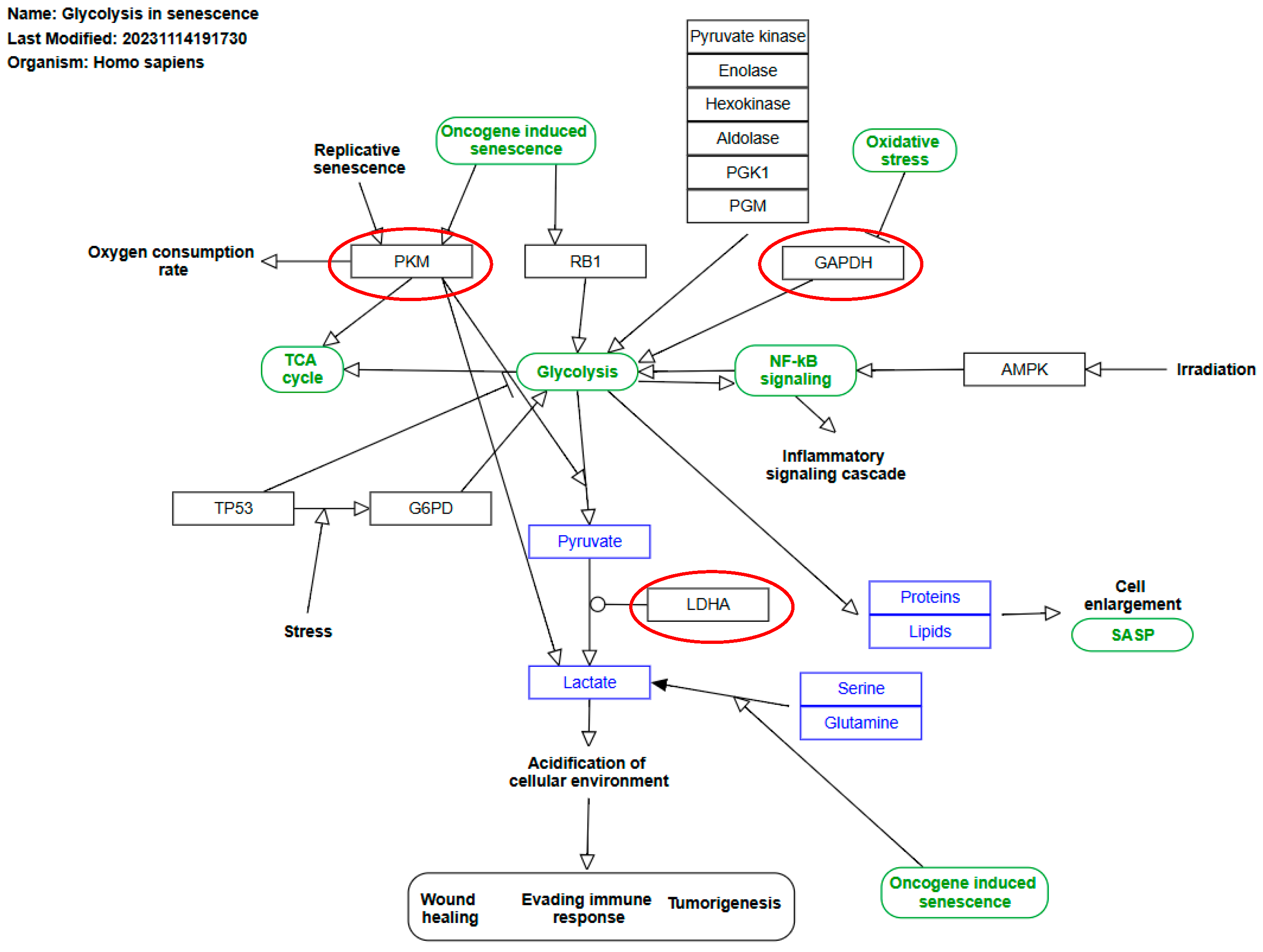
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Senescence Induction
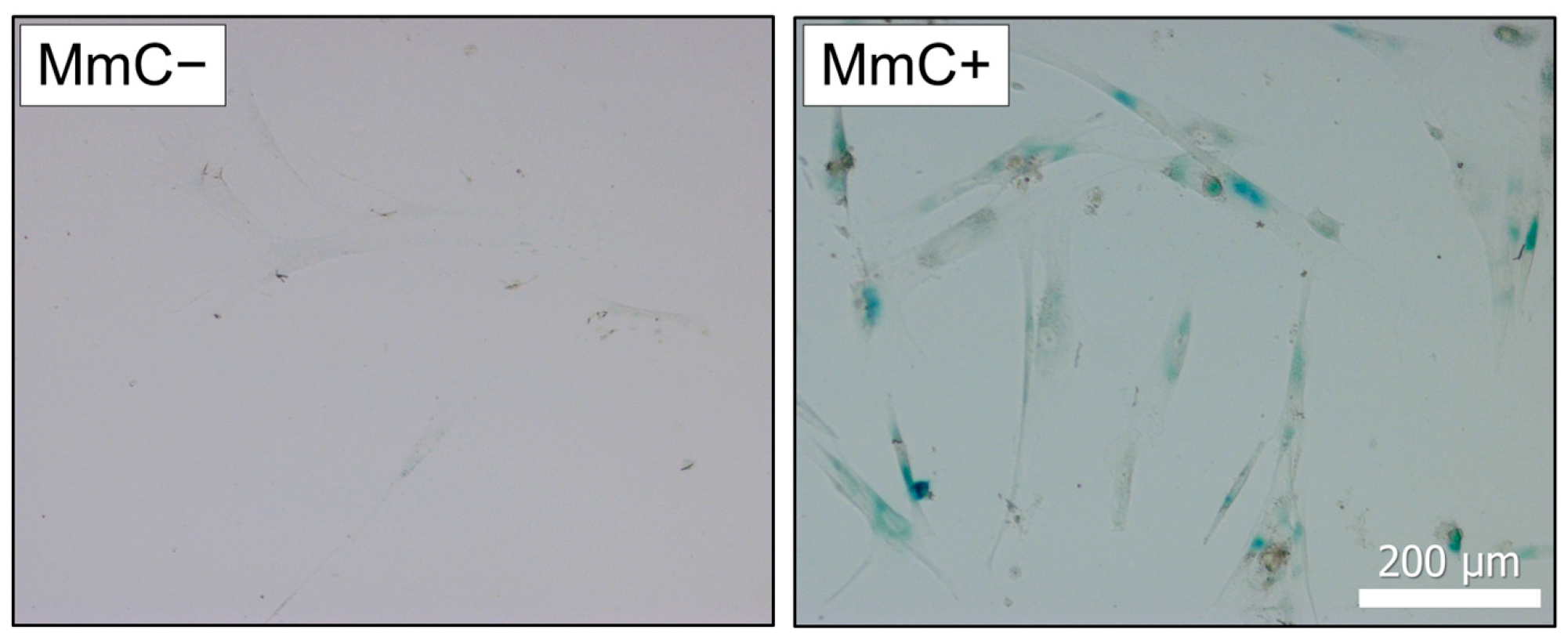
2.2. Senescence-Associated β-Galactosidase (SA-β-Gal) Staining
2.3. Sample Preparation for Proteomic Analysis
2.4. LC-MS/MS Proteomic Analysis
2.5. ELISA and Quantitative PCR Analysis
2.6. Data Processing and Bioinformatics
3. Results
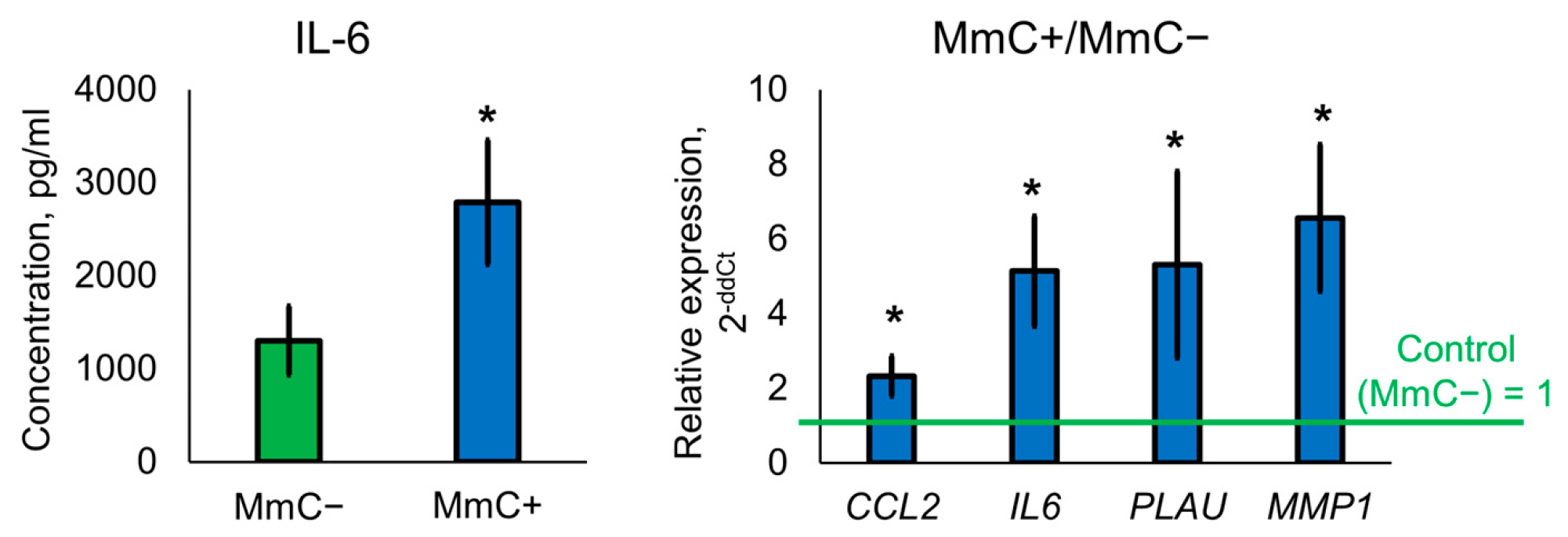
3.1. Induction of Cellular Senescence
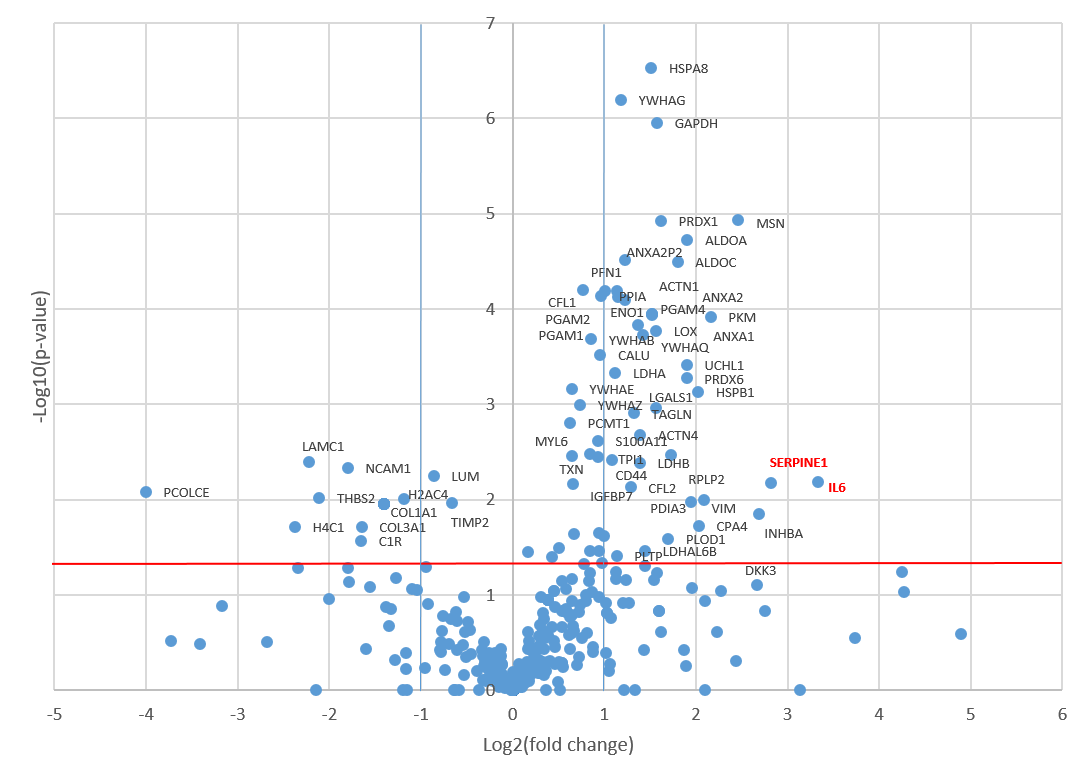
3.2. Proteomic Profiling of the Senescent MSC Secretome
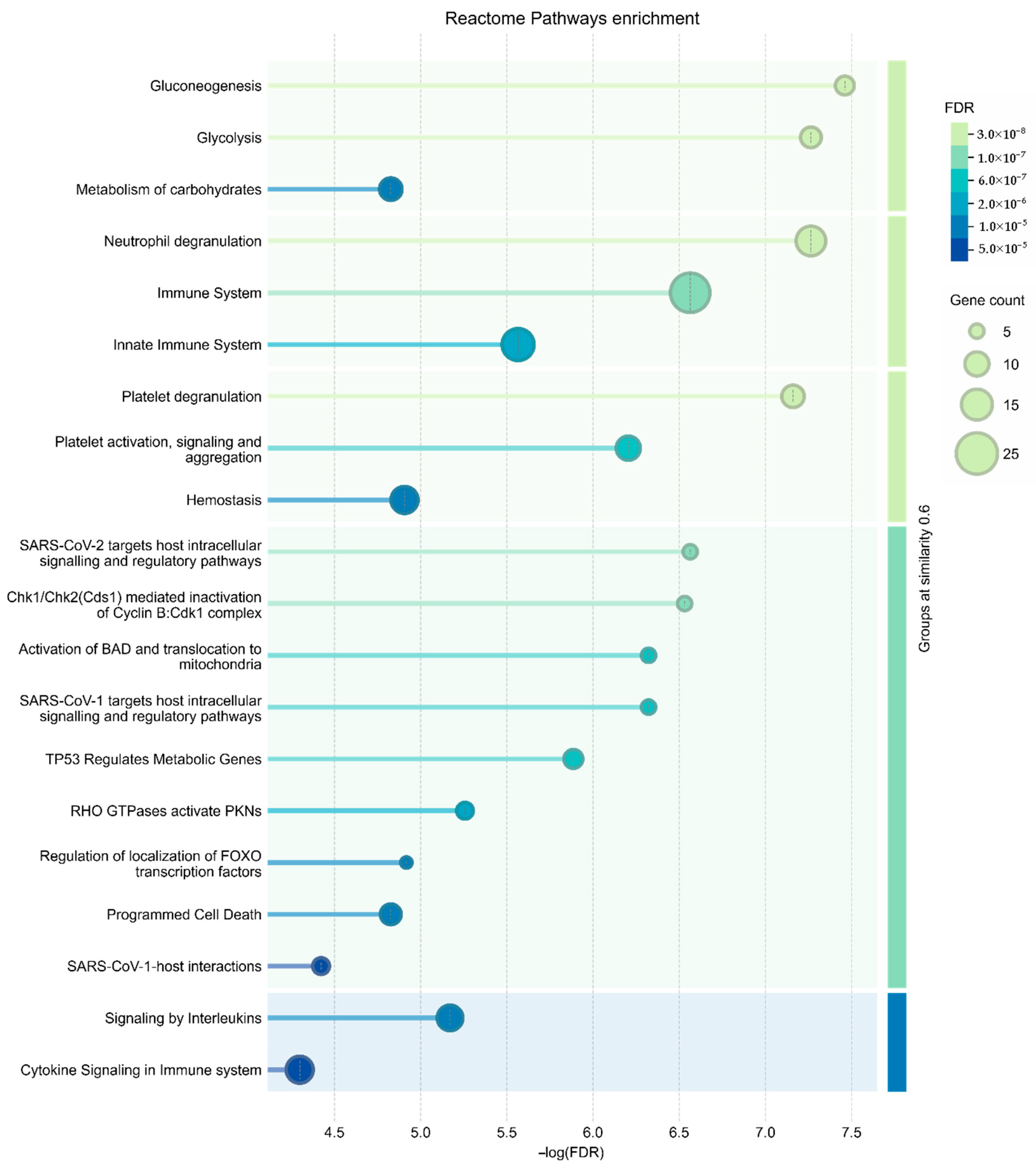
3.3. Functional Annotation of the Senescent MSC Secretome
3.4. Analysis of Proteins Downregulated in the Senescent MSC Secretome
3.5. ELISA and qPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraile, M.; Eiro, N.; Costa, L.A.; Martín, A.; Vizoso, F.J. Aging and Mesenchymal Stem Cells: Basic Concepts, Challenges and Strategies. Biology 2022, 11, 1678. [Google Scholar] [CrossRef]
- Gudiño, V.; Salas, A. Promise of mesenchymal stem cell lysates in IBD therapy: Are the parts greater than the whole? Dig. Dis. Sci. 2021, 66, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Infante, A.; Rodríguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. 2018, 9, 244. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, R.X.; He, X.T.; Xu, X.Y.; Wang, J.; Chen, F.M. Influences of age-related changes in mesenchymal stem cells on macrophages during in-vitro culture. Stem Cell Res. 2017, 8, 153. [Google Scholar] [CrossRef]
- Siraj, Y.; Galderisi, U.; Alessio, N. Senescence induces fundamental changes in the secretome of mesenchymal stromal cells (MSCs): Implications for the therapeutic use of MSCs and their derivates. Front. Bioeng. Biotechnol. 2023, 11, 1148761. [Google Scholar] [CrossRef]
- McKenna, E.; Traganos, F.; Zhao, H.; Darzynkiewicz, Z. Persistent DNA damage caused by low levels of mitomycin C induces irreversible cell senescence. Cell Cycle 2012, 11, 3132–3140. [Google Scholar] [CrossRef] [PubMed]
- Alili, L.; Diekmann, J.; Giesen, M.; Holtkötter, O.; Brenneisen, P. A drug-induced accelerated senescence (DIAS) is a possibility to study aging in time lapse. Age 2014, 36, 9658. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kang, M.; Yang, Y.; Zhang, H.; Zhang, Y.; Wu, Y.; Denslin, V.; Othman, R.B.; Yang, Z.; Han, J. Comparative analysis of serum and serum-free medium cultured mesenchymal stromal cells for cartilage repair. Int. J. Mol. Sci. 2024, 25, 10627. [Google Scholar] [CrossRef]
- Zwerschke, W.; Mazurek, S.; Stockl, P.; Hutter, E.; Eigenbrodt, E.; Jansen-Durr, P. Metabolic analysis of senescent human fibroblasts reveals a role for AMP in cellular senescence. Biochem. J. 2003, 376 Pt 2, 403–411. [Google Scholar] [CrossRef]
- Wiley, C.D.; Campisi, J. From ancient pathways to aging cells-connecting metabolism and cellular senescence. Cell Metab. 2016, 23, 1013–1021. [Google Scholar] [CrossRef]
- Sabbatinelli, J.; Prattichizzo, F.; Olivieri, F.; Procopio, A.D.; Rippo, M.R.; Giuliani, A. Where Metabolism Meets Senescence: Focus on Endothelial Cells. Front. Physiol. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Nacarelli, T.; Sell, C. Targeting metabolism in cellular senescence, a role for intervention. Mol. Cell Endocrinol. 2017, 455, 83–92. [Google Scholar] [CrossRef]
- Jiang, P.; Du, W.; Mancuso, A.; Wellen, K.E.; Yang, X. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature 2013, 493, 689–693. [Google Scholar] [CrossRef]
- Abbas, T.; Olivier, M.; Lopez, J.; Houser, S.; Xiao, G.; Kumar, G.S.; Tomasz, M.; Bargonetti, J. Differential activation of p53 by the various adducts of mitomycin C. J. Biol. Chem. 2002, 277, 40513–40519. [Google Scholar] [CrossRef]
- Liu, B.; Wang, C.; Weng, Z.; Yang, Y.; Zhao, H.; Zhang, Y.; Fei, Q.; Shi, Y.; Zhang, C. Glycolytic enzyme PKM2 regulates cell senescence but not inflammation in the process of osteoarthritis. Acta Biochim. Biophys. Sin. 2023, 55, 1425–1433. [Google Scholar] [CrossRef]
- Guillamat-Prats, R. Role of Mesenchymal Stem/Stromal Cells in Coagulation. Int. J. Mol. Sci. 2022, 23, 10393. [Google Scholar] [CrossRef] [PubMed]
- Prišlin, M.; Butorac, A.; Bertoša, R.; Kunić, V.; Ljolje, I.; Kostešić, P.; Vlahović, D.; Naletilić, Š.; Turk, N.; Brnić, D. In vitro aging alters the gene expression and secretome composition of canine adipose-derived mesenchymal stem cells. Front. Vet. Sci. 2024, 11, 1387174. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.E. PAI-1 and TGF-β: Unmasking the real driver of TGF-β-induced vascular pathology. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 679–680. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, A.D.; Albornoz, F.; Griffin, J.P.; Crandall, D.L.; Elokdah, H.; Fogo, A.B.; Vaughan, D.E.; Brown, N.J. Pharmacological inhibition and genetic deficiency of PAI-1 attenuates angiotensin II/salt-induced aortic remodeling. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 365–371. [Google Scholar] [CrossRef]
- Samarakoon, R.; Higgins, S.P.; Higgins, C.E.; Higgins, P.J. The TGF-β1/p53/PAI-1 Signaling Axis in Vascular Senescence: Role of Caveolin-1. Biomolecules 2019, 9, 341. [Google Scholar] [CrossRef]
- Liu, J.; Si, Z.; Liu, J.; Zhang, X.; Xie, C.; Zhao, W.; Wang, A.; Xia, Z. Machine learning identifies novel coagulation genes as diagnostic and immunological biomarkers in ischemic stroke. Aging 2024, 16, 6314–6333. [Google Scholar] [CrossRef]
- Cann, K.L.; Hicks, G.G. Regulation of the cellular DNA double-strand break response. Biochem. Cell Biol. 2007, 85, 663–674. [Google Scholar] [CrossRef]
- Ortiz-Montero, P.; Londono-Vallejo, A.; Vernot, J.P. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun. Signal. 2017, 15, 17. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and age-related diseases: Role of inflammation triggers and cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Özcan, S.; Alessio, N.; Acar, M.B.; Mert, E.; Omerli, F.; Peluso, G.; Galderisi, U. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging 2016, 8, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Valentijn, F.A.; Falke, L.L.; Nguyen, T.Q.; Goldschmeding, R. Cellular senescence in the aging and diseased kidney. J. Cell Commun. Signal. 2018, 12, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, D.; Kashirina, D.; Ezdakova, M.; Larina, I.; Buravkova, L.; Ratushnyy, A. Senescence-Associated Alterations in Matrisome of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2024, 25, 5332. [Google Scholar] [CrossRef]
- Kortlever, R.M.; Higgins, P.J.; Bernards, R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nature 2006, 8, 877–884. [Google Scholar] [CrossRef]
- Elzi, D.J.; Lai, Y.; Song, M.; Hakala, K.; Weintraub, S.T.; Shiio, Y. Plasminogen activator inhibitor 1—Insulin-like growth factor binding protein 3 cascade regulates stress-induced senescence. Proc. Natl. Acad. Sci. USA 2012, 109, 12052–12057. [Google Scholar] [CrossRef]
- Hiebert, P.; Wietecha, M.S.; Cangkrama, M.; Haertel, E.; Mavrogonatou, E.; Stumpe, M.; Steenbock, H.; Grossi, S.; Beer, H.-D.; Angel, P.; et al. Nrf2-Mediated Fibroblast Reprogramming Drives Cellular Senescence by Targeting the Matrisome. Dev. Cell 2018, 46, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Siraj, Y.; Aprile, D.; Alessio, N.; Peluso, G.; Di Bernardo, G.; Galderisi, U. IGFBP7 is a key component of the senescence-associated secretory phenotype (SASP) that induces senescence in healthy cells by modulating the insulin, IGF, and activin A pathways. Cell Commun. Signal. 2024, 22, 540. [Google Scholar] [CrossRef] [PubMed]
- Yodoi, J.; Matsuo, Y.; Tian, H.; Masutani, H.; Inamoto, T. Anti-Inflammatory Thioredoxin Family Proteins for Medicare, Healthcare and Aging Care. Nutrients 2017, 9, 1081. [Google Scholar] [CrossRef]
- Guillén, M.I.; Tofiño-Vian, M.; Silvestre, A.; Castejón, M.A.; Alcaraz, M.J. Role of peroxiredoxin 6 in the chondroprotective effects of microvesicles from human adipose tissue-derived mesenchymal stem cells. J. Orthop. Transl. 2021, 30, 61–69. [Google Scholar] [CrossRef]
- van Genderen, H.O.; Kenis, H.; Hofstra, L.; Narula, J.; Reutelingsperger, C.P. Extracellular annexin A5: Functions of phosphatidylserine-binding and two-dimensional crystallization. Biochim. Biophys. Acta 2008, 1783, 953–963. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Jiang, C.; Zheng, M.; Han, M.; Fang, Q.; Liu, Y.; Li, R.; Zhong, L.; Li, Z. ANXA2 promotes osteogenic differentiation and inhibits cellular senescence of periodontal ligament cells (PDLCs) in high glucose conditions. PeerJ 2024, 12, e18064. [Google Scholar] [CrossRef] [PubMed]
- Doshi, B.M.; Hightower, L.E.; Lee, J. HSPB1, actin filament dynamics, and aging cells. Ann. N. Y. Acad. Sci. 2010, 1197, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi-Hotta, M.; Hasegawa, S.; Hasebe, Y.; Inoue, Y.; Okuno, R.; Arima, M.; Iwata, Y.; Sugiura, K.; Akamatsu, H. Increase in inhibin beta A/Activin-A expression in the human epidermis and the suppression of epidermal stem/progenitor cell proliferation with aging. J. Dermatol. Sci. 2022, 106, 150–158. [Google Scholar] [CrossRef]
- Chen, S.; Gong, Y.; Shen, Y.; Liu, Y.; Fu, Y.; Dai, Y.; Rehman, A.U.; Tang, L.; Liu, H. INHBA is a novel mediator regulating cellular senescence and immune evasion in colorectal cancer. J. Cancer 2021, 12, 5938–5949. [Google Scholar] [CrossRef]
- Mor-Vaknin, N.; Punturieri, A.; Sitwala, K.; Markovitz, D.M. Vimentin is secreted by activated macrophages. Nat. Cell Biol. 2003, 5, 59–63. [Google Scholar] [CrossRef]
- Muñoz-Descalzo, S.; de Navascues, J.; Arias, A.M. Wnt-Notch signalling: An integrated mechanism regulating transitions between cell states. Bioessays 2012, 34, 110–118. [Google Scholar] [CrossRef]
- Moll, G.; Ankrum, J.A.; Olson, S.D.; Nolta, J.A. Improved MSC Minimal Criteria to Maximize Patient Safety: A Call to Embrace Tissue Factor and Hemocompatibility Assessment of MSC Products. Stem Cells Transl. Med. 2022, 11, 2–13. [Google Scholar] [CrossRef]
- George, M.J.; Prabhakara, K.; Toledano-Furman, N.E.; Wang, Y.W.; Gill, B.S.; Wade, C.E.; Olson, S.D.; Cox, C.S., Jr. Clinical Cellular Therapeutics Accelerate Clot Formation. Stem Cells Transl. Med. 2018, 7, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Samsonraj, R.M.; Law, S.F.; Chandra, A.; Pignolo, R.J. An unbiased proteomics approach to identify the senescence-associated secretory phenotype of human bone marrow-derived mesenchymal stem cells. Bone Rep. 2023, 18, 101674. [Google Scholar] [CrossRef] [PubMed]
- Marote, A.; Santos, D.; Mendes-Pinheiro, B.; Serre-Miranda, C.; Anjo, S.I.; Vieira, J.; Ferreira-Antunes, F.; Correia, J.S.; Borges-Pereira, C.; Pinho, A.G.; et al. Cellular Aging Secretes: A Comparison of Bone-Marrow-Derived and Induced Mesenchymal Stem Cells and Their Secretome Over Long-Term Culture. Stem Cell Rev. Rep. 2023, 19, 248–263. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashirina, D.; Matveeva, D.; Ezdakova, M.; Brzhozovskiy, A.; Kononikhin, A.; Pastushkova, L.; Larina, I.; Nikolaev, E.; Buravkova, L.; Ratushnyy, A. Influence of Stress-Induced Senescence on the Secretome of Primary Mesenchymal Stromal Cells. Biomolecules 2025, 15, 1734. https://doi.org/10.3390/biom15121734
Kashirina D, Matveeva D, Ezdakova M, Brzhozovskiy A, Kononikhin A, Pastushkova L, Larina I, Nikolaev E, Buravkova L, Ratushnyy A. Influence of Stress-Induced Senescence on the Secretome of Primary Mesenchymal Stromal Cells. Biomolecules. 2025; 15(12):1734. https://doi.org/10.3390/biom15121734
Chicago/Turabian StyleKashirina, Daria, Diana Matveeva, Mariia Ezdakova, Alexander Brzhozovskiy, Alexey Kononikhin, Ludmila Pastushkova, Irina Larina, Evgeny Nikolaev, Ludmila Buravkova, and Andrey Ratushnyy. 2025. "Influence of Stress-Induced Senescence on the Secretome of Primary Mesenchymal Stromal Cells" Biomolecules 15, no. 12: 1734. https://doi.org/10.3390/biom15121734
APA StyleKashirina, D., Matveeva, D., Ezdakova, M., Brzhozovskiy, A., Kononikhin, A., Pastushkova, L., Larina, I., Nikolaev, E., Buravkova, L., & Ratushnyy, A. (2025). Influence of Stress-Induced Senescence on the Secretome of Primary Mesenchymal Stromal Cells. Biomolecules, 15(12), 1734. https://doi.org/10.3390/biom15121734







