Embryological Divergence and Molecular Mechanisms in Thoracic and Abdominal Aortic Aneurysms: Bridging Developmental Biology and Clinical Insights
Abstract
1. Introduction
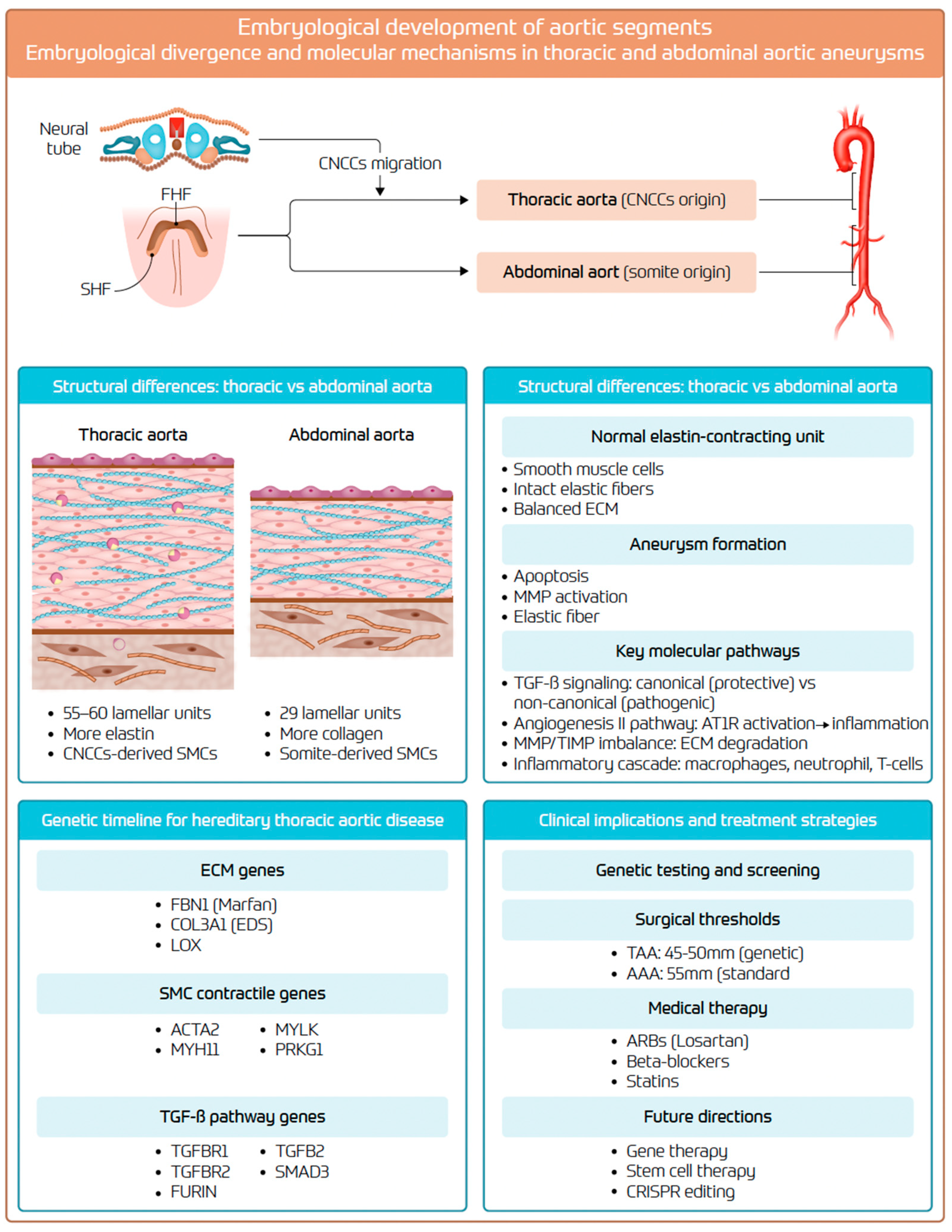
2. Embryological Development and Structural Differences
2.1. Embryological Development and Organogenesis
2.2. Aortic Wall: Structural and Histological Differences
3. Molecular and Cellular Mechanisms of Aneurysm Formation
3.1. Endothelial Cell Dysfunction
3.2. Smooth-Muscle-Cell Apoptosis and Phenotypic Switching
3.3. Pro-Inflammatory Cells
3.4. MMPs and Tissue Inhibitors of MMPs
3.5. AT-II Pathway
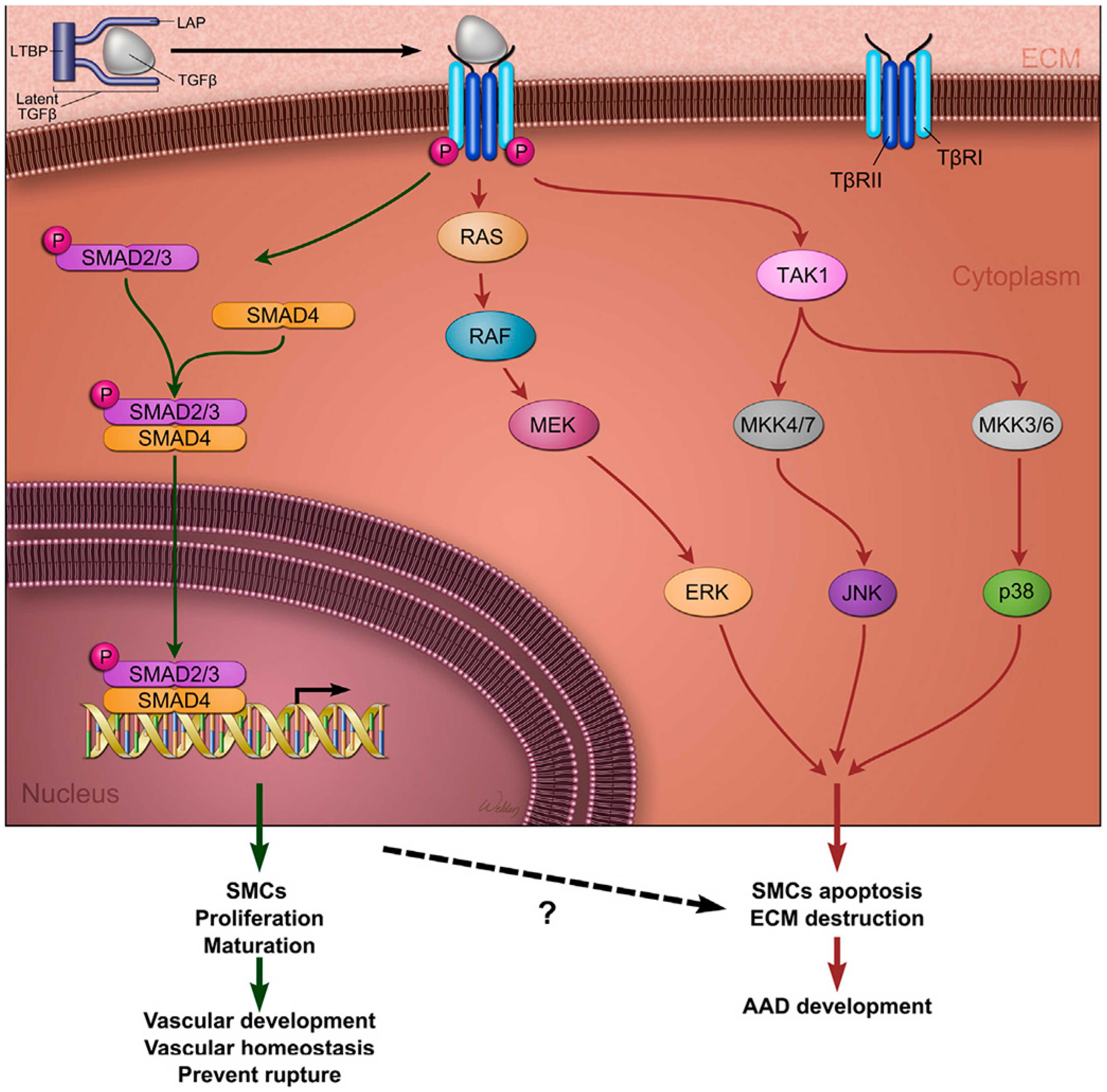
3.6. TGF-β Pathway
3.7. Atherosclerosis-Driven Inflammation
3.8. MicroRNAs (miRNAs)
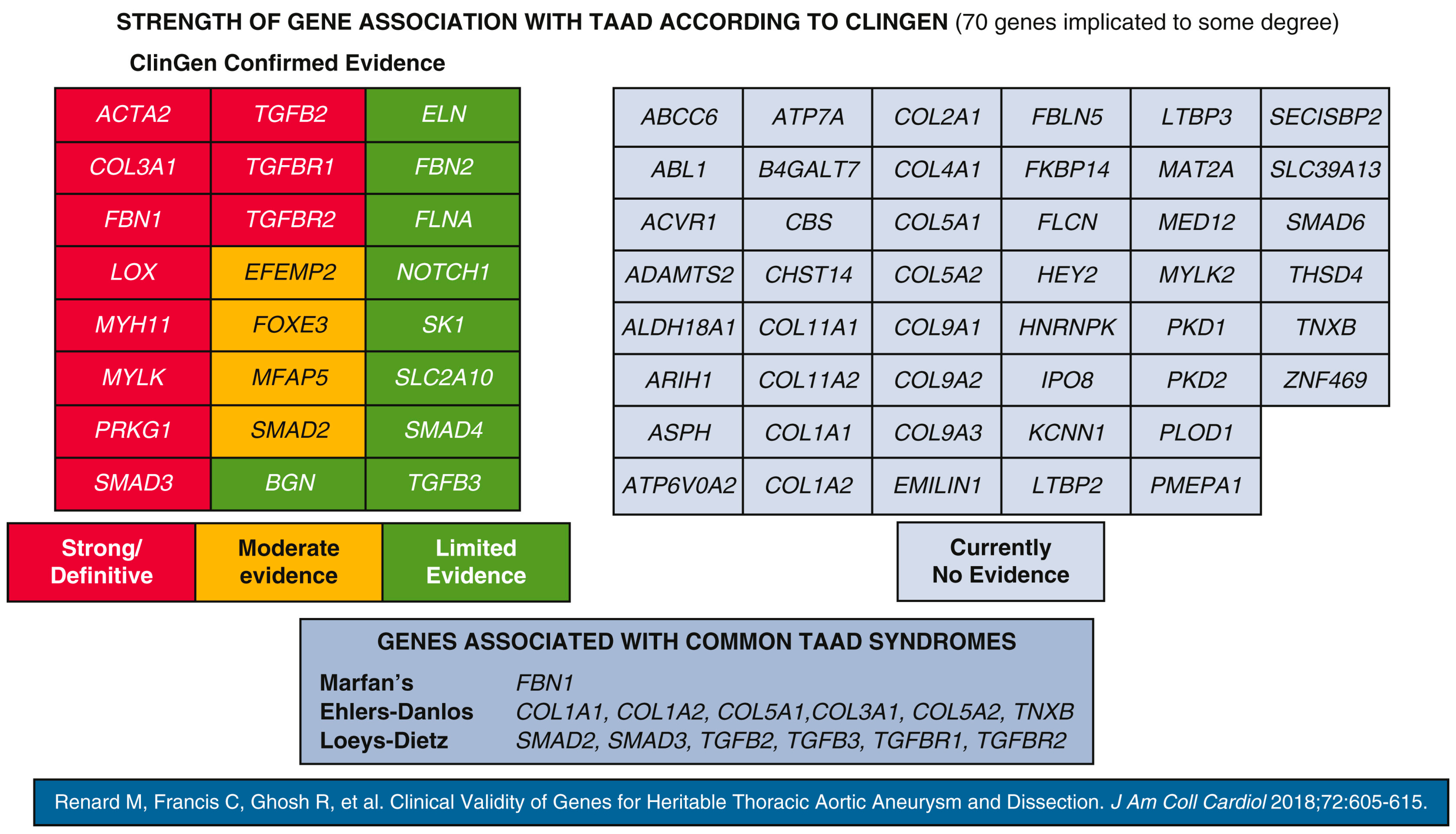
3.9. Genetic Predisposition
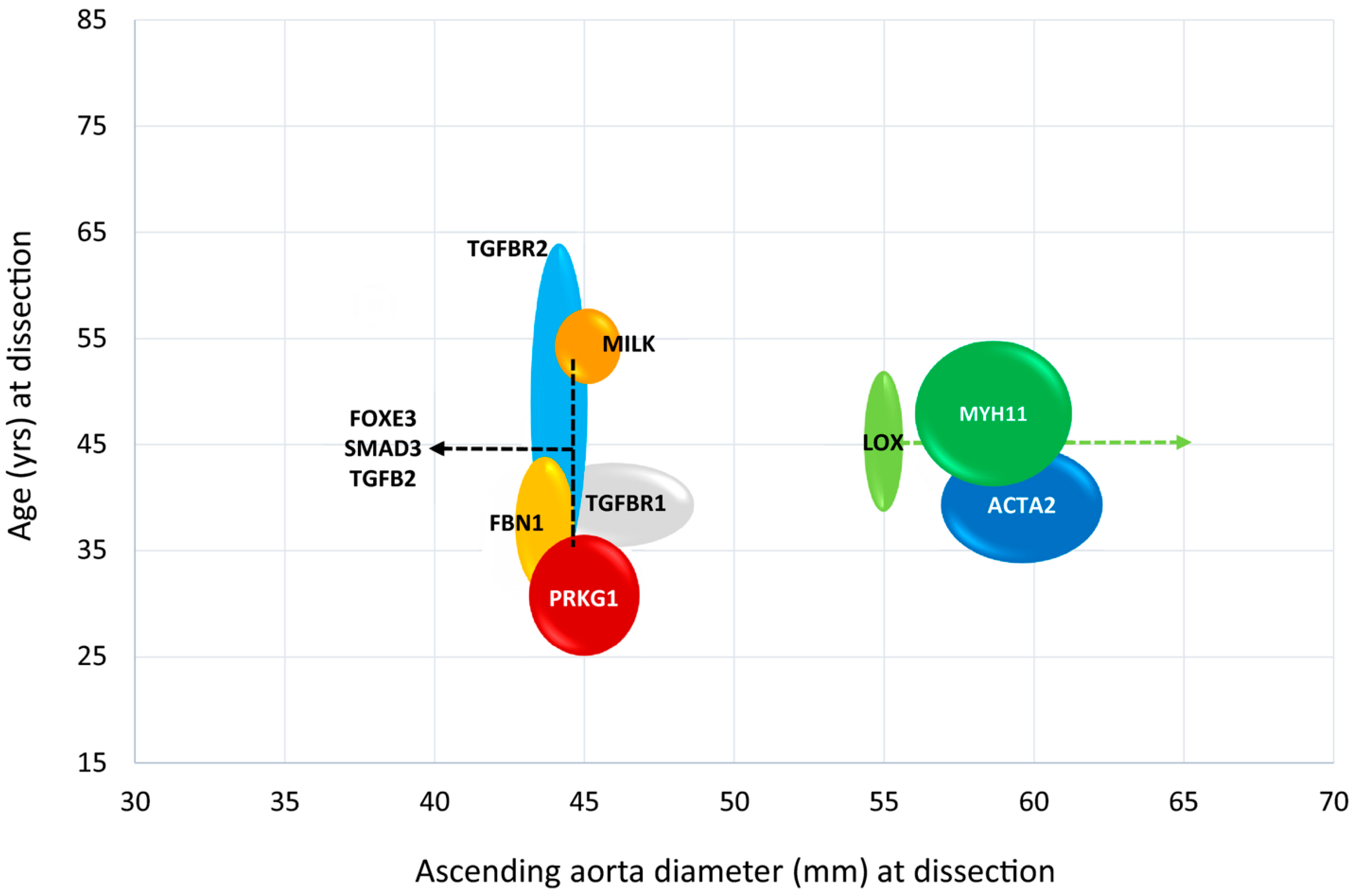
3.10. Pathogenic Variants in Genes Encoding ECM Components
3.11. Pathogenic Variants in TGF-β Signalling Pathway Genes
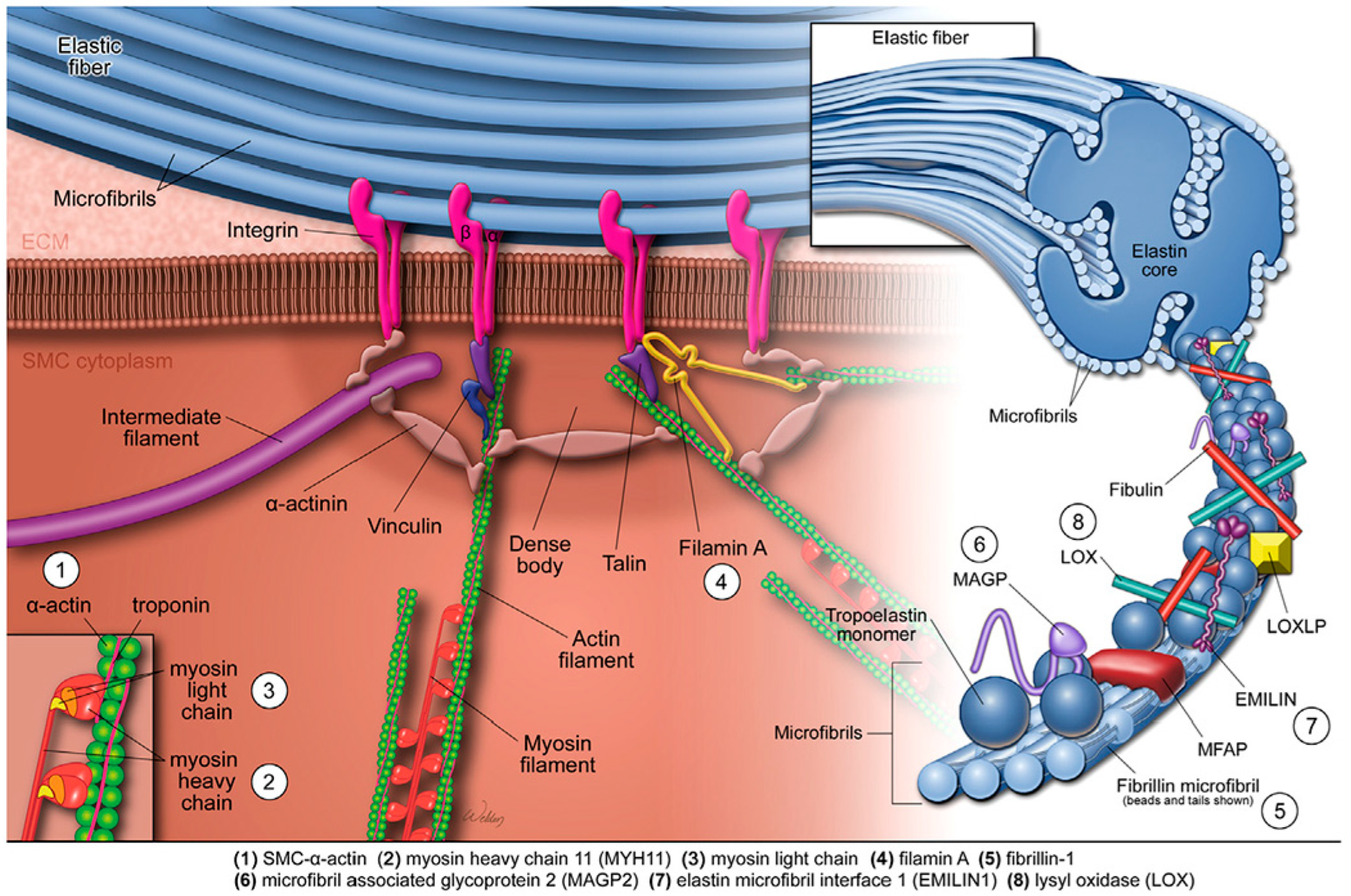
3.12. Pathogenic Defects in Genes Coding for the SMC Contractile Unit
4. Clinical Implications
5. Knowledge Gaps and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, X.; Wang, Z.; Shen, Z.; Lei, F.; Liu, Y.M.; Chen, Z.; Qin, J.J.; Liu, H.; Ji, Y.X.; Zhang, P.; et al. Projection of global burden and risk factors for aortic aneurysm—Timely warning for greater emphasis on managing blood pressure. Ann. Med. 2022, 54, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; You, Y.; Yin, Z.; Bao, Q.; Lei, S.; Yu, J.; Xie, C.; Ye, F.; Xie, X. Burden of Aortic Aneurysm and Its Attributable Risk Factors from 1990 to 2019: An Analysis of the Global Burden of Disease Study 2019. Front. Cardiovasc. Med. 2022, 9, 901225. [Google Scholar] [CrossRef] [PubMed]
- Oladokun, D.; Patterson, B.O.; Sobocinski, J.; Karthikesalingam, A.; Loftus, I.; Thompson, M.M.; Holt, P.J. Systematic Review of the Growth Rates and Influencing Factors in Thoracic Aortic Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 674–681. [Google Scholar] [CrossRef]
- Lindholt, J.S.; Sogaard, R. Population screening and intervention for vascular disease in Danish men (VIVA): A randomised controlled trial. Lancet 2017, 390, 2256–2265. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Hultgren, R.; Linne, A.; Holst, J.; Gottsater, A.; Langenskiold, M.; Smidfelt, K.; Björck, M.; Svensjö, S.; Swedish Aneurysm Screening Study Group (SASS). Outcome of the Swedish Nationwide Abdominal Aortic Aneurysm Screening Program. Circulation 2016, 134, 1141–1148. [Google Scholar] [CrossRef]
- Oliver-Williams, C.; Sweeting, M.J.; Turton, G.; Parkin, D.; Cooper, D.; Rodd, C.; Thompson, S.G.; Earnshaw, J.J. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br. J. Surg. 2018, 105, 68–74. [Google Scholar] [CrossRef]
- Marcaccio, C.L.; Schermerhorn, M.L. Epidemiology of abdominal aortic aneurysms. Semin. Vasc. Surg. 2021, 34, 29–37. [Google Scholar] [CrossRef]
- Czerny, M.; Grabenwoger, M.; Berger, T.; Aboyans, V.; Della Corte, A.; Chen, E.P.; Desai, N.D.; Dumfarth, J.; Elefteriades, J.A.; Etz, C.D.; et al. EACTS/STS Guidelines for diagnosing and treating acute and chronic syndromes of the aortic organ. Eur. J. Cardio-Thorac. Surg. 2024, 65, ezad426. [Google Scholar] [CrossRef] [PubMed]
- Obel, L.M.; Diederichsen, A.C.; Steffensen, F.H.; Frost, L.; Lambrechtsen, J.; Busk, M.; Urbonaviciene, G.; Egstrup, K.; Karon, M.; Rasmussen, L.M.; et al. Population-Based Risk Factors for Ascending, Arch, Descending, and Abdominal Aortic Dilations for 60–74-Year-Old Individuals. J. Am. Coll. Cardiol. 2021, 78, 201–211. [Google Scholar] [CrossRef]
- Bonser, R.S.; Ranasinghe, A.M.; Loubani, M.; Evans, J.D.; Thalji, N.M.; Bachet, J.E.; Carrel, T.P.; Czerny, M.; Di Bartolomeo, R.; Grabenwöger, M.; et al. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J. Am. Coll. Cardiol. 2011, 58, 2455–2474. [Google Scholar] [CrossRef]
- Pacini, D.; Di Marco, L.; Fortuna, D.; Belotti, L.M.; Gabbieri, D.; Zussa, C.; Pigini, F.; Contini, A.; Barattoni, M.C.; De Palma, R.; et al. Acute aortic dissection: Epidemiology and outcomes. Int. J. Cardiol. 2013, 167, 2806–2812. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Nakai, M.; Yano, T.; Matsuyama, M.; Yoshino, H.; Miyamoto, Y.; Sumita, Y.; Matsuda, H.; Inoue, Y.; Okita, Y.; et al. Population-based incidence and outcomes of acute aortic dissection in Japan. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 701–709. [Google Scholar]
- Pinard, A.; Jones, G.T.; Milewicz, D.M. Genetics of Thoracic and Abdominal Aortic Diseases. Circ. Res. 2019, 124, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zafar, M.A.; Liu, Y.; Chen, J.F.; Li, Y.; Ziganshin, B.A.; Ellauzi, H.; Mukherjee, S.K.; Rizzo, J.A.; Elefteriades, J.A. Fate of the unoperated ascending thoracic aortic aneurysm: Three-decade experience from the Aortic Institute at Yale University. Eur. Heart J. 2023, 44, 4579–4588. [Google Scholar] [CrossRef]
- Harik, L.; Leith, J.; Rahouma, M.; Cancelli, G.; Rossi, C.S.; Soletti, G.J.; Balaram, S.K.; Lau, C.; Girardi, L.; Gaudino, M. Association between social vulnerability and clinical outcomes after proximal aortic surgery. Eur. J. Cardio-Thorac. Surg. 2025, 67, ezaf217. [Google Scholar] [CrossRef] [PubMed]
- Mazzolai, L.; Teixido-Tura, G.; Lanzi, S.; Boc, V.; Bossone, E.; Brodmann, M.; Bura-Riviere, A.; De Backer, J.; Deglise, S.; Della Corte, A.; et al. 2024 ESC Guidelines for the management of peripheral arterial and aortic diseases. Eur. Heart J. 2024, 45, 3538–3700. [Google Scholar] [CrossRef]
- Isselbacher, E.M. Thoracic and abdominal aortic aneurysms. Circulation 2005, 111, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Elefteriades, J.A.; Zafar, M.A.; Ziganshin, B.A. Genetics of aortic aneurysm disease: 10 key points for the practitioner. JTCVS Open 2024, 21, 58–63. [Google Scholar] [CrossRef]
- Liyew, W.A.; Adane, F.; Wondemagegn, A.T.; Tsehay, B.; Deml, Y.A.; Abdu, H.M.; Animaw, Z. Roles of cardiac neural crest cells in cardiovascular development and associated congenital defects-an integrated review. Transl. Res. Anat. 2024, 36, 100304. [Google Scholar] [CrossRef]
- Waldo, K.L.; Hutson, M.R.; Ward, C.C.; Zdanowicz, M.; Stadt, H.A.; Kumiski, D.; Abu-Issa, R.; Kirby, M.L. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev. Biol. 2005, 281, 78–90. [Google Scholar] [CrossRef]
- Majesky, M.W. Developmental basis of vascular smooth muscle diversity. Arter. Thromb. Vasc. Biol. 2007, 27, 1248–1258. [Google Scholar] [CrossRef]
- Kirby, M.L.; Waldo, K.L. Neural crest and cardiovascular patterning. Circ. Res. 1995, 77, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Majesky, M.W.; Dong, X.R.; Hoglund, V.; Mahoney, W.M., Jr.; Daum, G. The adventitia: A dynamic interface containing resident progenitor cells. Arter. Thromb. Vasc. Biol. 2011, 31, 1530–1539. [Google Scholar] [CrossRef]
- Shen, Y.H.; LeMaire, S.A. Molecular pathogenesis of genetic and sporadic aortic aneurysms and dissections. Curr. Probl. Surg. 2017, 54, 95–155. [Google Scholar] [CrossRef]
- Wolinsky, H.; Glagov, S. A lamellar unit of aortic medial structure and function in mammals. Circ. Res. 1967, 20, 99–111. [Google Scholar] [CrossRef]
- Wagenseil, J.E.; Mecham, R.P. Elastin in large artery stiffness and hypertension. J. Cardiovasc. Transl. Res. 2012, 5, 264–273. [Google Scholar] [CrossRef]
- Wolinsky, H.; Glagov, S. Comparison of abdominal and thoracic aortic medial structure in mammals. Deviation of man from the usual pattern. Circ. Res. 1969, 25, 677–686. [Google Scholar] [CrossRef]
- Ruddy, J.M.; Jones, J.A.; Spinale, F.G.; Ikonomidis, J.S. Regional heterogeneity within the aorta: Relevance to aneurysm disease. J. Thorac. Cardiovasc. Surg. 2008, 136, 1123–1130. [Google Scholar] [CrossRef]
- Wolinsky, H. Comparison of medial growth of human thoracic and abdominal aortas. Circ. Res. 1970, 27, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Ejiri, J.; Inoue, N.; Tsukube, T.; Munezane, T.; Hino, Y.; Kobayashi, S.; Hirati, K.-I.; Kawashima, S.; Imajoh-Ohmi, S.; Hayashi, Y.; et al. Oxidative stress in the pathogenesis of thoracic aortic aneurysm: Protective role of statin and angiotensin II type 1 receptor blocker. Cardiovasc. Res. 2003, 59, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.X.; Zhang, W.M.; Zhang, H.J.; Li, T.T.; Wang, Y.L.; Qin, Y.W.; Gu, H.; Du, J. Mechanical stretch-induced endoplasmic reticulum stress, apoptosis and inflammation contribute to thoracic aortic aneurysm and dissection. J. Pathol. 2015, 236, 373–383. [Google Scholar] [CrossRef]
- Domagala, D.; Data, K.; Szyller, H.; Farzaneh, M.; Mozdziak, P.; Wozniak, S.; Zabel, M.; Dziegiel, P.; Kempisty, B. Cellular, Molecular and Clinical Aspects of Aortic Aneurysm-Vascular Physiology and Pathophysiology. Cells 2024, 13, 274. [Google Scholar] [CrossRef]
- Rateri, D.L.; Moorleghen, J.J.; Balakrishnan, A.; Owens, A.P., 3rd; Howatt, D.A.; Subramanian, V.; Poduri, A.; Charnigo, R.; Cassis, L.A.; Daugherty, A. Endothelial cell-specific deficiency of Ang II type 1a receptors attenuates Ang II-induced ascending aortic aneurysms in LDL receptor-/- mice. Circ. Res. 2011, 108, 574–581. [Google Scholar] [CrossRef]
- Fan, L.M.; Douglas, G.; Bendall, J.K.; McNeill, E.; Crabtree, M.J.; Hale, A.B.; Mai, A.; Li, J.M.; McAteer, M.A.; Schneider, J.E.; et al. Endothelial cell-specific reactive oxygen species production increases susceptibility to aortic dissection. Circulation 2014, 129, 2661–2672. [Google Scholar] [CrossRef]
- DeRoo, E.; Stranz, A.; Yang, H.; Hsieh, M.; Se, C.; Zhou, T. Endothelial Dysfunction in the Pathogenesis of Abdominal Aortic Aneurysm. Biomolecules 2022, 12, 509. [Google Scholar] [CrossRef] [PubMed]
- Malashicheva, A.; Kostina, D.; Kostina, A.; Irtyuga, O.; Voronkina, I.; Smagina, L.; Ignatieva, E.; Gavriluk, N.; Uspensky, V.; Moiseeva, O.; et al. Phenotypic and Functional Changes of Endothelial and Smooth Muscle Cells in Thoracic Aortic Aneurysms. Int. J. Vasc. Med. 2016, 2016, 3107879. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, C.; Yao, F.; Ding, Q.; Liu, H.; Luo, C.; Wang, D.; Huang, J.; Li, Z.; Schen, Y.; et al. Targeting endothelial tight junctions to predict and protect thoracic aortic aneurysm and dissection. Eur. Heart J. 2023, 44, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.X.; Shapiro, M.; Trogan, E.; Fisher, E.A. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc. Natl. Acad. Sci. USA 2003, 100, 13531–13536. [Google Scholar] [CrossRef]
- Lin, C.J.; Keating, C.; Roth, R.; Caliskan, Y.; Nazzal, M.; Exil, V.; DiPaolo, R.; Verma, D.R.; Harjai, K.; Zayed, M.; et al. Distinct Patterns of Smooth Muscle Phenotypic Modulation in Thoracic and Abdominal Aortic Aneurysms. J. Cardiovasc. Dev. Dis. 2024, 11, 349. [Google Scholar] [CrossRef]
- Hu, Y.; Cai, Z.; He, B. Smooth Muscle Heterogeneity and Plasticity in Health and Aortic Aneurysmal Disease. Int. J. Mol. Sci. 2023, 24, 11701. [Google Scholar] [CrossRef]
- Chen, P.Y.; Qin, L.; Li, G.; Malagon-Lopez, J.; Wang, Z.; Bergaya, S.; Gujja, S.; Caulk, A.W.; Murtada, S.I.; Zhang, X.; et al. Smooth Muscle Cell Reprogramming in Aortic Aneurysms. Cell Stem Cell 2020, 26, 542–557.e11. [Google Scholar] [CrossRef] [PubMed]
- Mellak, S.; Ait-Oufella, H.; Esposito, B.; Loyer, X.; Poirier, M.; Tedder, T.F.; Tedgui, A.; Mallat, Z.; Potteaux, S. Angiotensin II mobilizes spleen monocytes to promote the development of abdominal aortic aneurysm in Apoe−/− mice. Arter. Thromb. Vasc. Biol. 2015, 35, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Furubayashi, K.; Takai, S.; Jin, D.; Miyazaki, M.; Katsumata, T.; Inagaki, S.; Kimura, M.; Tanaka, K.; Nishimoto, M.; Fukumoto, H. Chymase activates promatrix metalloproteinase-9 in human abdominal aortic aneurysm. Clin. Chim. Acta 2008, 388, 214–216. [Google Scholar] [CrossRef]
- Elkhal, A.; Rodriguez Cetina Biefer, H.; Heinbokel, T.; Uehara, H.; Quante, M.; Seyda, M.; Schuitenmaker, J.M.; Krenzien, F.; Camacho, V.; de la Fuente, M.A.; et al. NAD(+) regulates Treg cell fate and promotes allograft survival via a systemic IL-10 production that is CD4(+) CD25(+) Foxp3(+) T cells independent. Sci. Rep. 2016, 6, 22325. [Google Scholar] [CrossRef]
- Galis, Z.S.; Khatri, J.J. Matrix Metalloproteinases in Vascular Remodeling and Atherogenesis. Circ. Res. 2002, 90, 251–262. [Google Scholar] [CrossRef]
- Rabkin, S.W. Differential expression of MMP-2, MMP-9 and TIMP proteins in thoracic aortic aneurysm—Comparison with and without bicuspid aortic valve: A meta-analysis. Vasa 2014, 43, 433–442. [Google Scholar] [CrossRef]
- Goodall, S.; Crowther, M.; Hemingway, D.M.; Bell, P.R.; Thompson, M.M. Ubiquitous elevation of matrix metalloproteinase-2 expression in the vasculature of patients with abdominal aneurysms. Circulation 2001, 104, 304–309. [Google Scholar] [CrossRef]
- Martin-Alonso, M.; Garcia-Redondo, A.B.; Guo, D.; Camafeita, E.; Martinez, F.; Alfranca, A.; Mendez-Berbero, N.; Pollan, A.; Sanchez-Camacho, C.; Denhardt, D.T.; et al. Deficiency of MMP17/MT4-MMP proteolytic activity predisposes to aortic aneurysm in mice. Circ. Res. 2015, 117, e13–e26. [Google Scholar] [CrossRef]
- Ito, S.; Lu, H.S.; Daugherty, A.; Sawada, H. Embryonic Heterogeneity of Smooth Muscle Cells in the Complex Mechanisms of Thoracic Aortic Aneurysms. Genes 2022, 13, 1618. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, J.; Yang, Z.; Zhang, H.; Wang, Y.; Liu, J.; Wang, S.; Shi, J.; Tong, X. SERCA2 dysfunction accelerates angiotensin II-induced aortic aneurysm and atherosclerosis by induction of oxidative stress in aortic smooth muscle cells. J. Mol. Cell. Cardiol. 2025, 200, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Das, A.A.; Waldeck-Weiermair, M.; Yadav, S.; Spyropoulos, F.; Pandey, A.; Dutta, T.; Convington, T.A.; Michel, T. Differential aortic aneurysm formation provoked by chemogenetic oxidative stress. J. Clin. Investig. 2025, 135, e188743. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, S.W. Mitochondrial dysfunction in cardiovascular disease. Free Radic. Biol. Med. 2005, 38, 1278–1295. [Google Scholar] [CrossRef]
- Habashi, J.P.; Judge, D.P.; Holm, T.M.; Cohn, R.D.; Loeys, B.L.; Cooper, T.K.; Myers, L.; Klein, E.C.; Liu, G.; Calvi, C.; et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006, 312, 117–121. [Google Scholar] [CrossRef]
- El-Hamamsy, I.; Yacoub, M.H. Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat. Rev. Cardiol. 2009, 6, 771–786. [Google Scholar] [CrossRef]
- Dai, J.; Losy, F.; Guinault, A.M.; Pages, C.; Anegon, I.; Desgranges, P.; Becquemin, J.P.; Allaire, E. Overexpression of transforming growth factor-beta1 stabilizes already-formed aortic aneurysms: A first approach to induction of functional healing by endovascular gene therapy. Circulation 2005, 112, 1008–1015. [Google Scholar] [CrossRef]
- Holm, T.M.; Habashi, J.P.; Doyle, J.J.; Bedja, D.; Chen, Y.; van Erp, C.; Lindsay, M.E.; Kim, D.; Schoenhoff, F.; Cohn, R.D.; et al. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 2011, 332, 358–361. [Google Scholar] [CrossRef]
- Iske, J.; El Fatimy, R.; Nian, Y.; Ghouzlani, A.; Eskandari, S.K.; Cetina Biefer, H.R.; Vasudevan, A.; Elkhal, A. NAD(+) prevents septic shock-induced death by non-canonical inflammasome blockade and IL-10 cytokine production in macrophages. Elife 2024, 12, RP88686. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.R.; Bruneval, P.; Angelini, A.; Bartoloni, G.; Basso, C.; Batoroeva, L.; Buja, L.M.; Butany, J.; d’Amati, G.; Fallon, J.T.; et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology: I. Inflammatory diseases. Cardiovasc. Pathol. 2015, 24, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Alcorn, H.G.; Wolfson, S.K., Jr.; Sutton-Tyrrell, K.; Kuller, L.H.; O’Leary, D. Risk factors for abdominal aortic aneurysms in older adults enrolled in The Cardiovascular Health Study. Arter. Thromb. Vasc. Biol. 1996, 16, 963–970. [Google Scholar] [CrossRef]
- Forsdahl, S.H.; Singh, K.; Solberg, S.; Jacobsen, B.K. Risk factors for abdominal aortic aneurysms: A 7-year prospective study: The Tromso Study, 1994–2001. Circulation 2009, 119, 2202–2208. [Google Scholar] [CrossRef]
- Naydeck, B.L.; Sutton-Tyrrell, K.; Schiller, K.D.; Newman, A.B.; Kuller, L.H. Prevalence and risk factors for abdominal aortic aneurysms in older adults with and without isolated systolic hypertension. Am. J. Cardiol. 1999, 83, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, S.H.; Forsdahl, S.H.; Singh, K.; Jacobsen, B.K. Atherosclerosis in abdominal aortic aneurysms: A causal event or a process running in parallel? The Tromso study. Arter. Thromb. Vasc. Biol. 2010, 30, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, H.; Howatt, D.A.; Balakrishnan, A.; Moorleghen, J.J.; Sorci-Thomas, M.; Cassis, L.A.; Daugherty, A. Associations of ApoAI and ApoB-containing lipoproteins with AngII-induced abdominal aortic aneurysms in mice. Arter. Thromb. Vasc. Biol. 2015, 35, 1826–1834. [Google Scholar] [CrossRef]
- Maegdefessel, L.; Spin, J.M.; Tsao, P.S. New ways to dismantle a ticking time bomb: MicroRNA 712/205 and abdominal aortic aneurysm development. Arter. Thromb. Vasc. Biol. 2014, 34, 1339–1340. [Google Scholar] [CrossRef] [PubMed]
- Merk, D.R.; Chin, J.T.; Dake, B.A.; Maegdefessel, L.; Miller, M.O.; Kimura, N.; Tsao, P.S.; Iosef, C.; Berry, G.J.; Mohr, F.W.; et al. miR-29b participates in early aneurysm development in Marfan syndrome. Circ. Res. 2012, 110, 312–324. [Google Scholar] [CrossRef]
- Winski, G.; Chernogubova, E.; Busch, A.; Eken, S.M.; Jin, H.; Lindquist Liljeqvist, M.; Khan, T.; Backlund, A.; Poloschi, V.; Roy, J.; et al. MicroRNA-15a-5p mediates abdominal aortic aneurysm progression and serves as a potential diagnostic and prognostic circulating biomarker. Commun Med. 2025, 5, 218. [Google Scholar] [CrossRef]
- Cho, M.J.; Lee, M.R.; Park, J.G. Aortic aneurysms: Current pathogenesis and therapeutic targets. Exp. Mol. Med. 2023, 55, 2519–2530. [Google Scholar] [CrossRef]
- Bararu Bojan Bararu, I.; Plesoianu, C.E.; Badulescu, O.V.; Vladeanu, M.C.; Badescu, M.C.; Iliescu, D.; Bojan, A.; Ciocoiu, M. Molecular and Cellular Mechanisms Involved in Aortic Wall Aneurysm Development. Diagnostics 2023, 13, 253. [Google Scholar] [CrossRef]
- Ziganshin, B.A.; Bailey, A.E.; Coons, C.; Dykas, D.; Charilaou, P.; Tanriverdi, L.H.; Lie, L.; Tranquilli, M.; Bale, A.E.; Elefteriades, J.A. Routine Genetic Testing for Thoracic Aortic Aneurysm and Dissection in a Clinical Setting. Ann. Thorac. Surg. 2015, 100, 1604–1611. [Google Scholar] [CrossRef]
- Wahlgren, C.M.; Larsson, E.; Magnusson, P.K.; Hultgren, R.; Swedenborg, J. Genetic and environmental contributions to abdominal aortic aneurysm development in a twin population. J. Vasc. Surg. 2010, 51, 3–7. [Google Scholar] [CrossRef]
- Liu, H.; ASIJ; de Bruin, J.L.; Verhagen, H.J.M.; Roos-Hesselink, J.W.; Bekkers, J.A.; Brüggenwirth, H.T.; van Beusekom, H.M.M.; Majoor-Krakauer, D.F. Whole aorta imaging shows increased risk for thoracic aortic aneurysms and dilatations in relatives of abdominal aortic aneurysm patients. J. Vasc. Surg. 2025, 81, 557–565.e7. [Google Scholar] [CrossRef]
- Roychowdhury, T.; Klarin, D.; Levin, M.G.; Spin, J.M.; Rhee, Y.H.; Deng, A.; Headley, C.A.; Tsao, N.L.; Gellatly, C.; Zuber, V.; et al. Genome-wide association meta-analysis identifies risk loci for abdominal aortic aneurysm and highlights PCSK9 as a therapeutic target. Nat. Genet. 2023, 55, 1831–1842. [Google Scholar] [CrossRef]
- Zheng, S.; Tsao, P.S.; Pan, C. Abdominal aortic aneurysm and cardiometabolic traits share strong genetic susceptibility to lipid metabolism and inflammation. Nat. Commun. 2024, 15, 5652. [Google Scholar] [CrossRef]
- Golledge, J.; Lu, H.S.; Shah, S. Protein convertase subtilisin/kexin type 9 as a drug target for abdominal aortic aneurysm. Curr. Opin. Lipidol. 2024, 35, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Renard, M.; Francis, C.; Ghosh, R.; Scott, A.F.; Witmer, P.D.; Ades, L.C.; Andelfinger, G.U.; Arnaud, P.; Boileau, C.; Callewaert, B.L.; et al. Clinical Validity of Genes for Heritable Thoracic Aortic Aneurysm and Dissection. J. Am. Coll. Cardiol. 2018, 72, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Pinard, A.; Salgado, D.; Desvignes, J.P.; Rai, G.; Hanna, N.; Arnaud, P.; Guien, C.; Martinez, M.; Faivre, L.; Jondeau, G.; et al. WES/WGS Reporting of Mutations from Cardiovascular “Actionable” Genes in Clinical Practice: A Key Role for UMD Knowledgebases in the Era of Big Databases. Hum. Mutat. 2016, 37, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.W.; Au Yeung, K.; Sandor, G.G.; Judge, D.P.; Dietz, H.C.; van Breemen, C. Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in the thoracic aortic aneurysm in Marfan syndrome. Circ. Res. 2007, 101, 512–522. [Google Scholar] [CrossRef]
- Neptune, E.R.; Frischmeyer, P.A.; Arking, D.E.; Myers, L.; Bunton, T.E.; Gayraud, B.; Ramirez, F.; Sakai, L.Y.; Dietz, H.C. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003, 33, 407–411. [Google Scholar] [CrossRef]
- Walker, S.; Bunyan, D.J.; Thomas, H.B.; Kesim, Y.; Kershaw, C.J.; Holloway, J.; Wai, H.; Day, M.; Smith, C.L.; Hawkes, G.; et al. Utility of genome sequencing and group-enrichment to support splice variant interpretation in Marfan syndrome. Genet. Med. 2025, 27, 101477. [Google Scholar] [CrossRef]
- Guo, D.C.; Regalado, E.S.; Gong, L.; Duan, X.; Santos-Cortez, R.L.; Arnaud, P.; Ren, Z.; Cai, B.; Hostetler, E.M.; Moran, R.; et al. LOX Mutations Predispose to Thoracic Aortic Aneurysms and Dissections. Circ. Res. 2016, 118, 928–934. [Google Scholar] [CrossRef]
- Gyftopoulos, A.; Ziganshin, B.A.; Elefteriades, J.A.; Ochoa Chaar, C.I. Comparison of Genes Associated with Thoracic and Abdominal Aortic Aneurysms. AORTA 2023, 11, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Superti-Furga, A.; Gugler, E.; Gitzelmann, R.; Steinmann, B. Ehlers-Danlos syndrome type IV: A multi-exon deletion in one of the two COL3A1 alleles affecting structure, stability, and processing of type III procollagen. J. Biol. Chem. 1988, 263, 6226–6232. [Google Scholar] [CrossRef] [PubMed]
- Monroe, G.R.; Harakalova, M.; van der Crabben, S.N.; Majoor-Krakauer, D.; Bertoli-Avella, A.M.; Moll, F.L.; Oranen, B.I.; Dooijes, D.; Vink, A.; Knoers, N.V.; et al. Familial Ehlers-Danlos syndrome with lethal arterial events caused by a mutation in COL5A1. Am. J. Med. Genet. A 2015, 167, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, U.; Hata, R.; McKusick, V.A.; Shinkai, H.; Hoyme, H.E.; Pyeritz, R.E.; Byers, P.H. Rare autosomal recessive cardiac valvular form of Ehlers-Danlos syndrome results from mutations in the COL1A2 gene that activate the nonsense-mediated RNA decay pathway. Am. J. Hum. Genet. 2004, 74, 917–930. [Google Scholar] [CrossRef]
- Loeys, B.L.; Chen, J.; Neptune, E.R.; Judge, D.P.; Podowski, M.; Holm, T.; Mayers, J.; Leitch, C.C.; Katsanis, N.; Sharifi, N.; et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005, 37, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, S.; Kwartler, C.S.; Lafont, A.L.; Liang, Y.Y.; Fadulu, V.T.; Duraisamy, S.; Willing, M.; Estrera, A.; Safi, H.; Hannibal, M.V.; et al. TGFBR2 mutations alter smooth muscle cell phenotype and predispose to thoracic aortic aneurysms and dissections. Cardiovasc. Res. 2010, 88, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Baas, A.F.; Medic, J.; van’t Slot, R.; de Vries, J.P.; van Sambeek, M.R.; Geelkerken, B.H.; Boll, B.P.; Grobbee, D.E.; Wijmenga, C.; Ruigrok, Y.M.; et al. Association study of single nucleotide polymorphisms on chromosome 19q13 with abdominal aortic aneurysm. Angiology 2010, 61, 243–247. [Google Scholar] [CrossRef]
- He, Z.; IJpma, A.S.; Vreeken, D.; Heijsman, D.; Rosier, K.; Verhagen, H.J.M.; de Bruin, J.L.; Bruggenwirth, H.T.; Roos-Hesselink, J.W.; Bekkers, J.A.; et al. The proprotein convertase FURIN is a novel aneurysm predisposition gene impairing TGF-beta signalling. Cardiovasc. Res. 2024, 120, 2278–2292. [Google Scholar] [CrossRef]
- Plaimauer, B.; Mohr, G.; Wernhart, W.; Himmelspach, M.; Dorner, F.; Schlokat, U. ‘Shed’ furin: Mapping of the cleavage determinants and identification of its C-terminus. Biochem. J. 2001, 354, 689–695. [Google Scholar] [CrossRef]
- International Consortium for Blood Pressure Genome-Wide Association Studies; Ehret, G.B.; Munroe, P.B.; Rice, K.M.; Bochud, M.; Johnson, A.D.; Chasman, D.I.; Smith, A.V.; Tobin, M.D.; Verwoert, C.; et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011, 478, 103–109. [Google Scholar] [CrossRef]
- Guo, D.C.; Pannu, H.; Tran-Fadulu, V.; Papke, C.L.; Yu, R.K.; Avidan, N.; Bourgeois, S.; Estrera, A.L.; Safi, H.J.; Sparks, E.; et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat. Genet. 2007, 39, 1488–1493. [Google Scholar] [CrossRef]
- Milewicz, D.M.; Ostergaard, J.R.; Ala-Kokko, L.M.; Khan, N.; Grange, D.K.; Mendoza-Londono, R.; Bradley, T.J.; Haskins Olney, A.; Ades, L.; Maher, J.F.; et al. De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am. J. Med. Genet. A 2010, 152A, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Shalata, A.; Mahroom, M.; Milewicz, D.M.; Limin, G.; Kassum, F.; Badarna, K.; Tarabeih, N.; Assy, N.; Fell, R.; Cohen, H.; et al. Fatal thoracic aortic aneurysm and dissection in a large family with a novel MYLK gene mutation: Delineation of the clinical phenotype. Orphanet J. Rare Dis. 2018, 13, 41. [Google Scholar] [CrossRef]
- Guo, D.C.; Regalado, E.; Casteel, D.E.; Santos-Cortez, R.L.; Gong, L.; Kim, J.J.; Dyack, S.; Horne, A.G.; Chang, G.; Jondeau, G.; et al. Recurrent gain-of-function mutation in PRKG1 causes thoracic aortic aneurysms and acute aortic dissections. Am. J. Hum. Genet. 2013, 93, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Stark, Z.; Dolman, L.; Manolio, T.A.; Ozenberger, B.; Hill, S.L.; Caulfied, M.J.; Levy, Y.; Glazer, D.; Wilson, J.; Lawler, M.; et al. Integrating Genomics into Healthcare: A Global Responsibility. Am. J. Hum. Genet. 2019, 104, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Kuang, S.Q.; Geng, L.; Prakash, S.K.; Cao, J.M.; Guo, S.; Villamizar, C.; Kwartler, C.S.; Peters, A.M.; Brasier, A.R.; Milewich, D.M. Aortic remodeling after transverse aortic constriction in mice is attenuated with AT1 receptor blockade. Arter. Thromb. Vasc. Biol. 2013, 33, 2172–2179. [Google Scholar] [CrossRef]
- Pitcher, A.; Spata, E.; Emberson, J.; Davies, K.; Halls, H.; Holland, L.; Wilson, K.; Reith, C.; Child, A.H.; Clayton, T.; et al. Angiotensin receptor blockers and beta blockers in Marfan syndrome: An individual patient data meta-analysis of randomised trials. Lancet 2022, 400, 822–831. [Google Scholar] [CrossRef]
- Turnbull, I.C.; Hadri, L.; Rapti, K.; Sadek, M.; Liang, L.; Shin, H.J.; Costa, K.D.; Marin, M.M.; Hajjar, R.J.; Faries, P.L. Aortic implantation of mesenchymal stem cells after aneurysm injury in a porcine model. J. Surg. Res. 2011, 170, e179–e188. [Google Scholar] [CrossRef]
- Hashizume, R.; Yamawaki-Ogata, A.; Ueda, Y.; Wagner, W.R.; Narita, Y. Mesenchymal stem cells attenuate angiotensin II-induced aortic aneurysm growth in apolipoprotein E-deficient mice. J. Vasc. Surg. 2011, 54, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; Saucy, F.; de Blic, R.; Dai, J.; Mohand, F.; Rouard, H.; Ricco, J.B.; Becquemin, J.P.; Gervais, M.; Allaire, E. Bone marrow mesenchymal stem cells stabilize already-formed aortic aneurysms more efficiently than vascular smooth muscle cells in a rat model. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 666–672. [Google Scholar] [CrossRef]
- Verhagen, J.M.A.; Kempers, M.; Cozijnsen, L.; Bouma, B.J.; Duijnhouwer, A.L.; Post, J.G.; Hilhorst-Hofstee, Y.; Bekkers, S.C.A.M.; Kerstjens-Frederikse, W.S.; van Brakel, T.J.; et al. Expert consensus recommendations on the cardiogenetic care for patients with thoracic aortic disease and their first-degree relatives. Int. J. Cardiol. 2018, 258, 243–248. [Google Scholar] [CrossRef]
- Mariscalco, G.; Debiec, R.; Elefteriades, J.A.; Samani, N.J.; Murphy, G.J. Systematic Review of Studies That Have Evaluated Screening Tests in Relatives of Patients Affected by Nonsyndromic Thoracic Aortic Disease. J. Am. Heart Assoc. 2018, 7, e009302. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Masson, E.; Zou, W.B.; Genin, E.; Cooper, D.N.; Le Gac, G.; Fichou, Y.; Pu, N.; Rebours, V.; Ferec, C.; Liao, Z.; et al. Expanding ACMG variant classification guidelines into a general framework. Hum. Genom. 2022, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, A.; Pirruccello, J.P.; Ellinor, P.T.; Lindsay, M.E. Using Genomics to Identify Novel Therapeutic Targets for Aortic Disease. Arter. Thromb. Vasc. Biol. 2024, 44, 334–351. [Google Scholar] [CrossRef]
- Liu, N.; Olson, E.N. CRISPR Modeling and Correction of Cardiovascular Disease. Circ. Res. 2022, 130, 1827–1850. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Hemelrijck, M.; Risteski, P.; Rings, L.; Milojevic, M.; Rodríguez Cetina Biefer, H.; Dzemali, O. Embryological Divergence and Molecular Mechanisms in Thoracic and Abdominal Aortic Aneurysms: Bridging Developmental Biology and Clinical Insights. Biomolecules 2025, 15, 1654. https://doi.org/10.3390/biom15121654
Van Hemelrijck M, Risteski P, Rings L, Milojevic M, Rodríguez Cetina Biefer H, Dzemali O. Embryological Divergence and Molecular Mechanisms in Thoracic and Abdominal Aortic Aneurysms: Bridging Developmental Biology and Clinical Insights. Biomolecules. 2025; 15(12):1654. https://doi.org/10.3390/biom15121654
Chicago/Turabian StyleVan Hemelrijck, Mathias, Petar Risteski, Laura Rings, Milan Milojevic, Héctor Rodríguez Cetina Biefer, and Omer Dzemali. 2025. "Embryological Divergence and Molecular Mechanisms in Thoracic and Abdominal Aortic Aneurysms: Bridging Developmental Biology and Clinical Insights" Biomolecules 15, no. 12: 1654. https://doi.org/10.3390/biom15121654
APA StyleVan Hemelrijck, M., Risteski, P., Rings, L., Milojevic, M., Rodríguez Cetina Biefer, H., & Dzemali, O. (2025). Embryological Divergence and Molecular Mechanisms in Thoracic and Abdominal Aortic Aneurysms: Bridging Developmental Biology and Clinical Insights. Biomolecules, 15(12), 1654. https://doi.org/10.3390/biom15121654








