Altered Short Non-Coding RNA Landscape in the Hippocampus of a Mouse Model of CDKL5 Deficiency Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Ethical Approval
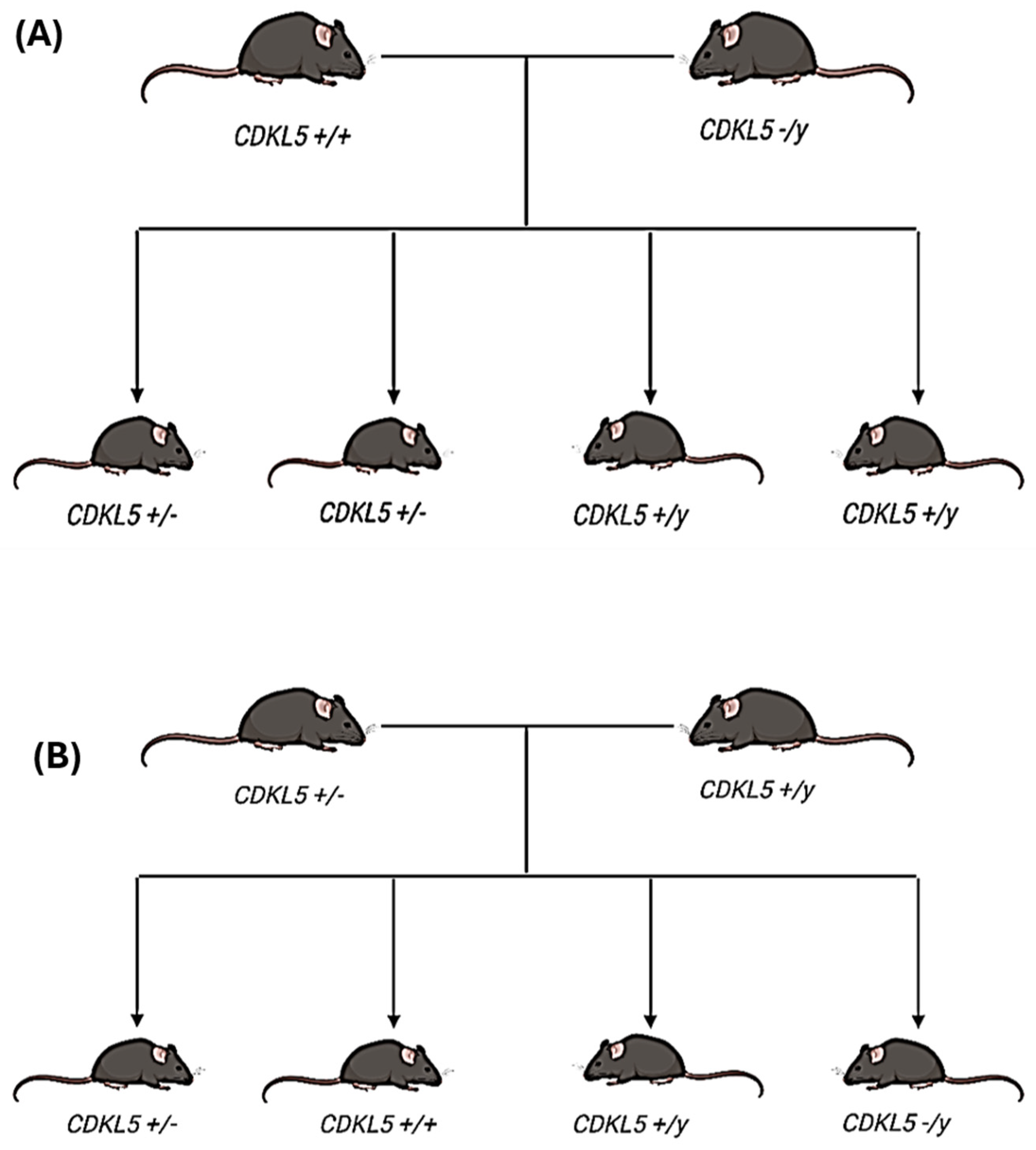
2.2. Animal Model Used in the Study
2.3. RNA Extraction
2.4. Small RNA Sequencing of Hippocampal Tissue
2.5. Analysis of Individual miRNA Expression
2.6. Statistical Analysis
3. Results
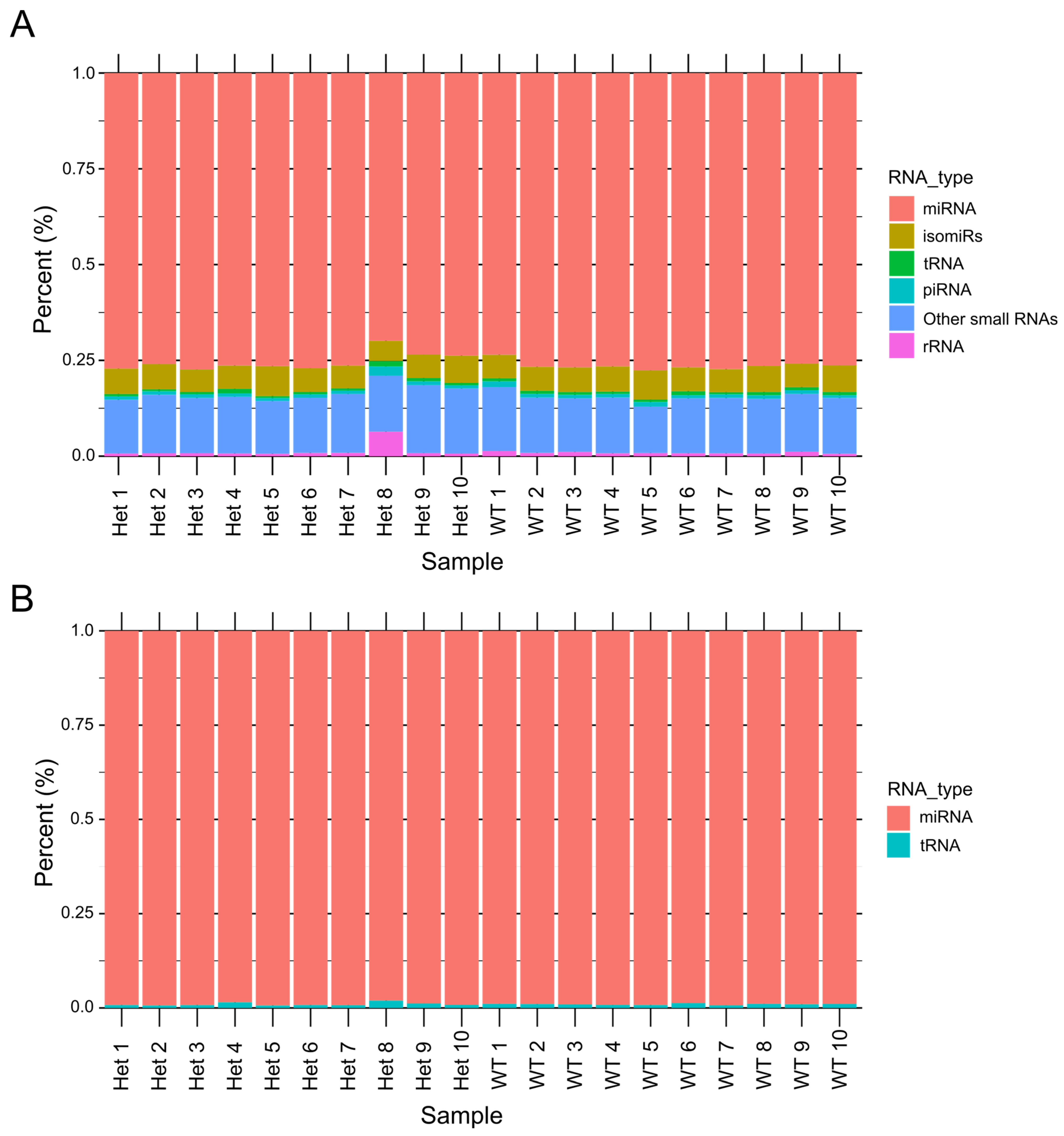
3.1. Distribution of Small RNAs in CDD Model Samples
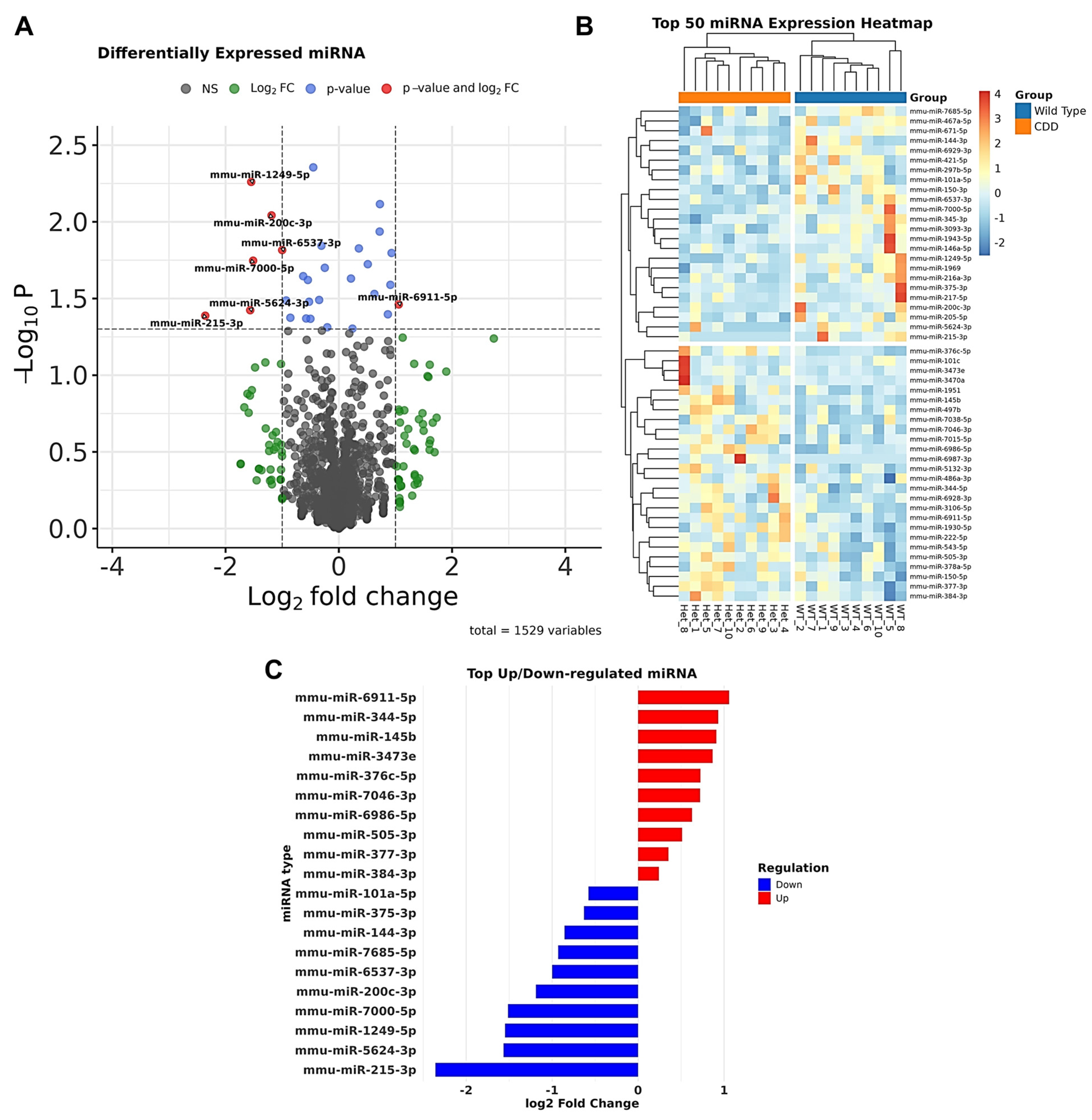
3.1.1. Dysregulated microRNAs in CDD
3.1.2. Validation of Dysregulated miRNAs and Their Target Networks in CDD
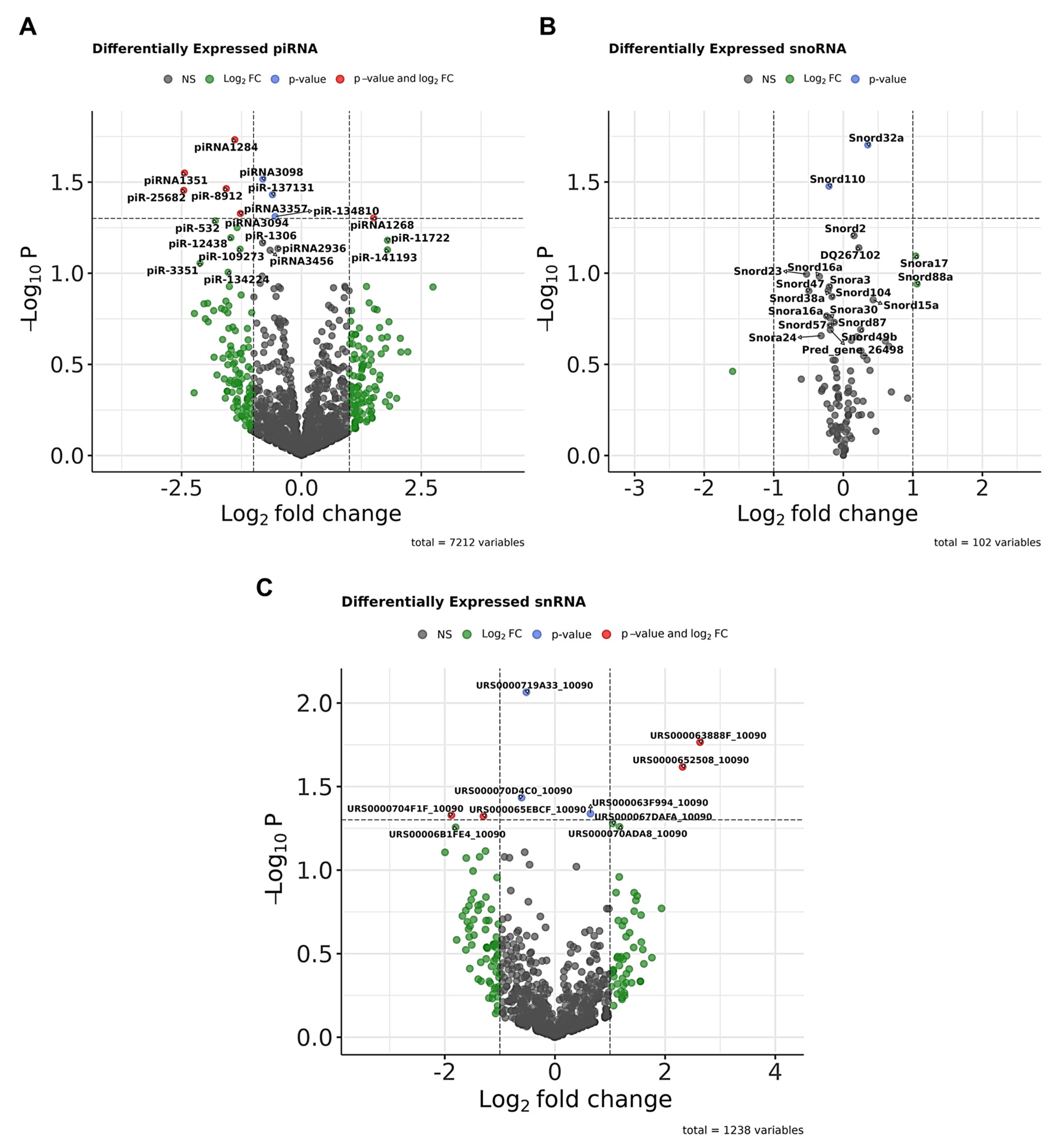
3.2. Transfer RNAs in CDD Mice
3.3. Other Small ncRNAs
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leonard, H.; Downs, J.; Benke, T.A.; Swanson, L.; Olson, H.; Demarest, S. CDKL5 deficiency disorder: Clinical features, diagnosis, and management. Lancet Neurol. 2022, 21, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Demarest, S.T.; Olson, H.E.; Moss, A.; Pestana-Knight, E.; Zhang, X.; Parikh, S.; Swanson, L.C.; Riley, K.D.; Bazin, G.A.; Angione, K.; et al. CDKL5 deficiency disorder: Relationship between genotype, epilepsy, cortical visual impairment, and development. Epilepsia 2019, 60, 1733–1742. [Google Scholar] [CrossRef]
- Fehr, S.; Wilson, M.; Downs, J.; Williams, S.; Murgia, A.; Sartori, S.; Vecchi, M.; Ho, G.; Polli, R.; Psoni, S.; et al. The CDKL5 disorder is an independent clinical entity associated with early-onset encephalopathy. Eur. J. Hum. Genet. 2013, 21, 266–273. [Google Scholar] [CrossRef]
- Daniels, C.; Greene, C.; Smith, L.; Pestana-Knight, E.; Demarest, S.; Zhang, B.; Benke, T.A.; Poduri, A.; Olson, H.E. CDKL5 deficiency disorder and other infantile-onset genetic epilepsies. Dev. Med. Child Neurol. 2024, 66, 456–468. [Google Scholar] [CrossRef]
- Lindy, A.S.; Stosser, M.B.; Butler, E.; Downtain-Pickersgill, C.; Shanmugham, A.; Retterer, K.; Brandt, T.; Richard, G.; McKnight, D.A. Diagnostic outcomes for genetic testing of 70 genes in 8565 patients with epilepsy and neurodevelopmental disorders. Epilepsia 2018, 59, 1062–1071. [Google Scholar] [CrossRef]
- Demarest, S.; Pestana-Knight, E.M.; Olson, H.E.; Downs, J.; Marsh, E.D.; Kaufmann, W.E.; Partridge, C.-A.; Leonard, H.; Gwadry-Sridhar, F.; Frame, K.E.; et al. Severity assessment in CDKL5 deficiency disorder. Pediatr. Neurol. 2019, 97, 38–42. [Google Scholar] [CrossRef]
- Benke, T.A.; Demarest, S.; Angione, K.; Downs, J.; Leonard, H.; Saldaris, J.; Marsh, E.D.; Olson, H.; Haviland, I. CDKL5 deficiency disorder. In GeneReviews®; University of Washington: Seattle, WA, USA, 2024. [Google Scholar]
- Amin, S.; Monaghan, M.; Aledo-Serrano, A.; Bahi-Buisson, N.; Chin, R.F.; Clarke, A.J.; Cross, J.H.; Demarest, S.; Devinsky, O.; Downs, J.; et al. International consensus recommendations for the assessment and management of individuals with CDKL5 deficiency disorder. Front. Neurol. 2022, 13, 874695. [Google Scholar] [CrossRef]
- Van Bergen, N.J.; Massey, S.; Quigley, A.; Rollo, B.; Harris, A.R.; Kapsa, R.M.; Christodoulou, J. CDKL5 deficiency disorder: Molecular insights and mechanisms of pathogenicity to fast-track therapeutic development. Biochem. Soc. Trans. 2022, 50, 1207–1224. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Sueyoshi, N.; Inazu, T.; Kameshita, I. Cyclin-Dependent Kinase-Like 5 (CDKL5): Possible Cellular Signalling Targets and Involvement in CDKL5 Deficiency Disorder. Neural Plast. 2020, 2020, 6970190. [Google Scholar] [CrossRef] [PubMed]
- Kilstrup-Nielsen, C.; Rusconi, L.; La Montanara, P.; Ciceri, D.; Bergo, A.; Bedogni, F.; Landsberger, N. What we know and would like to know about CDKL5 and its involvement in epileptic encephalopathy. Neural Plast. 2012, 2012, 728267. [Google Scholar] [CrossRef]
- Szafranski, P.; Golla, S.; Jin, W.; Fang, P.; Hixson, P.; Matalon, R.; Kinney, D.; Bock, H.-G.; Craigen, W.; Smith, J.L.; et al. Neurodevelopmental and neurobehavioral characteristics in males and females with CDKL5 duplications. Eur. J. Hum. Genet. 2015, 23, 915–921. [Google Scholar] [CrossRef]
- Hao, S.; Wang, Q.; Tang, B.; Wu, Z.; Yang, T.; Tang, J. CDKL5 deficiency augments inhibitory input into the dentate gyrus that can be reversed by deep brain stimulation. J. Neurosci. 2021, 41, 9031–9046. [Google Scholar] [CrossRef]
- Olson, H.E.; Daniels, C.I.; Haviland, I.; Swanson, L.C.; Greene, C.A.; Denny, A.M.M.; Demarest, S.T.; Pestana-Knight, E.; Zhang, X.; Moosa, A.N.; et al. Current neurologic treatment and emerging therapies in CDKL5 deficiency disorder. J. Neurodev. Disord. 2021, 13, 40. [Google Scholar] [CrossRef]
- Hong, W.; Haviland, I.; Pestana-Knight, E.; Weisenberg, J.L.; Demarest, S.; Marsh, E.D.; Olson, H.E. CDKL5 Deficiency Disorder-Related Epilepsy: A Review of Current and Emerging Treatment. CNS Drugs. 2022, 36, 591–604. [Google Scholar] [CrossRef]
- Voronin, G.; Narasimhan, J.; Gittens, J.; Sheedy, J.; Lipari, P.; Peters, M.; DeMarco, S.; Cao, L.; Varganov, Y.; Kim, M.J.; et al. Preclinical studies of gene replacement therapy for CDKL5 deficiency disorder. Mol. Ther. 2024, 32, 3331–3345. [Google Scholar] [CrossRef]
- Muñoz, I.M.; E Morgan, M.; Peltier, J.; Weiland, F.; Gregorczyk, M.; Brown, F.C.; Macartney, T.; Toth, R.; Trost, M.; Rouse, J. Phosphoproteomic screening identifies physiological substrates of the CDKL5 kinase. EMBO J. 2018, 37, e99559. [Google Scholar] [CrossRef]
- Liao, W.; Lee, K.Z. CDKL5-mediated developmental tuning of neuronal excitability and concomitant regulation of transcriptome. Hum. Mol. Genet. 2023, 32, 3276–3298. [Google Scholar] [CrossRef]
- Huang, J.; Eilbeck, K.; Smith, B.; Blake, J.A.; Dou, D.; Huang, W.; Natale, D.A.; Ruttenberg, A.; Huan, J.; Zimmermann, M.T.; et al. The development of non-coding RNA ontology. Int. J. Data Min. Bioinform. 2016, 15, 214–232. [Google Scholar] [CrossRef] [PubMed]
- Ulitsky, I. Interactions between short and long noncoding RNAs. FEBS Lett. 2018, 592, 2874–2883. [Google Scholar] [CrossRef] [PubMed]
- Dupuis-Sandoval, F.; Poirier, M.; Scott, M.S. The emerging landscape of small nucleolar RNAs in cell biology. Wiley Interdiscip. Rev. RNA 2015, 6, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Luteijn, M.J.; Ketting, R.F. PIWI-interacting RNAs: From generation to transgenerational epigenetics. Nat. Rev. Genet. 2013, 14, 523–534. [Google Scholar] [CrossRef]
- Brennan, G.P.; Henshall, D.C. MicroRNAs as regulators of brain function and targets for treatment of epilepsy. Nat. Rev. Neurol. 2020, 16, 506–519. [Google Scholar] [CrossRef]
- Henshall, D.C.; Hamer, H.M.; Pasterkamp, R.J.; Goldstein, D.B.; Kjems, J.; Prehn, J.H.M.; Schorge, S.; Lamottke, K.; Rosenow, F. MicroRNAs in epilepsy: Pathophysiology and clinical utility. Lancet Neurol. 2016, 15, 1368–1376. [Google Scholar] [CrossRef]
- Mohr, A.M.; Mott, J.L. Overview of microRNA biology. In Seminars in Liver Disease; Thieme Medical Publishers: Teningen, Germany, 2015; pp. 3–11. [Google Scholar]
- Guo, H.; Ingolia, N.T.; Weissman, J.S.; Bartel, D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010, 466, 835–840. [Google Scholar] [CrossRef]
- Helwak, A.; Kudla, G.; Dudnakova, T.; Tollervey, D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 2013, 153, 654–665. [Google Scholar] [CrossRef]
- Mathew, B.A.; Katta, M.; Ludhiadch, A.; Singh, P.; Munshi, A. Role of tRNA-derived fragments in neurological disorders: A review. Mol. Neurobiol. 2023, 60, 655–671. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, R.; Li, S.; Jin, X. tRNA Modifications and Dysregulation: Implications for Brain Diseases. Brain Sci. 2024, 14, 633. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, X.; Pu, W.; Peng, Y. tRNA-derived small non-coding RNAs in human disease. Cancer Lett. 2018, 419, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Tan, W.; Zhou, Y. Transfer RNA-derived small RNAs: Potential applications as novel biomarkers for disease diagnosis and prognosis. Ann. Transl. Med. 2020, 8, 1092. [Google Scholar] [CrossRef] [PubMed]
- Blaze, J.; Akbarian, S. The tRNA regulome in neurodevelopmental and neuropsychiatric disease. Mol. Psychiatry. 2022, 27, 3204–3213. [Google Scholar] [CrossRef]
- Ivanov, P.; Emara, M.M.; Villen, J.; Gygi, S.P.; Anderson, P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol. Cell 2011, 43, 613–623. [Google Scholar] [CrossRef]
- Emara, M.M.; Ivanov, P.; Hickman, T.; Dawra, N.; Tisdale, S.; Kedersha, N.; Hu, G.-F.; Anderson, P. Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. J. Biol. Chem. 2010, 285, 10959–10968. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, M.; Cao, Z.; Li, X.; Zhang, Y.; Shi, J.; Feng, G.-H.; Peng, H.; Zhang, X.; Zhang, Y.; et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 2016, 351, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Dhahbi, J.M.; Spindler, S.R.; Atamna, H.; Yamakawa, A.; Boffelli, D.; Mote, P.; Martin, D.I.K. 5′ tRNA halves are present as abundant complexes in serum, concentrated in blood cells, and modulated by aging and calorie restriction. BMC Genomics 2013, 14, 298. [Google Scholar] [CrossRef] [PubMed]
- Ghildiyal, M.; Zamore, P.D. Small silencing RNAs: An expanding universe. Nat. Rev. Genet. 2009, 10, 94–108. [Google Scholar] [CrossRef]
- Kuscu, C.; Kumar, P.; Kiran, M.; Su, Z.; Malik, A.; Dutta, A. tRNA fragments (tRFs) guide Ago to regulate gene expression post-transcriptionally in a Dicer-independent manner. RNA 2018, 24, 1093–1105. [Google Scholar] [CrossRef]
- Keam, S.P.; Hutvagner, G. tRNA-derived fragments (tRFs): Emerging new roles for an ancient RNA in the regulation of gene expression. Life 2015, 5, 1638–1651. [Google Scholar] [CrossRef]
- Sobala, A.; Hutvagner, G. Transfer RNA-derived fragments: Origins, processing, and functions. Wiley Interdiscip. Rev. RNA 2011, 2, 853–862. [Google Scholar] [CrossRef]
- Dieci, G.; Preti, M.; Montanini, B. Eukaryotic snoRNAs: A paradigm for gene expression flexibility. Genomics 2009, 94, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Huang, Z.; Du, Y.; Wen, J.; Lu, B.; Zhao, Y. snoRNAs: Functions and mechanisms in biological processes, and roles in tumor pathophysiology. Cell Death Discov. 2022, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Ozata, D.M.; Gainetdinov, I.; Zoch, A.; O’Carroll, D.; Zamore, P.D. PIWI-interacting RNAs: Small RNAs with big functions. Nat. Rev. Genet. 2019, 20, 89–108. [Google Scholar] [CrossRef]
- Wang, X.; Ramat, A.; Simonelig, M.; Liu, M.F. Emerging roles and functional mechanisms of PIWI-interacting RNAs. Nat. Rev. Mol. Cell Biol. 2023, 24, 123–141. [Google Scholar] [CrossRef]
- Stoyko, D.; Genzor, P.; Haase, A.D. Hierarchical length and sequence preferences establish a single major piRNA 3′-end. Iscience 2022, 25, 104427. [Google Scholar] [CrossRef] [PubMed]
- Perera, B.P.; Tsai, Z.T.-Y.; Colwell, M.L.; Jones, T.R.; Goodrich, J.M.; Wang, K.; Sartor, M.A.; Faulk, C.; Dolinoy, D.C. Somatic expression of piRNA and associated machinery in the mouse identifies short, tissue-specific piRNA. Epigenetics 2019, 14, 504–521. [Google Scholar] [CrossRef] [PubMed]
- Czech, B.; Munafò, M.; Ciabrelli, F.; Eastwood, E.L.; Fabry, M.H.; Kneuss, E.; Hannon, G.J. piRNA-guided genome defense: From biogenesis to silencing. Annu. Rev. Genet. 2018, 52, 131–157. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Zhou, H.; Zhang, P.; Song, T.; Ying, Z.; Yu, H.; Li, Y.; Zhao, Y.; Zeng, X.; et al. piRBase: Integrating piRNA annotation in all aspects. Nucleic Acids Res. 2022, 50, D265–D272. [Google Scholar] [CrossRef]
- Aalto, A.P.; Pasquinelli, A.E. Small non-coding RNAs mount a silent revolution in gene expression. Curr. Opin. Cell Biol. 2012, 24, 333–340. [Google Scholar] [CrossRef]
- Redis, R.S.; Calin, G.A. SnapShot: Non-coding RNAs and metabolism. Cell Metab. 2017, 25, 220. [Google Scholar] [CrossRef]
- Nicolas, F.E. Role of ncRNAs in development, diagnosis and treatment of human cancer. Recent Pat Anticancer. Drug Discov. 2017, 12, 128–135. [Google Scholar] [CrossRef]
- Karnati, H.K.; Panigrahi, M.K.; Gutti, R.K.; Greig, N.H.; Tamargo, I.A. miRNAs: Key players in neurodegenerative disorders and epilepsy. J. Alzheimer’s Dis. 2015, 48, 563–580. [Google Scholar] [CrossRef]
- Nwaobi, S.E.; Lin, E.; Peramsetty, S.R.; Olsen, M.L. DNA methylation functions as a critical regulator of Kir4.1 expression during CNS development. Glia 2014, 62, 411–427. [Google Scholar] [CrossRef]
- Winek, K.; Soreq, H. Emerging roles of transfer RNA fragments in the CNS. Brain 2025, 148, 2631–2645. [Google Scholar] [CrossRef]
- McArdle, H.; Hogg, M.C.; Bauer, S.; Rosenow, F.; Prehn, J.H.M.; Adamson, K.; Henshall, D.C.; Spain, E. Quantification of tRNA fragments by electrochemical direct detection in small volume biofluid samples. Sci. Rep. 2020, 10, 7516. [Google Scholar] [CrossRef] [PubMed]
- Gioiosa, S.; Gasparini, S.; Presutti, C.; Rinaldi, A.; Castrignanò, T.; Mannironi, C. Integrated gene expression and alternative splicing analysis in human and mouse models of Rett syndrome. Sci. Rep. 2025, 15, 2778. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Niswander, L.A. m5C methylated lncRncr3–MeCP2 interaction restricts miR124a-initiated neurogenesis. Nat. Commun. 2024, 15, 5136. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Tang, D.; Carninci, P. piRNAs warrant investigation in Rett Syndrome: An omics perspective. Dis. Markers 2012, 33, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Petazzi, P.; Sandoval, J.; Szczesna, K.; Jorge, O.C.; Roa, L.; Sayols, S.; Gomez, A.; Huertas, D.; Esteller, M. Dysregulation of the long non-coding RNA transcriptome in a Rett syndrome mouse model. RNA Biol. 2013, 10, 1197–1203. [Google Scholar] [CrossRef]
- Horvath, P.M.; Piazza, M.K.; Kavalali, E.T.; Monteggia, L.M. MeCP2 loss-of-function dysregulates microRNAs regionally and disrupts excitatory/inhibitory synaptic transmission balance. Hippocampus 2022, 32, 610–623. [Google Scholar] [CrossRef]
- Lou, S.; Tihagam, R.D.; Wasko, U.N.; Equbal, Z.; Venkatesan, S.; Braczyk, K.; Przanowski, P.; Koo, B.I.; Saltani, I.; Singh, A.T.; et al. Targeting microRNA-dependent control of X chromosome inactivation improves the Rett Syndrome phenotype. Nat. Commun. 2025, 16, 6169. [Google Scholar] [CrossRef]
- Wang, I.-T.J.; Allen, M.; Goffin, D.; Zhu, X.; Fairless, A.H.; Brodkin, E.S.; Siegel, S.J.; Marsh, E.D.; Blendy, J.A.; Zhou, Z. Loss of CDKL5 disrupts kinome profile and event-related potentials leading to autistic-like phenotypes in mice. Proc. Natl. Acad. Sci. USA 2012, 109, 21516–21521. [Google Scholar] [CrossRef]
- Heiland, M.; Connolly, N.M.C.; Mamad, O.; Nguyen, N.T.; Kesavan, J.C.; Langa, E.; Fanning, K.; Sanfeliu, A.; Yan, Y.; Su, J.; et al. MicroRNA-335-5p suppresses voltage-gated sodium channel expression and may be a target for seizure control. Proc. Natl. Acad. Sci. USA 2023, 120, e2216658120. [Google Scholar] [CrossRef]
- Rishik, S.; Hirsch, P.; Grandke, F.; Fehlmann, T.; Keller, A. miRNATissueAtlas 2025: An update to the uniformly processed and annotated human and mouse non-coding RNA tissue atlas. Nucleic Acids Res. 2025, 53, D129–D137. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 366, eaav1741. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, E.; Yuan, L.; Seong, E.; Ligon, C.; DeKorver, N.; Gurumurthy, C.; Arikkath, J. Neuron-type specific loss of CDKL5 leads to alterations in mTOR signaling and synaptic markers. Mol. Neurobiol. 2019, 56, 4151–4162. [Google Scholar] [CrossRef]
- Zhu, Z.-A.; Li, Y.-Y.; Xu, J.; Xue, H.; Feng, X.; Zhu, Y.-C.; Xiong, Z.-Q. CDKL5 deficiency in adult glutamatergic neurons alters synaptic activity and causes spontaneous seizures via TrkB signaling. Cell Rep. 2023, 42, 113202. [Google Scholar] [CrossRef]
- Silvestre, M.; Dempster, K.; Mihaylov, S.R.; Claxton, S.; Ultanir, S.K. Cell type-specific expression, regulation and compensation of CDKL5 activity in mouse brain. Mol. Psychiatry 2024, 29, 1844–1856. [Google Scholar] [CrossRef]
- Eddy, S.R. Non–coding RNA genes and the modern RNA world. Nat. Rev. Genet. 2001, 2, 919–929. [Google Scholar] [CrossRef]
- Chen, Y.; Mateski, J.; Gerace, L.; Wheeler, J.; Burl, J.; Prakash, B.; Svedin, C.; Amrick, R.; Adams, B.D. Non-coding RNAs and neuroinflammation: Implications for neurological disorders. Exp. Biol. Med. 2024, 249, 10120. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Liu, Z.; Shi, Z.S. Non-coding RNA in acute ischemic stroke: Mechanisms, biomarkers and therapeutic targets. Cell Transplant. 2018, 27, 1763–1777. [Google Scholar] [CrossRef]
- Salvatori, B.; Biscarini, S.; Morlando, M. Non-coding RNAs in nervous system development and disease. Front. Cell Dev. Biol. 2020, 8, 273. [Google Scholar] [CrossRef]
- Siqueira, E.; Velasco, C.D.; Tarrasón, A.; Soler, M.; Srinivas, T.; Setién, F.; Oliveira-Mateos, C.; Casado-Pelaez, M.; Martinez-Verbo, L.; Armstrong, J.; et al. NEAT1-mediated regulation of proteostasis and mRNA localization impacts autophagy dysregulation in Rett syndrome. Nucleic Acids Res. 2025, 53, gkaf074. [Google Scholar] [CrossRef]
- Cheng, T.L.; Qiu, Z. MeCP2: Multifaceted roles in gene regulation and neural development. Neurosci. Bull. 2014, 30, 601–609. [Google Scholar] [CrossRef]
- Szulwach, K.E.; Li, X.; Smrt, R.D.; Li, Y.; Luo, Y.; Lin, L.; Santistevan, N.J.; Li, W.; Zhao, X.; Jin, P. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J. Cell Biol. 2010, 189, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shin, B.C.; Thamotharan, S.; Devaskar, S.U. Differential methylation of the micro-RNA 7b gene targets postnatal maturation of murine neuronal Mecp2 gene expression. Dev. Neurobiol. 2014, 74, 407–425. [Google Scholar] [CrossRef]
- Gao, Y.; Su, J.; Guo, W.; Polich, E.D.; Magyar, D.P.; Xing, Y.; Li, H.; Smrt, R.D.; Chang, Q.; Zhao, X. Inhibition of miR-15a Promotes BDNF Expression and Rescues Dendritic Maturation Deficits in MeCP2-Deficient Neurons. Stem Cells 2015, 33, 1618–1629. [Google Scholar] [CrossRef]
- Marano, D.; Fioriniello, S.; D’Esposito, M.; Della Ragione, F. Transcriptomic and Epigenomic Landscape in Rett Syndrome. Biomolecules 2021, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- Urdinguio, R.G.; Fernández, A.F.; Lopez-Nieva, P.; Rossi, S.; Huertas, D.; Kulis, M.; Liu, C.-G.; Croce, C.M.; Calin, G.A.; Esteller, M. Disrupted microRNA expression caused by Mecp2 loss in a mouse model of Rett syndrome. Epigenetics 2010, 5, 656–663. [Google Scholar] [CrossRef]
- Henshall, D.C. MicroRNAs Fine-Tune Brain and Body Communication in Health and Disease. Brain-Body Connect. 2025, 1477, 311–337. [Google Scholar]
- Mari, F.; Azimonti, S.; Bertani, I.; Bolognese, F.; Colombo, E.; Caselli, R.; Scala, E.; Longo, I.; Grosso, S.; Pescucci, C.; et al. CDKL5 belongs to the same molecular pathway of MeCP2 and it is responsible for the early-onset seizure variant of Rett syndrome. Hum. Mol. Genet. 2005, 14, 1935–1946. [Google Scholar] [CrossRef]
- Carouge, D.; Host, L.; Aunis, D.; Zwiller, J.; Anglard, P. CDKL5 is a brain MeCP2 target gene regulated by DNA methylation. Neurobiol. Dis. 2010, 38, 414–424. [Google Scholar] [CrossRef]
- Ashhab, M.U.; Omran, A.; Gan, N.; Kong, H.; Peng, J.; Yin, F. microRNA s (9, 138, 181A, 221, and 222) and mesial temporal lobe epilepsy in developing brains. Transl. Neurosci. 2013, 4, 357–362. [Google Scholar] [CrossRef]
- Yousefi, M.J.; Rezvanimehr, A.; Saleki, K.; Mehrani, A.; Barootchi, E.; Ramezankhah, M.; Mazloomi, A.; Nateri, A.S.; Barootchi, S.; Rezaei, N. Inflammation-related microRNA alterations in epilepsy: A systematic review of human and animal studies. Rev. Neurosci. 2025. [Google Scholar] [CrossRef]
- Rajabi, M.; Kalantar, S.M.; Mojodi, E.; Salehi, M.; Firouzabadi, R.D.; Etemadifar, S.M.; Montazeri, F. Assessment of circulating miRNA-218, miRNA-222, and miRNA-146 as biomarkers of polycystic ovary syndrome in epileptic patients receiving valproic acid. Biomed. Res. Ther. 2023, 10, 5884–5895. [Google Scholar] [CrossRef]
- Zhang, C.-Z.; Zhang, J.-X.; Zhang, A.-L.; Shi, Z.-D.; Han, L.; Jia, Z.-F.; Yang, W.-D.; Wang, G.-X.; Jiang, T.; You, Y.-P.; et al. MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol. Cancer 2010, 9, 229. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Niu, J.Z. miR-222 regulates brain injury and inflammation following intracerebral hemorrhage by targeting ITGB8. Mol. Med. Rep. 2020, 21, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Shen, D.; Wang, Y.; Gong, L.; Tang, X.; Yu, B.; Gu, X.; Ding, F. microRNA-222 targeting PTEN promotes neurite outgrowth from adult dorsal root ganglion neurons following sciatic nerve transection. PLoS ONE 2012, 7, e44768. [Google Scholar]
- Szydlowska, K.; Bot, A.; Nizinska, K.; Olszewski, M.; Lukasiuk, K. Circulating microRNAs from plasma as preclinical biomarkers of epileptogenesis and epilepsy. Sci. Rep. 2024, 14, 708. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Hussen, B.M.; Abak, A.; Taheri, M.; Jalili Khoshnoud, R. Aberrant expression of miRNAs in epilepsy. Mol. Biol. Rep. 2022, 49, 5057–5074. [Google Scholar] [CrossRef]
- Wu, H.; Tao, J.; Chen, P.J.; Shahab, A.; Ge, W.; Hart, R.P.; Ruan, X.; Ruan, Y.; Sun, Y.E. Genome-wide analysis reveals methyl-CpG-binding protein 2-dependent regulation of microRNAs in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA 2010, 107, 18161–18166. [Google Scholar] [CrossRef]
- Li, P.; Cheng, Y.; Zhang, X.; Li, P.; Yang, C.; Tang, J.; Deng, X.; Yang, X.; Tao, J.; Lu, Q. MiR-200c promotes bladder cancer cell migration and invasion by directly targeting RECK. Onco Targets Ther. 2016, ume 9, 5091–5099. [Google Scholar] [CrossRef]
- McKiernan, R.C.; Jimenez-Mateos, E.M.; Bray, I.; Engel, T.; Brennan, G.P.; Sano, T.; Michalak, Z.; Moran, C.; Delanty, N.; Farrell, M.; et al. Reduced mature microRNA levels in association with dicer loss in human temporal lobe epilepsy with hippocampal sclerosis. PLoS ONE 2012, 7, e35921. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chi, X.; An, W. Downregulation of microRNA-200c-3p reduces damage of hippocampal neurons in epileptic rats by upregulating expression of RECK and inactivating the AKT signaling pathway. Chem. Biol. Interact. 2019, 307, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Higaki, S.; Muramatsu, M.; Matsuda, A.; Matsumoto, K.; Satoh, J.-I.; Michikawa, M.; Niida, S. Defensive effect of microRNA-200b/c against amyloid-beta peptide-induced toxicity in Alzheimer’s disease models. PLoS ONE 2018, 13, e0196929. [Google Scholar] [CrossRef] [PubMed]
- Galvani, G.; Mottolese, N.; Gennaccaro, L.; Loi, M.; Medici, G.; Tassinari, M.; Fuchs, C.; Ciani, E.; Trazzi, S. Inhibition of microglia overactivation restores neuronal survival in a mouse model of CDKL5 deficiency disorder. J. Neuroinflamm. 2021, 18, 155. [Google Scholar] [CrossRef]
- Dell’isola, G.B.; Perinelli, M.G.; Frulli, A.; D’onofrio, G.; Fattorusso, A.; Siciliano, M.; Ferrara, P.; Striano, P.; Verrotti, A. Exploring neurodevelopment in CDKL5 deficiency disorder: Current insights and future directions. Epilepsy Behav. 2025, 171, 110504. [Google Scholar] [CrossRef]
- De Rosa, R.; Valastro, S.; Cambria, C.; Barbiero, I.; Puricelli, C.; Tramarin, M.; Randi, S.; Bianchi, M.; Antonucci, F.; Kilstrup-Nielsen, C. Loss of CDKL5 Causes Synaptic GABAergic Defects That Can Be Restored with the Neuroactive Steroid Pregnenolone-Methyl-Ether. Int. J. Mol. Sci. 2022, 24, 68. [Google Scholar] [CrossRef]
- Glass, M.R.; Whye, D.; Anderson, N.C.; Wood, D.; Makhortova, N.R.; Polanco, T.; Kim, K.H.; Donovan, K.E.; Srinivasan, G.R.; Vaccaro, L.; et al. Excitatory Cortical Neurons from CDKL5 Deficiency Disorder Patient-Derived Organoids Show Early Hyperexcitability Not Identified in Neurogenin2 Induced Neurons. Neurobiol. Dis. 2025, 215, 107093. [Google Scholar] [CrossRef]
- Massey, S.; Ang, C.S.; Davidson, N.M.; Quigley, A.; Rollo, B.; Harris, A.R.; Kapsa, R.M.I.; Christodoulou, J.; Van Bergen, N.J. Novel CDKL5 targets identified in human iPSC-derived neurons. Cell Mol. Life Sci. 2024, 81, 347. [Google Scholar] [CrossRef]
- Sampedro-castañeda, M.; Baltussen, L.L.; Lopes, A.T.; Qiu, Y.; Sirvio, L.; Mihaylov, S.R.; Claxton, S.; Richardson, J.C.; Lignani, G. Epilepsy-linked kinase CDKL5 phosphor- altering inactivation kinetics and neuronal excitability. Nat. Commun. 2023, 14, 7830. [Google Scholar] [CrossRef]
- Baltussen, L.L.; Negraes, P.D.; Silvestre, M.; Claxton, S.; Moeskops, M.; Christodoulou, E.; Flynn, H.R.; Snijders, A.P.; Muotri, A.R.; Ultanir, S.K. Chemical genetic identification of CDKL5 substrates reveals its role in neuronal microtubule dynamics. EMBO J. 2018, 37, e99763. [Google Scholar] [CrossRef]
- Ricciardi, S.; Ungaro, F.; Hambrock, M.; Rademacher, N.; Stefanelli, G.; Brambilla, D.; Sessa, A.; Magagnotti, C.; Bachi, A.; Giarda, E.; et al. CDKL5 ensures excitatory synapse stability by reinforcing NGL-1–PSD95 interaction in the postsynaptic compartment and is impaired in patient iPSC-derived neurons. Nat. Cell Biol. 2012, 14, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Ghiretti, A.E.; Paradis, S. The GTPase Rem2 regulates synapse development and dendritic morphology. Dev. Neurobiol. 2011, 71, 374–389. [Google Scholar] [CrossRef]
- Kandratavicius, L.; Monteiro, M.R.; Hallak, J.E.; Carlotti, C.G.J.; Assirati, J.A.J.; Leite, J.P. Microtubule-associated proteins in mesial temporal lobe epilepsy with and without psychiatric comorbidities and their relation with granular cell layer dispersion. Biomed. Res. Int. 2013, 2013, 960126. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.R.; Chatterjee, M.; Taylor, A.M.; Gropman, A.L. SYNGAP1 Syndrome and the Brain Gene Registry. Genes 2025, 16, 405. [Google Scholar] [CrossRef]
- Green, J.L.; Dos Santos, W.F.; Fontana, A.C.K. Role of glutamate excitotoxicity and glutamate transporter EAAT2 in epilepsy: Opportunities for novel therapeutics development. Biochem. Pharmacol. 2021, 193, 114786. [Google Scholar] [CrossRef] [PubMed]
- Maximov, A.; Shin, O.H.; Liu, X.; Südhof, T.C. Synaptotagmin-12, a synaptic vesicle phosphoprotein that modulates spontaneous neurotransmitter release. J. Cell Biol. 2007, 176, 113–124. [Google Scholar] [CrossRef]
- Riva, M.; Ferreira, S.; Hayashi, K.; Saillour, Y.; Medvedeva, V.P.; Honda, T.; Hayashi, K.; Altersitz, C.; Albadri, S.; Rosello, M.; et al. De novo monoallelic Reelin missense variants cause dominant neuronal migration disorders via a dominant-negative mechanism. J. Clin. Invest. 2024, 134, e153097. [Google Scholar] [CrossRef]
- Kádková, A.; Murach, J.; Østergaard, M.; Malsam, A.; Malsam, J.; Lolicato, F.; Nickel, W.; Söllner, T.H.; Sørensen, J.B. SNAP25 disease mutations change the energy landscape for synaptic exocytosis due to aberrant SNARE interactions. Elife 2024, 12, RP88619. [Google Scholar] [CrossRef]
- Rehmsmeier, M.; Steffen, P.; Höchsmann, M.; Giegerich, R. Fast and effective prediction of microRNA/target duplexes. RNA 2004, 10, 1507–1517. [Google Scholar] [CrossRef]
- Vorozheykin, P.S.; Titov, I.I. Web server for prediction of miRNAs and their precursors and binding sites. Mol. Biol. 2015, 49, 755–761. [Google Scholar] [CrossRef]
- Christopher, A.F.; Kaur, R.P.; Kaur, G.; Kaur, A.; Gupta, V.; Bansal, P. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect. Clin. Res. 2016, 7, 68–74. [Google Scholar] [CrossRef]
- Hogg, M.C.; Raoof, R.; El Naggar, H.; Monsefi, N.; Delanty, N.; O’brien, D.F.; Bauer, S.; Rosenow, F.; Henshall, D.C.; Prehn, J.H. Elevation of plasma tRNA fragments precedes seizures in human epilepsy. J. Clin. investig. 2019, 129, 2946–2951. [Google Scholar] [CrossRef]
- Jirström, E.; Matveeva, A.; Baindoor, S.; Donovan, P.; Ma, Q.; Morrissey, E.P.; Arijs, I.; Boeckx, B.; Lambrechts, D.; Garcia-Munoz, A.; et al. Effects of ALS-associated 5’tiRNAGly-GCC on the transcriptomic and proteomic profile of primary neurons in vitro. Exp. Neurol. 2025, 385, 115128. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, A.E.; Pinkard, O.; Coller, J.M. tRNA metabolism and neurodevelopmental disorders. Annu. Rev. Genom. Hum. Genet. 2019, 20, 359–387. [Google Scholar] [CrossRef] [PubMed]
- Karijolich, J.; Yu, Y.T. Spliceosomal snRNA modifications and their function. RNA Biol. 2010, 7, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, R.; Lai, Y. Expression signature of ten small nuclear RNAs serves as novel biomarker for prognosis prediction of acute myeloid leukemia. Sci. Rep. 2023, 13, 18489. [Google Scholar] [CrossRef] [PubMed]
- Matera, A.G.; Terns, R.M.; Terns, M.P. Non-coding RNAs: Lessons from the small nuclear and small nucleolar RNAs. Nat. Rev. Mol. Cell Biol. 2007, 8, 209–220. [Google Scholar] [CrossRef]
- Watkins, N.J.; Bohnsack, M.T. The box C/D and H/ACA snoRNPs: Key players in the modification, processing and the dynamic folding of ribosomal RNA. Wiley Interdiscip. Rev. RNA 2012, 3, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Ruggero, D.; Pandolfi, P.P. Does the ribosome translate cancer? Nat. Rev. Cancer 2003, 3, 179–192. [Google Scholar] [CrossRef]
- Falaleeva, M.; Stamm, S. Processing of snoRNAs as a new source of regulatory non-coding RNAs: snoRNA fragments form a new class of functional RNAs. Bioessays 2013, 35, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Zhou, N.; Wu, K.; Guo, Y.; Tan, W.; Zhang, H.; Zhang, X.; Geng, G.; Pan, T.; Luo, H.; et al. A SnoRNA-derived piRNA interacts with human interleukin-4 pre-mRNA and induces its decay in nuclear exosomes. Nucleic Acids Res. 2015, 43, 10474–10491. [Google Scholar] [CrossRef]
- Zhao, P.-P.; Yao, M.-J.; Chang, S.-Y.; Gou, L.-T.; Liu, M.-F.; Qiu, Z.-L.; Yuan, X.-B. Novel function of PIWIL1 in neuronal polarization and migration via regulation of microtubule-associated proteins. Mol. Brain 2015, 8, 39. [Google Scholar] [CrossRef]
- Ghosheh, Y.; Seridi, L.; Ryu, T.; Takahashi, H.; Orlando, V.; Carninci, P.; Ravasi, T. Characterization of piRNAs across postnatal development in mouse brain. Sci. Rep. 2016, 6, 25039. [Google Scholar] [CrossRef]
- Nandi, S.; Chandramohan, D.; Fioriti, L.; Melnick, A.M.; Hébert, J.M.; Mason, C.E.; Rajasethupathy, P.; Kandel, E.R. Roles for small noncoding RNAs in silencing of retrotransposons in the mammalian brain. Proc. Natl. Acad. Sci. USA 2016, 113, 12697–12702. [Google Scholar] [CrossRef]
- Leighton, L.J.; Wei, W.; Marshall, P.R.; Ratnu, V.S.; Li, X.; Zajaczkowski, E.L.; Spadaro, P.A.; Khandelwal, N.; Kumar, A.; Bredy, T.W. Disrupting the hippocampal Piwi pathway enhances contextual fear memory in mice. Neurobiol. Learn. Mem. 2019, 161, 202–209. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, K.-M.; Pandey, R.R.; Homolka, D.; Reuter, M.; Janeiro, B.K.R.; Sachidanandam, R.; Fauvarque, M.-O.; McCarthy, A.A.; Pillai, R.S. PIWI slicing and EXD1 drive biogenesis of nuclear piRNAs from cytosolic targets of the mouse piRNA pathway. Mol. Cell 2016, 61, 138–152. [Google Scholar] [CrossRef] [PubMed]
- De Fazio, S.; Bartonicek, N.; Di Giacomo, M.; Abreu-Goodger, C.; Sankar, A.; Funaya, C.; Antony, C.; Moreira, P.N.; Enright, A.J.; O’cArroll, D. The endonuclease activity of Mili fuels piRNA amplification that silences LINE1 elements. Nature 2011, 480, 259–263. [Google Scholar] [CrossRef]
- Reuter, M.; Berninger, P.; Chuma, S.; Shah, H.; Hosokawa, M.; Funaya, C.; Antony, C.; Sachidanandam, R.; Pillai, R.S. Miwi catalysis is required for piRNA amplification-independent LINE1 transposon silencing. Nature 2011, 480, 264–267. [Google Scholar] [CrossRef]
- Baillie, J.K.; Barnett, M.W.; Upton, K.R.; Gerhardt, D.J.; Richmond, T.A.; De Sapio, F.; Brennan, P.M.; Rizzu, P.; Smith, S.; Fell, M.; et al. Somatic retrotransposition alters the genetic landscape of the human brain. Nature 2011, 479, 534–537. [Google Scholar] [CrossRef] [PubMed]
- de Thé, F.B.; Rekaik, H.; Peze-Heidsieck, E.; Massiani-Beaudoin, O.; Joshi, R.L.; Fuchs, J.; Prochiantz, A. Engrailed homeoprotein blocks degeneration in adult dopaminergic neurons through LINE-1 repression. EMBO J. 2018, 37, e97374. [Google Scholar] [CrossRef]
- Sun, W.; Samimi, H.; Gamez, M.; Zare, H.; Frost, B. Pathogenic tau-induced piRNA depletion promotes neuronal death through transposable element dysregulation in neurodegenerative tauopathies. Nat. Neurosci. 2018, 21, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Sommer, A.; Plötz, S.; Farrell, M.; Winner, B.; Grosch, J.; Winkler, J.; Riemenschneider, M.J. Sporadic Parkinson’s disease derived neuronal cells show disease-specific mRNA and small RNA signatures with abundant deregulation of piRNAs. Acta Neuropathol. Commun. 2018, 6, 58. [Google Scholar] [CrossRef]
- Zhan, L.; Chen, M.; Pang, T.; Li, X.; Long, L.; Liang, D.; Peng, L.; Sun, W.; Xu, E. Attenuation of Piwil2 induced by hypoxic postconditioning prevents cerebral ischemic injury by inhibiting CREB2 promoter methylation. Brain Pathol. 2023, 33, e13109. [Google Scholar] [CrossRef]
- Qiu, W.; Guo, X.; Lin, X.; Yang, Q.; Zhang, W.; Zhang, Y.; Zuo, L.; Zhu, Y.; Li, C.-S.R.; Ma, C.; et al. Transcriptome-wide piRNA profiling in human brains of Alzheimer’s disease. Neurobiol. Aging. 2017, 57, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Iossifov, I.; O’Roak, B.J.; Sanders, S.J.; Ronemus, M.; Krumm, N.; Levy, D.; Stessman, H.A.; Witherspoon, K.T.; Vives, L.; Patterson, K.E.; et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 2014, 515, 216–221. [Google Scholar] [CrossRef]
- Roy, J.; Sarkar, A.; Parida, S.; Ghosh, Z.; Mallick, B. Small RNA sequencing revealed dysregulated piRNAs in Alzheimer’s disease and their probable role in pathogenesis. Mol. Biosyst. 2017, 13, 565–576. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Astroglial cradle in the life of the synapse. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130595. [Google Scholar] [CrossRef]
- Allen, N.J.; Eroglu, C. Cell biology of astrocyte-synapse interactions. Neuron 2017, 96, 697–708. [Google Scholar] [CrossRef]
- El-Mansoury, B.; El Hiba, O.; Jayakumar, A.R. Physiology and Function of Glial Cells in Health and Disease; IGI Global: Hershey, PA, USA, 2023. [Google Scholar]
- Alsharafi, W.A.; Luo, Z.; Long, X.; Xie, Y.; Xiao, B. MicroRNA in glutamate receptor-dependent neurological diseases. Clin. Sci. 2017, 131, 1591–1604. [Google Scholar] [CrossRef]
- Chu, A.J.; Williams, J.M. Astrocytic MicroRNA in ageing, inflammation, and neurodegenerative disease. Front. Physiol. 2022, 12, 826697. [Google Scholar] [CrossRef]
- Marangon, D.; Castro e Silva, J.H.; Lecca, D. Neuronal and glial communication via non-coding RNAs: Messages in extracellular vesicles. Int. J. Mol. Sci. 2022, 24, 470. [Google Scholar] [CrossRef]
- Helmut, K.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- El-Mansoury, B.; Smimih, K.; Hamdan, Y.A.; Draoui, A.; Boulbaroud, S.; Jayakumar, A.R. Microglial Cells Function in the Central Nervous System: Beyond the Immune Function. In Physiology and Function of Glial Cells in Health and Disease; IGI Global: Hershey, PA, USA, 2024; pp. 60–82. [Google Scholar]
- El-Mansoury, B.; Smimih, K.; El Khiat, A.; Draoui, A.; Aimrane, A.; Chatoui, R.; Ferssiwi, A.; Bitar, A.; Gamrani, H.; Jayakumar, A.R.; et al. Short Working Memory Impairment Associated with Hippocampal Microglia Activation in Chronic Hepatic Encephalopathy. Metabolites 2024, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, A.D.; Fonken, L.K.; Watkins, L.R.; Nelson, R.J.; Popovich, P.G. MicroRNAs: Roles in regulating neuroinflammation. Neurosci 2018, 24, 221–245. [Google Scholar] [CrossRef]
- Freilich, R.W.; Woodbury, M.E.; Ikezu, T. Integrated expression profiles of mRNA and miRNA in polarized primary murine microglia. PLoS ONE 2013, 8, e79416. [Google Scholar] [CrossRef]
- Mottolese, N. Role of Neuroinflammation in the Pathophysiology of CDKL5 Deficiency Disorder. Ph.D. Thesis, ALMA Mater Studiorum Università di Bologna, Bologna, Italy, 2024. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Mansoury, B.; Hayes, A.; Egan, S.; Higgins, J.; Keane, S.B.; Langa, E.; Ghani, E.; Venø, M.T.; Heiland, M.; Henshall, D.C.; et al. Altered Short Non-Coding RNA Landscape in the Hippocampus of a Mouse Model of CDKL5 Deficiency Disorder. Biomolecules 2025, 15, 1612. https://doi.org/10.3390/biom15111612
El-Mansoury B, Hayes A, Egan S, Higgins J, Keane SB, Langa E, Ghani E, Venø MT, Heiland M, Henshall DC, et al. Altered Short Non-Coding RNA Landscape in the Hippocampus of a Mouse Model of CDKL5 Deficiency Disorder. Biomolecules. 2025; 15(11):1612. https://doi.org/10.3390/biom15111612
Chicago/Turabian StyleEl-Mansoury, Bilal, Adrian Hayes, Samuel Egan, Jordan Higgins, Stephen B. Keane, Elena Langa, Erva Ghani, Morten T. Venø, Mona Heiland, David C. Henshall, and et al. 2025. "Altered Short Non-Coding RNA Landscape in the Hippocampus of a Mouse Model of CDKL5 Deficiency Disorder" Biomolecules 15, no. 11: 1612. https://doi.org/10.3390/biom15111612
APA StyleEl-Mansoury, B., Hayes, A., Egan, S., Higgins, J., Keane, S. B., Langa, E., Ghani, E., Venø, M. T., Heiland, M., Henshall, D. C., & Mamad, O. (2025). Altered Short Non-Coding RNA Landscape in the Hippocampus of a Mouse Model of CDKL5 Deficiency Disorder. Biomolecules, 15(11), 1612. https://doi.org/10.3390/biom15111612






