Rapid In Situ Coating of Covered Stents with Highly Tough, Biocompatible Membrane for Emergency Coronary Artery Perforation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
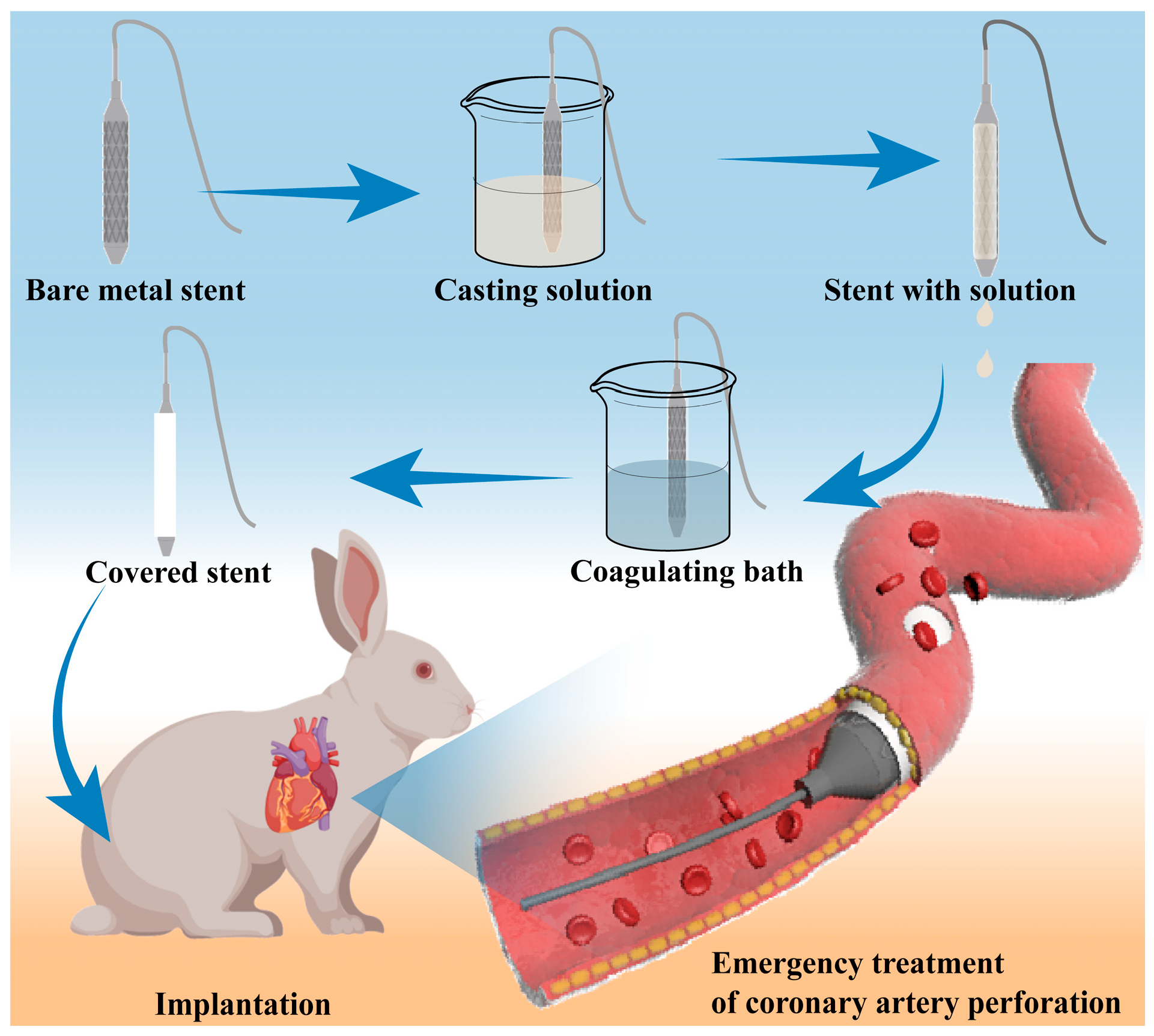
2.2. Preparation of Covered Stent
2.2.1. Preparation of Casting Solution
2.2.2. Coating of the Coronary Stent
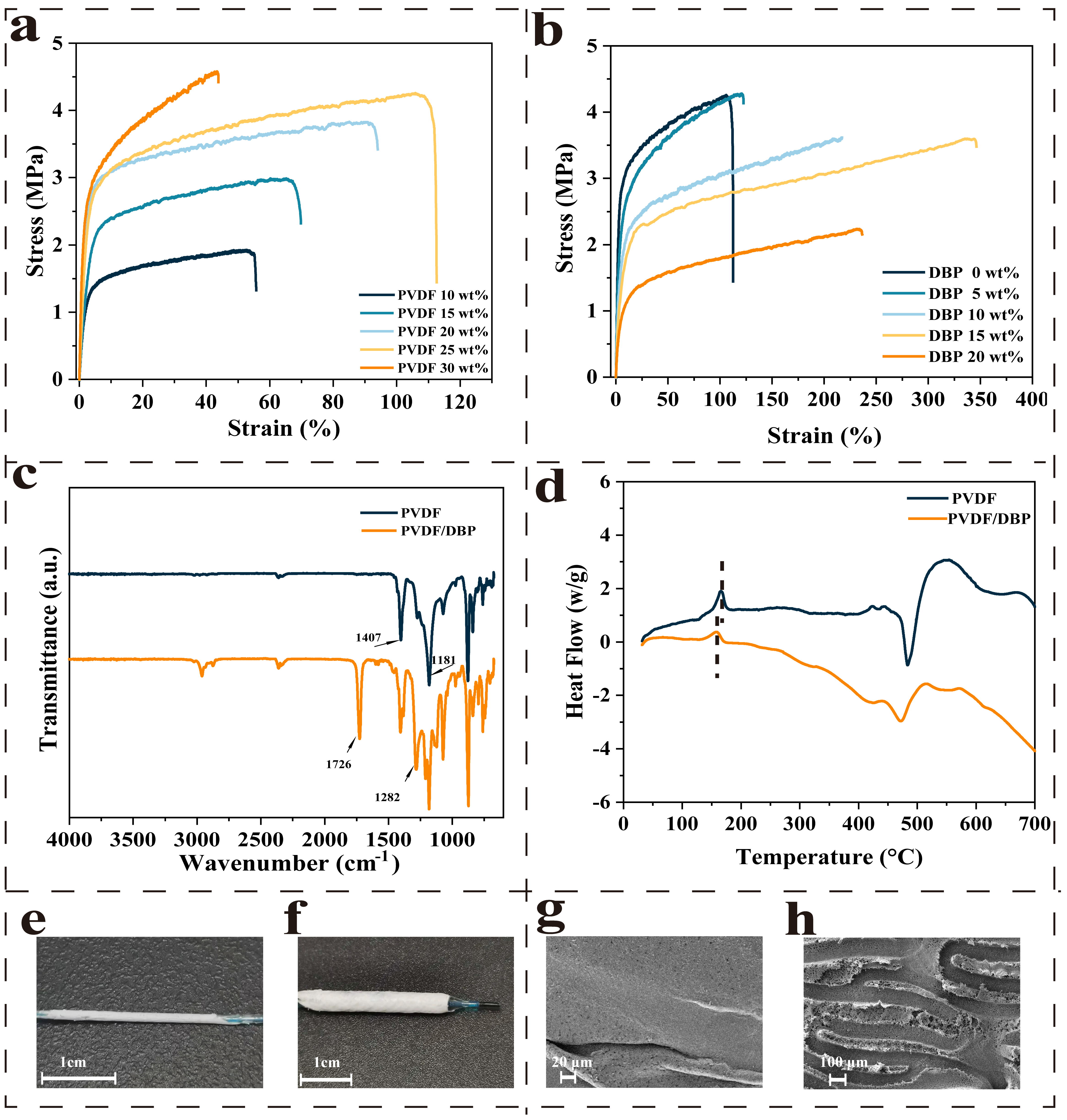
2.3. Mechanical Property of the Membrane
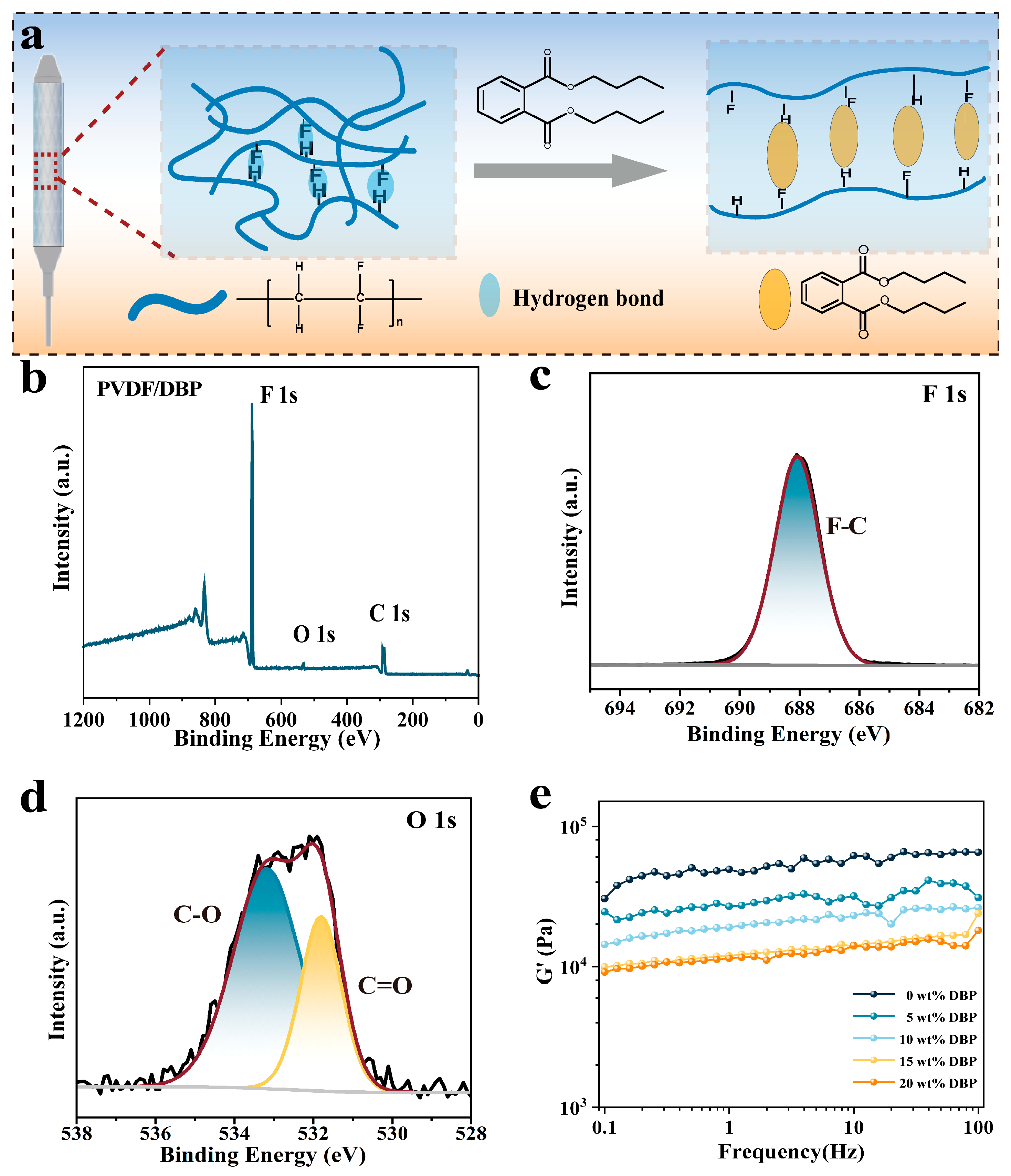
2.4. Chemical Characterization of the Membrane
2.5. Differential Scanning Calorimetry (DSC)
2.6. Scanning Electron Microscopy (SEM)
2.7. Storage Modulus
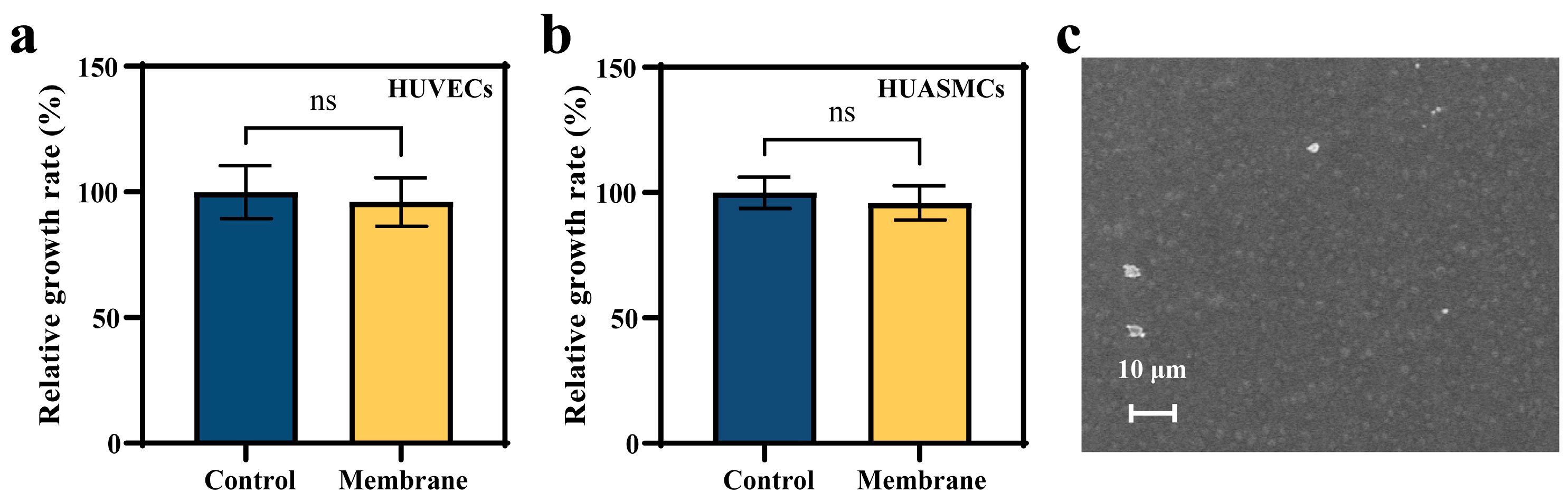
2.8. In Vitro Cell Experiment
2.8.1. MTT Assay
2.8.2. Scratch Test
2.8.3. Apoptosis Assay
2.9. Hemocompatibility
2.10. In Vivo Covered Stent Implantation in Rabbit
2.11. Statistical Analysis
3. Results and Discussion
3.1. Design of Covering Membranes
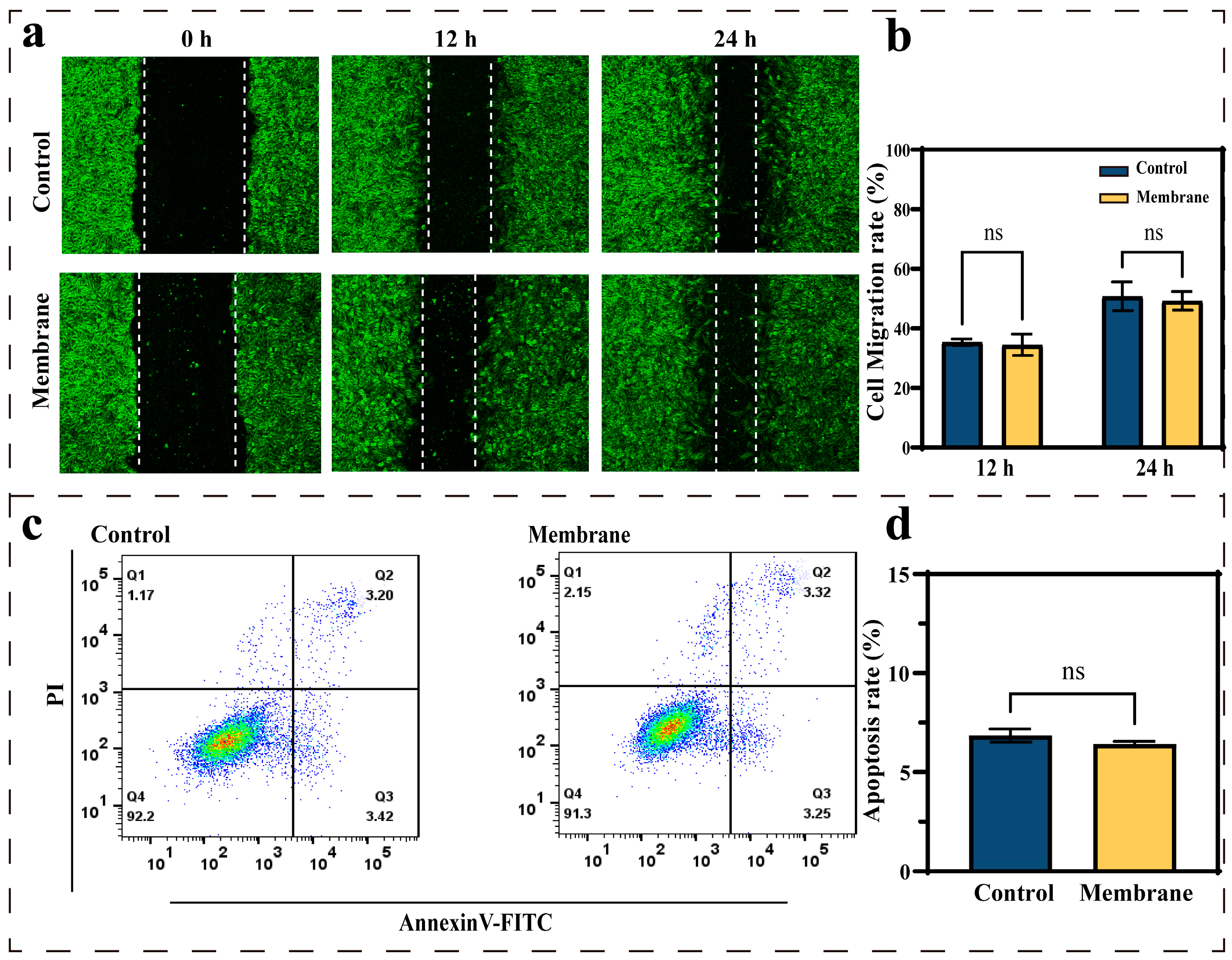
3.2. Effects of the Membrane Materials on In Vitro Biocompatibility
3.3. Covered Stent Deployment in the Rabbit Abdominal Aorta
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAP | Coronary artery perforation |
| PCI | Percutaneous coronary intervention |
| PVDF | polyvinylidene fluoride |
| DBP | Dibutyl phthalate |
| NIPS | Non-solvent induced phase separation |
References
- Strycek, M.; Jaworski, L.; Polasek, R.; Tomasov, P. Coronary artery perforation successfully treated with a second drug-eluting stent. J. Int. Med. Res. 2023, 51, 3000605231174998. [Google Scholar] [CrossRef]
- Shaikh, R.; Tomdio, A.N.; Lawson, B.D. Management of Coronary Artery Perforation in a Patient with Stemi. JACC 2024, 83, 3830. [Google Scholar] [CrossRef]
- Kandzari, D.E.; Sarao, R.C.; Waksman, R. Clinical Experience of the PK Papyrus Covered Stent in Patients With Coronary Artery Perforations: Results From a Multi-Center Humanitarian Device Exemption Survey. Cardiovasc. Revascularization Med. 2022, 43, 97–101. [Google Scholar] [CrossRef]
- Lemmert, M.E.; van Bommel, R.J.; Diletti, R.; Wilschut, J.M.; de Jaegere, P.P.; Zijlstra, F.; Daemen, J.; Van Mieghem, N.M. Clinical Characteristics and Management of Coronary Artery Perforations: A Single-Center 11-Year Experience and Practical Overview. J. Am. Heart Assoc. 2017, 6, e007049. [Google Scholar] [CrossRef]
- Harnek, J.; James, S.; Lagerqvist, B. Coronary Artery Perforation and Tamponade—Incidence, Risk Factors, Predictors and Outcomes From 12 Years’ Data of the SCAAR Registry. Circ. J. 2020, 84, 43–53. [Google Scholar] [CrossRef]
- Danek, B.A.; Karatasakis, A.; Tajti, P.; Sandoval, Y.; Karmpaliotis, D.; Alaswad, K.; Jaffer, F.; Yeh, R.W.; Kandzari, D.E.; Lembo, N.J.; et al. Incidence, Treatment, and Outcomes of Coronary Perforation During Chronic Total Occlusion Percutaneous Coronary Intervention. Am. J. Cardiol. 2017, 120, 1285–1292. [Google Scholar] [CrossRef]
- Solomonica, A.; Kerner, A.; Feld, Y.; Yalonetsky, S. Novel Technique for the Treatment of Coronary Artery Perforation. Can. J. Cardiol. 2020, 36, 1326.e1–1326.e3. [Google Scholar] [CrossRef]
- Khan, A.; Kumar, R.; Ali, R.; Fatima, K.; Abid, M.; Ali, R.; Meheshwari, G.; Amin, R.; Hasan, M.; Kasi, A.F.U.D. The prevalence, angiographic profile and clinical features, management, and outcomes of coronary artery perforation secondary to percutaneous coronary interventions in Pakistan: A retrospective cohort study. Ann. Med. Surg. 2023, 85, 2330–2335. [Google Scholar] [CrossRef]
- Kinnaird, T.; Anderson, R.; Ossei-Gerning, N.; Cockburn, J.; Sirker, A.; Ludman, P.; de Belder, M.; Johnson, T.W.; Copt, S.; Zaman, A.; et al. Coronary Perforation Complicating Percutaneous Coronary Intervention in Patients With a History of Coronary Artery Bypass Surgery: An Analysis of 309 Perforation Cases From the British Cardiovascular Intervention Society Database. Circ. Cardiovasc. Interv. 2017, 10, e005581. [Google Scholar] [CrossRef]
- Moroni, F.; Brilakis, E.S.; Azzalini, L. Chronic total occlusion percutaneous coronary intervention: Managing perforation complications. Expert Rev. Cardiovasc. Ther. 2021, 19, 71–87. [Google Scholar] [CrossRef]
- Harnek, J.; James, S.K.; Lagerqvist, B. Very long-term outcome of coronary covered stents: A report from the SCAAR registry. Eurointervention 2019, 14, 1660–1667. [Google Scholar] [CrossRef]
- Barbero, U.; Cerrato, E.; Secco, G.G.; Tedeschi, D.; Belliggiano, D.; Pavani, M.; Moncalvo, C.; Tomassini, F.; De Benedictis, M.; Doronzo, B.; et al. PK Papyrus coronary stent system: The ultrathin struts polyurethane-covered stent. Futur. Cardiol. 2020, 16, 405–411. [Google Scholar] [CrossRef]
- Farhatnia, Y.; Tan, A.; Motiwala, A.; Cousins, B.G.; Seifalian, A.M. Evolution of covered stents in the contemporary era: Clinical application, materials and manufacturing strategies using nanotechnology. Biotechnol. Adv. 2013, 31, 524–542. [Google Scholar] [CrossRef]
- Giannini, F.; Candilio, L.; Mitomo, S.; Ruparelia, N.; Chieffo, A.; Baldetti, L.; Ponticelli, F.; Latib, A.; Colombo, A. A Practical Approach to the Management of Complications During Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2018, 11, 1797–1810. [Google Scholar] [CrossRef]
- Secondo, M.T.S.; Rodrigues, L.D.; Ramos, L.P.M.; Bovolato, A.L.C.; Rodriguez-Sanchez, D.N.; Sobreira, M.L.; Moraes, M.P.D.; Bertanha, M. Evaluation of Biointegration and Inflammatory Response to Blood Vessels Produced by Tissue Engineering-Experimental Model in Rabbits. Biomolecules 2022, 12, 1776. [Google Scholar] [CrossRef]
- Morgan, G.J.; Ciuffreda, M.; Spadoni, I.; DeGiovanni, J. Optimus covered stent: Advanced covered stent technology for complex congenital heart disease. Congenit. Heart Dis. 2018, 13, 458–462. [Google Scholar] [CrossRef]
- Cohen, S.; Magal, S.; Yakov, I.; Sirabella, E.; Bitman, A.; Groisman, G.; Lotan, C. Tissue processing techniques for fabrication of covered stents for small-diameter vascular intervention. Acta Biomater. 2018, 65, 248–258. [Google Scholar] [CrossRef]
- Lee, W.C.; Hsueh, S.K.; Fang, C.Y.; Wu, C.J.; Hang, C.L.; Fang, H.Y. Clinical Outcomes Following Covered Stent for the Treatment of Coronary Artery Perforation. J. Interv. Cardiol. 2016, 29, 569–575. [Google Scholar] [CrossRef]
- Postalian, A.; Krajcer, Z. Pushing covered stents to the limit. Catheter. Cardiovasc. Interv. 2021, 97, 459–460. [Google Scholar] [CrossRef]
- Hou, R.X.; Wu, L.G.; Wang, J.; Yang, Z.L.; Tu, Q.F.; Zhang, X.C.; Huang, N. Surface-Degradable Drug-Eluting Stent with Anticoagulation, Antiproliferation, and Endothelialization Functions. Biomolecules 2019, 9, 69. [Google Scholar] [CrossRef]
- Shen, Y.H.; Yu, X.; Cui, J.; Yu, F.; Liu, M.Y.; Chen, Y.J.; Wu, J.L.; Sun, B.B.; Mo, X.M. Development of Biodegradable Polymeric Stents for the Treatment of Cardiovascular Diseases. Biomolecules 2022, 12, 1245. [Google Scholar] [CrossRef]
- Chashmi, F.S.; Khakbiz, M.; Zahedi, P.; Kabiri, M. Poly(lactic acid) Nanofibrous Scaffolds Containing Aspirin-loaded Zeolitic Imidazolate Frameworks: Morphology, Drug Release, Hemocompatibility and Shape Memory Studies. Fibers Polym. 2022, 23, 2103–2113. [Google Scholar] [CrossRef]
- Yu, Y.X.; Song, G.Z.; Dai, M.N.; Li, P.X.; Xu, J.M.; Yin, Y.; Wang, J.N. Tailoring silk-based covering material with matched mechanical properties for vascular tissue engineering. Sci. Rep. 2024, 14, 23347. [Google Scholar] [CrossRef]
- Chiu, Y.S.; Rinawati, M.; Chang, L.Y.; Aulia, S.; Shi, P.C.; Haw, S.C.; Huang, W.H.; Septiani, N.L.W.; Yuliarto, B.; Yeh, M.H. High-dielectric TiO2-mediated g-C3N4 enhanced self-polarized PVDF hybridized film for highly sensitive wearable triboelectric pressure sensors. Chem. Eng. J. 2025, 511, 161807. [Google Scholar] [CrossRef]
- Bhaskar, N.; Basu, B. Osteogenesis, hemocompatibility, and foreign body response of polyvinylidene difluoride-based composite reinforced with carbonaceous filler and higher volume of piezoelectric ceramic phase. Biomaterials 2023, 297, 122100. [Google Scholar] [CrossRef]
- Azimi, S.; Golabchi, A.; Nekookar, A.; Rabbani, S.; Amiri, M.H.; Asadi, K.; Abolhasani, M.M. Self-powered cardiac pacemaker by piezoelectric polymer nanogenerator implant. Nano Energy 2021, 83, 105781. [Google Scholar] [CrossRef]
- Huang, Z.X.; Li, L.W.; Huang, Y.Z.; Rao, W.X.; Jiang, H.W.; Wang, J.; Zhang, H.H.; He, H.Z.; Qu, J.P. Self-poled piezoelectric polymer composites via melt-state energy implantation. Nat. Commun. 2024, 15, 819. [Google Scholar] [CrossRef]
- Hu, R.H.; Liu, P.; Zhu, W.; Guo, X.; Liu, Z.; Jin, Z.X.; Yang, R.H.; Han, J.W.; Tian, H.L.; Ma, Y.M.; et al. Design, Fabrication, and Wearable Medical Application of a High-Resolution Flexible Capacitive Temperature Sensor Based on the Thermotropic Phase Transition Composites of PEO/PVDF-HFP/H3PO4. Adv. Funct. Mater. 2025, 35, 2417205. [Google Scholar] [CrossRef]
- Abdulghani, S.; Mitchell, G.R. Biomaterials for In Situ Tissue Regeneration: A Review. Biomolecules 2019, 9, 750. [Google Scholar] [CrossRef]
- Pullano, S.A.; Critello, C.D.; Bianco, M.G.; Menniti, M.; Fiorillo, A.S. PVDF Ultrasonic Sensors for In-Air Applications: A Review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2324–2335. [Google Scholar] [CrossRef]
- Pochivalov, K.V.; Basko, A.V.; Ilyasova, A.N.; Lebedeva, T.N.; Yurov, M.Y.; Bronnikov, S.V. Experimental phase diagram for the PVDF-DMAc-water ternary system with new topology: Method of construction, thermodynamics, and structure formation of membranes. Polymer 2023, 282, 126152. [Google Scholar] [CrossRef]
- González, N.; Fernández-Berridi, M.J. Application of Fourier transform infrared spectroscopy in the study of interactions between PVC and plasticizers: PVC/plasticizer compatibility versus chemical structure of plasticizer. J. Appl. Polym. Sci. 2006, 101, 1731–1737. [Google Scholar] [CrossRef]
- Mannion, A.M.; Bates, F.S.; Macosko, C.W. Synthesis and Rheology of Branched Multiblock Polymers Based on Polylactide. Macromolecules 2016, 49, 4587–4598. [Google Scholar] [CrossRef]
- Schneider, J.; Bourque, K.; Narayan, R. Moisture curable toughened poly(lactide) utilizing vinyltrimethoxysilane based crosslinks. Express Polym. Lett. 2016, 10, 799–809. [Google Scholar] [CrossRef]
- Zhou, Q.H.; Wang, Z.; Shen, H.J.; Zhu, Z.Y.; Liu, L.P.; Yang, L.B.; Cheng, L.N. Morphology and performance of PVDF TIPS microfiltration hollow fiber membranes prepared from PVDF/DBP/DOP systems for industrial application. J. Chem. Technol. Biotechnol. 2016, 91, 1697–1708. [Google Scholar] [CrossRef]
- Nechitailo, V.S. About the Polymer Free-Volume Theory. Int. J. Polym. Mater. Polym. Biomater. 1992, 16, 171–177. [Google Scholar] [CrossRef]
- Belibel, R.; Sali, S.; Marinval, N.; Garcia-Sanchez, A.; Barbaud, C.; Hlawaty, H. PDMMLA derivatives as a promising cardiovascular metallic stent coating: Physicochemical and biological evaluation. Mater. Sci. Eng. C 2020, 117, 111284. [Google Scholar] [CrossRef]
- Ai, F.R.; Liu, T.W.; Liu, Y.; Yang, K.; Liu, Y.S.; Wang, W.Y.; Yuan, F.S.; Dong, L.N.; Xin, H.B.; Wang, X.L. A 3D printed wound cooling system incorporated with injectable, adsorbable, swellable and broad spectrum antibacterial scaffolds for rapid hematischesis processing. J. Mater. Chem. B 2018, 6, 5940–5948. [Google Scholar] [CrossRef]
- Tang, H.Y.; Li, S.S.; Zhao, Y.; Liu, C.L.; Gu, X.N.; Fan, Y.B. A surface-eroding poly(1,3-trimethylene carbonate) coating for magnesium based cardiovascular stents with stable drug release and improved corrosion resistance. Bioact. Mater. 2022, 7, 144–153. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Fan, M.; Li, B.; Gao, G.; Jiang, Z. Rapid In Situ Coating of Covered Stents with Highly Tough, Biocompatible Membrane for Emergency Coronary Artery Perforation. Biomolecules 2025, 15, 1608. https://doi.org/10.3390/biom15111608
Ji Y, Fan M, Li B, Gao G, Jiang Z. Rapid In Situ Coating of Covered Stents with Highly Tough, Biocompatible Membrane for Emergency Coronary Artery Perforation. Biomolecules. 2025; 15(11):1608. https://doi.org/10.3390/biom15111608
Chicago/Turabian StyleJi, Yuan, Mingyue Fan, Bing Li, Guolin Gao, and Zaixing Jiang. 2025. "Rapid In Situ Coating of Covered Stents with Highly Tough, Biocompatible Membrane for Emergency Coronary Artery Perforation" Biomolecules 15, no. 11: 1608. https://doi.org/10.3390/biom15111608
APA StyleJi, Y., Fan, M., Li, B., Gao, G., & Jiang, Z. (2025). Rapid In Situ Coating of Covered Stents with Highly Tough, Biocompatible Membrane for Emergency Coronary Artery Perforation. Biomolecules, 15(11), 1608. https://doi.org/10.3390/biom15111608








