Lactobacilli-Derived Microbe-Associated Molecular Patterns (MAMPs) in Host Immune Modulation
Abstract
1. Introduction
2. Materials and Methods
3. Lactobacilli and the Art of Immune Modulation
3.1. Immune Effects of Live Probiotic Bacteria
3.1.1. Pro-Inflammatory Effects Under Resting Conditions
3.1.2. Anti-Inflammatory Response in Inflamed States
3.1.3. Administration of Live Lactobacilli
3.2. Immunomodulatory Effects by Non-Viable Forms
3.3. Host Determinants of the Pro-/Anti-Inflammatory “Switch”
3.3.1. PRR Abundance and Subcellular Routing
3.3.2. Pre-Activation State: Tolerance vs. Trained Immunity
3.3.3. Adaptors and PRR Crosstalk; Inflammasome Priming
3.3.4. Endogenous Negative Regulators
3.3.5. Immunometabolic Set-Point
3.3.6. Cytokine Milieu
3.3.7. Cell Type-Specific Signal Decoding
4. Lactobacilli Acts on PRR Pathways to Exert Immuno-Regulating Effects
4.1. General Overview of PRRs
4.2. Toll-like Receptors (TLRs)
4.2.1. Structure and Families
4.2.2. Cellular Distribution and Ligands
4.2.3. Signal Transduction
4.3. NOD-like Receptors (NLRs)
4.3.1. Structure and Classification
4.3.2. Ligand Recognition and Inflammasome Formation
4.3.3. Signal Transduction and Effector Activation
5. MAMPs of Lactobacilli and Their Immunomodulatory Effects
5.1. Peptidoglycan (PGN)
5.1.1. Signal Transduction and Effector Activation
5.1.2. Species- and Strain-Specific Effects
5.2. Lipoteichoic Acids (LTAs)
5.2.1. Structure and Key Features
5.2.2. Species- and Strain-Specific Effects
5.3. Exopolysaccharides (EPSs)
5.3.1. Structure and Key Features
5.3.2. Species- and Strain-Specific Effects
5.4. S-Layer Proteins (SLPs)
5.4.1. Structure and Key Features
5.4.2. Species- and Specific-Strain Effects
5.5. Pili
5.5.1. Structure and Key Features
5.5.2. Species- and Strain-Specific Effect
5.6. Lipoproteins (LPPs)
5.6.1. Structure and Key Features
5.6.2. Species- and Strain-Specific Effects
5.7. DNA and CpG-Rich Oligodeoxynucleotides
5.7.1. Structure and Key Features
5.7.2. Species- and Strain-Specific Effects
5.8. Membrane Vesicles (MVs)
5.8.1. Structure and Key Features
5.8.2. Species- and Strain-Specific Effects
5.9. Immunobiotic-like Particles (IBLPs)
6. How Do Lactobacillaceae MAMPs Differ from Those of Other Bacteria?
7. Main Challenges in Understanding Immunomodulation by Lactobacilli
8. Discussion
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MAMP | Microorganism-Associated Molecular Pattern |
| PRR | Pattern Recognition Receptor |
| TLR | Toll-Like Receptor |
| IL | Interleukin |
| TNF-α | Tumor Necrosis Factor α |
| Treg | Regulatory T-Cell |
| ROS | Reactive Oxygen Species |
| GRAS | Generally Recognized as Safe |
| LTA | Lipotheicoic Acid |
| PGN | Peptydoglycan |
| EPS | Exopolysaccharide |
| MAPK | Mitogen-Activated Protein Kinase |
| NLR | NOD-Like Receptor |
| Th | T Helper Cell |
| NO | Nitric Oxide |
| PGE2 | Prostaglandin E2 |
| iNOS | Inducible Nitric Oxide Syntase |
| IFN-γ | Interferon-γ |
| LPS | Lipopolysaccharide |
| NK | Natural Killer |
| DAI | Disease Activity Index |
| LGG | L. rhamnosus GG |
| DC | Dendritic Cell |
| CFS | Cell-Free Supernatant |
| ERK | Extracellular Signal-Regulated Kinase |
| JNK | c-jun N-Terminal Kinase-Dependent |
| CLR | C-Type Lectin Receptor |
| RLR | RIG-1-Like Receptor |
| ALR | AIM-2 Like Receptor |
| LRR | Leucine-Rich Repeat |
| TIR | Toll-Interleukin 1 Receptor |
| PAMP | Pathogen-Associated Molecular Pattern |
| DAMP | Damage-Associated Molecular Pattern |
| XAMP | Xeno-Associated Molecular Pattern |
| APC | Antigen-Presenting Cell |
| MD-2 | Myeloid Differentiation Factor 2 |
| LBP | LPS Binding Protein |
| MyD88 | Myeloid Differentiation Primary Response 88 |
| TRIF | TIR Domain Containing Adaptor-Inducing Interferon-β |
| TIRAP/MAL | TIR Domain Containing Adaptor Protein |
| DD | Death Domain |
| IRAK | IL-1 Receptor-Associated Kinase |
| TRAF | TNF Receptor-Associated Factor |
| TAK | TGF-β-Activated Kinase |
| IKK | IκB Kinase |
| IRF | IFN-Regulatory Factor |
| RIP | Receptor-Interacting Protein |
| TRAM | TRIF-Related Adaptor |
| BIR | Baculoviral Inhibitory Repeat-Like Domain |
| NAIP | NLR Family Apoptosis Inhibitory Protein |
| CARD | Caspase Activation and Recruitment Domain |
| PYR | Pyrin Domain |
| ASC | Apoptosis Associated Speck-Like Protein-Containing CARD |
| MDP | Muramyl Dipeptide |
| TnSS | Type n Secretion System |
| BMDM | Bone Marrow-Derived Macrophages |
| TA | Teichoic Acid |
| WT | Wild-Type |
| pLTA | LTA from L. plantarum |
| ETEC | Enterotoxigenic E. coli |
| rhLTA | LTA from L. rhamnosus |
| RSV | Respiratory Syncytial Virus |
| AM | Alveolar Macrophage |
| PSPG | Polysaccharide–Peptidoglycan Complex |
| PGH | Peptidoglycan Hydrolase |
| MoDC | Monocyte-Derived Dendritic Cell |
| cLTA | LTA from L. casei |
| PUFA | Poly-Unsaturated Fatty Acid |
| aLTA | LTA from L. acidophilus |
| sLTA | LTA from L. sakei |
| dLTA | LTA from L. delbrueckii |
| pEPS | EPS from L. plantarum |
| T-AOC | Total Antioxidant Capacity |
| SOD | Superoxide Dismutase |
| GHS-Px | Gluthatione Peroxidase |
| CAT | Catalase |
| rhEPS | EPS from L. rhamnosus |
| cEPS | EPS from L. casei |
| pcEPS | EPS from L. paracasei |
| dbEPS | EPS from L. delbrueckii spp. bulgaricus |
| aEPS | EPS from L. acidophilus |
| reEPS | EPS from L. reuteri |
| SLP | S-Layer Protein |
| ISG | Interferon I-Stimulated Gene |
| MIMP | Micro Integral Membrane Protein |
| gDNA | Genomic DNA |
| ODN | Oligodeoxynucleotide |
| MV | Membrane Vesicles |
| IBLP | Immunobiotic-Like Particle |
| EV | Extracellular Vesicles |
References
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Fuochi, V.; Cardile, V.; Petronio Petronio, G.; Furneri, P.M. Biological properties and production of bacteriocins-like-inhibitory substances by Lactobacillus sp. strains from human vagina. J. Appl. Microbiol. 2018, 126, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Fuochi, V.; Li Volti, G.; Furneri, P.M. Commentary: Lactobacilli Dominance and Vaginal pH: Why Is the Human Vaginal Microbiome Unique? Front. Microbiol. 2017, 8, 1815. [Google Scholar] [CrossRef] [PubMed]
- Laudani, S.; Torrisi, S.A.; Alboni, S.; Bastiaanssen, T.F.S.; Benatti, C.; Rivi, V.; Moloney, R.D.; Fuochi, V.; Furneri, P.M.; Drago, F.; et al. Gut microbiota alterations promote traumatic stress susceptibility associated with p-cresol-induced dopaminergic dysfunctions. Brain Behav. Immun. 2023, 107, 385–396. [Google Scholar] [CrossRef]
- Fuochi, V. Amensalistic Activity of Lactobacillus spp. Isolated from Human Samples. Ph.D. Thesis, Università degli Studi di Catania, Catania, Italy, 2016. [Google Scholar]
- Fuochi, V.; Li Volti, G.; Furneri, P.M. Probiotic Properties of Lactobacillus fermentum Strains Isolated from Human Oral Samples and Description of their Antibacterial Activity. Curr. Pharm. Biotechnol. 2017, 18, 138–149. [Google Scholar] [CrossRef]
- Fuochi, V. Antifungal Activity of Extracts Produced by Lactobacillus fermentum Strains and Analysis of Candida Albicans Yeast/Mold (Y/M) Switching. J. Clin. Gastroenterol. 2018, 52, S104. [Google Scholar]
- Rocha-Ramirez, L.M.; Hernandez-Chinas, U.; Moreno-Guerrero, S.S.; Ramirez-Pacheco, A.; Eslava, C.A. Probiotic Properties and Immunomodulatory Activity of Lactobacillus Strains Isolated from Dairy Products. Microorganisms 2021, 9, 825. [Google Scholar] [CrossRef]
- Stivala, A.; Carota, G.; Fuochi, V.; Furneri, P.M. Lactobacillus rhamnosus AD3 as a Promising Alternative for Probiotic Products. Biomolecules 2021, 11, 94. [Google Scholar] [CrossRef]
- Rastogi, S.; Singh, A. Gut microbiome and human health: Exploring how the probiotic genus Lactobacillus modulate immune responses. Front. Pharmacol. 2022, 13, 1042189. [Google Scholar] [CrossRef]
- Fuochi, V.; Spampinato, M.; Distefano, A.; Palmigiano, A.; Garozzo, D.; Zagni, C.; Rescifina, A.; Li Volti, G.; Furneri, P.M. Soluble peptidoglycan fragments produced by Limosilactobacillus fermentum with antiproliferative activity are suitable for potential therapeutic development: A preliminary report. Front. Mol. Biosci. 2023, 10, 1082526. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Garbacz, K. Anticancer activity of lactic acid bacteria. Semin. Cancer Biol. 2022, 86, 356–366. [Google Scholar] [CrossRef]
- Samanta, S. Potential Impacts of Prebiotics and Probiotics on Cancer Prevention. Anticancer Agents Med. Chem. 2022, 22, 605–628. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Wakai, K.; Tamakoshi, K.; Tokudome, S.; Toyoshima, H.; Watanabe, Y.; Hayakawa, N.; Suzuki, K.; Hashimoto, S.; Ito, Y.; et al. Diet and colorectal cancer mortality: Results from the Japan Collaborative Cohort Study. Nutr. Cancer 2004, 50, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Pala, V.; Sieri, S.; Berrino, F.; Vineis, P.; Sacerdote, C.; Palli, D.; Masala, G.; Panico, S.; Mattiello, A.; Tumino, R.; et al. Yogurt consumption and risk of colorectal cancer in the Italian European prospective investigation into cancer and nutrition cohort. Int. J. Cancer 2011, 129, 2712–2719. [Google Scholar] [CrossRef] [PubMed]
- Fuochi, V.; Coniglio, M.A.; Laghi, L.; Rescifina, A.; Caruso, M.; Stivala, A.; Furneri, P.M. Metabolic Characterization of Supernatants Produced by Lactobacillus spp. With In vitro Anti-Legionella Activity. Front. Microbiol. 2019, 10, 1403. [Google Scholar] [CrossRef]
- Tarique, M.; Abdalla, A.; Masad, R.; Al-Sbiei, A.; Kizhakkayil, J.; Osaili, T.; Olaimat, A.; Liu, S.-Q.; Fernandez-Cabezudo, M.; al-Ramadi, B. Potential probiotics and postbiotic characteristics including immunomodulatory effects of lactic acid bacteria isolated from traditional yogurt-like products. Lwt 2022, 159, 113207. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Delgado, S.; Sanchez, B.; Margolles, A.; Ruas-Madiedo, P.; Ruiz, L. Molecules Produced by Probiotics and Intestinal Microorganisms with Immunomodulatory Activity. Nutrients 2020, 12, 391. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C. Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat. Rev. Microbiol. 2010, 8, 171–184. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Li, Z.; Ma, K.; Zhang, C.; Chen, X.; Wang, G.; Yang, L.; Dong, M.; Rui, X.; Zhang, Q. Structural characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus helveticus LZ-R-5. Carbohydr. Polym. 2020, 235, 115977. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Jiang, J.; Xu, Q.; Zeng, N.; Lu, B.; Yuan, P.; Sun, K.; Zhou, H.; He, X. A probiotic bi-functional peptidoglycan hydrolase sheds NOD2 ligands to regulate gut homeostasis in female mice. Nat. Commun. 2023, 14, 3338. [Google Scholar] [CrossRef]
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and postbiotics of probiotic Lactobacilli, their positive effects on the host and action mechanisms: A review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef]
- Fong, F.L.Y.; Shah, N.P.; Kirjavainen, P.; El-Nezami, H. Mechanism of action of probiotic bacteria on intestinal and systemic immunities and antigen-presenting cells. Int. Rev. Immunol. 2016, 35, 179–188. [Google Scholar] [CrossRef]
- Metchnikoff, I.I. The Prolongation of Life: Optimistic Studies; Springer Publishing Company: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An overview of beneficial effects. Antonie Van Leeuwenhoek 2002, 82, 279–289. [Google Scholar] [CrossRef]
- Park, C.; Ji, S.Y.; Hwangbo, H.; Shin, S.Y.; Kim, M.Y.; Lee, K.; Kim, D.H.; Cho, B.R.; Lee, H.; Choi, Y.H.; et al. Enhancement of Immune Functions by Limosilactobacillus reuteri KBL346: In vitro and In Vivo Studies. Int. J. Mol. Sci. 2023, 25, 141. [Google Scholar] [CrossRef]
- Lee, J.; Jung, I.; Choi, J.W.; Lee, C.W.; Cho, S.; Choi, T.G.; Sohn, M.; Park, Y.I. Micronized and Heat-Treated Lactobacillus plantarum LM1004 Stimulates Host Immune Responses Via the TLR-2/MAPK/NF-kappaB Signalling Pathway In vitro and In Vivo. J. Microbiol. Biotechnol. 2019, 29, 704–712. [Google Scholar] [CrossRef]
- Chondrou, P.; Karapetsas, A.; Kiousi, D.E.; Vasileiadis, S.; Ypsilantis, P.; Botaitis, S.; Alexopoulos, A.; Plessas, S.; Bezirtzoglou, E.; Galanis, A. Assessment of the Immunomodulatory Properties of the Probiotic Strain Lactobacillus paracasei K5 In vitro and In Vivo. Microorganisms 2020, 8, 709. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cano, F.J.; Dong, H.; Yaqoob, P. In vitro immunomodulatory activity of Lactobacillus fermentum CECT5716 and Lactobacillus salivarius CECT5713: Two probiotic strains isolated from human breast milk. Immunobiology 2010, 215, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, M.; Nomoto, R.; Mizuno, M.; Osawa, R. An In vitro investigation of immunomodulatory properties of Lactobacillus plantarum and L. delbrueckii cells and their extracellular polysaccharides. Biosci. Microbiota Food Health 2017, 36, 101–110. [Google Scholar] [CrossRef]
- Salva, S.; Villena, J.; Alvarez, S. Immunomodulatory activity of Lactobacillus rhamnosus strains isolated from goat milk: Impact on intestinal and respiratory infections. Int. J. Food Microbiol. 2010, 141, 82–89. [Google Scholar] [CrossRef]
- Min, F.; Hu, J.; Huang, T.; Huang, Y.; Nie, S.; Xiong, T.; Xie, M. Effects of Lactobacillus casei NCU011054 on immune response and gut microbiota of cyclophosphamide induced immunosuppression mice. Food Chem. Toxicol. 2023, 174, 113662. [Google Scholar] [CrossRef]
- Michels, M.; Jesus, G.F.A.; Abatti, M.R.; Corneo, E.; Cucker, L.; de Medeiros Borges, H.; da Silva Matos, N.; Rocha, L.B.; Dias, R.; Simon, C.S.; et al. Effects of different probiotic strains B. lactis, L. rhamnosus and L. reuteri on brain-intestinal axis immunomodulation in an en-dotoxin-induced inflammation. Mol. Neurobiol. 2022, 59, 5168–5178. [Google Scholar] [CrossRef]
- Qiao, J.; Sun, Z.; Liang, D.; Li, H. Lactobacillus salivarius alleviates inflammation via NF-kappaB signaling in ETEC K88-induced IPEC-J2 cells. J. Anim. Sci. Biotechnol. 2020, 11, 76. [Google Scholar] [CrossRef]
- Wong, W.Y.; Chan, B.D.; Sham, T.T.; Lee, M.M.; Chan, C.O.; Chau, C.T.; Mok, D.K.; Kwan, Y.W.; Tai, W.C. Lactobacillus casei Strain Shirota Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice by Increasing Taurine-Conjugated Bile Acids and Inhibiting NF-kappaB Signaling via Stabilization of IkappaBalpha. Front. Nutr. 2022, 9, 816836. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, S.; Yan, X.; Zhang, X.; Hao, Y.; Jiang, L.; Dai, Z. Living, Heat-Killed Limosilactobacillus mucosae and Its Cell-Free Supernatant Differentially Regulate Colonic Serotonin Receptors and Immune Response in Experimental Colitis. Nutrients 2024, 16, 468. [Google Scholar] [CrossRef]
- Mata Forsberg, M.; Bjorkander, S.; Pang, Y.; Lundqvist, L.; Ndi, M.; Ott, M.; Escriba, I.B.; Jaeger, M.C.; Roos, S.; Sverremark-Ekstrom, E. Extracellular Membrane Vesicles from Lactobacilli Dampen IFN-gamma Responses in a Monocyte-Dependent Manner. Sci. Rep. 2019, 9, 17109. [Google Scholar] [CrossRef]
- Jung, J.Y.; Shin, J.S.; Lee, S.G.; Rhee, Y.K.; Cho, C.W.; Hong, H.D.; Lee, K.T. Lactobacillus sakei K040706 evokes immunostimulatory effects on macrophages through TLR 2-mediated activation. Int. Immunopharmacol. 2015, 28, 88–96. [Google Scholar] [CrossRef]
- Si, W.; Liang, H.; Bugno, J.; Xu, Q.; Ding, X.; Yang, K.; Fu, Y.; Weichselbaum, R.R.; Zhao, X.; Wang, L. Lactobacillus rhamnosus GG induces cGAS/STING- dependent type I interferon and improves response to immune checkpoint blockade. Gut 2022, 71, 521–533. [Google Scholar] [CrossRef]
- Wang, X.; Tang, J.; Zhang, S.; Zhang, N. Effects of Lactiplantibacillus plantarum 19-2 on immunomodulatory function and gut microbiota in mice. Front. Microbiol. 2022, 13, 926756. [Google Scholar] [CrossRef]
- Johansson, M.A.; Bjorkander, S.; Mata Forsberg, M.; Qazi, K.R.; Salvany Celades, M.; Bittmann, J.; Eberl, M.; Sverremark-Ekstrom, E. Probiotic Lactobacilli Modulate Staphylococcus aureus-Induced Activation of Conventional and Unconventional T cells and NK Cells. Front. Immunol. 2016, 7, 273. [Google Scholar] [CrossRef]
- Yamasaki-Yashiki, S.; Kawashima, F.; Saika, A.; Hosomi, R.; Kunisawa, J.; Katakura, Y. RNA-Based Anti-Inflammatory Effects of Membrane Vesicles Derived from Lactiplantibacillus plantarum. Foods 2024, 13, 967. [Google Scholar] [CrossRef]
- Holowacz, S.; Blondeau, C.; Guinobert, I.; Guilbot, A.; Hidalgo, S.; Bisson, J.F. Lactobacillus salivarius LA307 and Lactobacillus rhamnosus LA305 attenuate skin inflammation in mice. Benef. Microbes 2018, 9, 299–309. [Google Scholar] [CrossRef]
- Park, S.W.; Choi, Y.H.; Gho, J.Y.; Kang, G.A.; Kang, S.S. Synergistic Inhibitory Effect of Lactobacillus Cell Lysates and Butyrate on Poly I:C-Induced IL-8 Production in Human Intestinal Epithelial Cells. Probiotics Antimicrob. Proteins 2024, 16, 1–12. [Google Scholar] [CrossRef]
- Kim, M.Y.; Hyun, I.K.; An, S.; Kim, D.; Kim, K.H.; Kang, S.S. In vitro anti-inflammatory and antibiofilm activities of bacterial lysates from lactobacilli against oral pathogenic bacteria. Food Funct. 2022, 13, 12755–12765. [Google Scholar] [CrossRef]
- McClure, R.; Massari, P. TLR-Dependent Human Mucosal Epithelial Cell Responses to Microbial Pathogens. Front. Immunol. 2014, 5, 386. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Wallace, T.D.; Bradley, S.; Buckley, N.D.; Green-Johnson, J.M. Interactions of lactic acid bacteria with human intestinal epithelial cells: Effects on cytokine production. J. Food Prot. 2003, 66, 466–472. [Google Scholar] [CrossRef]
- Borruel, N.; Casellas, F.; Antolin, M.; Llopis, M.; Carol, M.; Espiin, E.; Naval, J.; Guarner, F.; Malagelada, J.R. Effects of nonpathogenic bacteria on cytokine secretion by human intestinal mucosa. Am. J. Gastroenterol. 2003, 98, 865–870. [Google Scholar] [CrossRef]
- Paturi, G.; Phillips, M.; Jones, M.; Kailasapathy, K. Immune enhancing effects of Lactobacillus acidophilus LAFTI L10 and Lactobacillus paracasei LAFTI L26 in mice. Int. J. Food Microbiol. 2007, 115, 115–118. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Zanoni, I.; Ostuni, R.; Marek, L.R.; Barresi, S.; Barbalat, R.; Barton, G.M.; Granucci, F.; Kagan, J.C. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 2011, 147, 868–880. [Google Scholar] [CrossRef]
- Stack, J.; Doyle, S.L.; Connolly, D.J.; Reinert, L.S.; O’Keeffe, K.M.; McLoughlin, R.M.; Paludan, S.R.; Bowie, A.G. TRAM is required for TLR2 endosomal signaling to type I IFN induction. J. Immunol. 2014, 193, 6090–6102. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collins, P.E.; Carmody, R.J. The Regulation of Endotoxin Tolerance and its Impact on Macrophage Activation. Crit. Rev. Immunol. 2015, 35, 293–323. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.; van der Meer, J.W.; Mhlanga, M.M.; Mulder, W.J. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Gantner, B.N.; Simmons, R.M.; Canavera, S.J.; Akira, S.; Underhill, D.M. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 2003, 197, 1107–1117. [Google Scholar] [CrossRef]
- Okai, N.; Masuta, Y.; Otsuka, Y.; Hara, A.; Masaki, S.; Kamata, K.; Minaga, K.; Honjo, H.; Kudo, M.; Watanabe, T. Crosstalk between NOD2 and TLR2 suppresses the development of TLR2-mediated experimental colitis. J. Clin. Biochem. Nutr. 2024, 74, 146–153. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hernandez, L.D.; Galán, J.E.; Janeway, C.A.; Medzhitov, R.; Flavell, R.A. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 2002, 110, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Malynn, B.A. A20: Linking a complex regulator of ubiquitylation to immunity and human disease. Nat. Rev. Immunol. 2012, 12, 774–785. [Google Scholar] [CrossRef]
- Wald, D.; Qin, J.; Zhao, Z.; Qian, Y.; Naramura, M.; Tian, L.; Towne, J.; Sims, J.E.; Stark, G.R.; Li, X. SIGIRR, a negative regulator of Toll-like receptor–interleukin 1 receptor signaling. Nat. Immunol. 2003, 4, 920–927. [Google Scholar] [CrossRef]
- Zhang, G.; Ghosh, S. Negative regulation of toll-like receptor-mediated signaling by Tollip. J. Biol. Chem. 2002, 277, 7059–7065. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef]
- Hoque, R.; Farooq, A.; Ghani, A.; Gorelick, F.; Mehal, W.Z. Lactate reduces liver and pancreatic injury in toll-like receptor–and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 2014, 146, 1763–1774. [Google Scholar] [CrossRef]
- Yang, K.; Xu, J.; Fan, M.; Tu, F.; Wang, X.; Ha, T.; Williams, D.L.; Li, C. Lactate Suppresses Macrophage Pro-Inflammatory Response to LPS Stimulation by Inhibition of YAP and NF-κB Activation via GPR81-Mediated Signaling. Front. Immunol. 2020, 11, 587913. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.-g. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.L.; Siddiqui, K.R.; Arancibia-Cárcamo, C.V.; Hall, J.; Sun, C.-M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β–and retinoic acid–dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Mucida, D.; Park, Y.; Kim, G.; Turovskaya, O.; Scott, I.; Kronenberg, M.; Cheroutre, H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 2007, 317, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Cario, E.; Gerken, G.; Podolsky, D.K. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 2004, 127, 224–238. [Google Scholar] [CrossRef]
- Eisenbarth, S. Dendritic cell subsets in T cell programming: Location dictates function. Nat. Rev. Immunol. 2019, 19, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, R.; Piccolo, V.; Barozzi, I.; Polletti, S.; Termanini, A.; Bonifacio, S.; Curina, A.; Prosperini, E.; Ghisletti, S.; Natoli, G. Latent enhancers activated by stimulation in differentiated cells. Cell 2013, 152, 157–171. [Google Scholar] [CrossRef]
- Jia, D.J.; Wang, Q.W.; Hu, Y.Y.; He, J.M.; Ge, Q.W.; Qi, Y.D.; Chen, L.Y.; Zhang, Y.; Fan, L.N.; Lin, Y.F.; et al. Lactobacillus johnsonii alleviates colitis by TLR1/2-STAT3 mediated CD206(+) macrophages(IL-10) activation. Gut Microbes 2022, 14, 2145843. [Google Scholar] [CrossRef]
- Chen, Y.; Guan, W.; Zhang, N.; Wang, Y.; Tian, Y.; Sun, H.; Li, X.; Wang, Y.; Liu, J. Lactobacillus plantarum Lp2 improved LPS-induced liver injury through the TLR-4/MAPK/NFkappaB and Nrf2-HO-1/CYP2E1 pathways in mice. Food Nutr. Res. 2022, 66, 10-29219. [Google Scholar] [CrossRef]
- Onishi, K.; Mochizuki, J.; Sato, A.; Goto, A.; Sashihara, T. Total RNA and genomic DNA of Lactobacillus gasseri OLL2809 induce interleukin-12 production in the mouse macrophage cell line J774.1 via toll-like receptors 7 and 9. BMC Microbiol. 2020, 20, 217. [Google Scholar] [CrossRef] [PubMed]
- Kingma, S.D.; Li, N.; Sun, F.; Valladares, R.B.; Neu, J.; Lorca, G.L. Lactobacillus johnsonii N6.2 stimulates the innate immune response through Toll-like receptor 9 in Caco-2 cells and increases intestinal crypt Paneth cell number in biobreeding diabetes-prone rats. J. Nutr. 2011, 141, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Wang, X.; Huang, J.; Feng, X.; Shi, C.; Yang, W.; Jiang, Y.; Cao, X.; Wang, J.; et al. Lactobacillus plantarum NC8 and its metabolite acetate alleviate type 1 diabetes via inhibiting NLRP3. Microb. Pathog. 2023, 182, 106237. [Google Scholar] [CrossRef]
- Li, P.; Chen, G.; Zhang, J.; Pei, C.; Chen, Y.; Gong, J.; Deng, S.; Cai, K.; Li, H.; Wang, D.; et al. Live Lactobacillus acidophilus alleviates ulcerative colitis via the SCFAs/mitophagy/NLRP3 inflammasome axis. Food Funct. 2022, 13, 2985–2997. [Google Scholar] [CrossRef]
- Suzuki, H.; Yamazaki, T.; Ohshio, K.; Sugamata, M.; Yoshikawa, M.; Kanauchi, O.; Morita, Y. A Specific Strain of Lactic Acid Bacteria, Lactobacillus paracasei, Inhibits Inflammasome Activation In vitro and Prevents Inflammation-Related Disorders. J. Immunol. 2020, 205, 811–821. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Du, C.; Liu, Q.; Yang, D.; Chen, L.; Zhu, Q.; Wang, Z. Effects of dietary supplementation with Lactobacillus acidophilus on the performance, intestinal physical barrier function, and the expression of NOD-like receptors in weaned piglets. PeerJ 2018, 6, e6060. [Google Scholar] [CrossRef] [PubMed]
- Udayan, S.; Butto, L.F.; Rossini, V.; Velmurugan, J.; Martinez-Lopez, M.; Sancho, D.; Melgar, S.; O’Toole, P.W.; Nally, K. Macrophage cytokine responses to commensal Gram-positive Lactobacillus salivarius strains are TLR2-independent and Myd88-dependent. Sci. Rep. 2021, 11, 5896. [Google Scholar] [CrossRef]
- Sameer, A.S.; Nissar, S. Toll-Like Receptors (TLRs): Structure, Functions, Signaling, and Role of Their Polymorphisms in Colorectal Cancer Susceptibility. Biomed. Res. Int. 2021, 2021, 1157023. [Google Scholar] [CrossRef]
- Behzadi, P.; Garcia-Perdomo, H.A.; Karpinski, T.M. Toll-Like Receptors: General Molecular and Structural Biology. J. Immunol. Res. 2021, 2021, 9914854. [Google Scholar] [CrossRef]
- Wells, J.M. Immunomodulatory mechanisms of lactobacilli. Microb. Cell Fact. 2011, 10 (Suppl. S1), S17. [Google Scholar] [CrossRef]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate immune pattern recognition: A cell biological perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- Caplan, I.F.; Maguire-Zeiss, K.A. Toll-Like Receptor 2 Signaling and Current Approaches for Therapeutic Modulation in Synucleinopathies. Front. Pharmacol. 2018, 9, 417. [Google Scholar] [CrossRef]
- De Nardo, D. Toll-like receptors: Activation, signalling and transcriptional modulation. Cytokine 2015, 74, 181–189. [Google Scholar] [CrossRef]
- Jeon, D.; Hill, E.; McNeel, D.G. Toll-like receptor agonists as cancer vaccine adjuvants. Hum. Vaccin. Immunother. 2024, 20, 2297453. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Farhat, K.; Riekenberg, S.; Heine, H.; Debarry, J.; Lang, R.; Mages, J.; Buwitt-Beckmann, U.; Roschmann, K.; Jung, G.; Wiesmuller, K.H.; et al. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J. Leukoc. Biol. 2008, 83, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, P.A.; Sriskandan, S. Mammalian Toll-like receptors: To immunity and beyond. Clin. Exp. Immunol. 2005, 140, 395–407. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Zhao, Y.; Ma, X.; Yi, H. Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses. J. Zhejiang Univ. Sci. B 2021, 22, 609–632. [Google Scholar] [CrossRef]
- Gewirtz, A.T.; Navas, T.A.; Lyons, S.; Godowski, P.J.; Madara, J.L. Cutting edge: Bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 2001, 167, 1882–1885. [Google Scholar] [CrossRef]
- Patinote, C.; Karroum, N.B.; Moarbess, G.; Cirnat, N.; Kassab, I.; Bonnet, P.A.; Deleuze-Masquefa, C. Agonist and antagonist ligands of toll-like receptors 7 and 8: Ingenious tools for therapeutic purposes. Eur. J. Med. Chem. 2020, 193, 112238. [Google Scholar] [CrossRef]
- Bauer, S.; Wagner, H. Bacterial CpG-DNA licenses TLR9. Curr. Top. Microbiol. Immunol. 2002, 270, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Kawagoe, T.; Sato, S.; Matsushita, K.; Kato, H.; Matsui, K.; Kumagai, Y.; Saitoh, T.; Kawai, T.; Takeuchi, O.; Akira, S. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat. Immunol. 2008, 9, 684–691. [Google Scholar] [CrossRef]
- Wicherska-Pawlowska, K.; Wrobel, T.; Rybka, J. Toll-Like Receptors (TLRs), NOD-Like Receptors (NLRs), and RIG-I-Like Receptors (RLRs) in Innate Immunity. TLRs, NLRs, and RLRs Ligands as Immunotherapeutic Agents for Hematopoietic Diseases. Int. J. Mol. Sci. 2021, 22, 13397. [Google Scholar] [CrossRef]
- Meylan, E.; Burns, K.; Hofmann, K.; Blancheteau, V.; Martinon, F.; Kelliher, M.; Tschopp, J. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat. Immunol. 2004, 5, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, S.; Sato, S.; Yamamoto, M.; Hirotani, T.; Kato, H.; Takeshita, F.; Matsuda, M.; Coban, C.; Ishii, K.J.; Kawai, T.; et al. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-alpha induction. J. Exp. Med. 2005, 201, 915–923. [Google Scholar] [CrossRef]
- Almeida-da-Silva, C.L.C.; Savio, L.E.B.; Coutinho-Silva, R.; Ojcius, D.M. The role of NOD-like receptors in innate immunity. Front. Immunol. 2023, 14, 1122586. [Google Scholar] [CrossRef] [PubMed]
- Rosenstiel, P.; Till, A.; Schreiber, S. NOD-like receptors and human diseases. Microbes Infect. 2007, 9, 648–657. [Google Scholar] [CrossRef]
- Chen, G.; Shaw, M.H.; Kim, Y.G.; Nunez, G. NOD-like receptors: Role in innate immunity and inflammatory disease. Annu. Rev. Pathol. 2009, 4, 365–398. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Hara, H.; Seregin, S.S.; Yang, D.; Fukase, K.; Chamaillard, M.; Alnemri, E.S.; Inohara, N.; Chen, G.Y.; Nunez, G. The NLRP6 Inflammasome Recognizes Lipoteichoic Acid and Regulates Gram-Positive Pathogen Infection. Cell 2018, 175, 1651–1664.e14. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, J.S.; Nahm, M.H. NOD-Like Receptors in Infection, Immunity, and Diseases. Yonsei Med. J. 2016, 57, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Damm, A.; Lautz, K.; Kufer, T.A. Roles of NLRP10 in innate and adaptive immunity. Microbes Infect. 2013, 15, 516–523. [Google Scholar] [CrossRef]
- Chamaillard, M.; Hashimoto, M.; Horie, Y.; Masumoto, J.; Qiu, S.; Saab, L.; Ogura, Y.; Kawasaki, A.; Fukase, K.; Kusumoto, S.; et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 2003, 4, 702–707. [Google Scholar] [CrossRef]
- Platnich, J.M.; Muruve, D.A. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. [Google Scholar] [CrossRef]
- Rohde, M. The Gram-Positive Bacterial Cell Wall. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Neuhaus, F.C.; Baddiley, J. A continuum of anionic charge: Structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 2003, 67, 686–723. [Google Scholar] [CrossRef]
- Schleifer, K.H.; Kandler, O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 1972, 36, 407–477. [Google Scholar] [CrossRef]
- Vollmer, W.; Blanot, D.; de Pedro, M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef]
- Bersch, K.L.; DeMeester, K.E.; Zagani, R.; Chen, S.; Wodzanowski, K.A.; Liu, S.; Mashayekh, S.; Reinecker, H.C.; Grimes, C.L. Bacterial Peptidoglycan Fragments Differentially Regulate Innate Immune Signaling. ACS Cent. Sci. 2021, 7, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Pan, D.; Guo, Y.; Sun, Y.; Zeng, X. Peptidoglycan diversity and anti-inflammatory capacity in Lactobacillus strains. Carbohydr. Polym. 2015, 128, 130–137. [Google Scholar] [CrossRef]
- Irazoki, O.; Hernandez, S.B.; Cava, F. Peptidoglycan Muropeptides: Release, Perception, and Functions as Signaling Molecules. Front. Microbiol. 2019, 10, 500. [Google Scholar] [CrossRef]
- Zeuthen, L.H.; Fink, L.N.; Frokiaer, H. Toll-like receptor 2 and nucleotide-binding oligomerization domain-2 play divergent roles in the recognition of gut-derived lactobacilli and bifidobacteria in dendritic cells. Immunology 2008, 124, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, X.; Li, X. Peptidoglycan-based immunomodulation. Appl. Microbiol. Biotechnol. 2022, 106, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shi, Y.H.; Le, G.W.; Ma, X.Y. Distinct immune response induced by peptidoglycan derived from Lactobacillus sp. World J. Gastroenterol. 2005, 11, 6330–6337. [Google Scholar] [CrossRef]
- Salva, S.; Tiscornia, I.; Gutierrez, F.; Alvarez, S.; Bollati-Fogolin, M. Lactobacillus rhamnosus postbiotic-induced immunomodulation as safer alternative to the use of live bacteria. Cytokine 2021, 146, 155631. [Google Scholar] [CrossRef]
- Huang, J.; Li, J.; Li, Q.; Li, L.; Zhu, N.; Xiong, X.; Li, G. Peptidoglycan derived from Lactobacillus rhamnosus MLGA up-regulates the expression of chicken beta-defensin 9 without triggering an inflammatory response. Innate Immun. 2020, 26, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Kolling, Y.; Salva, S.; Villena, J.; Alvarez, S. Are the immunomodulatory properties of Lactobacillus rhamnosus CRL1505 peptidoglycan common for all Lactobacilli during respiratory infection in malnourished mice? PLoS ONE 2018, 13, e0194034. [Google Scholar] [CrossRef] [PubMed]
- Kolling, Y.; Salva, S.; Villena, J.; Marranzino, G.; Alvarez, S. Non-viable immunobiotic Lactobacillus rhamnosus CRL1505 and its peptidoglycan improve systemic and respiratory innate immune response during recovery of immunocompromised-malnourished mice. Int. Immunopharmacol. 2015, 25, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Clua, P.; Tomokiyo, M.; Raya Tonetti, F.; Islam, M.A.; Garcia Castillo, V.; Marcial, G.; Salva, S.; Alvarez, S.; Takahashi, H.; Kurata, S.; et al. The Role of Alveolar Macrophages in the Improved Protection against Respiratory Syncytial Virus and Pneumococcal Superinfection Induced by the Peptidoglycan of Lactobacillus rhamnosus CRL1505. Cells 2020, 9, 1653. [Google Scholar] [CrossRef]
- Zelaya, H.; Arellano-Arriagada, L.; Fukuyama, K.; Matsumoto, K.; Marranzino, G.; Namai, F.; Salva, S.; Alvarez, S.; Aguero, G.; Kitazawa, H.; et al. Lacticaseibacillus rhamnosus CRL1505 Peptidoglycan Modulates the Inflammation-Coagulation Response Triggered by Poly(I:C) in the Respiratory Tract. Int. J. Mol. Sci. 2023, 24, 16907. [Google Scholar] [CrossRef]
- Raya Tonetti, F.; Clua, P.; Fukuyama, K.; Marcial, G.; Sacur, J.; Marranzino, G.; Tomokiyo, M.; Vizoso-Pinto, G.; Garcia-Cancino, A.; Kurata, S.; et al. The Ability of Postimmunobiotics from L. rhamnosus CRL1505 to Protect against Respiratory Syncytial Virus and Pneumococcal Super-Infection Is a Strain-Dependent Characteristic. Microorganisms 2022, 10, 2185. [Google Scholar] [CrossRef]
- Belkacem, N.; Serafini, N.; Wheeler, R.; Derrien, M.; Boucinha, L.; Couesnon, A.; Cerf-Bensussan, N.; Gomperts Boneca, I.; Di Santo, J.P.; Taha, M.K.; et al. Lactobacillus paracasei feeding improves immune control of influenza infection in mice. PLoS ONE 2017, 12, e0184976. [Google Scholar] [CrossRef]
- Li, A.L.; Sun, Y.Q.; Du, P.; Meng, X.C.; Guo, L.; Li, S.; Zhang, C. The Effect of Lactobacillus actobacillus Peptidoglycan on Bovine beta-Lactoglobulin-Sensitized Mice via TLR2/NF-kappaB Pathway. Iran. J. Allergy Asthma Immunol. 2017, 16, 147–158. [Google Scholar]
- Wu, Z.; Pan, D.D.; Guo, Y.; Zeng, X. Structure and anti-inflammatory capacity of peptidoglycan from Lactobacillus acidophilus in RAW-264.7 cells. Carbohydr. Polym. 2013, 96, 466–473. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, S.H.; Kim, M.G.; Lee, H.J.; Kim, G.B. Lactobacillus plantarum CAU1055 ameliorates inflammation in lipopolysaccharide-induced RAW264.7 cells and a dextran sulfate sodium-induced colitis animal model. J. Dairy Sci. 2019, 102, 6718–6725. [Google Scholar] [CrossRef]
- Song, X.; Li, F.; Zhang, M.; Xia, Y.; Ai, L.; Wang, G. Effect of D-Ala-Ended Peptidoglycan Precursors on the Immune Regulation of Lactobacillus plantarum Strains. Front. Immunol. 2021, 12, 825825. [Google Scholar] [CrossRef]
- Canto, E.; Moga, E.; Ricart, E.; Garcia-Bosch, O.; Garcia-Planella, E.; Juarez, C.; Vidal, S. MDP-Induced selective tolerance to TLR4 ligands: Impairment in NOD2 mutant Crohn’s disease patients. Inflamm. Bowel Dis. 2009, 15, 1686–1696. [Google Scholar] [CrossRef]
- Kim, D.; Choi, H.; Oh, H.; Lee, J.; Hwang, Y.; Kang, S.S. Mutanolysin-Digested Peptidoglycan of Lactobacillus reuteri Promotes the Inhibition of Porphyromonas gingivalis Lipopolysaccharide-Induced Inflammatory Responses through the Regulation of Signaling Cascades via TLR4 Suppression. Int. J. Mol. Sci. 2023, 25, 42. [Google Scholar] [CrossRef]
- Matsumoto, S.; Hara, T.; Hori, T.; Mitsuyama, K.; Nagaoka, M.; Tomiyasu, N.; Suzuki, A.; Sata, M. Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. Clin. Exp. Immunol. 2005, 140, 417–426. [Google Scholar] [CrossRef]
- Matsumoto, S.; Hara, T.; Nagaoka, M.; Mike, A.; Mitsuyama, K.; Sako, T.; Yamamoto, M.; Kado, S.; Takada, T. A component of polysaccharide peptidoglycan complex on Lactobacillus induced an improvement of murine model of inflammatory bowel disease and colitis-associated cancer. Immunology 2009, 128, e170–e180. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.; Muzsai, S.; Regulski, K.; Szendi-Szatmari, T.; Czimmerer, Z.; Rajnavolgyi, E.; Chapot-Chartier, M.P.; Bacsi, A. The Phagocytosis of Lacticaseibacillus casei and Its Immunomodulatory Properties on Human Monocyte-Derived Dendritic Cells Depend on the Expression of Lc-p75, a Bacterial Peptidoglycan Hydrolase. Int. J. Mol. Sci. 2022, 23, 7620. [Google Scholar] [CrossRef] [PubMed]
- Macho Fernandez, E.; Valenti, V.; Rockel, C.; Hermann, C.; Pot, B.; Boneca, I.G.; Grangette, C. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut 2011, 60, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Hatano, S.; Hirose, Y.; Yamamoto, Y.; Murosaki, S.; Yoshikai, Y. Scavenger receptor for lipoteichoic acid is involved in the potent ability of Lactobacillus plantarum strain L-137 to stimulate production of interleukin-12p40. Int. Immunopharmacol. 2015, 25, 321–331. [Google Scholar] [CrossRef]
- Matsuguchi, T.; Takagi, A.; Matsuzaki, T.; Nagaoka, M.; Ishikawa, K.; Yokokura, T.; Yoshikai, Y. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin. Diagn. Lab. Immunol. 2003, 10, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Claes, I.J.; Segers, M.E.; Verhoeven, T.L.; Dusselier, M.; Sels, B.F.; De Keersmaecker, S.C.; Vanderleyden, J.; Lebeer, S. Lipoteichoic acid is an important microbe-associated molecular pattern of Lactobacillus rhamnosus GG. Microb. Cell Fact. 2012, 11, 161. [Google Scholar] [CrossRef]
- Kaji, R.; Kiyoshima-Shibata, J.; Nagaoka, M.; Nanno, M.; Shida, K. Bacterial teichoic acids reverse predominant IL-12 production induced by certain lactobacillus strains into predominant IL-10 production via TLR2-dependent ERK activation in macrophages. J. Immunol. 2010, 184, 3505–3513. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ahmadi, S.; Nagpal, R.; Jain, S.; Mishra, S.P.; Kavanagh, K.; Zhu, X.; Wang, Z.; McClain, D.A.; Kritchevsky, S.B.; et al. Lipoteichoic acid from the cell wall of a heat killed Lactobacillus paracasei D3-5 ameliorates aging-related leaky gut, inflammation and improves physical and cognitive functions: From C. elegans to mice. Geroscience 2020, 42, 333–352. [Google Scholar] [CrossRef]
- Saber, R.; Zadeh, M.; Pakanati, K.C.; Bere, P.; Klaenhammer, T.; Mohamadzadeh, M. Lipoteichoic acid-deficient Lactobacillus acidophilus regulates downstream signals. Immunotherapy 2011, 3, 337–347. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.Y.; Kim, H.; Chung, D.K. Differential role of lipoteichoic acids isolated from Staphylococcus aureus and Lactobacillus plantarum on the aggravation and alleviation of atopic dermatitis. Microb. Pathog. 2020, 147, 104360. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.; Kim, H.R.; Kim, H.; Chung, D.K. In vitro and in vivo downregulation of C3 by lipoteichoic acid isolated from Lactobacillus plantarum K8 suppressed cytokine-mediated complement system activation. FEMS Microbiol. Lett. 2016, 363, fnw140. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, H.; Jung, B.J.; Kim, N.R.; Park, J.E.; Chung, D.K. Lipoteichoic acid isolated from Lactobacillus plantarum suppresses LPS-mediated atherosclerotic plaque inflammation. Mol. Cells 2013, 35, 115–124. [Google Scholar] [CrossRef]
- Kim, H.; Jung, B.J.; Jung, J.H.; Kim, J.Y.; Chung, S.K.; Chung, D.K. Lactobacillus plantarum lipoteichoic acid alleviates TNF-alpha-induced inflammation in the HT-29 intestinal epithelial cell line. Mol. Cells 2012, 33, 479–486. [Google Scholar] [CrossRef]
- Mizuno, H.; Arce, L.; Tomotsune, K.; Albarracin, L.; Funabashi, R.; Vera, D.; Islam, M.A.; Vizoso-Pinto, M.G.; Takahashi, H.; Sasaki, Y.; et al. Lipoteichoic Acid Is Involved in the Ability of the Immunobiotic Strain Lactobacillus plantarum CRL1506 to Modulate the Intestinal Antiviral Innate Immunity Triggered by TLR3 Activation. Front. Immunol. 2020, 11, 571. [Google Scholar] [CrossRef]
- Lu, Q.; Guo, Y.; Yang, G.; Cui, L.; Wu, Z.; Zeng, X.; Pan, D.; Cai, Z. Structure and Anti-Inflammation Potential of Lipoteichoic Acids Isolated from Lactobacillus Strains. Foods 2022, 11, 1610. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Kang, S.S.; Woo, S.J.; Park, O.J.; Ahn, K.B.; Song, K.D.; Lee, H.K.; Yun, C.H.; Han, S.H. Lipoteichoic Acid of Probiotic Lactobacillus plantarum Attenuates Poly I:C-Induced IL-8 Production in Porcine Intestinal Epithelial Cells. Front. Microbiol. 2017, 8, 1827. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.H.; Baik, J.E.; Yang, J.S.; Kang, S.S.; Im, J.; Yun, C.H.; Kim, D.W.; Lee, K.; Chung, D.K.; Ju, H.R.; et al. Differential immunostimulatory effects of Gram-positive bacteria due to their lipoteichoic acids. Int. Immunopharmacol. 2009, 9, 127–133. [Google Scholar] [CrossRef]
- Lee, B.; Yin, X.; Griffey, S.M.; Marco, M.L. Attenuation of Colitis by Lactobacillus casei BL23 Is Dependent on the Dairy Delivery Matrix. Appl. Environ. Microbiol. 2015, 81, 6425–6435. [Google Scholar] [CrossRef] [PubMed]
- Smelt, M.J.; de Haan, B.J.; Bron, P.A.; van Swam, I.; Meijerink, M.; Wells, J.M.; Kleerebezem, M.; Faas, M.M.; de Vos, P. The impact of Lactobacillus plantarum WCFS1 teichoic acid D-alanylation on the generation of effector and regulatory T-cells in healthy mice. PLoS ONE 2013, 8, e63099. [Google Scholar] [CrossRef]
- Claes, I.; Lebeer, S.; Shen, C.; Verhoeven, T.; Dilissen, E.; De Hertogh, G.; Bullens, D.; Ceuppens, J.; Van Assche, G.; Vermeire, S. Impact of lipoteichoic acid modification on the performance of the probiotic Lactobacillus rhamnosus GG in experimental colitis. Clin. Exp. Immunol. 2010, 162, 306–314. [Google Scholar] [CrossRef]
- Cui, L.; Zeng, H.; Hou, M.; Li, Z.; Mu, C.; Zhu, W.; Hang, S. Lactiplantibacillus plantarum L47 and inulin alleviate enterotoxigenic Escherichia coli induced ileal inflammation in piglets by upregulating the levels of alpha-linolenic acid and 12,13-epoxyoctadecenoic acid. Anim. Nutr. 2023, 14, 370–382. [Google Scholar] [CrossRef]
- Hong, Y.F.; Kim, H.; Kim, H.R.; Gim, M.G.; Chung, D.K. Different immune regulatory potential of Lactobacillus plantarum and Lactobacillus sakei isolated from Kimchi. J. Microbiol. Biotechnol. 2014, 24, 1629–1635. [Google Scholar] [CrossRef]
- Pradhan, D.; Gulati, G.; Avadhani, R.; H, M.R.; Soumya, K.; Kumari, A.; Gupta, A.; Dwivedi, D.; Kaushik, J.K.; Grover, S. Postbiotic Lipoteichoic acid of probiotic Lactobacillus origin ameliorates inflammation in HT-29 cells and colitis mice. Int. J. Biol. Macromol. 2023, 236, 123962. [Google Scholar] [CrossRef]
- Jeong, J.H.; Jang, S.; Jung, B.J.; Jang, K.S.; Kim, B.G.; Chung, D.K.; Kim, H. Differential immune-stimulatory effects of LTAs from different lactic acid bacteria via MAPK signaling pathway in RAW 264.7 cells. Immunobiology 2015, 220, 460–466. [Google Scholar] [CrossRef]
- Noh, S.Y.; Kang, S.S.; Yun, C.H.; Han, S.H. Lipoteichoic acid from Lactobacillus plantarum inhibits Pam2CSK4-induced IL-8 production in human intestinal epithelial cells. Mol. Immunol. 2015, 64, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.E.; Kim, H.; Chung, D.K. Lipoteichoic Acid Isolated from Lactobacillus plantarum Maintains Inflammatory Homeostasis through Regulation of Th1- and Th2-Induced Cytokines. J. Microbiol. Biotechnol. 2019, 29, 151–159. [Google Scholar] [CrossRef]
- Friedrich, A.D.; Campo, V.E.; Cela, E.M.; Morelli, A.E.; Shufesky, W.J.; Tckacheva, O.A.; Leoni, J.; Paz, M.L.; Larregina, A.T.; Gonzalez Maglio, D.H. Oral administration of lipoteichoic acid from Lactobacillus rhamnosus GG overcomes UVB-induced immunosuppression and impairs skin tumor growth in mice. Eur. J. Immunol. 2019, 49, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, A.D.; Leoni, J.; Paz, M.L.; Maglio, D.H.G. Lipoteichoic Acid from Lacticaseibacillus rhamnosus GG Modulates Dendritic Cells and T Cells in the Gut. Nutrients 2022, 14, 723. [Google Scholar] [CrossRef] [PubMed]
- Mohamadzadeh, M.; Pfeiler, E.A.; Brown, J.B.; Zadeh, M.; Gramarossa, M.; Managlia, E.; Bere, P.; Sarraj, B.; Khan, M.W.; Pakanati, K.C.; et al. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4623–4630. [Google Scholar] [CrossRef]
- Khan, M.W.; Zadeh, M.; Bere, P.; Gounaris, E.; Owen, J.; Klaenhammer, T.; Mohamadzadeh, M. Modulating intestinal immune responses by lipoteichoic acid-deficient Lactobacillus acidophilus. Immunotherapy 2012, 4, 151–161. [Google Scholar] [CrossRef]
- Chen, K.; Luo, H.; Li, Y.; Han, X.; Gao, C.; Wang, N.; Lu, F.; Wang, H. Lactobacillus paracasei TK1501 fermented soybeans alleviate dextran sulfate sodium-induced colitis by regulating intestinal cell function. J. Sci. Food Agric. 2023, 103, 5422–5431. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Kaur, N.; Dey, P. Bacterial exopolysaccharides as emerging bioactive macromolecules: From fundamentals to applications. Res. Microbiol. 2023, 174, 104024. [Google Scholar] [CrossRef]
- Chaisuwan, W.; Jantanasakulwong, K.; Wangtueai, S.; Phimolsiripol, Y.; Chaiyaso, T.; Techapun, C.; Phongthai, S.; You, S.; Regenstein, J.M.; Seesuriyachan, P. Microbial exopolysaccharides for immune enhancement: Fermentation, modifications and bioactivities. Food Biosci. 2020, 35, 100564. [Google Scholar] [CrossRef]
- Shao, L.; Wu, Z.; Zhang, H.; Chen, W.; Ai, L.; Guo, B. Partial characterization and immunostimulatory activity of exopolysaccharides from Lactobacillus rhamnosus KF5. Carbohydr. Polym. 2014, 107, 51–56. [Google Scholar] [CrossRef]
- Tang, W.; Dong, M.; Wang, W.; Han, S.; Rui, X.; Chen, X.; Jiang, M.; Zhang, Q.; Wu, J.; Li, W. Structural characterization and antioxidant property of released exopolysaccharides from Lactobacillus delbrueckii ssp. bulgaricus SRFM-1. Carbohydr. Polym. 2017, 173, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, H.J.; Kim, S.A.; Park, S.D.; Shim, J.J.; Lee, J.L. Exopolysaccharide from Lactobacillus plantarum HY7714 Protects against Skin Aging through Skin-Gut Axis Communication. Molecules 2021, 26, 1651. [Google Scholar] [CrossRef]
- Wang, X.; Tian, J.; Zhang, X.; Tang, N.; Rui, X.; Zhang, Q.; Dong, M.; Li, W. Characterization and Immunological Activity of Exopolysaccharide from Lacticaseibacillus paracasei GL1 Isolated from Tibetan Kefir Grains. Foods 2022, 11, 3330. [Google Scholar] [CrossRef]
- Min, W.H.; Fang, X.B.; Wu, T.; Fang, L.; Liu, C.L.; Wang, J. Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J. Biosci. Bioeng. 2019, 127, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.; Sato, A.; Goto, A.; Nakamura, M.; Ogawa, M.; Chiba, Y.; Hemmi, J.; Kano, H.; Takeda, K.; Okumura, K.; et al. Enhanced natural killer cell activation by exopolysaccharides derived from yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J. Dairy Sci. 2016, 99, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Lee, J.; Park, S.; Kwon, O.H.; Seo, J.; Roh, S. Exopolysaccharide Isolated from Lactobacillus plantarum L-14 Has Anti-Inflammatory Effects via the Toll-Like Receptor 4 Pathway in LPS-Induced RAW 264.7 Cells. Int. J. Mol. Sci. 2020, 21, 9283. [Google Scholar] [CrossRef]
- Khedr, O.M.S.; El-Sonbaty, S.M.; Moawed, F.S.M.; Kandil, E.I.; Abdel-Maksoud, B.E. Lactobacillus acidophilus ATCC 4356 Exopolysaccharides Suppresses Mediators of Inflammation through the Inhibition of TLR2/STAT-3/P38-MAPK Pathway in DEN-Induced Hepatocarcinogenesis in Rats. Nutr. Cancer 2022, 74, 1037–1047. [Google Scholar] [CrossRef]
- Xu, X.; Qiao, Y.; Peng, Q.; Shi, B.; Dia, V.P. Antioxidant and Immunomodulatory Properties of Partially purified Exopolysaccharide from Lactobacillus casei Isolated from Chinese Northeast Sauerkraut. Immunol. Investig. 2022, 51, 748–765. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Wu, Q.; Gao, N.; Wang, Z.; Yang, Y.; Shan, A. Exopolysaccharides of Lactobacillus rhamnosus GG ameliorate Salmonella typhimurium-induced intestinal inflammation via the TLR4/NF-kappaB/MAPK pathway. J. Anim. Sci. Biotechnol. 2023, 14, 23. [Google Scholar] [CrossRef]
- Kissova, Z.; Mudronova, D.; Link, R.; Tkacikova, L. Immunomodulatory effect of probiotic exopolysaccharides in a porcine In vitro co-culture model mimicking the intestinal environment on ETEC infection. Vet. Res. Commun. 2024, 48, 705–724. [Google Scholar] [CrossRef]
- Kissova, Z.; Tkacikova, L.; Mudronova, D.; Bhide, M.R. Immunomodulatory Effect of Lactobacillus reuteri (Limosilactobacillus reuteri) and Its Exopolysaccharides Investigated on Epithelial Cell Line IPEC-J2 Challenged with Salmonella typhimurium. Life 2022, 12, 1955. [Google Scholar] [CrossRef]
- Wang, X.; Shao, C.; Liu, L.; Guo, X.; Xu, Y.; Lu, X. Optimization, partial characterization and antioxidant activity of an exopolysaccharide from Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 2017, 103, 1173–1184. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Guo, H.; Cheng, Q.; Abbas, Z.; Tong, Y.; Yang, T.; Zhou, Y.; Zhang, H.; Wei, X.; et al. Optimization of Exopolysaccharide Produced by Lactobacillus plantarum R301 and Its Antioxidant and Anti-Inflammatory Activities. Foods 2023, 12, 2481. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Wu, J.M.; Wu, W.T.; Lin, J.W.; Liang, Y.T.; Hong, Z.Z.; Jia, X.Z.; Liu, D.M. Structural, antioxidant, and immunomodulatory activities of an acidic exopolysaccharide from Lactiplantibacillus plantarum DMDL 9010. Front. Nutr. 2022, 9, 1073071. [Google Scholar] [CrossRef]
- Li, J.Y.; Jin, M.M.; Meng, J.; Gao, S.M.; Lu, R.R. Exopolysaccharide from Lactobacillus planterum LP6: Antioxidation and the effect on oxidative stress. Carbohydr. Polym. 2013, 98, 1147–1152. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Li, D.; Zhao, Y.; Zhang, X.; Zeng, X.; Yang, Z.; Li, S. Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int. J. Biol. Macromol. 2013, 54, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Tseng, K.C.; Chiang, S.S.; Lee, B.H.; Hsu, W.H.; Pan, T.M. Immunomodulatory and antioxidant potential of Lactobacillus exopolysaccharides. J. Sci. Food Agric. 2011, 91, 2284–2291. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Mehwish, H.M.; Kitazawa, H.; Barba, F.J.; Berthelot, L.; Umair, M.; Zhu, Q.; He, Z.; Zhao, L. Techno-functional properties and immunomodulatory potential of exopolysaccharide from Lactiplantibacillus plantarum MM89 isolated from human breast milk. Food Chem. 2022, 377, 131954. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cui, Y.; Wang, X.; Yue, F.; Shan, Y.; Liu, B.; Zhou, Y.; Yi, Y.; Lu, X. Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 2019, 128, 480–492. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Pan, W.; Shen, X.; He, Y.; Yin, H.; Zhou, K.; Zou, L.; Chen, S.; Liu, S. Exopolysaccharides produced by yogurt-texture improving Lactobacillus plantarum RS20D and the immunoregulatory activity. Int. J. Biol. Macromol. 2019, 121, 342–349. [Google Scholar] [CrossRef]
- Yilmaz, T.; Simsek, O. Potential Health Benefits of Ropy Exopolysaccharides Produced by Lactobacillus plantarum. Molecules 2020, 25, 3293. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Song, Y.; Sun, M.; Wang, A.; Jiang, S.; Mu, G.; Tuo, Y. Exopolysaccharide Produced by Lactiplantibacillus plantarum-12 Alleviates Intestinal Inflammation and Colon Cancer Symptoms by Modulating the Gut Microbiome and Metabolites of C57BL/6 Mice Treated by Azoxymethane/Dextran Sulfate Sodium Salt. Foods 2021, 10, 3060. [Google Scholar] [CrossRef]
- Zhou, X.; Qi, W.; Hong, T.; Xiong, T.; Gong, D.; Xie, M.; Nie, S. Exopolysaccharides from Lactobacillus plantarum NCU116 Regulate Intestinal Barrier Function via STAT3 Signaling Pathway. J. Agric. Food Chem. 2018, 66, 9719–9727. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Sun, M.; Mu, G.; Tuo, Y. Exopolysaccharide secreted by Lactiplantibacillus plantarum Y12 showed inhibitory effect on the pathogenicity of Shigella flexneri in vitro and in vivo. Int. J. Biol. Macromol. 2024, 261, 129478. [Google Scholar] [CrossRef]
- Tao, T.; Zhang, L.; Yu, T.; Ma, J.; Lu, S.; Ren, J.; Li, X.; Guo, X. Exopolysaccharide production by Lactobacillus plantarum T10 is responsible for the probiotic activity in enhancing intestinal barrier function in vitro and in vivo. Food Funct. 2024, 15, 3583–3599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Xiao, Y.; Wang, H.; Zhang, H.; Lu, W. Interspecific differences and mechanisms of Lactobacillus-derived anti-inflammatory exopolysaccharides. Int. J. Biol. Macromol. 2024, 263, 130313. [Google Scholar] [CrossRef]
- Chen, L.; Gu, Q.; Zhou, T. Statistical Optimization of Novel Medium to Maximize the Yield of Exopolysaccharide From Lacticaseibacillus rhamnosus ZFM216 and Its Immunomodulatory Activity. Front. Nutr. 2022, 9, 924495. [Google Scholar] [CrossRef]
- Han, J.; Xia, W.; Wang, D.; Wang, Y.; Liu, Z.; Wu, Z. Characterization of an exopolysaccharide synthesized by Lacticaseibacillus rhamnosus B6 and its immunomodulatory activity in vitro. Int. J. Biol. Macromol. 2024, 264, 130576. [Google Scholar] [CrossRef]
- Ciszek-Lenda, M.; Nowak, B.; Srottek, M.; Walczewska, M.; Gorska-Fraczek, S.; Gamian, A.; Marcinkiewicz, J. Further studies on immunomodulatory effects of exopolysaccharide isolated from Lactobacillus rhamnosus KL37C. Cent. Eur. J. Immunol. 2013, 38, 289–298. [Google Scholar] [CrossRef]
- Li, L.; Jiang, Y.J.; Yang, X.Y.; Liu, Y.; Wang, J.Y.; Man, C.X. Immunoregulatory effects on Caco-2 cells and mice of exopolysaccharides isolated from Lactobacillus acidophilus NCFM. Food Funct. 2014, 5, 3261–3268. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Dong, W.; Wan, K.; Zhang, L.; Li, C.; Zhang, L.; Liu, N. Exopolysaccharide Produced by Lactobacillus plantarum Induces Maturation of Dendritic Cells in BALB/c Mice. PLoS ONE 2015, 10, e0143743. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Dasriya, V.L.; Nataraj, B.H.; Nagpal, R.; Behare, P.V. Lacticaseibacillus rhamnosus-Derived Exopolysaccharide Attenuates D-Galactose-Induced Oxidative Stress and Inflammatory Brain Injury and Modulates Gut Microbiota in a Mouse Model. Microorganisms 2022, 10, 2046. [Google Scholar] [CrossRef]
- Wan, C.; Qian, W.W.; Liu, W.; Pi, X.; Tang, M.T.; Wang, X.L.; Gu, Q.; Li, P.; Zhou, T. Exopolysaccharide from Lactobacillus rhamnosus ZFM231 alleviates DSS-induced colitis in mice by regulating gut microbiota. J. Sci. Food Agric. 2022, 102, 7087–7097. [Google Scholar] [CrossRef]
- Srutkova, D.; Kozakova, H.; Novotna, T.; Gorska, S.; Hermanova, P.P.; Hudcovic, T.; Svabova, T.; Sinkora, M.; Schwarzer, M. Exopolysaccharide from Lacticaseibacillus rhamnosus induces IgA production in airways and alleviates allergic airway inflammation in mouse model. Eur. J. Immunol. 2023, 53, e2250135. [Google Scholar] [CrossRef]
- Patten, D.A.; Leivers, S.; Chadha, M.J.; Maqsood, M.; Humphreys, P.N.; Laws, A.P.; Collett, A. The structure and immunomodulatory activity on intestinal epithelial cells of the EPSs isolated from Lactobacillus helveticus sp. Rosyjski and Lactobacillus acidophilus sp. 5e2. Carbohydr. Res. 2014, 384, 119–127. [Google Scholar] [CrossRef]
- Noda, M.; Danshiitsoodol, N.; Kanno, K.; Uchida, T.; Sugiyama, M. The Exopolysaccharide Produced by Lactobacillus paracasei IJH-SONE68 Prevents and Ameliorates Inflammatory Responses in DSS-Induced Ulcerative Colitis. Microorganisms 2021, 9, 2243. [Google Scholar] [CrossRef] [PubMed]
- Kissova, Z.; Schusterova, P.; Mudronova, D.; Novotny, J.; Tkacikova, L. Exopolysaccharides from Limosilactobacillus reuteri: Their influence on In vitro activation of porcine monocyte-derived dendritic cells—Brief report. Vet. Res. Commun. 2024, 48, 3315–3321. [Google Scholar] [CrossRef]
- Ksonzekova, P.; Bystricky, P.; Vlckova, S.; Patoprsty, V.; Pulzova, L.; Mudronova, D.; Kubaskova, T.; Csank, T.; Tkacikova, L. Exopolysaccharides of Lactobacillus reuteri: Their influence on adherence of E. coli to epithelial cells and inflammatory response. Carbohydr. Polym. 2016, 141, 10–19. [Google Scholar] [CrossRef]
- Prete, R.; Dell’Orco, F.; Sabatini, G.; Montagano, F.; Battista, N.; Corsetti, A. Improving the Antioxidant and Anti-Inflammatory Activity of Fermented Milks with Exopolysaccharides-Producing Lactiplantibacillus plantarum Strains. Foods 2024, 13, 1663. [Google Scholar] [CrossRef]
- Quach, N.T.; Nguyen, T.T.A.; Vu, T.H.N.; Nguyen, T.T.N.; Tran, X.K.; Chu, N.H.; Ta, T.T.T.; Chu, H.H.; Phi, Q.T. New insight into protective effect against oxidative stress and biosynthesis of exopolysaccharides produced by Lacticaseibacillus paracasei NC4 from fermented eggplant. Curr. Genet. 2024, 70, 7. [Google Scholar] [CrossRef] [PubMed]
- Fagan, R.P.; Fairweather, N.F. Biogenesis and functions of bacterial S-layers. Nat. Rev. Microbiol. 2014, 12, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, M.; Guo, Y.; Xun, M.; Wang, W.; Wu, Z.; Pan, D. Purification of Lactobacillus acidophilus surface-layer protein and its immunomodulatory effects on RAW264.7 cells. J. Sci. Food Agric. 2017, 97, 4204–4209. [Google Scholar] [CrossRef] [PubMed]
- Gerbino, E.; Carasi, P.; Mobili, P.; Serradell, M.A.; Gomez-Zavaglia, A. Role of S-layer proteins in bacteria. World J. Microbiol. Biotechnol. 2015, 31, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Yokota, K.; Igimi, S.; Kajikawa, A. Comparative analysis of immunological properties of S-layer proteins isolated from Lactobacillus strains. Microbiology 2019, 165, 188–196. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Xu, S.; Pan, J.; Zhang, Q.; Lu, R. Surface-Layer Protein from Lactobacillus acidophilus NCFM Inhibits Lipopolysaccharide-Induced Inflammation through MAPK and NF-kappaB Signaling Pathways in RAW264.7 Cells. J. Agric. Food Chem. 2018, 66, 7655–7662. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Niu, Y.; Zhang, X.; Lu, R. Surface-layer protein from Lactobacillus acidophilus NCFM attenuates tumor necrosis factor-alpha-induced intestinal barrier dysfunction and inflammation. Int. J. Biol. Macromol. 2019, 136, 27–34. [Google Scholar] [CrossRef]
- Bumgardner, S.A.; Zhang, L.; LaVoy, A.S.; Andre, B.; Frank, C.B.; Kajikawa, A.; Klaenhammer, T.R.; Dean, G.A. Nod2 is required for antigen-specific humoral responses against antigens orally delivered using a recombinant Lactobacillus vaccine platform. PLoS ONE 2018, 13, e0196950. [Google Scholar] [CrossRef]
- Konstantinov, S.R.; Smidt, H.; de Vos, W.M.; Bruijns, S.C.; Singh, S.K.; Valence, F.; Molle, D.; Lortal, S.; Altermann, E.; Klaenhammer, T.R.; et al. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc. Natl. Acad. Sci. USA 2008, 105, 19474–19479. [Google Scholar] [CrossRef]
- Klotz, C.; Goh, Y.J.; O’Flaherty, S.; Barrangou, R. S-layer associated proteins contribute to the adhesive and immunomodulatory properties of Lactobacillus acidophilus NCFM. BMC Microbiol. 2020, 20, 248. [Google Scholar] [CrossRef]
- Klotz, C.; Goh, Y.J.; O’Flaherty, S.; Johnson, B.; Barrangou, R. Deletion of S-Layer Associated Ig-Like Domain Protein Disrupts the Lactobacillus acidophilus Cell Surface. Front. Microbiol. 2020, 11, 345. [Google Scholar] [CrossRef]
- Prado Acosta, M.; Ruzal, S.M.; Cordo, S.M. S-layer proteins from Lactobacillus sp. inhibit bacterial infection by blockage of DC-SIGN cell receptor. Int. J. Biol. Macromol. 2016, 92, 998–1005. [Google Scholar] [CrossRef]
- Li, P.; Yu, Q.; Ye, X.; Wang, Z.; Yang, Q. Lactobacillus S-layer protein inhibition of Salmonella-induced reorganization of the cytoskeleton and activation of MAPK signalling pathways in Caco-2 cells. Microbiology 2011, 157, 2639–2646. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.; Yu, Q.; Yang, Q. Lactobacillus acidophilus S-layer protein-mediated inhibition of Salmonella-induced apoptosis in Caco-2 cells. Biochem. Biophys. Res. Commun. 2011, 409, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Huang, L.; Zhu, L.; Mou, C.; Hou, Q.; Yu, Q. Inhibition of H9N2 Virus Invasion into Dendritic Cells by the S-Layer Protein from L. acidophilus ATCC 4356. Front. Cell. Infect. Microbiol. 2016, 6, 137. [Google Scholar] [CrossRef]
- Kobatake, E.; Kabuki, T. S-Layer Protein of Lactobacillus helveticus SBT2171 Promotes Human beta-Defensin 2 Expression via TLR2-JNK Signaling. Front. Microbiol. 2019, 10, 2414. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.R.; Cui, Y.J.; Liu, J.X.; Luo, X.; Wang, H.F. Lactobacillus rhamnosus GG components, SLP, gDNA and CpG, exert protective effects on mouse macrophages upon lipopolysaccharide challenge. Lett. Appl. Microbiol. 2020, 70, 118–127. [Google Scholar] [CrossRef]
- Gao, K.; Wang, C.; Liu, L.; Dou, X.; Liu, J.; Yuan, L.; Zhang, W.; Wang, H. Immunomodulation and signaling mechanism of Lactobacillus rhamnosus GG and its components on porcine intestinal epithelial cells stimulated by lipopolysaccharide. J. Microbiol. Immunol. Infect. 2017, 50, 700–713. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, X.; Hao, S.; Lu, Q.; Zhang, L.; Han, X.; Lu, W. Inhibition of Shigella sonnei-induced epithelial barrier disruption by surface-layer associated proteins of lactobacilli from Chinese fermented food. J. Dairy Sci. 2018, 101, 1834–1842. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Zhang, L.W.; Tuo, Y.F.; Guo, C.F.; Yi, H.X.; Li, J.Y.; Han, X.; Du, M. Inhibition of Shigella sonnei adherence to HT-29 cells by lactobacilli from Chinese fermented food and preliminary characterization of S-layer protein involvement. Res. Microbiol. 2010, 161, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Zhang, C.; Chi, H.; Liu, C.; Li, A.; Yu, W. Postbiotics derived from Lactobacillus plantarum 1.0386 ameliorate lipopolysaccharide-induced tight junction injury via MicroRNA-200c-3p mediated activation of the MLCK-MLC pathway in Caco-2 cells. Food Funct. 2022, 13, 11008–11020. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shen, T.; Moyer, M.P.; Qin, H. Identification of the Lactobacillus SLP domain that binds gastric mucin. Front. Biosci. 2011, 16, 2128–2143. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Yan, X.; Weng, W.; Yang, Y.; Gao, R.; Liu, M.; Pan, C.; Zhu, Q.; Li, H.; Wei, Q.; et al. Micro Integral Membrane Protein (MIMP), a Newly Discovered Anti-Inflammatory Protein of Lactobacillus plantarum, Enhances the Gut Barrier and Modulates Microbiota and Inflammatory Cytokines. Cell Physiol. Biochem. 2018, 45, 474–490. [Google Scholar] [CrossRef]
- Proft, T.; Baker, E.N. Pili in Gram-negative and Gram-positive bacteria—Structure, assembly and their role in disease. Cell Mol. Life Sci. 2009, 66, 613–635. [Google Scholar] [CrossRef]
- Leser, T.; Baker, A. Molecular mechanisms of Lacticaseibacillus rhamnosus, LGG® probiotic function. Microorganisms 2024, 12, 794. [Google Scholar] [CrossRef]
- von Ossowski, I.; Reunanen, J.; Satokari, R.; Vesterlund, S.; Kankainen, M.; Huhtinen, H.; Tynkkynen, S.; Salminen, S.; de Vos, W.M.; Palva, A. Mucosal adhesion properties of the probiotic Lactobacillus rhamnosus GG SpaCBA and SpaFED pilin subunits. Appl. Environ. Microbiol. 2010, 76, 2049–2057. [Google Scholar] [CrossRef]
- Lebeer, S.; Claes, I.; Tytgat, H.L.; Verhoeven, T.L.; Marien, E.; von Ossowski, I.; Reunanen, J.; Palva, A.; Vos, W.M.; Keersmaecker, S.C.; et al. Functional analysis of Lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl. Environ. Microbiol. 2012, 78, 185–193. [Google Scholar] [CrossRef]
- von Ossowski, I.; Pietila, T.E.; Rintahaka, J.; Nummenmaa, E.; Makinen, V.M.; Reunanen, J.; Satokari, R.; de Vos, W.M.; Palva, I.; Palva, A. Using recombinant Lactococci as an approach to dissect the immunomodulating capacity of surface piliation in probiotic Lactobacillus rhamnosus GG. PLoS ONE 2013, 8, e64416. [Google Scholar] [CrossRef]
- Ganguli, K.; Collado, M.C.; Rautava, J.; Lu, L.; Satokari, R.; von Ossowski, I.; Reunanen, J.; de Vos, W.M.; Palva, A.; Isolauri, E.; et al. Lactobacillus rhamnosus GG and its SpaC pilus adhesin modulate inflammatory responsiveness and TLR-related gene expression in the fetal human gut. Pediatr. Res. 2015, 77, 528–535. [Google Scholar] [CrossRef]
- Tytgat, H.L.; Douillard, F.P.; Reunanen, J.; Rasinkangas, P.; Hendrickx, A.P.; Laine, P.K.; Paulin, L.; Satokari, R.; de Vos, W.M. Lactobacillus rhamnosus GG Outcompetes Enterococcus faecium via Mucus-Binding Pili: Evidence for a Novel and Heterospecific Probiotic Mechanism. Appl. Environ. Microbiol. 2016, 82, 5756–5762. [Google Scholar] [CrossRef] [PubMed]
- Vargas Garcia, C.E.; Petrova, M.; Claes, I.J.; De Boeck, I.; Verhoeven, T.L.; Dilissen, E.; von Ossowski, I.; Palva, A.; Bullens, D.M.; Vanderleyden, J.; et al. Piliation of Lactobacillus rhamnosus GG promotes adhesion, phagocytosis, and cytokine modulation in macrophages. Appl. Environ. Microbiol. 2015, 81, 2050–2062. [Google Scholar] [CrossRef]
- Ardita, C.S.; Mercante, J.W.; Kwon, Y.M.; Luo, L.; Crawford, M.E.; Powell, D.N.; Jones, R.M.; Neish, A.S. Epithelial adhesion mediated by pilin SpaC is required for Lactobacillus rhamnosus GG-induced cellular responses. Appl. Environ. Microbiol. 2014, 80, 5068–5077. [Google Scholar] [CrossRef]
- Tytgat, H.L.; van Teijlingen, N.H.; Sullan, R.M.; Douillard, F.P.; Rasinkangas, P.; Messing, M.; Reunanen, J.; Satokari, R.; Vanderleyden, J.; Dufrene, Y.F.; et al. Probiotic Gut Microbiota Isolate Interacts with Dendritic Cells via Glycosylated Heterotrimeric Pili. PLoS ONE 2016, 11, e0151824. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, K.; Ryu, K.H.; Ichikawa, R.; Masuda, A.; Kim, M.S.; Lee, H.; Chae, J.H.; Shimizu, T.; Saitoh, T.; Kuwano, K.; et al. Novel bacterial lipoprotein structures conserved in low-GC content gram-positive bacteria are recognized by Toll-like receptor 2. J. Biol. Chem. 2012, 287, 13170–13181. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Gotz, F. Lipoproteins of Gram-Positive Bacteria: Key Players in the Immune Response and Virulence. Microbiol. Mol. Biol. Rev. 2016, 80, 891–903. [Google Scholar] [CrossRef]
- Jin, M.S.; Kim, S.E.; Heo, J.Y.; Lee, M.E.; Kim, H.M.; Paik, S.G.; Lee, H.; Lee, J.O. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 2007, 130, 1071–1082. [Google Scholar] [CrossRef]
- Takeuchi, O.; Kawai, T.; Muhlradt, P.F.; Morr, M.; Radolf, J.D.; Zychlinsky, A.; Takeda, K.; Akira, S. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 2001, 13, 933–940. [Google Scholar] [CrossRef]
- Lee, I.-C.; van Swam, I.I.; Boeren, S.; Vervoort, J.; Meijerink, M.; Taverne, N.; Starrenburg, M.; Bron, P.A.; Kleerebezem, M. Lipoproteins Contribute to the Anti-inflammatory Capacity of Lactobacillus plantarum WCFS1. Front. Microbiol. 2020, 11, 1822. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Yun, C.H.; Han, S.H.; Song, K.D.; Kang, S.S. Inhibitory Effect of Lipoteichoic Acid Derived from Three Lactobacilli on Flagellin-Induced IL-8 Production in Porcine Peripheral Blood Mononuclear Cells. Probiotics Antimicrob. Proteins 2021, 13, 72–79. [Google Scholar] [CrossRef]
- Kim, B.; Shynlova, O.; Lye, S. Probiotic Lactobacillus rhamnosus GR-1 is a unique prophylactic agent that suppresses infection-induced myometrial cell responses. Sci. Rep. 2019, 9, 4698. [Google Scholar] [CrossRef] [PubMed]
- Dalpke, A.; Frank, J.; Peter, M.; Heeg, K. Activation of toll-like receptor 9 by DNA from different bacterial species. Infect. Immun. 2006, 74, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Rachmilewitz, D.; Katakura, K.; Karmeli, F.; Hayashi, T.; Reinus, C.; Rudensky, B.; Akira, S.; Takeda, K.; Lee, J.; Takabayashi, K.; et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 2004, 126, 520–528. [Google Scholar] [CrossRef]
- Iliev, I.D.; Tohno, M.; Kurosaki, D.; Shimosato, T.; He, F.; Hosoda, M.; Saito, T.; Kitazawa, H. Immunostimulatory oligodeoxynucleotide containing TTTCGTTT motif from Lactobacillus rhamnosus GG DNA potentially suppresses OVA-specific IgE production in mice. Scand. J. Immunol. 2008, 67, 370–376. [Google Scholar] [CrossRef]
- Kant, R.; de Vos, W.M.; Palva, A.; Satokari, R. Immunostimulatory CpG motifs in the genomes of gut bacteria and their role in human health and disease. J. Med. Microbiol. 2014, 63, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, Y.; Satho, T.; Hyakutake, M.; Irie, K.; Mishima, K.; Miake, F.; Kashige, N. The anti-inflammatory effects of a high-frequency oligodeoxynucleotide from the genomic DNA of Lactobacillus casei. Int. Immunopharmacol. 2014, 23, 139–147. [Google Scholar] [CrossRef]
- Bouladoux, N.; Hall, J.A.; Grainger, J.R.; dos Santos, L.M.; Kann, M.G.; Nagarajan, V.; Verthelyi, D.; Belkaid, Y. Regulatory role of suppressive motifs from commensal DNA. Mucosal Immunol. 2012, 5, 623–634. [Google Scholar] [CrossRef]
- Shigemori, S.; Namai, F.; Ogita, T.; Sato, T.; Shimosato, T. Oral priming with oligodeoxynucleotide particles from Lactobacillus rhamnosus GG attenuates symptoms of dextran sodium sulfate-induced acute colitis in mice. Anim. Sci. J. 2020, 91, e13468. [Google Scholar] [CrossRef]
- Luyer, M.D.; Buurman, W.A.; Hadfoune, M.; Speelmans, G.; Knol, J.; Jacobs, J.A.; Dejong, C.H.; Vriesema, A.J.; Greve, J.W. Strain-specific effects of probiotics on gut barrier integrity following hemorrhagic shock. Infect. Immun. 2005, 73, 3686–3692. [Google Scholar] [CrossRef]
- Hotte, N.S.; Salim, S.Y.; Tso, R.H.; Albert, E.J.; Bach, P.; Walker, J.; Dieleman, L.A.; Fedorak, R.N.; Madsen, K.L. Patients with inflammatory bowel disease exhibit dysregulated responses to microbial DNA. PLoS ONE 2012, 7, e37932. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.N.; Leary, D.H.; Sullivan, C.J.; Oh, E.; Walper, S.A. Isolation and characterization of Lactobacillus-derived membrane vesicles. Sci. Rep. 2019, 9, 877. [Google Scholar] [CrossRef]
- Hu, R.; Lin, H.; Wang, M.; Zhao, Y.; Liu, H.; Min, Y.; Yang, X.; Gao, Y.; Yang, M. Lactobacillus reuteri-derived extracellular vesicles maintain intestinal immune homeostasis against lipopolysaccharide-induced inflammatory responses in broilers. J. Anim. Sci. Biotechnol. 2021, 12, 25. [Google Scholar] [CrossRef]
- Pang, Y.; Ermann Lundberg, L.; Mata Forsberg, M.; Ahl, D.; Bysell, H.; Pallin, A.; Sverremark-Ekstrom, E.; Karlsson, R.; Jonsson, H.; Roos, S. Extracellular membrane vesicles from Limosilactobacillus reuteri strengthen the intestinal epithelial integrity, modulate cytokine responses and antagonize activation of TRPV1. Front. Microbiol. 2022, 13, 1032202. [Google Scholar] [CrossRef]
- Champagne-Jorgensen, K.; Mian, M.F.; McVey Neufeld, K.A.; Stanisz, A.M.; Bienenstock, J. Membrane vesicles of Lacticaseibacillus rhamnosus JB-1 contain immunomodulatory lipoteichoic acid and are endocytosed by intestinal epithelial cells. Sci. Rep. 2021, 11, 13756. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, Y.; Saika, A.; Nagatake, T.; Matsunaga, A.; Kunisawa, J.; Katakura, Y.; Yamasaki-Yashiki, S. Mechanisms underlying enhanced IgA production in Peyer’s patch cells by membrane vesicles derived from Lactobacillus sakei. Biosci. Biotechnol. Biochem. 2021, 85, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Kurata, A.; Kiyohara, S.; Imai, T.; Yamasaki-Yashiki, S.; Zaima, N.; Moriyama, T.; Kishimoto, N.; Uegaki, K. Characterization of extracellular vesicles from Lactiplantibacillus plantarum. Sci. Rep. 2022, 12, 13330. [Google Scholar] [CrossRef]
- Yamasaki-Yashiki, S.; Miyoshi, Y.; Nakayama, T.; Kunisawa, J.; Katakura, Y. IgA-enhancing effects of membrane vesicles derived from Lactobacillus sakei subsp. sakei NBRC15893. Biosci. Microbiota Food Health 2019, 38, 23–29. [Google Scholar] [CrossRef]
- Champagne-Jorgensen, K.; Jose, T.A.; Stanisz, A.M.; Mian, M.F.; Hynes, A.P.; Bienenstock, J. Bacterial membrane vesicles and phages in blood after consumption of Lacticaseibacillus rhamnosus JB-1. Gut Microbes 2021, 13, 1993583. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Kuhn, T.; Koch, M.; Fuhrmann, G. Stimulation of Probiotic Bacteria Induces Release of Membrane Vesicles with Augmented Anti-inflammatory Activity. ACS Appl. Bio Mater. 2021, 4, 3739–3748. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, M.; De, X.; Sun, T.; Bai, Z.; Luo, J.; Wang, F.; Ge, J. Bacterium-like particles derived from probiotics: Progress, challenges and prospects. Front. Immunol. 2023, 14, 1263586. [Google Scholar] [CrossRef]
- Raya Tonetti, F.; Arce, L.; Salva, S.; Alvarez, S.; Takahashi, H.; Kitazawa, H.; Vizoso-Pinto, M.G.; Villena, J. Immunomodulatory Properties of Bacterium-Like Particles Obtained From Immunobiotic Lactobacilli: Prospects for Their Use as Mucosal Adjuvants. Front. Immunol. 2020, 11, 15. [Google Scholar] [CrossRef]
- Guo, Z.; Ren, H.; Chang, Q.; Liu, R.; Zhou, X.; Xue, K.; Sun, T.; Luo, J.; Wang, F.; Ge, J. Lactobacilli-derived adjuvants combined with immunoinformatics-driven multi-epitope antigens based approach protects against Clostridium perfringens in a mouse model. Int. J. Biol. Macromol. 2024, 267, 131475. [Google Scholar] [CrossRef]
- Shida, K.; Kiyoshima-Shibata, J.; Kaji, R.; Nagaoka, M.; Nanno, M. Peptidoglycan from lactobacilli inhibits interleukin—12 production by macrophages induced by Lactobacillus casei through Toll—like receptor 2—dependent and independent mechanisms. Immunology 2009, 128, e858–e869. [Google Scholar] [CrossRef] [PubMed]
- Brott, A.S.; Clarke, A.J. Peptidoglycan O-acetylation as a virulence factor: Its effect on lysozyme in the innate immune system. Antibiotics 2019, 8, 94. [Google Scholar] [CrossRef]
- Sychantha, D.; Brott, A.S.; Jones, C.S.; Clarke, A.J. Mechanistic pathways for peptidoglycan O-acetylation and de-O-acetylation. Front. Microbiol. 2018, 9, 2332. [Google Scholar] [CrossRef]
- Kang, S.-S.; Sim, J.-R.; Yun, C.-H.; Han, S.H. Lipoteichoic acids as a major virulence factor causing inflammatory responses via Toll-like receptor 2. Arch. Pharmacal Res. 2016, 39, 1519–1529. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; López, P.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Suárez, A.; Margolles, A.; Ruas-Madiedo, P. Immune modulation capability of exopolysaccharides synthesised by lactic acid bacteria and bifidobacteria. Probiotics Antimicrob. Proteins 2012, 4, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides produced by lactic acid bacteria: From health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar] [CrossRef]
- Konieczna, P.; Schiavi, E.; Ziegler, M.; Groeger, D.; Healy, S.; Grant, R.; O’Mahony, L. Human dendritic cell DC-SIGN and TLR-2 mediate complementary immune regulatory activities in response to Lactobacillus rhamnosus JB-1. PLoS ONE 2015, 10, e0120261. [Google Scholar] [CrossRef] [PubMed]
- Prado Acosta, M.; Geoghegan, E.M.; Lepenies, B.; Ruzal, S.; Kielian, M.; Martinez, M.G. Surface (S) layer proteins of Lactobacillus acidophilus block virus infection via DC-SIGN interaction. Front. Microbiol. 2019, 10, 810. [Google Scholar] [CrossRef]
- Basset, A.; Zhang, F.; Benes, C.; Sayeed, S.; Herd, M.; Thompson, C.; Golenbock, D.T.; Camilli, A.; Malley, R. Toll-like receptor (TLR) 2 mediates inflammatory responses to oligomerized RrgA pneumococcal pilus type 1 protein. J. Biol. Chem. 2013, 288, 2665–2675. [Google Scholar] [CrossRef]
- Bertorello, S.; Cei, F.; Fink, D.; Niccolai, E.; Amedei, A. The future exploring of gut microbiome-immunity interactions: From in vivo/vitro models to in silico innovations. Microorganisms 2024, 12, 1828. [Google Scholar] [CrossRef] [PubMed]
- Metwaly, A.; Kriaa, A.; Hassani, Z.; Carraturo, F.; Druart, C.; Arnauts, K.; Wilmes, P.; Walter, J.; Rosshart, S. A Consensus Statement on establishing causality, therapeutic applications and the use of preclinical models in microbiome research. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 343–356. [Google Scholar] [CrossRef]
- Griffin, M.E.; Hang, H.C. Microbial mechanisms to improve immune checkpoint blockade responsiveness. Neoplasia 2022, 31, 100818. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lambris, J.D. More than complementing Tolls: Complement–Toll—like receptor synergy and crosstalk in innate immunity and inflammation. Immunol. Rev. 2016, 274, 233–244. [Google Scholar] [CrossRef]
- Sanz, Y.; Moya-Pérez, A. Microbiota, inflammation and obesity. In Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease; Springer: New York, NY, USA, 2014; pp. 291–317. [Google Scholar]
- Struijk, E.A.; Heraclides, A.; Witte, D.R.; Soedamah-Muthu, S.S.; Geleijnse, J.M.; Toft, U.; Lau, C.J. Dairy product intake in relation to glucose regulation indices and risk of type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 822–828. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef]
- Wang, H.; Livingston, K.A.; Fox, C.S.; Meigs, J.B.; Jacques, P.F. Yogurt consumption is associated with better diet quality and metabolic profile in American men and women. Nutr. Res. 2013, 33, 18–26. [Google Scholar] [CrossRef]
- Cai, J.; Zhao, C.; Du, Y.; Zhang, Y.; Zhao, M.; Zhao, Q. Comparative efficacy and tolerability of probiotics for antibiotic-associated diarrhea: Systematic review with network meta-analysis. United Eur. Gastroenterol. J. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Huang, I.F.; Lin, I.C.; Liu, P.F.; Cheng, M.F.; Liu, Y.C.; Hsieh, Y.D.; Chen, J.J.; Chen, C.L.; Chang, H.W.; Shu, C.W. Lactobacillus acidophilus attenuates Salmonella-induced intestinal inflammation via TGF-beta signaling. BMC Microbiol. 2015, 15, 203. [Google Scholar] [CrossRef]
- Rokana, N.; Singh, R.; Mallappa, R.H.; Batish, V.K.; Grover, S. Modulation of intestinal barrier function to ameliorate Salmonella infection in mice by oral administration of fermented milks produced with Lactobacillus plantarum MTCC 5690—A probiotic strain of Indian gut origin. J. Med. Microbiol. 2016, 65, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.Z.; Yap, C.; Lytvyn, L.; Lo, C.K.; Beardsley, J.; Mertz, D.; Johnston, B.C. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst. Rev. 2017, 12, CD006095. [Google Scholar] [CrossRef]
- Francavilla, R.; Lionetti, E.; Castellaneta, S.P.; Magista, A.M.; Maurogiovanni, G.; Bucci, N.; De Canio, A.; Indrio, F.; Cavallo, L.; Ierardi, E.; et al. Inhibition of Helicobacter pylori infection in humans by Lactobacillus reuteri ATCC 55730 and effect on eradication therapy: A pilot study. Helicobacter 2008, 13, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Tongtawee, T.; Dechsukhum, C.; Leeanansaksiri, W.; Kaewpitoon, S.; Kaewpitoon, N.; Loyd, R.A.; Matrakool, L.; Panpimanmas, S. Improved Helicobacter pylori Eradication Rate of Tailored Triple Therapy by Adding Lactobacillus delbrueckii and Streptococcus thermophilus in Northeast Region of Thailand: A Prospective Randomized Controlled Clinical Trial. Gastroenterol. Res. Pr. 2015, 2015, 518018. [Google Scholar] [CrossRef]
- Kwon, H.K.; Kim, G.C.; Kim, Y.; Hwang, W.; Jash, A.; Sahoo, A.; Kim, J.E.; Nam, J.H.; Im, S.H. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin. Immunol. 2013, 146, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Shen, L. Probiotics as potential therapeutic options for Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2021, 105, 7721–7730. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.; Vora, A.; Kaur, G.; Akhtar, J. Dysbiosis and Alzheimer’s disease: Role of probiotics, prebiotics and synbiotics. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 2911–2923. [Google Scholar] [CrossRef]
- Gazerani, P. Probiotics for Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 4121. [Google Scholar] [CrossRef]
- Partty, A.; Kalliomaki, M.; Wacklin, P.; Salminen, S.; Isolauri, E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: A randomized trial. Pediatr. Res. 2015, 77, 823–828. [Google Scholar] [CrossRef]
- Vogel, D.Y.; Glim, J.E.; Stavenuiter, A.W.; Breur, M.; Heijnen, P.; Amor, S.; Dijkstra, C.D.; Beelen, R.H. Human macrophage polarization In vitro: Maturation and activation methods compared. Immunobiology 2014, 219, 695–703. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Carol, M.; Borruel, N.; Antolin, M.; Llopis, M.; Casellas, F.; Guarner, F.; Malagelada, J.R. Modulation of apoptosis in intestinal lymphocytes by a probiotic bacteria in Crohn’s disease. J. Leukoc. Biol. 2006, 79, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Peng, K.; Xiao, S.; Long, Y.; Yu, Q. The role of Lactobacillus in inflammatory bowel disease: From actualities to prospects. Cell Death Discov. 2023, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Lee, S.Y.; Kim, N.R.; Lee, H.Y.; Ko, M.Y.; Jung, B.J.; Kim, C.M.; Lee, J.M.; Park, J.H.; Han, S.H.; et al. Lactobacillus plantarum lipoteichoic acid down-regulated Shigella flexneri peptidoglycan-induced inflammation. Mol. Immunol. 2011, 48, 382–391. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, X.; Tong, L.; Liu, Q.; Liang, X.; Bu, Y.; Gong, P.; Liu, T.; Zhang, L.; Xia, Y.; et al. Effect of Extracellular Vesicles Derived From Lactobacillus plantarum Q7 on Gut Microbiota and Ulcerative Colitis in Mice. Front. Immunol. 2021, 12, 777147. [Google Scholar] [CrossRef]
- Riehl, T.E.; Alvarado, D.; Ee, X.; Zuckerman, A.; Foster, L.; Kapoor, V.; Thotala, D.; Ciorba, M.A.; Stenson, W.F. Lactobacillus rhamnosus GG protects the intestinal epithelium from radiation injury through release of lipoteichoic acid, macrophage activation and the migration of mesenchymal stem cells. Gut 2019, 68, 1003–1013. [Google Scholar] [CrossRef]
- Nowak, B.; Srottek, M.; Ciszek-Lenda, M.; Skalkowska, A.; Gamian, A.; Gorska, S.; Marcinkiewicz, J. Exopolysaccharide from Lactobacillus rhamnosus KL37 Inhibits T Cell-dependent Immune Response in Mice. Arch. Immunol. Ther. Exp. 2020, 68, 17. [Google Scholar] [CrossRef]
- Noda, M.; Sultana, N.; Hayashi, I.; Fukamachi, M.; Sugiyama, M. Exopolysaccharide Produced by Lactobacillus paracasei IJH-SONE68 Prevents and Improves the Picryl Chloride-Induced Contact Dermatitis. Molecules 2019, 24, 2970. [Google Scholar] [CrossRef]
- Kawanabe-Matsuda, H.; Takeda, K.; Nakamura, M.; Makino, S.; Karasaki, T.; Kakimi, K.; Nishimukai, M.; Ohno, T.; Omi, J.; Kano, K.; et al. Dietary Lactobacillus-Derived Exopolysaccharide Enhances Immune-Checkpoint Blockade Therapy. Cancer Discov. 2022, 12, 1336–1355. [Google Scholar] [CrossRef]
- Lu, S.; Xu, J.; Zhao, Z.; Guo, Y.; Zhang, H.; Jurutka, P.W.; Huang, D.; Cao, C.; Cheng, S. Dietary Lactobacillus rhamnosus GG extracellular vesicles enhance antiprogrammed cell death 1 (anti-PD-1) immunotherapy efficacy against colorectal cancer. Food Funct. 2023, 14, 10314–10328. [Google Scholar] [CrossRef]
- Bender, M.J.; McPherson, A.C.; Phelps, C.M.; Pandey, S.P.; Laughlin, C.R.; Shapira, J.H.; Medina Sanchez, L.; Rana, M.; Richie, T.G.; Mims, T.S.; et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell 2023, 186, 1846–1862 e1826. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Yen, H.R.; Lu, W.L.; Ho, H.H.; Lin, W.Y.; Kuo, Y.W.; Huang, Y.Y.; Tsai, S.Y.; Lin, H.C. Adjuvant Probiotics of Lactobacillus salivarius subsp. salicinius AP-32, L. johnsonii MH-68, and Bifidobacterium animalis subsp. lactis CP-9 Attenuate Glycemic Levels and Inflammatory Cytokines in Patients With Type 1 Diabetes Mellitus. Front. Endocrinol. 2022, 13, 754401. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, H.; Li, X.; Li, D.; Sun, Y.; Yang, L.; Ma, Y.; Chan, E.C.Y. Lactobacillus paracasei IMC 502 ameliorates type 2 diabetes by mediating gut microbiota-SCFA-hormone/inflammation pathway in mice. J. Sci. Food Agric. 2023, 103, 2949–2959. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Tsai, J.J.; Lin, S.L.; Lin, M.Y. Lactobacillus casei MYL01 modulates the proinflammatory state induced by ethanol in an In vitro model. J. Dairy Sci. 2014, 97, 2009–2016. [Google Scholar] [CrossRef]
- Nimgampalle, M.; Kuna, Y. Anti-Alzheimer Properties of Probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s Disease induced Albino Rats. J. Clin. Diagn. Res. 2017, 11, KC01–KC05. [Google Scholar] [CrossRef]
- Del Piano, M.; Balzarini, M.; Carmagnola, S.; Pagliarulo, M.; Tari, R.; Nicola, S.; Deidda, F.; Pane, M. Assessment of the capability of a gelling complex made of tara gum and the exopolysaccharides produced by the microorganism Streptococcus thermophilus ST10 to prospectively restore the gut physiological barrier: A pilot study. J. Clin. Gastroenterol. 2014, 48 (Suppl. S1), S56–S61. [Google Scholar] [CrossRef] [PubMed]
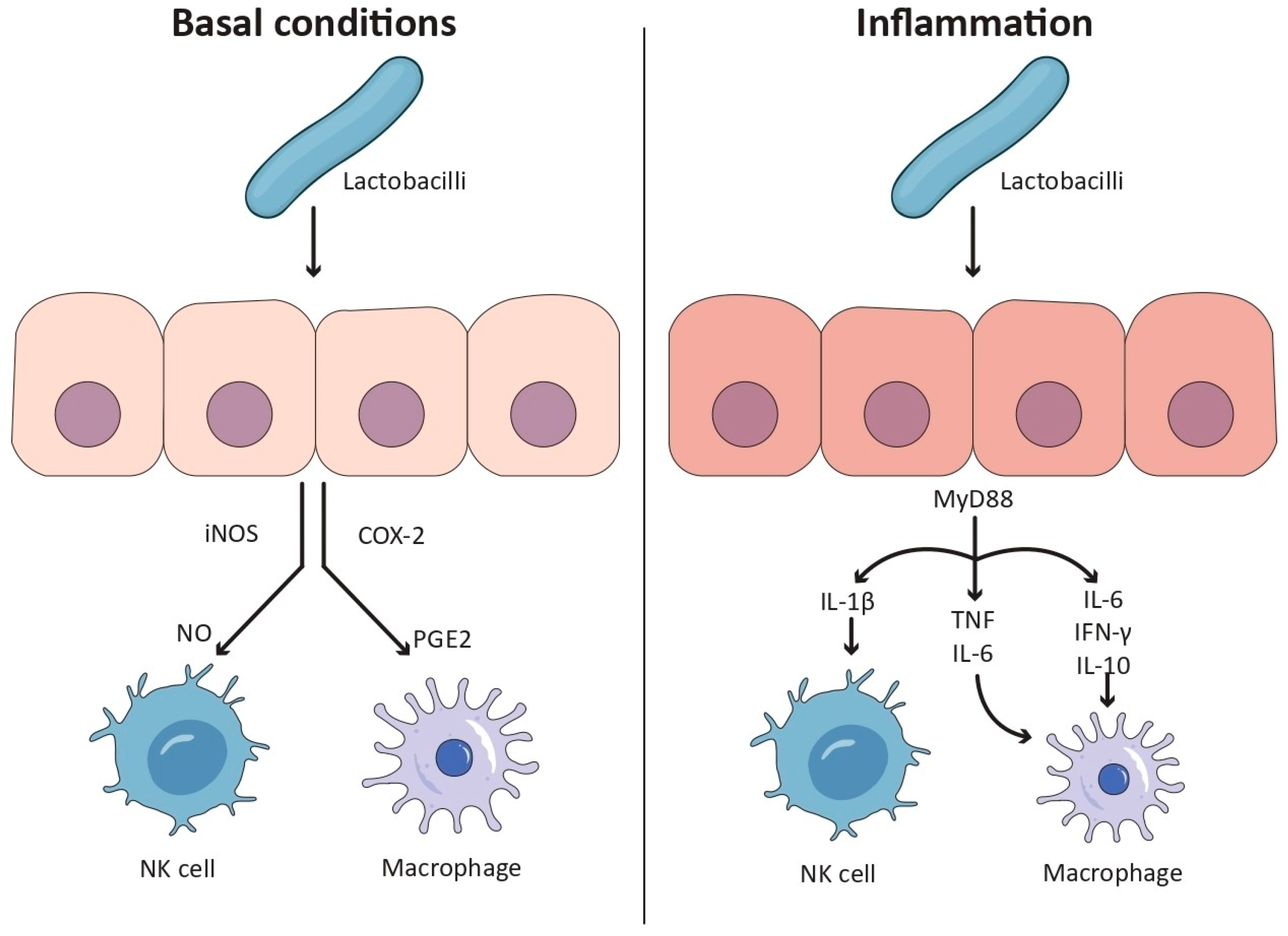
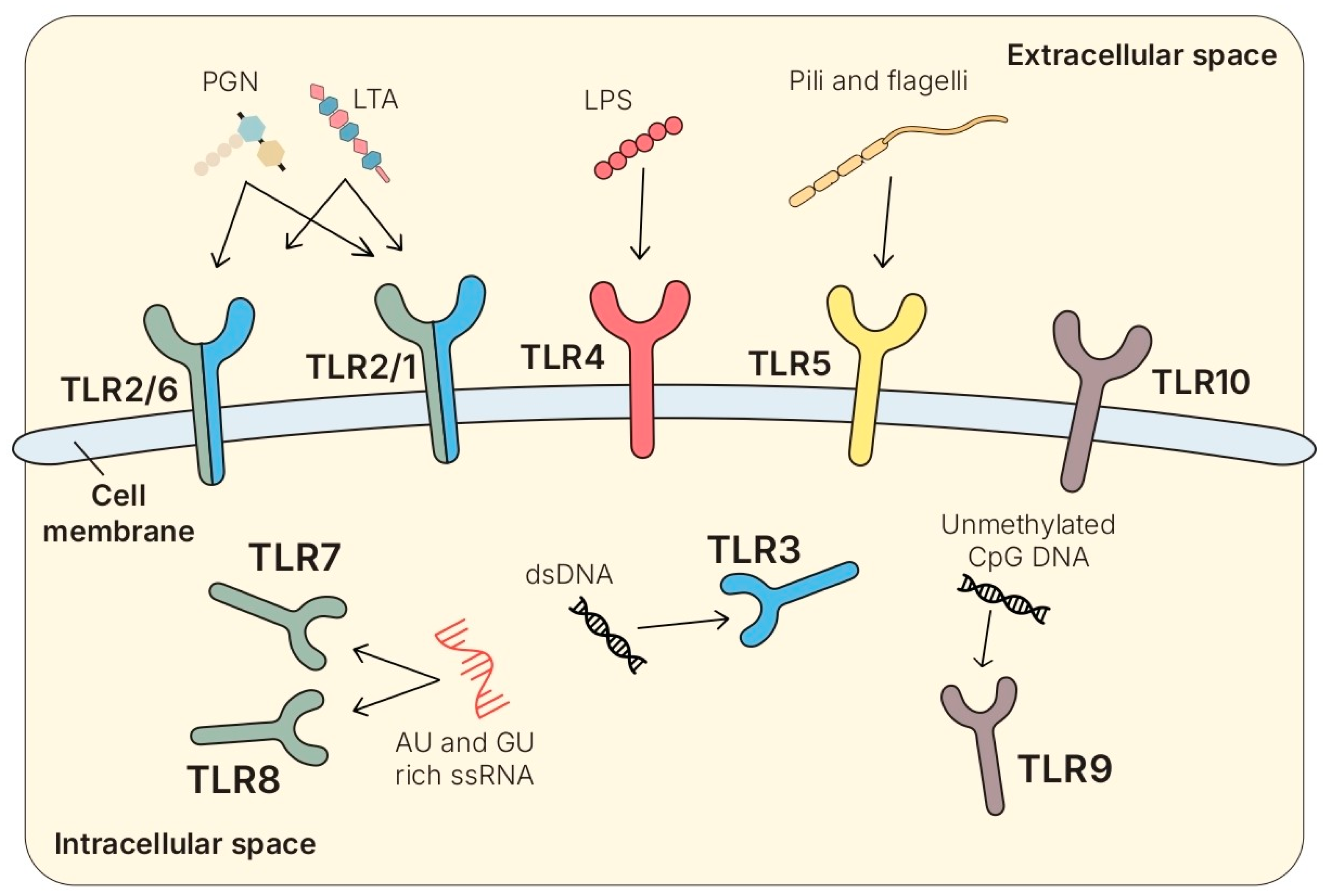
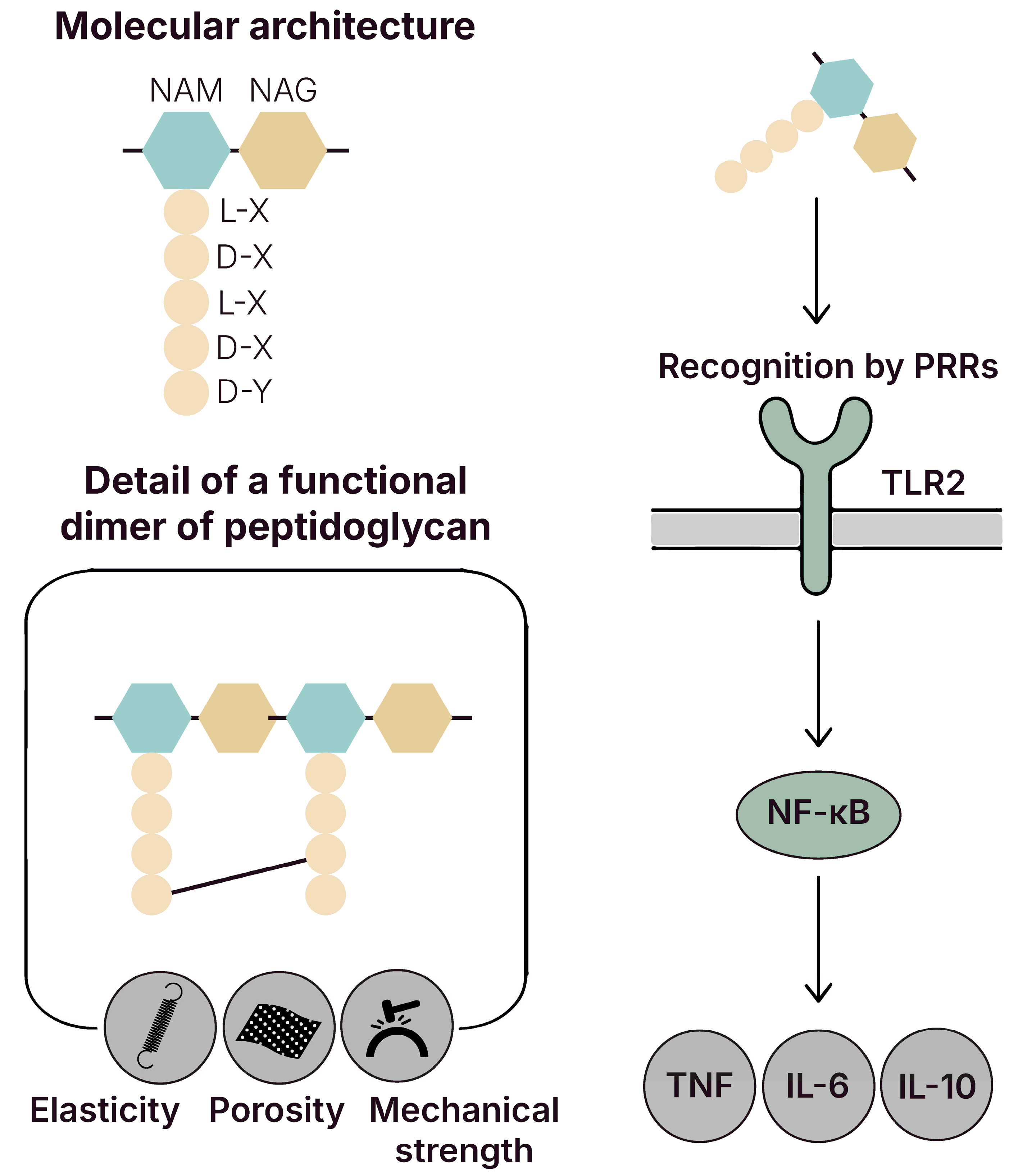
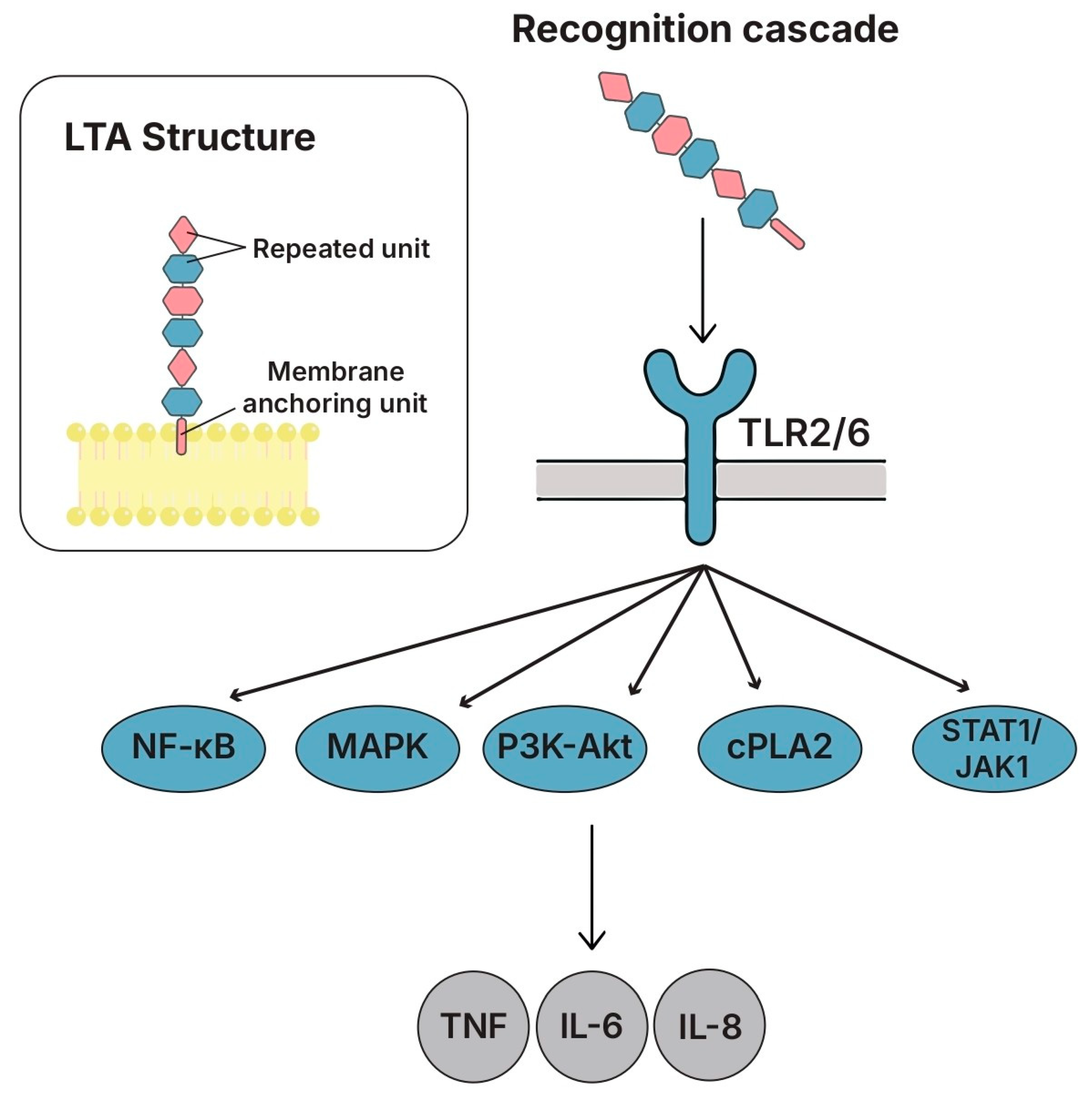

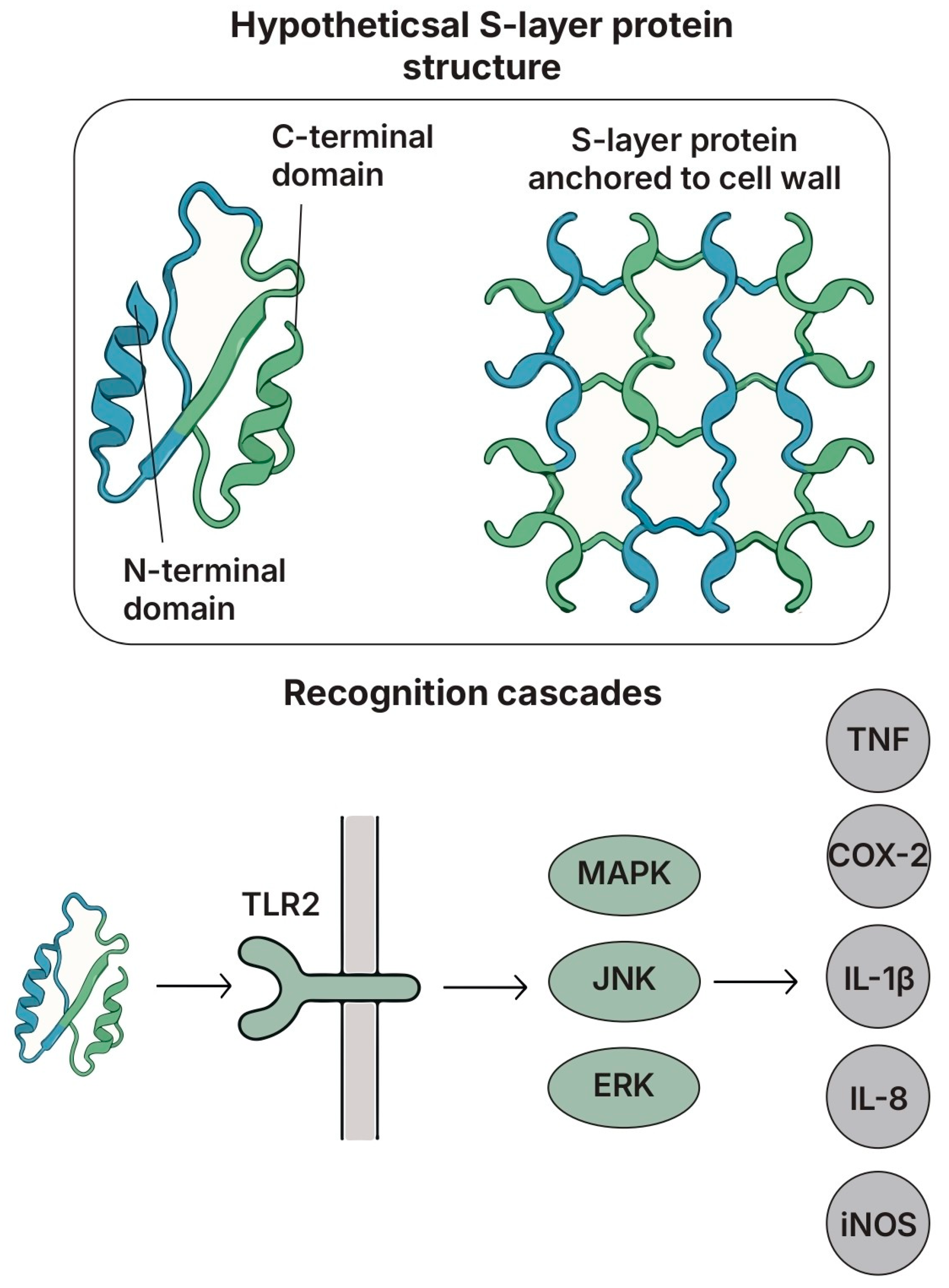
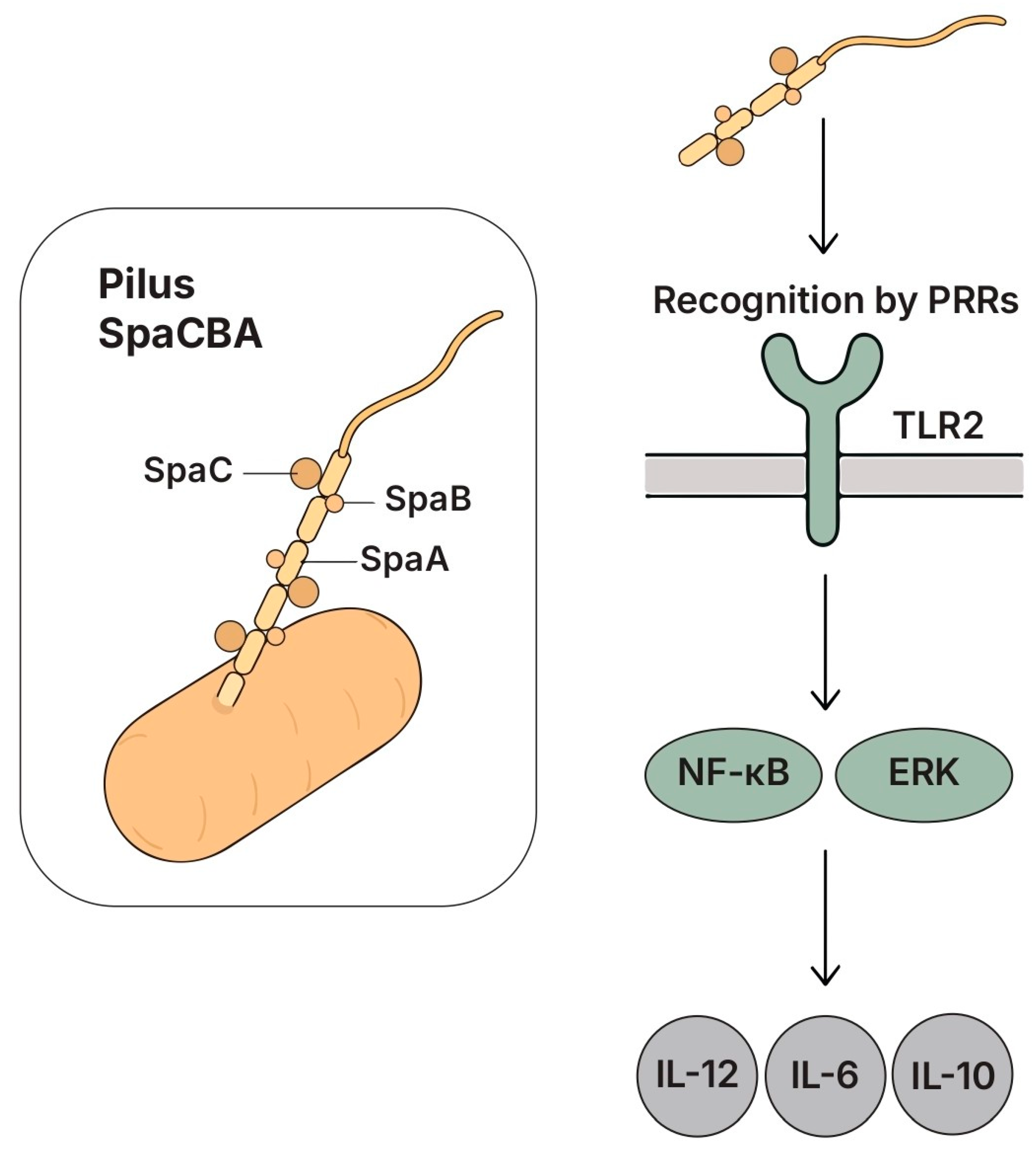
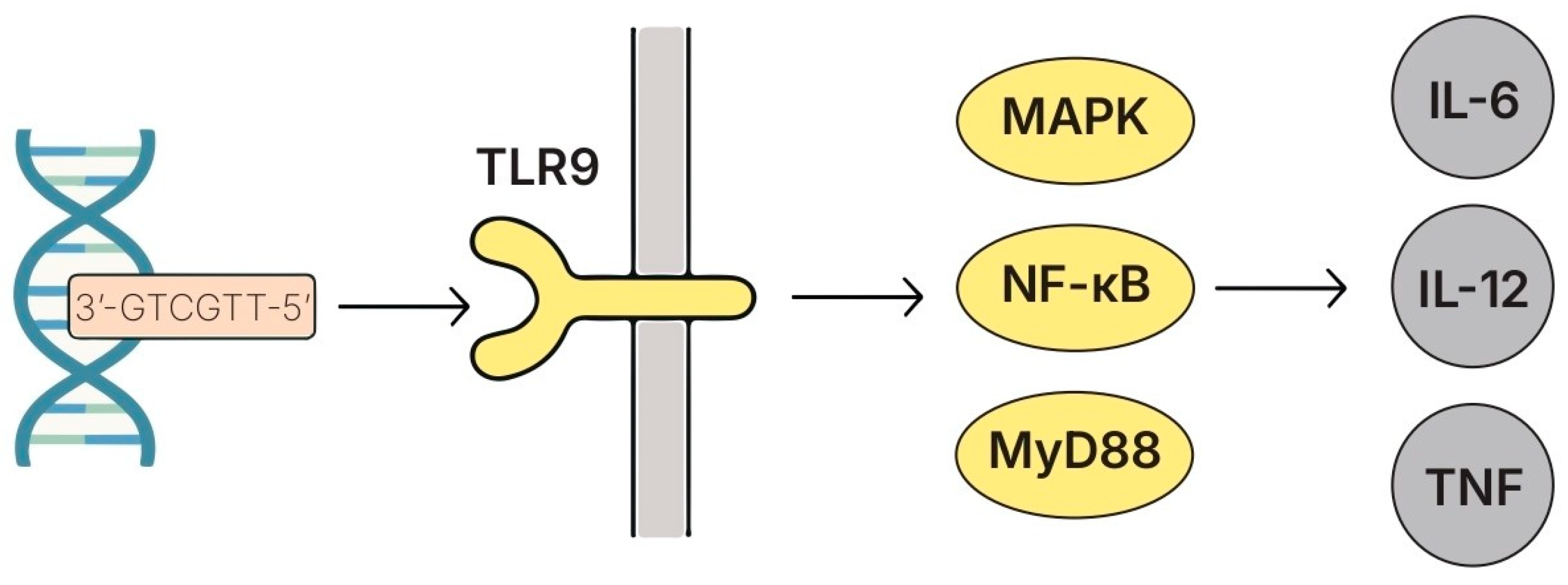
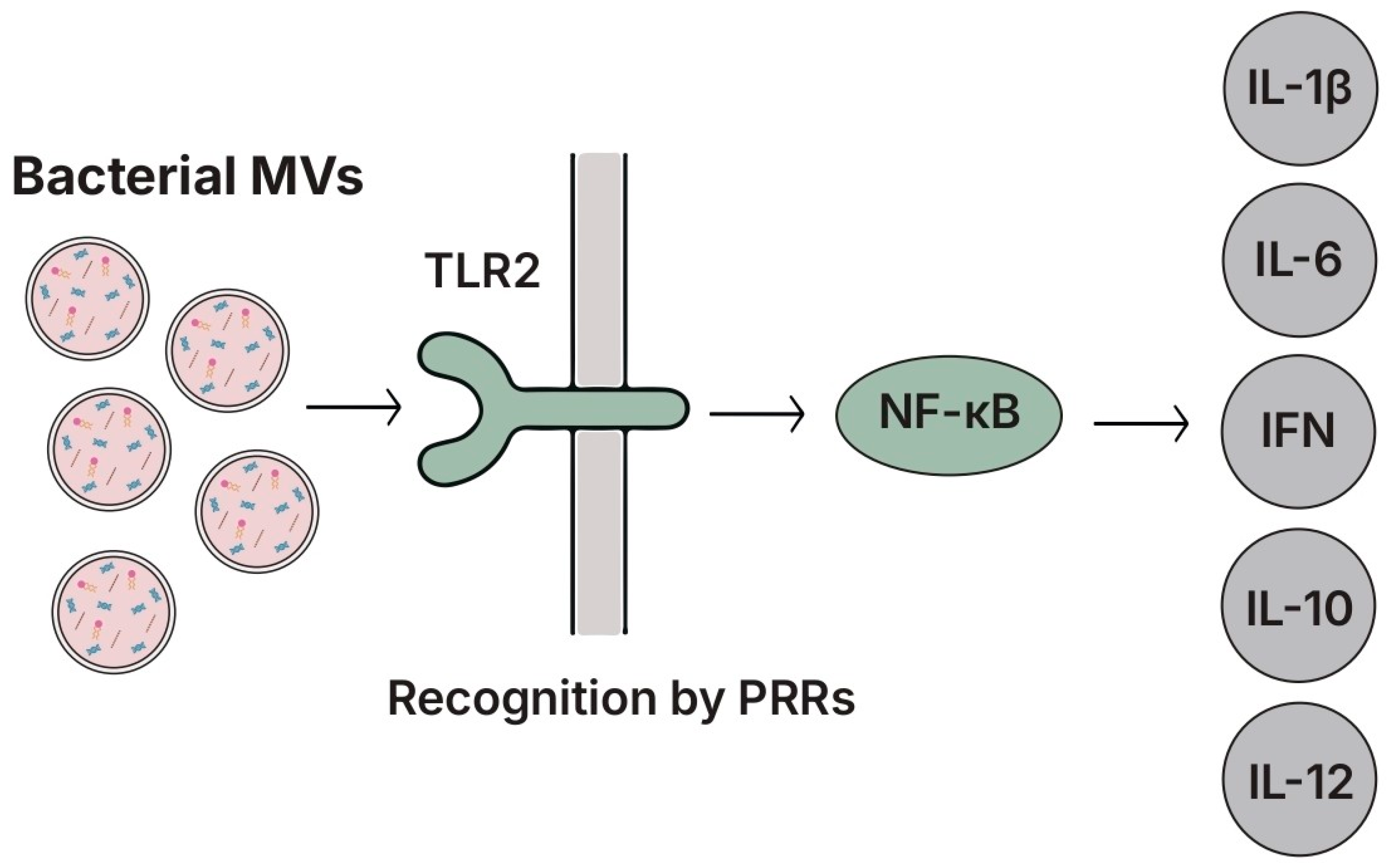
| MAMP | Lactobacilli: Recurrent Feature | Comparison with Other Genera and Functional Implication | Refs. |
|---|---|---|---|
| Peptidoglycan | Muropeptide patterns and cell-wall modifications (e.g., O-acetylation via OatA, amidation) tune NOD1/NOD2 sensing and lysozyme susceptibility; in several lactobacilli strains, PGN/fragments tend to dampen IL-12 and NF-κB outputs, favoring regulatory/barrier-supportive profiles. | In pathogens, PGN modifications often promote lysozyme evasion and strong NOD/TLR priming, and the outcome is a pro-inflammatory bias. | [24,118,274,275,276] |
| Lipoteichoic acid | The degree of D-alanine substitution (operon dlt) acts as a “rheostat”: reduced D-alanylation attenuates TLR2 signaling and inflammation. Lactobacilli LTA can increase IL-10 and reduce MAPK/NF-κB in epithelial/monocytic models. | In many Gram-positive pathogens, highly D-alanylated LTA drives robust TLR2-dependent activation and pro-inflammatory cytokines. | [154,277] |
| Exopolysaccharides | Frequently acidic/branched, high-MW polymers that modulate TLR2/TLR4–MAPK/STAT pathways, lower pro-inflammatory cytokines, and strengthen tight junctions (barrier-protective profile). | Pathogen EPS/capsules favor adhesion/biofilm and immune evasion; outputs are often pro-inflammatory or broadly suppressive without barrier-repair features. | [25,278,279] |
| S-layer | S-layer of L. acidophilus (SlpA) is a DC-SIGN ligand and cooperates with TLR2 to reshape DC functions and T-cell output (regulatory bias). Recent work reinforces lectin-PRR (DC-SIGN) engagement and immunoregulation in Lactobacilli. | S-layers are not ubiquitous among commensals; in pathogens, S-layers are often linked to innate stimulation (via TLR/CLR) and/or evasion. A DC-SIGN-centric, tolerogenic signature is more typical of lactobacilli. | [221,280,281] |
| Pili | SpaCBA pili of L. rhamnosus GG mediate high-affinity mucus adhesion and can modulate epithelial/DC cytokines (e.g., IL-8); mutant/heterologous systems reveal a contribution of pili to non-pathogenic DC/IEC crosstalk. | In pathogens (e.g., pneumococcus, streptococcus), pili act as pro-inflammatory MAMPs (often TLR2-dependent), boosting IL-8/TNF-α and virulence. | [238,239,282] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furnari, S.; Ciantia, R.; Garozzo, A.; Furneri, P.M.; Fuochi, V. Lactobacilli-Derived Microbe-Associated Molecular Patterns (MAMPs) in Host Immune Modulation. Biomolecules 2025, 15, 1609. https://doi.org/10.3390/biom15111609
Furnari S, Ciantia R, Garozzo A, Furneri PM, Fuochi V. Lactobacilli-Derived Microbe-Associated Molecular Patterns (MAMPs) in Host Immune Modulation. Biomolecules. 2025; 15(11):1609. https://doi.org/10.3390/biom15111609
Chicago/Turabian StyleFurnari, Salvatore, Ruben Ciantia, Adriana Garozzo, Pio Maria Furneri, and Virginia Fuochi. 2025. "Lactobacilli-Derived Microbe-Associated Molecular Patterns (MAMPs) in Host Immune Modulation" Biomolecules 15, no. 11: 1609. https://doi.org/10.3390/biom15111609
APA StyleFurnari, S., Ciantia, R., Garozzo, A., Furneri, P. M., & Fuochi, V. (2025). Lactobacilli-Derived Microbe-Associated Molecular Patterns (MAMPs) in Host Immune Modulation. Biomolecules, 15(11), 1609. https://doi.org/10.3390/biom15111609







