Nanotechnology-Based Delivery Systems and Retinal Pigment Epithelium: Advances, Targeting Approaches, and Translational Challenges
Abstract
1. Introduction
2. Materials and Methods
3. Therapeutics Delivery to the RPE
4. Nanomedicine Approaches for RPE-Related Diseases
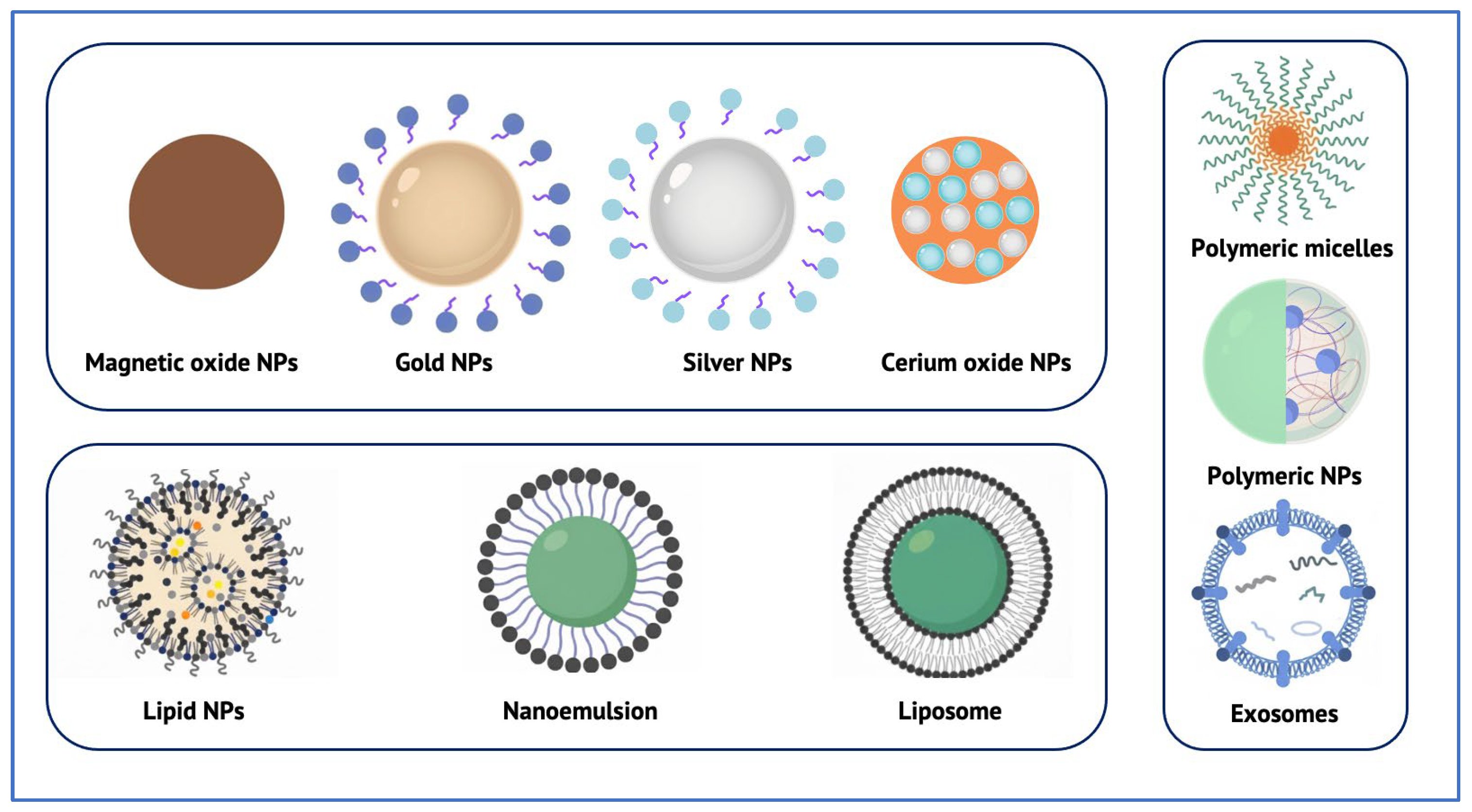
5. Classes of Nanotechnology Systems Reaching the RPE
| Nanocarrier Class | Typical Therapeutic Cargo | Common Routes of Ocular Administration | Advantages | Limitations/Considerations |
|---|---|---|---|---|
| Polymeric nanoparticles (e.g., PLGA, PEG–PLGA, chitosan-, HA-modified) | Anti-VEGF drugs, siRNA, miRNA, proteins [48] | Intravitreal; Subretinal [49,50] Systemic [68] | Sustained release; Reduced injection frequency; Tunable surface chemistry; Good cellular internalization [49,50] | Size-dependent drug loading; Risk of burst release; Limited long-term safety data for very small particles; Potential for off-target accumulation [51] |
| Lipid-based nanoparticles (SLNs, NLCs) | Hydrophobic and hydrophilic drugs; mRNA; Plasmids [52,53] | Intravitreal [53]; Subretinal [55]; Topical [69] | High biocompatibility; Suitable for gene delivery; Good loading efficiency [52,53] | Challenges in large-scale reproducible manufacturing; Storage instability; Risk of rapid clearance [56] |
| Metallic and inorganic nanoparticles (e.g., Gold, Cerium oxide, Mesoporous silica) | Anti-VEGF drugs; Antioxidants; Enzyme mimetics [58] | Intravitreal [58]; Systemic [70]; Topical [71] | Potential dose sparing; Intrinsic bioactivity (e.g., ROS scavenging); Long-lasting effects [60] | Non-biodegradable core may accumulate; Limited data on long-term ocular safety; Risk of lysosomal persistence [61] |
| Liposomes | Anti-VEGF drugs; Steroids; Proteins [62] | Intravitreal [62]; Subretinal [63]; Systemic [68] | Co-encapsulation of hydrophilic & hydrophobic agents; Generally low toxicity; Potential for sustained release [62] | Sensitive to pH and enzymatic degradation; Rapid vitreous clearance; May still require repeated injections [63] |
| Polymeric micelles | Hydrophobic small molecules; peptides [64] | Intravitreal [64] | High solubilization capacity; Customizable targeting ligands; Good stability in solution [64] | Possible premature drug leakage; Requires cross-linking for durable release [64] |
| Extracellular vesicles/Exosomes | siRNA; miRNA; proteins; antioxidants [65] | Intravitreal [65] | Endogenous biocompatibility; Low immunogenicity; Natural tropism to retinal cells [65] | Scalability and purification challenges; Cargo loading variability; Stability and dosing protocols not yet standardized [66] |
6. Targeting Strategies for the RPE
6.1. Physicochemical Tuning
6.2. Ligand–Receptor-Mediated Targeting
| Ligand | Target Receptor/Mechanism | Nanoparticle Example | Key Outcome |
|---|---|---|---|
| Hyaluronic acid (HA) | CD44 | HA-modified LNPs | Preferential localization in RPE–choroid complex after IVT injection; uptake influenced by HA molecular weight and surface density [69,80] |
| Hyaluronic acid (HA) | CD44 | HA-coated gold nanoparticles (topical) | Reached retina after eye-drop administration; noninvasive delivery potential [71] |
| Cleavable peptide linker (cathepsin D–sensitive) | Cathepsin D (lysosomal enzyme, highly expressed in RPE) | Peptide-modified nanoparticles | Enabled selective intracellular drug release in RPE cells, reducing off-target effects [81] |
| RGD peptide (arginine–glycine–aspartic acid) | Integrins (αvβ3, others) | RGD-functionalized PEI nanoparticles | Selective accumulation in RPE and CNV regions; sustained release and therapeutic effect in CNV mouse model [83] |
6.3. Pathology-Responsive Targeting
7. Therapeutic Payloads Delivered to and Through the RPE
7.1. Small Molecules: Steroids and Antioxidants
7.2. Biologics: Anti-Angiogenic Agents
7.3. Nucleic Acids and Gene-Editing Cargo
8. Challenges and Translational Considerations
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Li, W.; Chen, M.; Cao, Y.; Lu, W.; Li, X. The retinal pigment epithelium: Functions and roles in ocular diseases. Fundam. Res. 2024, 4, 1710–1718. [Google Scholar] [CrossRef]
- Sparrrow, J.R.; Hicks, D.; Hamel, C.P. The Retinal Pigment Epithelium in Health and Disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y. Ranibizumab for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Bennett, J.; A Wellman, J.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65 -mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, D.; Sahoo, N.K.; Mariappan, I.; Narayanan, R. Gene therapy in retinal diseases: A review. Indian J. Ophthalmol. 2021, 69, 2257–2265. [Google Scholar] [CrossRef]
- Mostafa, M.; Al Fatease, A.; Alany, R.G.; Abdelkader, H. Recent Advances of Ocular Drug Delivery Systems: Prominence of Ocular Implants for Chronic Eye Diseases. Pharmaceutics 2023, 15, 1746. [Google Scholar] [CrossRef] [PubMed]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Xu, C.; Peng, A.; Qin, H.; Yao, K. Advancements in age-related macular degeneration treatment: From traditional anti-VEGF to emerging therapies in gene, stem cell, and nanotechnology. Biochem. Pharmacol. 2025, 236, 116902. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Luo, L.-J.; Yang, C.-J.; Lai, J.-Y. Highly Retina-Permeating and Long-Acting Resveratrol/Metformin Nanotherapeutics for Enhanced Treatment of Macular Degeneration. ACS Nano 2023, 17, 168–183. [Google Scholar] [CrossRef]
- Huang, K.; Deng, H.; Wang, S.; Zhang, F.; Huang, G.; Wang, L.; Liu, J.; Zhao, X.; Ren, H.; Yang, G.; et al. Melanin-Like Nanomedicine Functions as a Novel RPE Ferroptosis Inhibitor to Ameliorate Retinal Degeneration and Visual Impairment in Dry Age-Related Macular Degeneration. Adv. Healthc. Mater. 2024, 13, e2401613. [Google Scholar] [CrossRef]
- Cao, D.; Zhu, J.; Guo, Y.; Zhou, Y.; Zeng, J.; Tu, Y.; Zhao, Z.; Xie, L.; Song, E.; Zhu, M.; et al. Dynamically covalent lipid nanoparticles mediate CRISPR-Cas9 genome editing against choroidal neovascularization in mice. Sci. Adv. 2025, 11, eadj0006. [Google Scholar] [CrossRef]
- Gabai, A.; Zeppieri, M.; Finocchio, L.; Salati, C. Innovative Strategies for Drug Delivery to the Ocular Posterior Segment. Pharmaceutics 2023, 15, 1862. [Google Scholar] [CrossRef]
- Gade, S.; So, Y.; Mishra, D.; Baviskar, S.M.; Assiri, A.A.; Glover, K.; Sheshala, R.; Vora, L.K.; Thakur, R.R.S. Ocular Drug Delivery: Emerging Approaches and Advances. Pharmaceutics 2025, 17, 599. [Google Scholar] [CrossRef]
- Hancock, S.E.; Wan, C.-R.; Fisher, N.E.; Andino, R.V.; Ciulla, T.A. Biomechanics of suprachoroidal drug delivery: From benchtop to clinical investigation in ocular therapies. Expert Opin. Drug Deliv. 2021, 18, 777–788. [Google Scholar] [CrossRef]
- Wu, K.Y.; Fujioka, J.K.; Gholamian, T.; Zaharia, M.; Tran, S.D. Suprachoroidal Injection: A Novel Approach for Targeted Drug Delivery. Pharmaceuticals 2023, 16, 1241. [Google Scholar] [CrossRef]
- Wu, K.Y.; Gao, A.; Giunta, M.; Tran, S.D. What’s New in Ocular Drug Delivery: Advances in Suprachoroidal Injection since 2023. Pharmaceuticals 2024, 17, 1007. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chau, Y. Intravitreal nanoparticles for retinal delivery. Drug Discov. Today 2019, 24, 1510–1523. [Google Scholar] [CrossRef]
- Bohley, M.; Dillinger, A.E.; Tamm, E.R.; Goepferich, A. Targeted drug delivery to the retinal pigment epithelium: Untapped therapeutic potential for retinal diseases. Drug Discov. Today 2022, 27, 2497–2509. [Google Scholar] [CrossRef]
- Sahu, A.; Patel, A.R.; Shetty, K.H.; Shah, D.O.; Willcox, M.D.; Maulvi, F.A.; Desai, D.T. Revolutionizing age-related macular degeneration treatment: Advances and future directions in non-invasive retinal drug delivery systems. Int. J. Pharm. 2025, 683, 126009. [Google Scholar] [CrossRef] [PubMed]
- Israilevich, R.N.; Mansour, H.; Patel, S.N.; Garg, S.J.; Klufas, M.A.; Yonekawa, Y.; Regillo, C.D.; Hsu, J. Risk of Endophthalmitis Based on Cumulative Number of Anti-VEGF Intravitreal Injections. Ophthalmology 2024, 131, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Patel, S.N.; Chaudhary, V.; Garg, S.J. Complications of intravitreal injections: 2022. Curr. Opin. Ophthalmol. 2022, 33, 137–146. [Google Scholar] [CrossRef]
- Koirala, A.; Conley, S.M.; Naash, M.I. A review of therapeutic prospects of non-viral gene therapy in the retinal pigment epithelium. Biomaterials 2013, 34, 7158–7167. [Google Scholar] [CrossRef]
- Irigoyen, C.; Alonso, A.A.; Sanchez-Molina, J.; Rodríguez-Hidalgo, M.; Lara-López, A.; Ruiz-Ederra, J. Subretinal Injection Techniques for Retinal Disease: A Review. J. Clin. Med. 2022, 11, 4717. [Google Scholar] [CrossRef]
- Miller, C.W.; Rabljenovic, A.; Papproth, C.; Sciulli, H.; Platt, S.; Miller, D.G. Characteristics and Risks of Endophthalmitis after 25-gauge Vitrectomy Surgery over a 14-year Period. Ophthalmol. Retin. 2024, 9, 556–563. [Google Scholar] [CrossRef]
- Prado, D.A.; Acosta-Acero, M.; Maldonado, R.S. Gene therapy beyond luxturna: A new horizon of the treatment for inherited retinal disease. Curr. Opin. Ophthalmol. 2020, 31, 147–154. [Google Scholar] [CrossRef]
- Ramsay, E.; Hagström, M.; Vellonen, K.-S.; Boman, S.; Toropainen, E.; del Amo, E.M.; Kidron, H.; Urtti, A.; Ruponen, M. Role of retinal pigment epithelium permeability in drug transfer between posterior eye segment and systemic blood circulation. Eur. J. Pharm. Biopharm. 2019, 143, 18–23. [Google Scholar] [CrossRef]
- Campbell, M.; Humphries, P. The blood-retina barrier: Tight junctions and barrier modulation. Adv. Exp. Med. Biol. 2012, 763, 70–84. [Google Scholar]
- Li, S.; Chen, L.; Fu, Y. Nanotechnology-based ocular drug delivery systems: Recent advances and future prospects. J. Nanobiotechnol. 2023, 21, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Mainardes, R.M.; Silva, L.P. Drug Delivery Systems: Past, Present, and Future. Curr. Drug Targets 2004, 5, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Rehan, F.; Zhang, M.; Fang, J.; Greish, K. Therapeutic Applications of Nanomedicine: Recent Developments and Future Perspectives. Molecules 2024, 29, 2073. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Cheng, N.; Sun, X.; Yan, L.; Li, W. The application of nanomedicine in clinical settings. Front. Bioeng. Biotechnol. 2023, 11, 1219054. [Google Scholar] [CrossRef]
- Chaurasia, S.S.; Lim, R.R.; Lakshminarayanan, R.; Mohan, R.R. Nanomedicine Approaches for Corneal Diseases. J. Funct. Biomater. 2015, 6, 277–298. [Google Scholar] [CrossRef]
- Jiang, S.; Franco, Y.L.; Zhou, Y.; Chen, J. Nanotechnology in retinal drug delivery. Int. J. Ophthalmol. 2018, 11, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gao, Y.; Liu, A.; Zhai, G. A review of nanocarrier-mediated drug delivery systems for posterior segment eye disease: Challenges analysis and recent advances. J. Drug Target. 2021, 29, 687–702. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, M.; Shen, W.; Xu, Y.; Shao, A.; Xu, P.; Yao, K.; Han, H.; Ye, J. Recent Advances in Nanomedicine for Ocular Fundus Neovascularization Disease Management. Adv. Healthc. Mater. 2024, 13, e2304626. [Google Scholar] [CrossRef]
- Mahaling, B.; Low, S.W.Y.; Ch, S.; Addi, U.R.; Ahmad, B.; Connor, T.B.; Mohan, R.R.; Biswas, S.; Chaurasia, S.S. Next-Generation Nanomedicine Approaches for the Management of Retinal Diseases. Pharmaceutics 2023, 15, 2005. [Google Scholar] [CrossRef]
- Nikolaidou, A.; Spyratou, E.; Sandali, A.; Gianni, T.; Platoni, K.; Lamprogiannis, L.; Efstathopoulos, E.P. Utilization of Nanoparticles for Treating Age-Related Macular Degeneration. Pharmaceuticals 2025, 18, 162. [Google Scholar] [CrossRef]
- Maya-Vetencourt, J.F.; Manfredi, G.; Mete, M.; Colombo, E.; Bramini, M.; Di Marco, S.; Shmal, D.; Mantero, G.; Dipalo, M.; Rocchi, A.; et al. Subretinally injected semiconducting polymer nanoparticles rescue vision in a rat model of retinal dystrophy. Nat. Nanotechnol. 2020, 15, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for ocular drug delivery: Current status and translational opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef] [PubMed]
- Ranta, V.-P.; Mannermaa, E.; Lummepuro, K.; Subrizi, A.; Laukkanen, A.; Antopolsky, M.; Murtomäki, L.; Hornof, M.; Urtti, A. Barrier analysis of periocular drug delivery to the posterior segment. J. Control Release 2010, 148, 42–48. [Google Scholar] [CrossRef]
- Biasella, F.; Plössl, K.; Baird, P.N.; Weber, B.H.F. The extracellular microenvironment in immune dysregulation and inflammation in retinal disorders. Front. Immunol. 2023, 14, 1147037. [Google Scholar] [CrossRef]
- Niu, P.; Wu, Y.; Zeng, F.; Zhang, S.; Liu, S.; Gao, H. Development of nanodrug-based eye drops with good penetration properties and ROS responsiveness for controllable release to treat fungal keratitis. NPG Asia Mater. 2023, 15, 1–15. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, N.K. Inflammation and retinal degenerative diseases. Neural Regen. Res. 2023, 18, 513–518. [Google Scholar] [CrossRef]
- Caban, M.; Owczarek, K.; Lewandowska, U. The Role of Metalloproteinases and Their Tissue Inhibitors on Ocular Diseases: Focusing on Potential Mechanisms. Int. J. Mol. Sci. 2022, 23, 4256. [Google Scholar] [CrossRef]
- Huang, K.; Liu, X.; Lv, Z.; Zhang, D.; Zhou, Y.; Lin, Z.; Guo, J. MMP9-Responsive Graphene Oxide Quantum Dot-Based Nano-in-Micro Drug Delivery System for Combinatorial Therapy of Choroidal Neovascularization. Small 2023, 19, 2207335. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, L.; Zhang, W.; Wang, X.; Geng, Y.; Zhang, Y.; Wang, L.; Zhang, W.; Zhang, Y.J.; Xiao, S.; et al. A transistor-like pH-sensitive nanodetergent for selective cancer therapy. Nat. Nanotechnol. 2022, 17, 541–551. [Google Scholar] [CrossRef]
- Sun, J.G.; Jiang, Q.; Zhang, X.-P.; Shan, K.; Liu, B.-H.; Zhao, C.; Yan, B. Mesoporous silica nanoparticles as a delivery system for improving antiangiogenic therapy. Int. J. Nanomed. 2019, 14, 1489–1501. [Google Scholar] [CrossRef]
- Deng, S.; Gigliobianco, M.R.; Censi, R.; Di Martino, P. Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status, Challenges and Opportunities. Nanomaterials 2020, 10, 847. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, N.; Zhao, X.; Su, X.; Liu, Z. Recent advancements in polymer science for retinal diseases: New frontiers in drug delivery systems. APL Bioeng. 2025, 9, 020902. [Google Scholar] [CrossRef] [PubMed]
- Varshochian, R.; Riazi-Esfahani, M.; Jeddi-Tehrani, M.; Mahmoudi, A.R.; Aghazadeh, S.; Mahbod, M.; Movassat, M.; Atyabi, F.; Sabzevari, A.; Dinarvand, R. Albuminated Plga Nanoparticles for Ocular Neovascularization Treatment. J. Biomed. Mater. Res. A 2015, 103, 3148–3156. [Google Scholar] [CrossRef]
- del Pozo-Rodríguez, A.; Delgado, D.; Gascón, A.R.; Solinís, M.Á. Lipid Nanoparticles as Drug/Gene Delivery Systems to the Retina. J. Ocul. Pharmacol. Ther. 2013, 29, 173–188. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Deng, Z.; Ji, D. Advances in the development of lipid nanoparticles for ophthalmic therapeutics. Biomed. Pharmacother. 2024, 178, 117108. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Jozic, A.; Su, G.L.-N.; Herrera-Barrera, M.; Curtis, A.; Arrizabalaga, S.; Tschetter, W.; Ryals, R.C.; Sahay, G. Lipid nanoparticles with PEG-variant surface modifications mediate genome editing in the mouse retina. Nat. Commun. 2023, 14, 1–12. [Google Scholar] [CrossRef]
- Finn, J.D.; Smith, A.R.; Patel, M.C.; Shaw, L.; Youniss, M.R.; van Heteren, J.; Dirstine, T.; Ciullo, C.; Lescarbeau, R.; Seitzer, J.; et al. A Single Administration of CRISPR/Cas9 Lipid Nanoparticles Achieves Robust and Persistent In Vivo Genome Editing. Cell Rep. 2018, 22, 2227–2235. [Google Scholar] [CrossRef]
- Samaridou, E.; Heyes, J.; Lutwyche, P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Adv. Drug Deliv. Rev. 2020, 154–155, 37–63. [Google Scholar] [CrossRef] [PubMed]
- Giannaccini, M.; Giannini, M.; Calatayud, M.P.; Goya, G.F.; Cuschieri, A.; Dente, L.; Raffa, V. Magnetic Nanoparticles as Intraocular Drug Delivery System to Target Retinal Pigmented Epithelium (RPE). Int. J. Mol. Sci. 2014, 15, 1590–1605. [Google Scholar] [CrossRef]
- Liu, S.; Yan, Z.; Huang, Z.; Yang, H.; Li, J. Smart Nanocarriers for the Treatment of Retinal Diseases. ACS Appl. Bio Mater. 2024, 7, 2070–2085. [Google Scholar] [CrossRef]
- Yang, B.; Li, G.; Liu, J.; Li, X.; Zhang, S.; Sun, F.; Liu, W. Nanotechnology for Age-Related Macular Degeneration. Pharmaceutics 2021, 13, 2035. [Google Scholar] [CrossRef]
- Huang, L.; Liang, W.; Zhou, K.; Wassel, R.A.; Ridge, Z.D.; Ma, J.-X.; Wang, B. Therapeutic Effects of Fenofibrate Nano-Emulsion Eye Drops on Retinal Vascular Leakage and Neovascularization. Biology 2021, 10, 1328. [Google Scholar] [CrossRef]
- Hagigit, T.; Abdulrazik, M.; Valamanesh, F.; Behar-Cohen, F.; Benita, S. Ocular antisense oligonucleotide delivery by cationic nanoemulsion for improved treatment of ocular neovascularization: An in-vivo study in rats and mice. J. Control Release 2012, 160, 225–231. [Google Scholar] [CrossRef]
- Dhahir, R.K.; Al-Nima, A.M.; Al-Bazzaz, F. Nanoemulsions as Ophthalmic Drug Delivery Systems. Turk. J. Pharm. Sci. 2021, 18, 652–664. [Google Scholar] [CrossRef]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control Release 2017, 248, 96–116. [Google Scholar] [CrossRef]
- Osipova, O.; Sharoyko, V.; Zashikhina, N.; Zakharova, N.; Tennikova, T.; Urtti, A.; Korzhikova-Vlakh, E. Amphiphilic Polypeptides for VEGF siRNA Delivery into Retinal Epithelial Cells. Pharmaceutics 2020, 12, 39. [Google Scholar] [CrossRef]
- Yavuz, B.; Pehlivan, S.B.; Unlü, N. Dendrimeric Systems and Their Applications in Ocular Drug Delivery. Sci. World J. 2013, 2013, 732340. [Google Scholar] [CrossRef]
- Seah, I.; Zhao, X.; Lin, Q.; Liu, Z.; Su, S.Z.Z.; Yuen, Y.S.; Hunziker, W.; Lingam, G.; Loh, X.J.; Su, X. Use of biomaterials for sustained delivery of anti-VEGF to treat retinal diseases. Eye 2020, 34, 1341–1356. [Google Scholar] [CrossRef]
- Chen, C.-W.; Lu, D.-W.; Yeh, M.-K.; Shiau, C.-Y.; Chiang, C.-H. Novel RGD-lipid conjugate-modified liposomes for enhancing siRNA delivery in human retinal pigment epithelial cells. Int. J. Nanomed. 2011, 6, 2567–2580. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Tian, Y.; Liu, Y.; Li, D.; Zhang, H.; Yang, Y.; Qi, J.; Wang, H.; Gan, L. Hyaluronic acid-modified cationic niosomes for ocular gene delivery: Improving transfection efficiency in retinal pigment epithelium. J. Pharm. Pharmacol. 2018, 70, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, J.H.; Kim, K.W.; Kim, M.H.; Yu, Y.S. Intravenously administered gold nanoparticles pass through the blood–retinal barrier depending on the particle size, and induce no retinal toxicity. Nanotechnology 2009, 20, 505101. [Google Scholar] [CrossRef] [PubMed]
- Laradji, A.; Karakocak, B.B.; Kolesnikov, A.V.; Kefalov, V.J.; Ravi, N. Hyaluronic Acid-Based Gold Nanoparticles for the Topical Delivery of Therapeutics to the Retina and the Retinal Pigment Epithelium. Polymers 2021, 13, 3324. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Camacho, R.M.; Blanco-Llamero, C.; da Ana, R.; Fuertes, M.A.; Señoráns, F.J.; Silva, A.M.; García, M.L.; Souto, E.B. Therapeutic Approaches for Age-Related Macular Degeneration. Int. J. Mol. Sci. 2022, 23, 11769. [Google Scholar] [CrossRef] [PubMed]
- A Kelley, R.; Conley, S.M.; Makkia, R.; Watson, J.N.; Han, Z.; Cooper, M.J.; I Naash, M. DNA nanoparticles are safe and nontoxic in non-human primate eyes. Int. J. Nanomed. 2018, 13, 1361–1379. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, J.; Lu, A.; Gong, J.; Yang, Y.; Lin, X.; Li, M.; Xu, H. Nanoparticles in ocular applications and their potential toxicity. Front. Mol. Biosci. 2022, 9, 931759. [Google Scholar] [CrossRef]
- Koo, H.; Moon, H.; Han, H.; Na, J.H.; Huh, M.S.; Park, J.H.; Woo, S.J.; Park, K.H.; Kwon, I.C.; Kim, K.; et al. The movement of self-assembled amphiphilic polymeric nanoparticles in the vitreous and retina after intravitreal injection. Biomaterials 2012, 33, 3485–3493. [Google Scholar] [CrossRef]
- Lee, J.; Goh, U.; Lee, H.-J.; Kim, J.; Jeong, M.; Park, J.-H. Effective Retinal Penetration of Lipophilic and Lipid-Conjugated Hydrophilic Agents Delivered by Engineered Liposomes. Mol. Pharm. 2017, 14, 423–430. [Google Scholar] [CrossRef]
- Kambhampati, S.P.; Clunies-Ross, A.J.M.; Bhutto, I.; Mishra, M.K.; Edwards, M.; McLeod, D.S.; Kannan, R.M.; Lutty, G. Systemic and Intravitreal Delivery of Dendrimers to Activated Microglia/Macrophage in Ischemia/Reperfusion Mouse Retina. Investig. Opthalmology Vis. Sci. 2015, 56, 4413–4424. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef]
- Gan, L.; Wang, J.; Zhao, Y.; Chen, D.; Zhu, C.; Liu, J.; Gan, Y. Hyaluronan-modified core–shell liponanoparticles targeting CD44-positive retinal pigment epithelium cells via intravitreal injection. Biomaterials 2013, 34, 5978–5987. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Sarkhel, S.; Peltoniemi, J.; Broadbridge, R.; Tuomainen, M.; Auriola, S.; Urtti, A. Differentially cleaving peptides as a strategy for controlled drug release in human retinal pigment epithelial cells. J. Control Release 2017, 251, 37–48. [Google Scholar] [CrossRef]
- Suda, K.; Murakami, T.; Gotoh, N.; Fukuda, R.; Hashida, Y.; Hashida, M.; Tsujikawa, A.; Yoshimura, N. High-density lipoprotein mutant eye drops for the treatment of posterior eye diseases. J. Control Release 2017, 266, 301–309. [Google Scholar] [CrossRef]
- Singh, S.R.; Grossniklaus, H.E.; Kang, S.J.; Edelhauser, H.F.; Ambati, B.K.; Kompella, U.B. Intravenous transferrin, RGD peptide and dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009, 16, 645–659. [Google Scholar] [CrossRef]
- Elbedwehy, A.M.; Wu, J.; Na, H.-K.; Baek, A.; Jung, H.; Kwon, I.H.; Lee, S.W.; Kim, J.H.; Lee, T.G. ROS-responsive charge reversal mesoporous silica nanoparticles as promising drug delivery system for neovascular retinal diseases. J. Control Release 2024, 373, 224–239. [Google Scholar] [CrossRef]
- Giannaccini, M.; Pedicini, L.; De Matienzo, G.; Chiellini, F.; Dente, L.; Raffa, V. Magnetic nanoparticles: A strategy to target the choroidal layer in the posterior segment of the eye. Sci. Rep. 2017, 7, srep43092. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Schur, R.M.; Sears, A.E.; Gao, S.-Q.; Sun, W.; Naderi, A.; Kern, T.; Palczewski, K.; Lu, Z.-R. Stable Retinoid Analogue Targeted Dual pH-Sensitive Smart Lipid ECO/pDNA Nanoparticles for Specific Gene Delivery in the Retinal Pigment Epithelium. ACS Appl. Bio Mater. 2020, 3, 3078–3086. [Google Scholar] [CrossRef] [PubMed]
- Behroozi, F.; Abdkhodaie, M.-J.; Abandansari, H.S.; Satarian, L.; Ashtiani, M.K.; Jaafari, M.R.; Baharvand, H. Smart liposomal drug delivery for treatment of oxidative stress model in human embryonic stem cell-derived retinal pigment epithelial cells. Int. J. Pharm. 2018, 548, 62–72. [Google Scholar] [CrossRef]
- Iezzi, R.; Guru, B.R.; Glybina, I.V.; Mishra, M.K.; Kennedy, A.; Kannan, R.M. Dendrimer-based targeted intravitreal therapy for sustained attenuation of neuroinflammation in retinal degeneration. Biomaterials 2012, 33, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Sapowadia, A.; Ghanbariamin, D.; Zhou, L.; Zhou, Q.; Schmidt, T.; Tamayol, A.; Chen, Y. Biomaterial Drug Delivery Systems for Prominent Ocular Diseases. Pharmaceutics 2023, 15, 1959. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, A.; Sun, R.; Xu, J.; Yin, T.; He, H.; Gou, J.; Kong, J.; Zhang, Y.; Tang, X. Penetratin-modified lutein nanoemulsion in-situ gel for the treatment of age-related macular degeneration. Expert Opin. Drug Deliv. 2020, 17, 603–619. [Google Scholar] [CrossRef]
- Lim, C.; Kim, D.-W.; Sim, T.; Hoang, N.H.; Lee, J.W.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Preparation and characterization of a lutein loading nanoemulsion system for ophthalmic eye drops. J. Drug Deliv. Sci. Technol. 2016, 36, 168–174. [Google Scholar] [CrossRef]
- Garcia-Garcia, J.; Usategui-Martin, R.; Sanabria, M.R.; Fernandez-Perez, E.; Telleria, J.J.; Coco-Martin, R.M. Pathophysiology of Age-Related Macular Degeneration: Implications for Treatment. Ophthalmic Res. 2022, 65, 615–636. [Google Scholar] [CrossRef]
- Cao, F.; Liang, K.; Tang, W.-W.; Ni, Q.-Y.; Ji, Z.-Y.; Zha, C.-K.; Wang, Y.-K.; Jiang, Z.-X.; Hou, S.; Tao, L.-M.; et al. Polyvinylpyrrolidone-curcumin nanoparticles with immune regulatory and metabolism regulatory effects for the treatment of experimental autoimmune uveitis. J. Control Release 2024, 372, 551–570. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.E.; Agrón, E.; Domalpally, A.; Keenan, T.D.L.; Vitale, S.; Weber, C.; Smith, D.C.; Christen, W.; AREDS2 Research Group; et al. Long-term Outcomes of Adding Lutein/Zeaxanthin and ω-3 Fatty Acids to the AREDS Supplements on Age-Related Macular Degeneration Progression. JAMA Ophthalmol 2022, 140, 692–698. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, Y.E.; Kim, J. Mesoporous Cerium Oxide Nanoparticles with High Scavenging Properties of Reactive Oxygen Species for Treating Age-Related Macular Degeneration. Adv. NanoBiomed Res. 2023, 3, 2300062. [Google Scholar] [CrossRef]
- Pan, C.K.; Durairaj, C.; Kompella, U.B.; Agwu, O.; Oliver, S.C.; Quiroz-Mercado, H.; Mandava, N.; Olson, J.L. Comparison of Long-Acting Bevacizumab Formulations in the Treatment of Choroidal Neovascularization in a Rat Model. J. Ocul. Pharmacol. Ther. 2011, 27, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hurley, B.; Liu, Y.; Leonard, B.; Griffith, M. Controlled Release of Bevacizumab Through Nanospheres for Extended Treatment of Age-Related Macular Degeneration. Open Ophthalmol. J. 2012, 6, 54–58. [Google Scholar] [CrossRef]
- Janoria, K.G.; Gunda, S.; Boddu, S.H.S.; Mitra, A.K. Novel approaches to retinal drug delivery. Expert Opin. Drug Deliv. 2007, 4, 371–388. [Google Scholar] [CrossRef]
- Dai, J.; Zhou, X.; Bai, H.; Zeng, M.; Peng, Q.; Wu, Q. Pathogenic mechanisms and treatment advances in proliferative vitreoretinopathy: A review. Medicine 2025, 104, e44804. [Google Scholar] [CrossRef] [PubMed]
- Bejjani, R.A.; BenEzra, D.; Cohen, H.; Rieger, J.; Andrieu, C.; Jeanny, J.-C.; Gollomb, G.; Behar-Cohen, F.F. Nanoparticles for gene delivery to retinal pigment epithelial cells. Mol. Vis. 2005, 11, 124–132. [Google Scholar]
- Birch, D.G.; Liang, F.Q. Age-related macular degeneration: A target for nanotechnology derived medicines. Int. J. Nanomed. 2007, 2, 65–77. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Ma, Z.; Wang, J.; Zhang, Q. A Lipid Nanoparticle System Improves siRNA Efficacy in RPE Cells and a Laser-Induced Murine CNV Model. Investig. Opthalmol. Vis. Sci. 2011, 52, 4789. [Google Scholar] [CrossRef]
- Chen, C.W.; Yeh, M.K.; Shiau, C.Y.; Chiang, C.H.; Lu, D.W. Efficient downregulation of VEGF in retinal pigment epithelial cells by integrin ligand-labeled liposome-mediated siRNA delivery. Int. J. Nanomed. 2013, 8, 2613–2627. [Google Scholar] [CrossRef]
- Chambers, C.Z.; Soo, G.L.; Engel, A.L.; Glass, I.A.; Frassetto, A.; Martini, P.G.V.; Cherry, T.J.; the Birth Defects Research Laboratory (BDRL). Lipid Nanoparticle-Mediated Delivery of mRNA into the Mouse and Human Retina and Other Ocular Tissues. Transl. Vis. Sci. Technol. 2024, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Barrera, M.; Ryals, R.C.; Gautam, M.; Jozic, A.; Landry, M.; Korzun, T.; Gupta, M.; Acosta, C.; Stoddard, J.; Reynaga, R.; et al. Peptide-guided lipid nanoparticles deliver mRNA to the neural retina of rodents and nonhuman primates. Sci. Adv. 2023, 9, eadd4623. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.-Q.; Quiambao, A.B.; Fitzgerald, J.B.; Cooper, M.J.; Conley, S.M.; Naash, M.I. Ocular Delivery of Compacted DNA-Nanoparticles Does Not Elicit Toxicity in the Mouse Retina. PLoS ONE 2009, 4, e7410. [Google Scholar] [CrossRef]
- Cai, X.; Nash, Z.; Conley, S.M.; Fliesler, S.J.; Cooper, M.J.; Naash, M.I. A Partial Structural and Functional Rescue of a Retinitis Pigmentosa Model with Compacted DNA Nanoparticles. PLoS ONE 2009, 4, e5290. [Google Scholar] [CrossRef] [PubMed]
- del Pozo-Rodríguez, A.; Delgado, D.; Solinís, M.; Gascón, A.; Pedraz, J. Solid lipid nanoparticles for retinal gene therapy: Transfection and intracellular trafficking in RPE cells. Int. J. Pharm. 2008, 360, 177–183. [Google Scholar] [CrossRef]
- Dubey, S.K.; Pradhan, R.; Hejmady, S.; Singhvi, G.; Choudhury, H.; Gorain, B.; Kesharwani, P. Emerging innovations in nano-enabled therapy against age-related macular degeneration: A paradigm shift. Int. J. Pharm. 2021, 600, 120499. [Google Scholar] [CrossRef]
- Sainz, V.; Conniot, J.; Matos, A.I.; Peres, C.; Zupanǒiǒ, E.; Moura, L.; Silva, L.C.; Florindo, H.F.; Gaspar, R.S. Regulatory aspects on nanomedicines. Biochem. Biophys. Res. Commun. 2015, 468, 504–510. [Google Scholar] [CrossRef]
- Rowe-Rendleman, C.L.; Durazo, S.A.; Kompella, U.B.; Rittenhouse, K.D.; Di Polo, A.; Weiner, A.L.; Grossniklaus, H.E.; Naash, M.I.; Lewin, A.S.; Horsager, A.; et al. Drug and Gene Delivery to the Back of the Eye: From Bench to Bedside. Investig. Opthalmology Vis. Sci. 2014, 55, 2714–2730. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
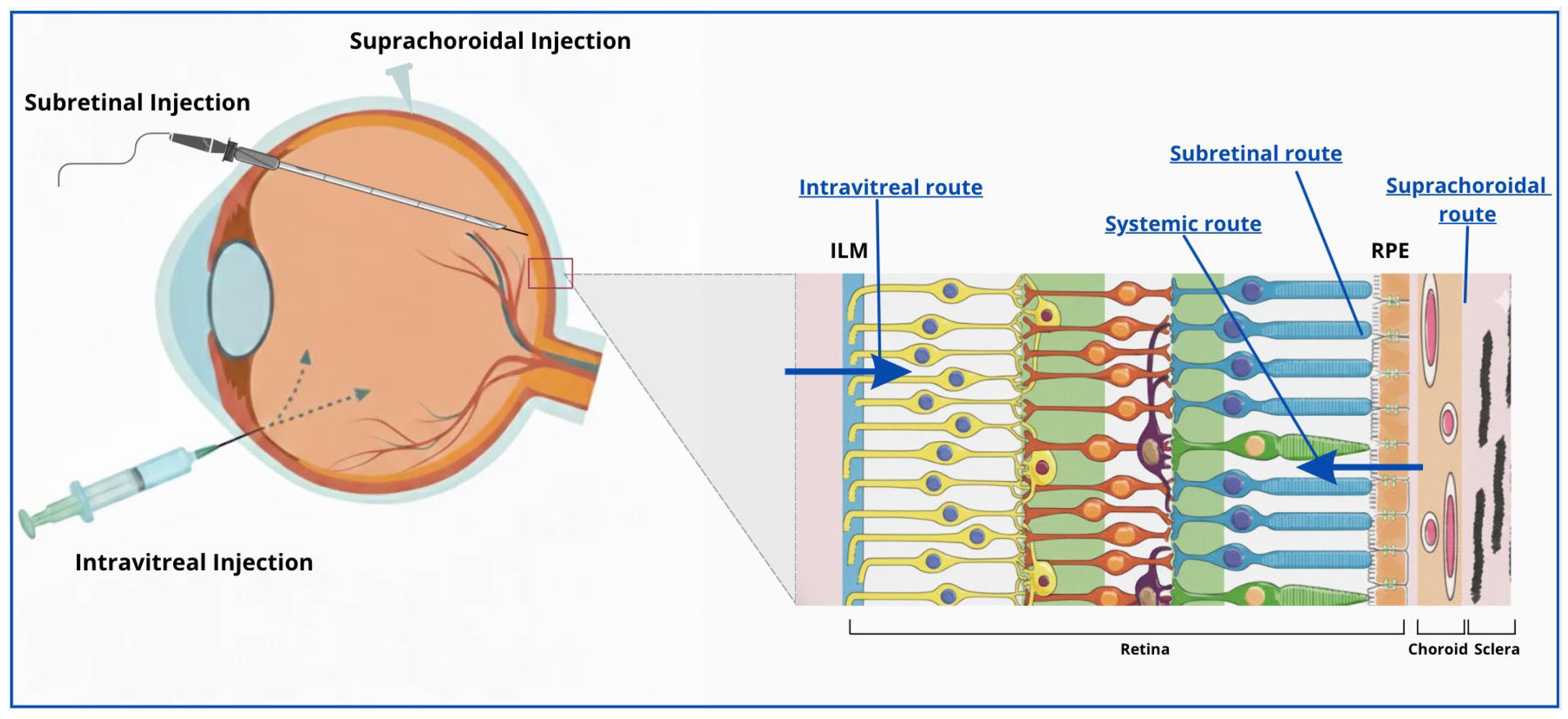
| Route of Administration | Anatomical Access | Advantages | Limitations |
|---|---|---|---|
| Topical (eye drops) | Corneal and conjunctival surface; limited diffusion beyond anterior chamber | Noninvasive, easy to use, high patient compliance | Extremely low posterior penetration; <5% reaches intraocular tissues; unsuitable for RPE targeting |
| Periocular | Subconjunctival, sub-Tenon’s | Moderately invasive; bypasses corneal barrier | Limited scleral permeability; rapid clearance by choroidal circulation; poor RPE bioavailability |
| Suprachoroidal | Space between sclera and choroid | Minimally invasive; localized posterior delivery; reduced anterior exposure; suitable for sustained-release formulations | Requires specialized microneedle device; nonuniform drug distribution; rapid clearance through choriocapillaris |
| Intravitreal | Pars Plana → Vitreous cavity | Clinically established; outpatient procedure; repeatable; low complication rate; suitable for chronic therapy | Diffusion barriers (vitreous and ILM); vector dilution; repeated injections required; limited RPE penetration |
| Subretinal | Pars Plana Vitrectomy → Potential space between photoreceptors and RPE | Direct access to target cells; high bioavailability; reduced immune activation; long-lasting effect after single procedure | Surgically invasive (requires vitrectomy); risk of retinal tears or detachment; limited treatment area; requires expert surgeon |
| Systemic (oral or intravenous) | Vascular access → choriocapillaris | Noninvasive; allows repeated dosing | Poor ocular bioavailability; high systemic exposure and toxicity risk; limited permeability across BM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardella, M.; Pellegrini, M.; Yu, A.C.; Adamo, G.G.; Mura, M.; Busin, M. Nanotechnology-Based Delivery Systems and Retinal Pigment Epithelium: Advances, Targeting Approaches, and Translational Challenges. Biomolecules 2025, 15, 1592. https://doi.org/10.3390/biom15111592
Nardella M, Pellegrini M, Yu AC, Adamo GG, Mura M, Busin M. Nanotechnology-Based Delivery Systems and Retinal Pigment Epithelium: Advances, Targeting Approaches, and Translational Challenges. Biomolecules. 2025; 15(11):1592. https://doi.org/10.3390/biom15111592
Chicago/Turabian StyleNardella, Michele, Marco Pellegrini, Angeli Christy Yu, Ginevra Giovanna Adamo, Marco Mura, and Massimo Busin. 2025. "Nanotechnology-Based Delivery Systems and Retinal Pigment Epithelium: Advances, Targeting Approaches, and Translational Challenges" Biomolecules 15, no. 11: 1592. https://doi.org/10.3390/biom15111592
APA StyleNardella, M., Pellegrini, M., Yu, A. C., Adamo, G. G., Mura, M., & Busin, M. (2025). Nanotechnology-Based Delivery Systems and Retinal Pigment Epithelium: Advances, Targeting Approaches, and Translational Challenges. Biomolecules, 15(11), 1592. https://doi.org/10.3390/biom15111592





