Decreased PPM1B Expression Drives PRMT5-Mediated Histone Modification in Lung Cancer Progression
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Biopsies and Tissue Samples
2.3. Protein Extraction
2.4. Western Blot Analysis
2.5. RNA Isolation from Lung Tissue
2.6. Semi-Quantitative Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.7. Immunohistochemistry
2.8. Immunofluorescent Staining
2.9. Kaplan–Meier Survival Analysis
2.10. Statistical Analysis
3. Results
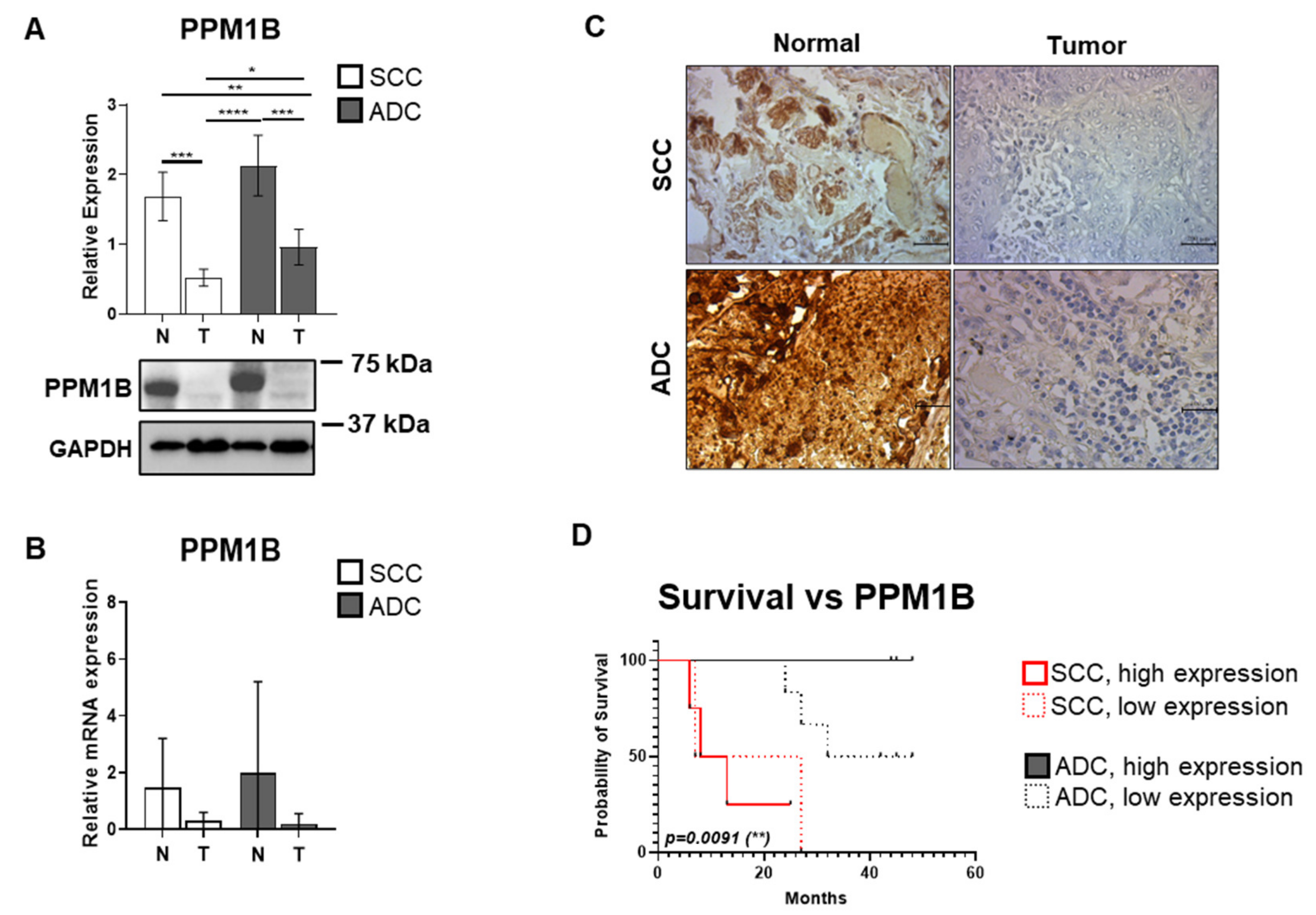
3.1. PPM1B Expression Is Significantly Reduced in Both ADC and SCC Samples
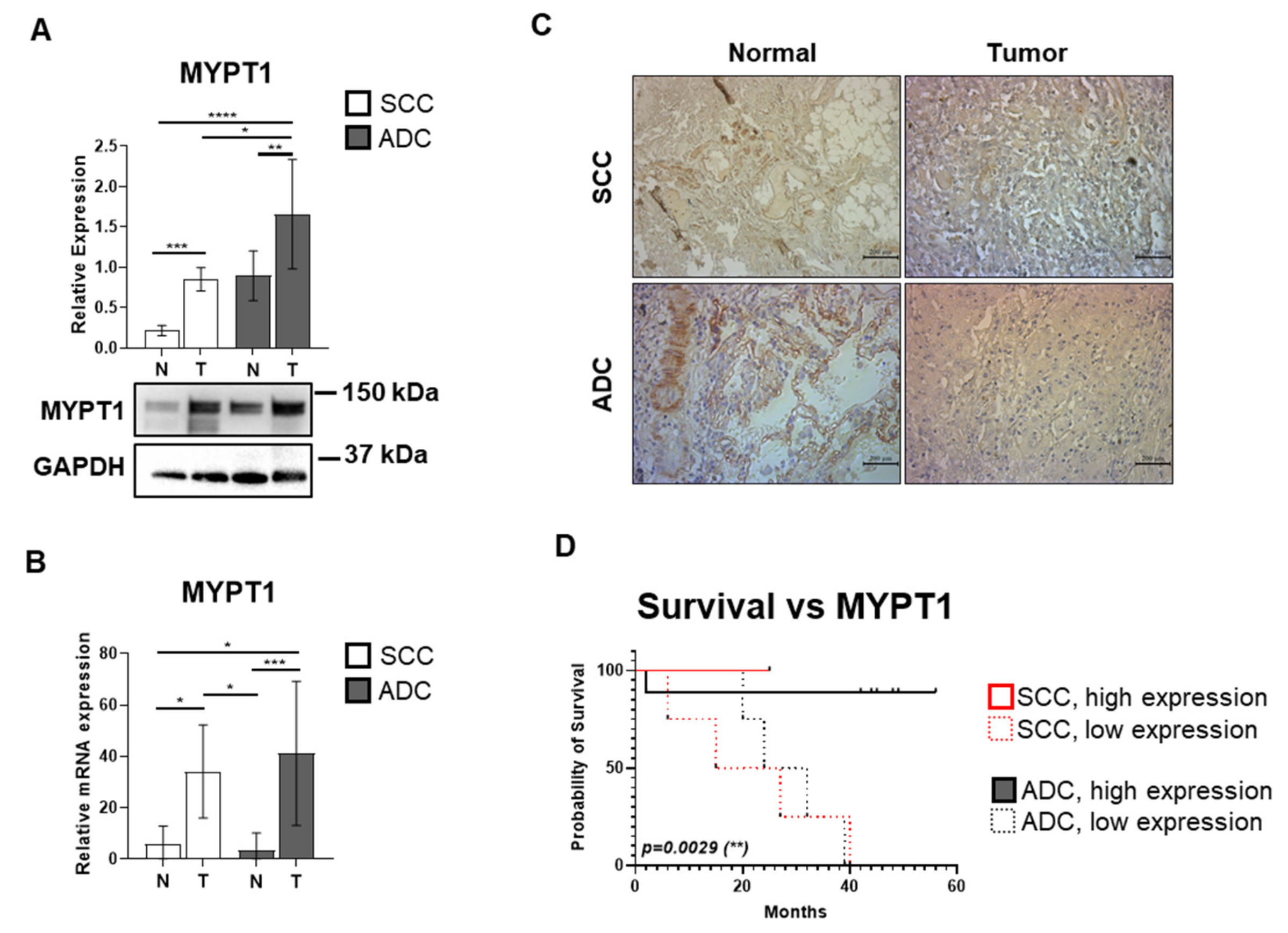
3.2. The Expression of the MYPT1 Regulatory Subunit of MP Is Elevated in ADC and SCC Tissues
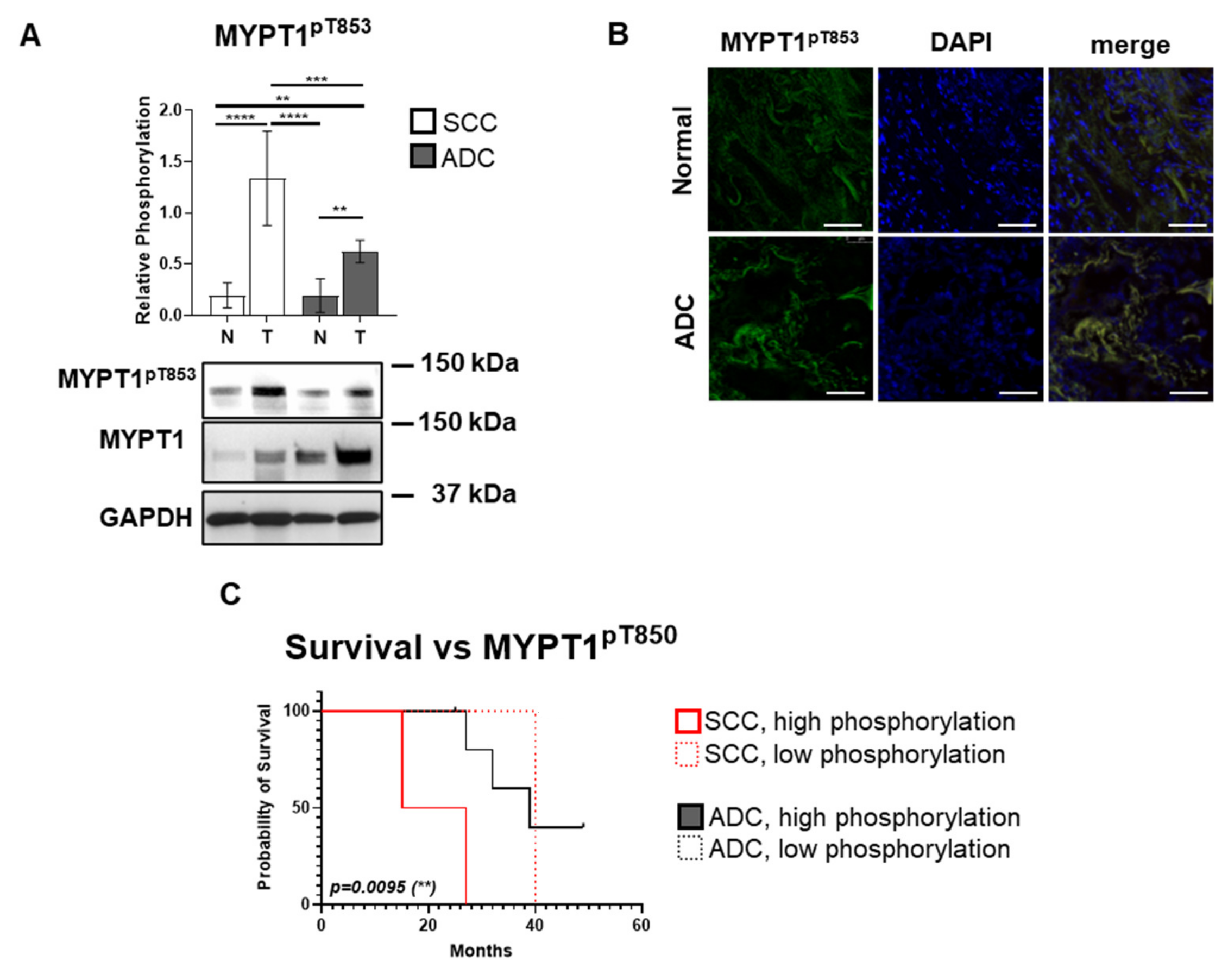
3.3. The Inhibitory MYPT1 Thr853 Phosphorylation Increases Significantly in Lung Cancer
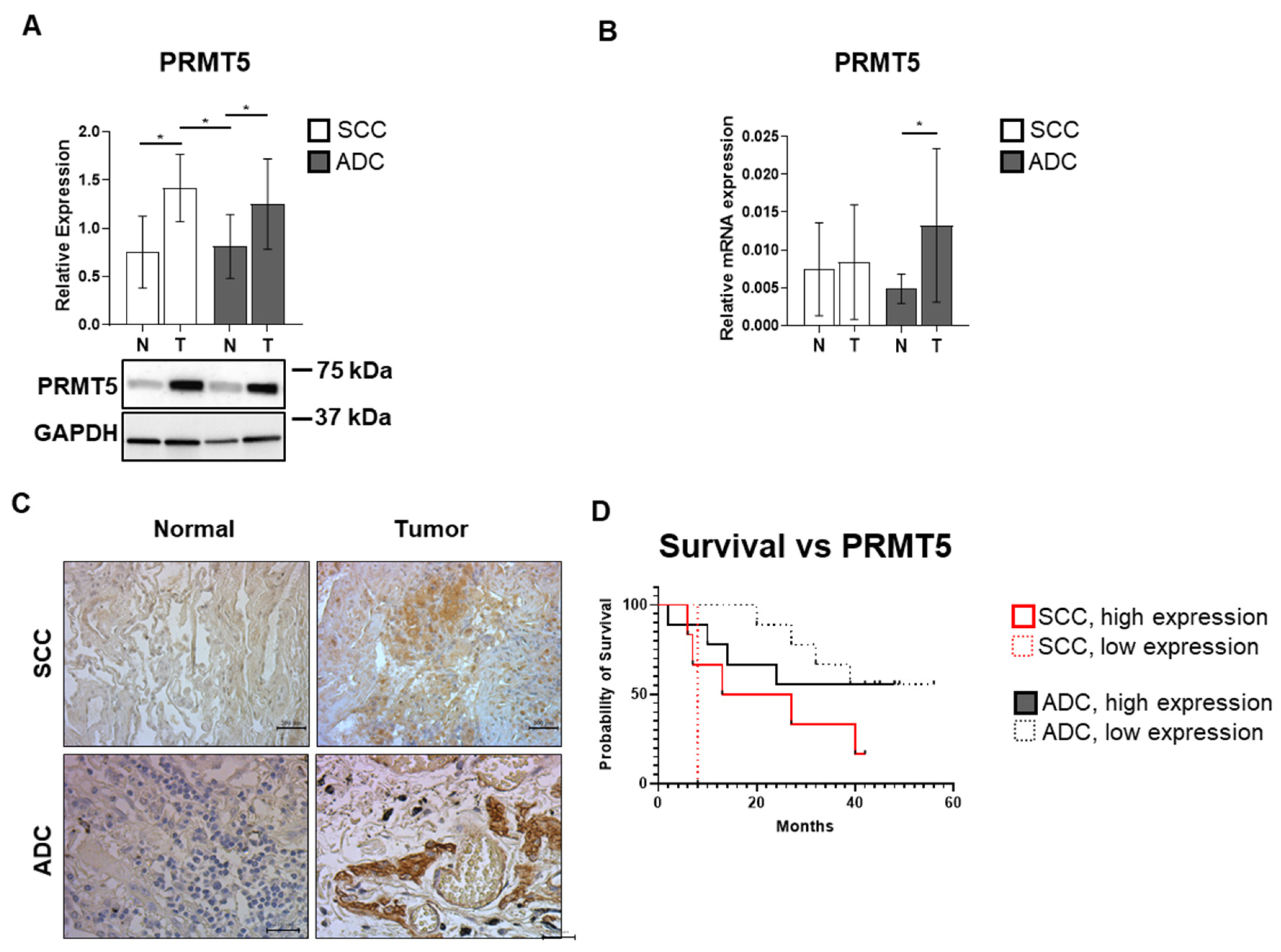
3.4. SCC and ADC Tissue Samples Exhibit Elevated PRMT5 Expression Levels
3.5. Activating PRMT5pT80 Phosphorylation Is Enhanced in SCC and ADC Lung Cancer
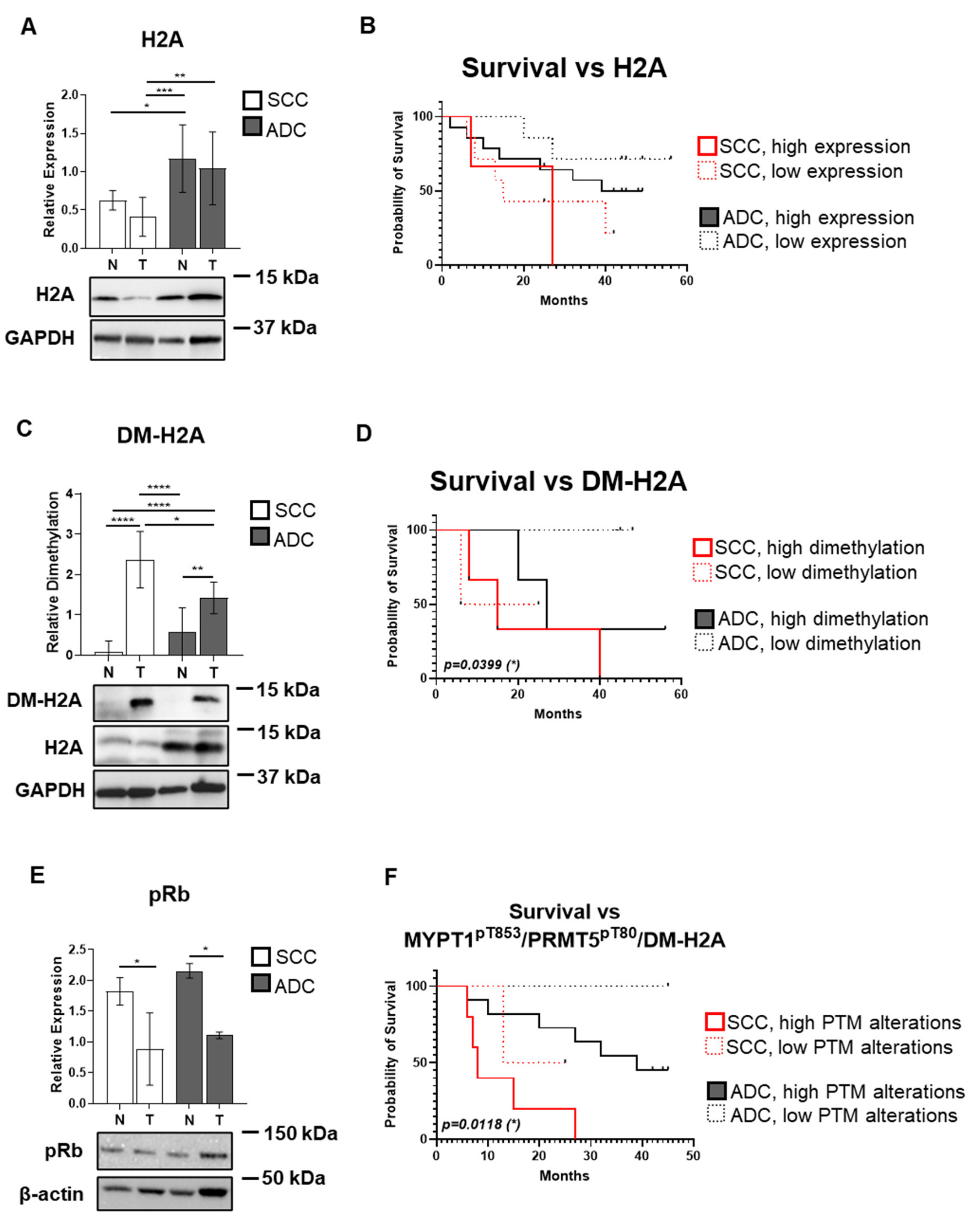
3.6. Symmetrical Dimethylation of H2A Protein Occurs in SCC and ADC Without Changes in Its Protein Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Adenocarcinoma |
| ANOVA | Analysis of Variance |
| BSA | Bovine Serum Albumin |
| DAB | 3,3′-Diaminobenzidine |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| DFS | Disease-Free Survival |
| DM | Dimethylation |
| EDTA | Ethylenediaminetetraacetic Acid |
| GAPDH | Glyceraldehyde 3-Phosphate Dehydrogenase |
| GDPR | General Data Protection Regulation |
| H2A | Histone H2A |
| HCC | Hepatocellular Carcinoma |
| IHC | Immunohistochemistry |
| MP | Myosin Phosphatase |
| MYPT1 | Myosin Phosphatase Targeting Subunit 1 (regulatory subunit of MP) |
| OS | Overall Survival |
| PP1c | Protein Phosphatase 1 catalytic subunit |
| PPM1B | Mg2+/Mn2+-dependent Protein Phosphatase 1B |
| pRb | retinoblastoma protein |
| PRMT5 | Protein Arginine Methyltransferase 5 |
| RT-qPCR | Reverse Transcription-quantitative Polymerase Chain Reaction |
| SCC | Squamous Cell Carcinoma |
| SD | Standard Deviation |
| SDS | Sodium Dodecyl-Sulfate |
| SCLC | Small Cell Lung Cancer |
| TBST | Tris-Buffered Saline with Tween 20 |
References
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar]
- Fois, S.S.; Paliogiannis, P.; Zinellu, A.; Fois, A.G.; Cossu, A.; Palmieri, G. Molecular epidemiology of the main druggable genetic alterations in non-small cell lung cancer. Int. J. Mol. Sci. 2021, 22, 612. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cote, M.L. Cancer progress and priorities: Lung cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef]
- de Sousa, V.M.L.; Carvalho, L. Heterogeneity in lung cancer. Pathobiology 2018, 85, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Kerpel-Fronius, A.; Monostori, Z.; Kovacs, G.; Ostoros, G.; Horvath, I.; Solymosi, D.; Pipek, O.; Szatmari, F.; Kovacs, A.; Markoczy, Z.; et al. Nationwide lung cancer screening with low-dose computed tomography: Implementation and first results of the hunchest screening program. Eur. Radiol. 2022, 32, 4457–4467. [Google Scholar] [CrossRef]
- Becker, N.; Motsch, E.; Trotter, A.; Heussel, C.P.; Dienemann, H.; Schnabel, P.A.; Kauczor, H.U.; Maldonado, S.G.; Miller, A.B.; Kaaks, R.; et al. Lung cancer mortality reduction by ldct screening-results from the randomized german lusi trial. Int. J. Cancer 2020, 146, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, U.; Silva, M.; Sestini, S.; Sabia, F.; Boeri, M.; Cantarutti, A.; Sverzellati, N.; Sozzi, G.; Corrao, G.; Marchiano, A. Prolonged lung cancer screening reduced 10-year mortality in the mild trial: New confirmation of lung cancer screening efficacy. Ann. Oncol. 2019, 30, 1162–1169. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 world health organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 who classification of lung tumors: Impact of advances since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef]
- Gibbons, D.L.; Byers, L.A.; Kurie, J.M. Smoking, p53 mutation, and lung cancer. Mol. Cancer Res. 2014, 12, 3–13. [Google Scholar] [CrossRef]
- Bhateja, P.; Chiu, M.; Wildey, G.; Lipka, M.B.; Fu, P.; Yang, M.C.L.; Ardeshir-Larijani, F.; Sharma, N.; Dowlati, A. Retinoblastoma mutation predicts poor outcomes in advanced non small cell lung cancer. Cancer Med. 2019, 8, 1459–1466. [Google Scholar] [CrossRef]
- Janne, P.A.; Riely, G.J.; Gadgeel, S.M.; Heist, R.S.; Ou, S.I.; Pacheco, J.M.; Johnson, M.L.; Sabari, J.K.; Leventakos, K.; Yau, E.; et al. Adagrasib in non-small-cell lung cancer harboring a kras(g12c) mutation. N. Engl. J. Med. 2022, 387, 120–131. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for lung cancers with kras p.G12c mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.T.; Vyse, S.; Huang, P.H. Rare epidermal growth factor receptor (egfr) mutations in non-small cell lung cancer. Semin. Cancer Biol. 2020, 61, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazieres, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab deruxtecan in her2-mutant non-small-cell lung cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef]
- Sholl, L.M.; Hirsch, F.R.; Hwang, D.; Botling, J.; Lopez-Rios, F.; Bubendorf, L.; Mino-Kenudson, M.; Roden, A.C.; Beasley, M.B.; Borczuk, A.; et al. The promises and challenges of tumor mutation burden as an immunotherapy biomarker: A perspective from the international association for the study of lung cancer pathology committee. J. Thorac. Oncol. 2020, 15, 1409–1424. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Kim, W.J. DNA methylation markers in lung cancer. Curr. Genom. 2021, 22, 79–87. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, Y.; Huang, W.; Gao, Y.; Xu, W.; Tao, J.; Yang, M.; Li, L.; Ping, W.; Shen, H.; et al. Non-invasive diagnosis of early-stage lung cancer using high-throughput targeted DNA methylation sequencing of circulating tumor DNA (ctdna). Theranostics 2019, 9, 2056–2070. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Liu, M.; Luo, J. Protein post-translational modifications in the regulation of cancer hallmarks. Cancer Gene Ther. 2023, 30, 529–547. [Google Scholar] [CrossRef]
- Kiss, A.; Erdodi, F.; Lontay, B. Myosin phosphatase: Unexpected functions of a long-known enzyme. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Motolani, A.; Martin, M.; Sun, M.Y.; Lu, T. The structure and functions of prmt5 in human diseases. Life 2021, 11, 1074. [Google Scholar] [CrossRef]
- Wolf, S.S. The protein arginine methyltransferase family: An update about function, new perspectives and the physiological role in humans. Cell. Mol. Life Sci. 2009, 66, 2109–2121. [Google Scholar] [CrossRef]
- Cai, C.; Gu, S.; Yu, Y.; Zhu, Y.; Zhang, H.; Yuan, B.; Shen, L.; Yang, B.; Feng, X.H. Prmt5 enables robust stat3 activation via arginine symmetric dimethylation of smad7. Adv. Sci. 2021, 8, 2003047. [Google Scholar] [CrossRef]
- Kong, G.M.; Yu, M.; Gu, Z.; Chen, Z.; Xu, R.M.; O’Bryant, D.; Wang, Z. Selective small-chemical inhibitors of protein arginine methyltransferase 5 with anti-lung cancer activity. PLoS ONE 2017, 12, e0181601. [Google Scholar] [CrossRef]
- Bajbouj, K.; Ramakrishnan, R.K.; Saber-Ayad, M.; Omar, H.A.; Saheb Sharif-Askari, N.; Shafarin, J.; Elmoselhi, A.B.; Ihmaid, A.; AlHaj Ali, S.; Alalool, A.; et al. Prmt5 selective inhibitor enhances therapeutic efficacy of cisplatin in lung cancer cells. Int. J. Mol. Sci. 2021, 22, 6131. [Google Scholar] [CrossRef]
- Hartshorne, D.J.; Ito, M.; Erdodi, F. Myosin light chain phosphatase: Subunit composition, interactions and regulation. J. Muscle Res. Cell Motil. 1998, 19, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Sipos, A.; Ivan, J.; Becsi, B.; Darula, Z.; Tamas, I.; Horvath, D.; Medzihradszky, K.F.; Erdodi, F.; Lontay, B. Myosin phosphatase and rhoa-activated kinase modulate arginine methylation by the regulation of protein arginine methyltransferase 5 in hepatocellular carcinoma cells. Sci. Rep. 2017, 7, 40590. [Google Scholar] [CrossRef] [PubMed]
- Keller, I.; Ungvari, A.; Major, E.; Horvath, D.; Konya, Z.; Toth, E.; Erdodi, F.; Kiss, A.; Lontay, B. Magnesium-dependent-protein phosphatase 1b regulates the protein arginine methyltransferase 5 through the modulation of myosin phosphatase. J. Biol. Chem. 2024, 301, 108107. [Google Scholar] [CrossRef]
- Paragh, G.; Nemeth, A.; Harangi, M.; Banach, M.; Fulop, P. Causes, clinical findings and therapeutic options in chylomicronemia syndrome, a special form of hypertriglyceridemia. Lipids Health Dis. 2022, 21, 21. [Google Scholar] [CrossRef]
- Asamura, H.; Chansky, K.; Crowley, J.; Goldstraw, P.; Rusch, V.W.; Vansteenkiste, J.F.; Watanabe, H.; Wu, Y.L.; Zielinski, M.; Ball, D.; et al. The international association for the study of lung cancer lung cancer staging project: Proposals for the revision of the n descriptors in the forthcoming 8th edition of the tnm classification for lung cancer. J. Thorac. Oncol. 2015, 10, 1675–1684. [Google Scholar] [CrossRef]
- Mazzone, P.J.; Lam, L. Evaluating the patient with a pulmonary nodule: A review. JAMA 2022, 327, 264–273. [Google Scholar] [CrossRef]
- Major, E.; Gyory, F.; Horvath, D.; Keller, I.; Tamas, I.; Uray, K.; Fulop, P.; Lontay, B. Smoothelin-like protein 1 regulates development and metabolic transformation of skeletal muscle in hyperthyroidism. Front. Endocrinol. 2021, 12, 751488. [Google Scholar] [CrossRef] [PubMed]
- Lontay, B.; Pal, B.; Serfozo, Z.; Koszeghy, A.; Szucs, G.; Rusznak, Z.; Erdodi, F. Protein phosphatase-1m and rho-kinase affect exocytosis from cortical synaptosomes and influence neurotransmission at a glutamatergic giant synapse of the rat auditory system. J. Neurochem. 2012, 123, 84–99. [Google Scholar] [CrossRef]
- Lontay, B.; Serfozo, Z.; Gergely, P.; Ito, M.; Hartshorne, D.J.; Erdodi, F. Localization of myosin phosphatase target subunit 1 in rat brain and in primary cultures of neuronal cells. J. Comp. Neurol. 2004, 478, 72–87. [Google Scholar] [CrossRef]
- Keller, I.; Ungvari, A.; Kinter, R.; Szalmas, F.; Kokai, E.; Lontay, B. Smoothelin-like protein 1 promotes insulin sensitivity and modulates the contractile properties of endometrial epithelial cells with insulin resistance. Front. Endocrinol. 2024, 15, 1375771. [Google Scholar] [CrossRef]
- Blumenthal, G.M.; Karuri, S.W.; Zhang, H.; Zhang, L.; Khozin, S.; Kazandjian, D.; Tang, S.; Sridhara, R.; Keegan, P.; Pazdur, R. Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: Us food and drug administration trial-level and patient-level analyses. J. Clin. Oncol. 2015, 33, 1008–1014. [Google Scholar] [CrossRef]
- Yue, D.; Liu, W.; Chen, C.; Zhang, T.; Ma, Y.; Cui, L.; Gu, Y.; Bei, T.; Zhao, X.; Zhang, B.; et al. Circulating tumor DNA predicts neoadjuvant immunotherapy efficacy and recurrence-free survival in surgical non-small cell lung cancer patients. Transl. Lung Cancer Res. 2022, 11, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Chang, A.N.; He, W.; Chen, C.P.; Qiao, Y.N.; Zhu, M.; Kamm, K.E.; Stull, J.T. Physiological signalling to myosin phosphatase targeting subunit-1 phosphorylation in ileal smooth muscle. J. Physiol. 2016, 594, 3209–3225. [Google Scholar] [CrossRef]
- Li, R.; Dou, J.; Bai, T.; Cai, B.; Liu, Y. Protein phosphatase ppm1b inhibits gastric cancer progression and serves as a favorable prognostic biomarker. Appl. Immunohistochem. Mol. Morphol. 2022, 30, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Kim, J.T.; Lee, S.J.; Hwang, Y.S.; Park, S.Y.; Kim, B.Y.; Yoo, J.; Hong, K.S.; Min, J.K.; Lee, C.H.; et al. Protein phosphatase 1b dephosphorylates rho guanine nucleotide dissociation inhibitor 1 and suppresses cancer cell migration and invasion. Cancer Lett. 2018, 417, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, J.; Tian, Y.; Feng, R. Pleckstrin-2-promoted ppm1b degradation plays an important role in transforming growth factor-β-induced breast cancer cell invasion and metastasis. Cancer Sci. 2023, 114, 2429–2444. [Google Scholar] [CrossRef]
- Li, Z.; Chen, R.; Li, Y.; Zhou, Q.; Zhao, H.; Zeng, K.; Zhao, B.; Lu, Z. A comprehensive overview of ppm1b: From biological functions to diseases. Eur. J. Pharmacol. 2023, 947, 175633. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gharib, T.G.; Huang, C.C.; Taylor, J.M.; Misek, D.E.; Kardia, S.L.; Giordano, T.J.; Iannettoni, M.D.; Orringer, M.B.; Hanash, S.M.; et al. Discordant protein and mrna expression in lung adenocarcinomas. Mol. Cell Proteom. 2002, 1, 304–313. [Google Scholar] [CrossRef]
- Jansson, M.D.; Lund, A.H. Microrna and cancer. Mol. Oncol. 2012, 6, 590–610. [Google Scholar] [CrossRef]
- Robichaud, N.; Sonenberg, N.; Ruggero, D.; Schneider, R.J. Translational control in cancer. Cold Spring Harb. Perspect. Biol. 2019, 11, a032896. [Google Scholar] [CrossRef]
- Wang, F.; Sun, Y. Overexpression of myosin phosphatase target subunit 1 (mypt1) inhibits tumor progression and metastasis of gastric cancer. Med. Sci. Monit. 2018, 24, 2508–2517. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhuo, Y.; Lin, Z.; Jiang, F.; Dai, Q.; Lu, J.; Dong, W.; Zhu, X.; Han, Z.; Zhong, W. Decreased expression of mypt1 contributes to tumor angiogenesis and poor patient prognosis in human prostate cancer. Curr. Mol. Med. 2018, 18, 100–108. [Google Scholar] [CrossRef]
- Hwang, J.W.; Cho, Y.; Bae, G.U.; Kim, S.N.; Kim, Y.K. Protein arginine methyltransferases: Promising targets for cancer therapy. Exp. Mol. Med. 2021, 53, 788–808. [Google Scholar] [CrossRef]
- Hu, R.; Zhou, B.; Chen, Z.; Chen, S.; Chen, N.; Shen, L.; Xiao, H.; Zheng, Y. Prmt5 inhibition promotes pd-l1 expression and immuno-resistance in lung cancer. Front. Immunol. 2021, 12, 722188. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makai, A.; Keller, I.; Szalmás, F.A.; Ungvári, Á.; Horváth, D.; Major, E.; Enyedi, A.; Takács, I.; Lontay, B. Decreased PPM1B Expression Drives PRMT5-Mediated Histone Modification in Lung Cancer Progression. Biomolecules 2025, 15, 1581. https://doi.org/10.3390/biom15111581
Makai A, Keller I, Szalmás FA, Ungvári Á, Horváth D, Major E, Enyedi A, Takács I, Lontay B. Decreased PPM1B Expression Drives PRMT5-Mediated Histone Modification in Lung Cancer Progression. Biomolecules. 2025; 15(11):1581. https://doi.org/10.3390/biom15111581
Chicago/Turabian StyleMakai, Attila, Ilka Keller, Fanni A. Szalmás, Ádám Ungvári, Dániel Horváth, Evelin Major, Attila Enyedi, István Takács, and Beáta Lontay. 2025. "Decreased PPM1B Expression Drives PRMT5-Mediated Histone Modification in Lung Cancer Progression" Biomolecules 15, no. 11: 1581. https://doi.org/10.3390/biom15111581
APA StyleMakai, A., Keller, I., Szalmás, F. A., Ungvári, Á., Horváth, D., Major, E., Enyedi, A., Takács, I., & Lontay, B. (2025). Decreased PPM1B Expression Drives PRMT5-Mediated Histone Modification in Lung Cancer Progression. Biomolecules, 15(11), 1581. https://doi.org/10.3390/biom15111581





