Unraveling the Transport Properties of RONS across Nitro-Oxidized Membranes
Abstract
1. Introduction
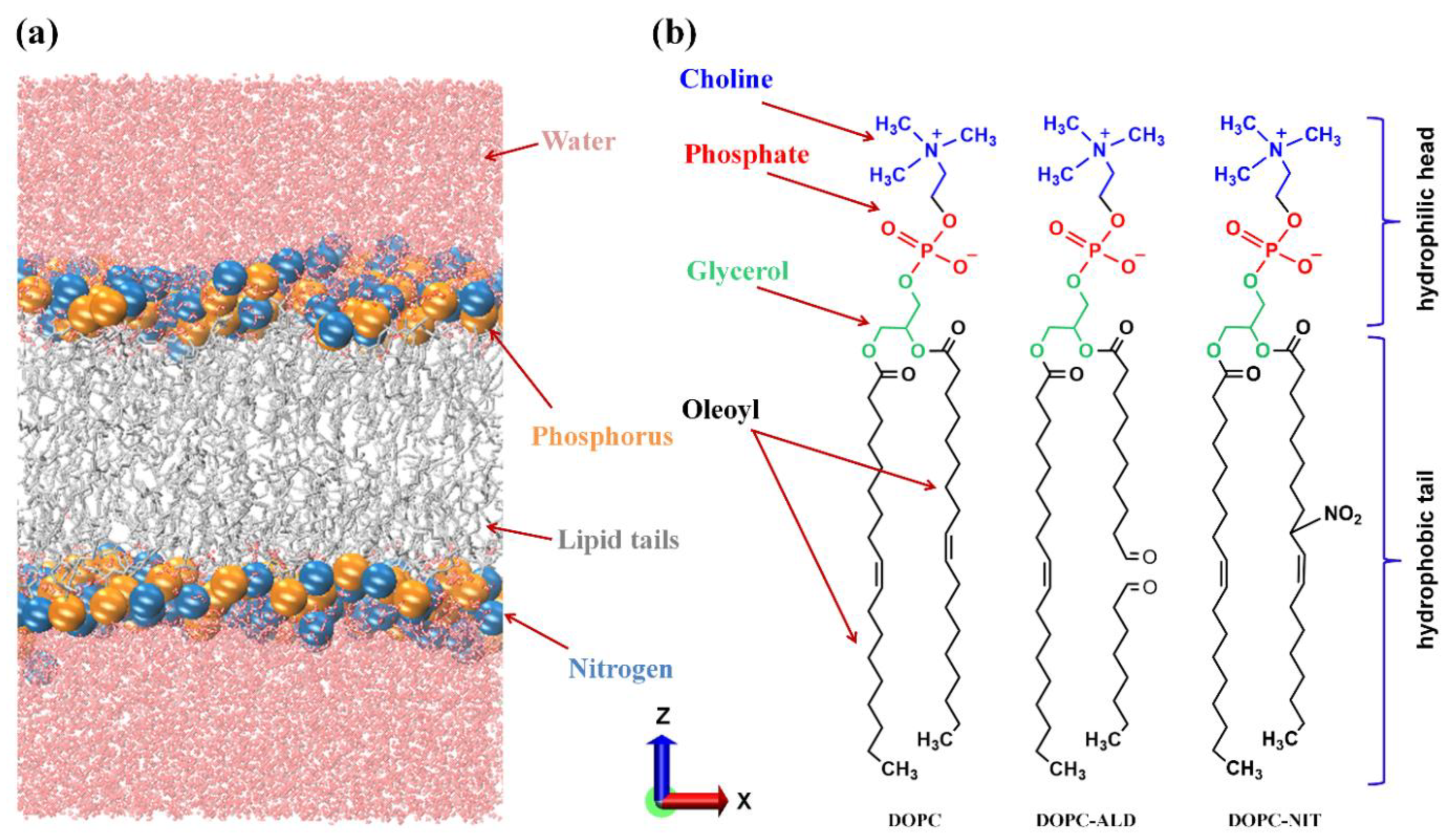
2. Simulation Setup
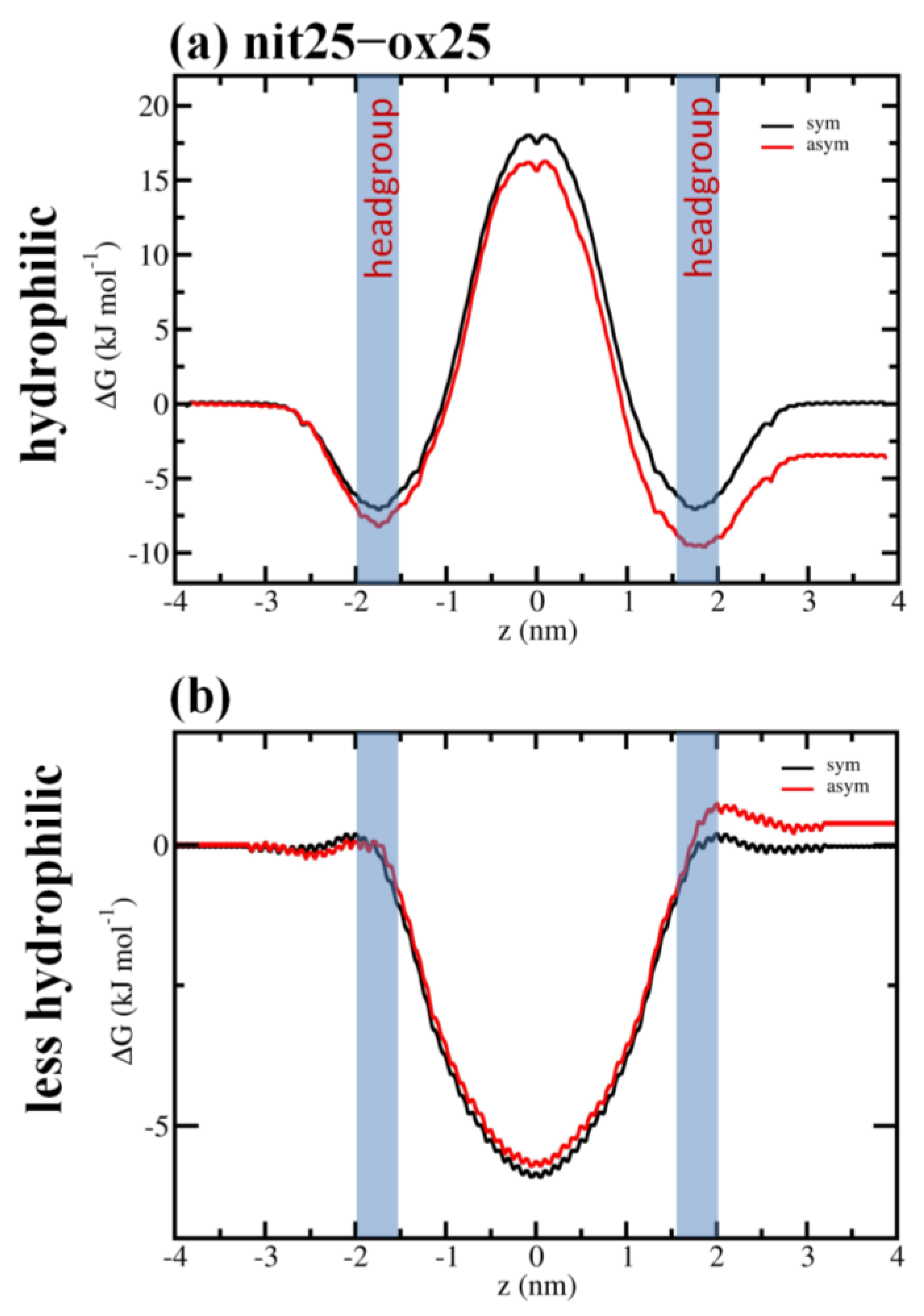
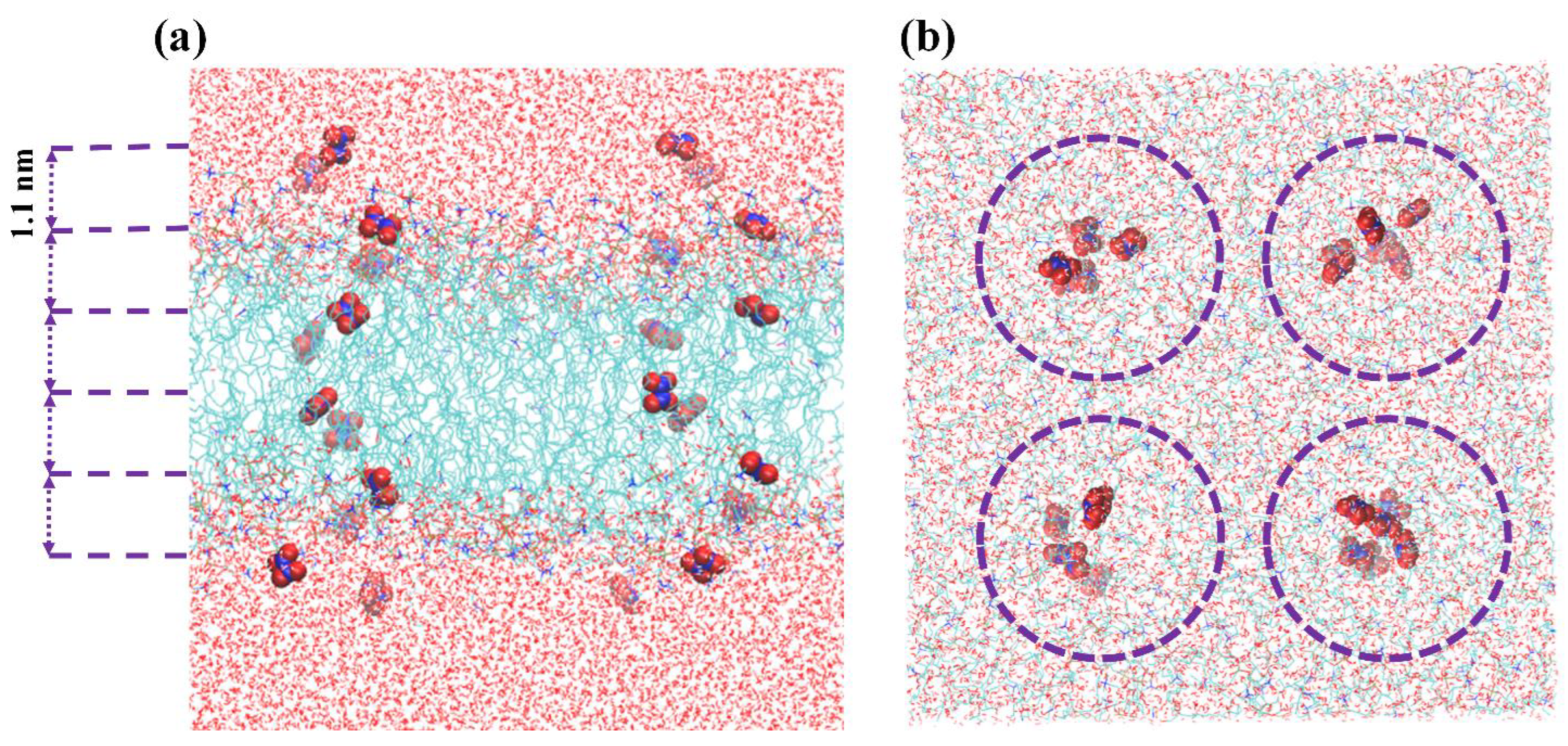
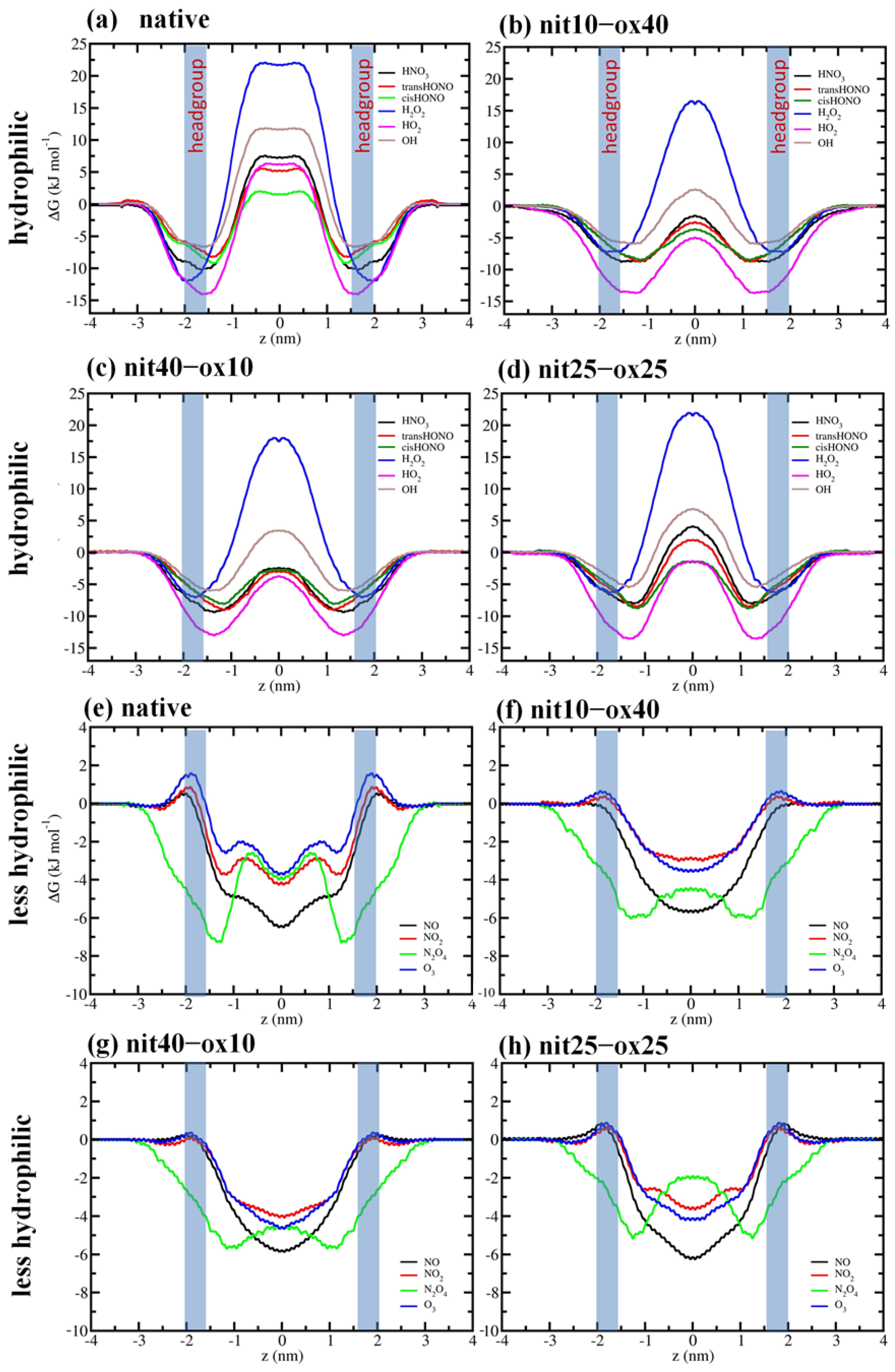
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Bárdos, L.; Baránková, H. Cold atmospheric plasma: Sources, processes, and applications. Thin Solid Film. 2010, 518, 6705–6713. [Google Scholar] [CrossRef]
- Benedikt, J.; Hefny, M.M.; Shaw, A.; Buckley, B.; Iza, F.; Schäkermann, S.; Bandow, J. The fate of plasma-generated oxygen atoms in aqueous solutions: Non-equilibrium atmospheric pressure plasmas as an efficient source of atomic O (aq). Phys. Chem. Chem. Phys. 2018, 20, 12037–12042. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, H.; Ishikawa, K.; Zhao, Q.-L.; Andocs, G.; Nojima, N.; Takeda, K.; Krishna, M.C.; Ishijima, T.; Matsuya, Y.; Hori, M. Free radical generation by non-equilibrium atmospheric pressure plasma in alcohol–water mixtures: An EPR-spin trapping study. J. Phys. D Appl. Phys. 2018, 51, 095202. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Polak, M.; Masur, K.; von Woedtke, T.; Winter, J.; Reuter, S. Plasma processes and plasma sources in medicine. Contrib. Plasma Phys. 2012, 52, 644–654. [Google Scholar] [CrossRef]
- Georgescu, N.; Lupu, A.R. Tumoral and normal cells treatment with high-voltage pulsed cold atmospheric plasma jets. IEEE Trans. Plasma Sci. 2010, 38, 1949–1955. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Domonkos, M.; Tichá, P.; Trejbal, J.; Demo, P. Applications of cold atmospheric pressure plasma technology in medicine, agriculture and food industry. Appl. Sci. 2021, 11, 4809. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.-D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Isbary, G.; Morfill, G.; Schmidt, H.; Georgi, M.; Ramrath, K.; Heinlin, J.; Karrer, S.; Landthaler, M.; Shimizu, T.; Steffes, B. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010, 163, 78–82. [Google Scholar] [CrossRef]
- Penkov, O.V.; Khadem, M.; Lim, W.-S.; Kim, D.-E. A review of recent applications of atmospheric pressure plasma jets for materials processing. J. Coat. Technol. Res. 2015, 12, 225–235. [Google Scholar] [CrossRef]
- Assadi, I.; Guesmi, A.; Baaloudj, O.; Zeghioud, H.; Elfalleh, W.; Benhammadi, N.; Khezami, L.; Assadi, A.A. Review on inactivation of airborne viruses using non-thermal plasma technologies: From MS2 to coronavirus. Environ. Sci. Pollut. Res. Int. 2021, 29, 4880–4892. [Google Scholar] [CrossRef]
- Tanaka, H.; Mizuno, M.; Ishikawa, K.; Toyokuni, S.; Kajiyama, H.; Kikkawa, F.; Hori, M. Molecular mechanisms of non-thermal plasma-induced effects in cancer cells. Biol. Chem. 2018, 400, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.G.; Paff, M.; Friedman, G.; Fridman, G.; Fridman, A.; Brooks, A.D. Control of methicillin-resistant Staphylococcus aureus in planktonic form and biofilms: A biocidal efficacy study of nonthermal dielectric-barrier discharge plasma. Am. J. Infect. Control 2010, 38, 293–301. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, G.; Obenchain, R.; Zhang, R.; Bai, F.; Fang, T.; Wang, H.; Lu, Y.; Wirz, R.E.; Gu, Z. Cold atmospheric plasma delivery for biomedical applications. Mater. Today 2022, 54, 153–188. [Google Scholar] [CrossRef]
- Misra, N.; Bhatt, S.; Arefi-Khonsari, F.; Kumar, V. State of the art in nonthermal plasma processing for biomedical applications: Can it help fight viral pandemics like COVID-19? Plasma Process. Polym. 2021, 18, 2000215. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; Van Dijk, J.; Zimmermann, J. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295–1301. [Google Scholar] [CrossRef]
- González-Mendoza, B.; López-Callejas, R.; Rodríguez-Méndez, B.G.; Eguiluz, R.P.; Mercado-Cabrera, A.; Valencia-Alvarado, R.; Betancourt-Ángeles, M.; de Lourdes Reyes-Frías, M.; Reboyo-Barrios, D.; Chávez-Aguilar, E. Healing of wounds in lower extremities employing a non-thermal plasma. Clin. Plasma Med. 2019, 16, 100094. [Google Scholar] [CrossRef]
- Lee, J.-S.; Shin, H.-S.; Seok, J.-W.; Jang, G.-W.; Beag, Y.-H. Surface Modification of Polystyrene (PS) by Atmospheric Pressure Plasma. J. Korean Vac. Soc. 2009, 18, 1–8. [Google Scholar] [CrossRef]
- Razzokov, J.; Fazliev, S.; Kodirov, A.; AttrI, P.; Chen, Z.; Shiratani, M. Mechanistic insight into permeation of plasma-generated species from vacuum into water bulk. Int. J. Mol. Sci. 2022, 23, 6330. [Google Scholar] [CrossRef]
- Van der Paal, J.; Hong, S.-H.; Yusupov, M.; Gaur, N.; Oh, J.-S.; Short, R.D.; Szili, E.J.; Bogaerts, A. How membrane lipids influence plasma delivery of reactive oxygen species into cells and subsequent DNA damage: An experimental and computational study. Phys. Chem. Chem. Phys. 2019, 21, 19327–19341. [Google Scholar] [CrossRef]
- Kaushik, N.; Kaushik, N.K.; Kim, C.H.; Choi, E.H. Oxidative stress and cell death induced in U-937 human monocytic cancer cell line by non-thermal atmospheric air plasma soft jet. Sci. Adv. Mater. 2014, 6, 1740–1751. [Google Scholar] [CrossRef]
- Xia, W.; Budge, S.M. Techniques for the analysis of minor lipid oxidation products derived from triacylglycerols: Epoxides, alcohols, and ketones. Compr. Rev. Food Sci. Food Saf. 2017, 16, 735–758. [Google Scholar] [CrossRef]
- Volinsky, R.; Cwiklik, L.; Jurkiewicz, P.; Hof, M.; Jungwirth, P.; Kinnunen, P.K. Oxidized phosphatidylcholines facilitate phospholipid flip-flop in liposomes. Biophys. J. 2011, 101, 1376–1384. [Google Scholar] [CrossRef]
- Elbaradei, A.; Wang, Z.; Malmstadt, N.J.L. Oxidation of Membrane Lipids Alters the Activity of the Human Serotonin 1A Receptor. Langmuir 2022, 38, 6798–6807. [Google Scholar] [CrossRef]
- Corvalan, N.A.; Caviglia, A.F.; Felsztyna, I.; Itri, R.; Lascano, R.J.L. Lipid hydroperoxidation effect on the dynamical evolution of the conductance process in bilayer lipid membranes: A condition toward criticality. Langmuir 2020, 36, 8883–8893. [Google Scholar] [CrossRef]
- Ouchi, Y.; Unoura, K.; Nabika, H. Role of oxidized lipids in permeation of H2O2 through a lipid membrane: Molecular mechanism of an inhibitor to promoter switch. Sci. Rep. 2019, 9, 12497. [Google Scholar] [CrossRef]
- De Rosa, R.; Spinozzi, F.; Itri, R. Hydroperoxide and carboxyl groups preferential location in oxidized biomembranes experimentally determined by small angle X-ray scattering: Implications in membrane structure. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 2299–2307. [Google Scholar] [CrossRef]
- Neto, A.J.; Cordeiro, R.M. Molecular simulations of the effects of phospholipid and cholesterol peroxidation on lipid membrane properties. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 2191–2198. [Google Scholar] [CrossRef]
- Singer, S.J.; Nicolson, G.L. The Fluid Mosaic Model of the Structure of Cell Membranes: Cell membranes are viewed as two-dimensional solutions of oriented globular proteins and lipids. Science 1972, 175, 720–731. [Google Scholar] [CrossRef]
- Razzokov, J.; Yusupov, M.; Vanuytsel, S.; Neyts, E.C.; Bogaerts, A. Phosphatidylserine flip-flop induced by oxidation of the plasma membrane: A better insight by atomic scale modeling. Plasma Process. Polym. 2017, 14, 1700013. [Google Scholar] [CrossRef]
- Yusupov, M.; Razzokov, J.; Cordeiro, R.M.; Bogaerts, A. Transport of reactive oxygen and nitrogen species across aquaporin: A molecular level picture. Oxidative Med. Cell. Longev. 2019, 2019, 2930504. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.C.; Yusupov, M.; Bogaerts, A.; Cordeiro, R.M. Biophysics. Distribution of lipid aldehydes in phase-separated membranes: A molecular dynamics study. Arch. Biochem. Biophys. 2022, 717, 109136. [Google Scholar] [CrossRef] [PubMed]
- Melo, T.; Domingues, P.; Ferreira, R.; Milic, I.; Fedorova, M.; Santos, S.M.; Segundo, M.A.; Domingues, M.R.M. Recent Advances on Mass Spectrometry Analysis of Nitrated Phospholipids. Anal. Chem. 2016, 88, 2622–2629. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.C.; Yusupov, M.; Bogaerts, A.; Cordeiro, R.M. How do nitrated lipids affect the properties of phospholipid membranes? Arch. Biochem. Biophys. 2020, 695, 108548. [Google Scholar] [CrossRef]
- Razzokov, J.; Yusupov, M.; Cordeiro, R.M.; Bogaerts, A. Atomic scale understanding of the permeation of plasma species across native and oxidized membranes. J. Phys. D Appl. Phys. 2018, 51, 365203. [Google Scholar] [CrossRef]
- Nasri, Z.; Ahmadi, M.; Striesow, J.; Ravandeh, M.; von Woedtke, T.; Wende, K.J.I. Insight into the Impact of Oxidative Stress on the Barrier Properties of Lipid Bilayer Models. Int. J. Mol. Sci. 2022, 23, 5932. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Yusupov, M.; Cordeiro, R.M.; Bogaerts, A. Unraveling the permeation of reactive species across nitrated membranes by computer simulations. Comput. Biol. Med. 2021, 136, 104768. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Bengtson, C.; Razzokov, J.; Smits, E.; Bogaerts, A. Modifying the tumour microenvironment: Challenges and future perspectives for anticancer plasma treatments. Cancers 2019, 11, 1920. [Google Scholar] [CrossRef]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D., Jr.; Pastor, R.W. Update of the CHARMM all-atom additive force field for lipids: Validation on six lipid types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar] [CrossRef]
- Kučerka, N.; Tristram-Nagle, S.; Nagle, J.F. Structure of fully hydrated fluid phase lipid bilayers with monounsaturated chains. J. Membr. Biol. 2006, 208, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Kučerka, N.; Nagle, J.F.; Sachs, J.N.; Feller, S.E.; Pencer, J.; Jackson, A.; Katsaras, J. Lipid bilayer structure determined by the simultaneous analysis of neutron and X-ray scattering data. Biophys. J. 2008, 95, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Schopfer, F.; Batthyany, C.; Baker, P.; Bonacci, G.; Cole, M.; Rudolph, V.; Groeger, A.; Rudolph, T.; Nadtochiy, S.; Brookes, P. Detection and quantification of protein adduction by electrophilic fatty acids: Mitochondrial generation of fatty acid nitroalkene derivatives. Free Radic. Biol. Med. 2009, 46, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Matosevic, S.; Paegel, B.M. Layer-by-layer cell membrane assembly. Nat. Chem. 2013, 5, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.E.; Ide, M.S.; Davis, R.J. Selective oxidation of alcohols and aldehydes over supported metal nanoparticles. Green Chem. 2013, 15, 17–45. [Google Scholar] [CrossRef]
- Yusupov, M.; Wende, K.; Kupsch, S.; Neyts, E.C.; Reuter, S.; Bogaerts, A. Effect of head group and lipid tail oxidation in the cell membrane revealed through integrated simulations and experiments. Sci. Rep. 2017, 7, 5761. [Google Scholar] [CrossRef]
- Runas, K.A.; Malmstadt, N. Low levels of lipid oxidation radically increase the passive permeability of lipid bilayers. Soft Matter 2015, 11, 499–505. [Google Scholar] [CrossRef]
- Bacellar, I.O.; Oliveira, M.C.; Dantas, L.S.; Costa, E.B.; Junqueira, H.C.; Martins, W.K.; Durantini, A.M.; Cosa, G.; Di Mascio, P.; Wainwright, M. Photosensitized membrane permeabilization requires contact-dependent reactions between photosensitizer and lipids. J. Am. Chem. Soc. 2018, 140, 9606–9615. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Yusupov, M.; Bogaerts, A.; Cordeiro, R.M. Lipid oxidation: Role of membrane phase-separated domains. J. Chem. Inf. Model. 2021, 61, 2857–2868. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 6th ed.; Garland Science: New York, NY, USA, 2014. [Google Scholar]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Kutzner, C.; Páll, S.; Fechner, M.; Esztermann, A.; de Groot, B.L.; Grubmüller, H. More bang for your buck: Improved use of GPU nodes for GROMACS 2018. J. Comput. Chem. 2019, 40, 2418–2431. [Google Scholar] [CrossRef] [PubMed]
- Oostenbrink, C.; Villa, A.; Mark, A.E.; Van Gunsteren, W.F. A biomolecular force field based on the free enthalpy of hydration and solvation: The GROMOS force-field parameter sets 53A5 and 53A6. J. Comput. Chem. 2004, 25, 1656–1676. [Google Scholar] [CrossRef]
- Wong-ekkabut, J.; Xu, Z.; Triampo, W.; Tang, I.M.; Peter Tieleman, D.; Monticelli, L. Effect of Lipid Peroxidation on the Properties of Lipid Bilayers: A Molecular Dynamics Study. Biophys. J. 2007, 93, 4225–4236. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, R.M. Reactive oxygen species at phospholipid bilayers: Distribution, mobility and permeation. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 438–444. [Google Scholar] [CrossRef]
- Cordeiro, R.M. Reactive oxygen and nitrogen species at phospholipid bilayers: Peroxynitrous acid and its homolysis products. J. Phys. Chem. B 2018, 122, 8211–8219. [Google Scholar] [CrossRef]
- Cordeiro, R.M.; Yusupov, M.; Razzokov, J.; Bogaerts, A. Parametrization and molecular dynamics simulations of nitrogen oxyanions and oxyacids for applications in atmospheric and biomolecular sciences. J. Phys. Chem. B 2020, 124, 1082–1089. [Google Scholar] [CrossRef]
- Torrie, G.M.; Valleau, J.P. Nonphysical sampling distributions in Monte Carlo free-energy estimation: Umbrella sampling. J. Comput. Phys. 1977, 23, 187–199. [Google Scholar] [CrossRef]
- Kästner, J. Umbrella sampling. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 932–942. [Google Scholar] [CrossRef]
- Kumar, S.; Rosenberg, J.M.; Bouzida, D.; Swendsen, R.H.; Kollman, P.A. The weighted histogram analysis method for free-energy calculations on biomolecules. I. The method. J. Comput. Chem. 1992, 13, 1011–1021. [Google Scholar] [CrossRef]
- Squadrito, G.L.; Postlethwait, E.M. On the hydrophobicity of nitrogen dioxide: Could there be a “lens” effect for NO2 reaction kinetics? Nitric Oxide 2009, 21, 104–109. [Google Scholar] [CrossRef]
- Hardy, M.; Zielonka, J.; Karoui, H.; Sikora, A.; Michalski, R.; Podsiadły, R.; Lopez, M.; Vasquez-Vivar, J.; Kalyanaraman, B.; Ouari, O.J.A.; et al. Detection and characterization of reactive oxygen and nitrogen species in biological systems by monitoring species-specific products. Antioxid. Redox Signal. 2018, 28, 1416–1432. [Google Scholar] [CrossRef] [PubMed]
- Goss, S.P.; Singh, R.J.; Hogg, N.; Kalyanaraman, B. Reactions of· NO,· NO2 and peroxynitrite in membranes: Physiological implications. Free Radic. Res. 1999, 31, 597–606. [Google Scholar] [CrossRef]
- Cox, A.P.; Brittain, A.H.; Finnigan, D.J. Microwave spectrum, structure, dipole moment and quadrupole coupling constants of cis and trans nitrous acids. Trans. Faraday Soc. 1971, 67, 2179–2194. [Google Scholar] [CrossRef]
- Cox, A.P.; Kuczkowski, R.L. The Microwave Spectrum, Structure, Dipole Moment, and Quadrupole Coupling Constants of trans-Nitrous Acid1a. J. Am. Chem. Soc. 1966, 88, 5071–5074. [Google Scholar] [CrossRef]
- Yusupov, M.; Van der Paal, J.; Neyts, E.C.; Bogaerts, A. Synergistic effect of electric field and lipid oxidation on the permeability of cell membranes. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.N.; Li, Q.; Lancaster, J.R., Jr.; Denicola, A. Acceleration of nitric oxide autoxidation and nitrosation by membranes. IUBMB Life 2007, 59, 243–248. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Lomnicka, M.; Hyde, J.S. Permeability of nitric oxide through lipid bilayer membranes. Free Radic. Res. 1996, 24, 343–349. [Google Scholar] [CrossRef]
- Möller, M.N.; Lancaster, J.R., Jr.; Denicola, A. The interaction of reactive oxygen and nitrogen species with membranes. Curr. Top. Membr. 2008, 61, 23–42. [Google Scholar]
- Denicola, A.; Souza, J.M.; Radi, R.; Lissi, E. Nitric oxide diffusion in membranes determined by fluorescence quenching. Arch. Biochem. Biophys. 1996, 328, 208–212. [Google Scholar] [CrossRef]
- Signorelli, S.; Möller, M.N.; Coitiño, E.L.; Denicola, A. Nitrogen dioxide solubility and permeation in lipid membranes. Arch. Biochem. Biophys. 2011, 512, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.K.; Hopwood, L.E.; Hyde, J.S. Is the mammalian cell plasma membrane a barrier to oxygen transport? J. Gen. Physiol. 1992, 100, 69–87. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Hyde, J.S.; Kusumi, A. Oxygen permeability of phosphatidylcholine--cholesterol membranes. Proc. Natl. Acad. Sci. USA 1989, 86, 4474–4478. [Google Scholar] [CrossRef] [PubMed]
- Widomska, J.; Raguz, M.; Subczynski, W.K. Oxygen permeability of the lipid bilayer membrane made of calf lens lipids. Biochim. Biophys. Acta (BBA)-Biomembr. 2007, 1768, 2635–2645. [Google Scholar] [CrossRef]
- Van der Paal, J.; Verheyen, C.; Neyts, E.C.; Bogaerts, A. Hampering effect of cholesterol on the permeation of reactive oxygen species through phospholipids bilayer: Possible explanation for plasma cancer selectivity. Sci. Rep. 2017, 7, 39526. [Google Scholar] [CrossRef] [PubMed]
| PLB Type | DOPC | Nitrated DOPC | Oxidized DOPC |
|---|---|---|---|
| native | 100% | - | - |
| nit10-ox40 | 50% | 10% | 40% |
| nit40-ox10 | 50% | 40% | 10% |
| nit25-ox25 | 50% | 25% | 25% |
| ΔG for Hydrophilic RONS (kJ·mol−1) | ||||
|---|---|---|---|---|
| Native | nit10–ox40 | nit40–ox10 | nit25–ox25 | |
| HNO3 | 17.5 ± 2 | 7.7 ± 2.1 | 7.3 ± 1.2 | 12.2 ± 1.1 |
| s-trans-HONO | 13.9 ± 2 | 6.1 ± 2.2 | 6.1 ± 1.2 | 10.6 ± 1.2 |
| s-cis-HONO | 11.3 ± 3 | 5.1 ± 2.1 | 5.6 ± 1.2 | 7.9 ± 1.3 |
| H2O2 | 34.0 ± 3 | 23.5 ± 2.1 | 25.7 ± 1.3 | 28.3 ± 1.1 |
| HO2 | 20.0 ± 3 | 8.8 ± 2.1 | 9.5 ± 1.1 | 12.5 ± 1.2 |
| OH | 18.5 ± 3 | 3.7 ± 2.3 | 9.4 ± 1.1 | 12.4 ± 1.3 |
| ΔG for Less Hydrophilic RONS (kJ·mol−1) | ||||
| Native | nit10–ox40 | nit40–ox10 | nit25–ox25 | |
| NO | 0.5 | 0.1 | 0.3 | 1.0 |
| NO2 | 0.9 | 0.4 | 0.1 | 0.6 |
| N2O4 | 4.7 | 1.7 | 1.2 | 3.4 |
| O3 | 1.6 | 0.8 | 0.5 | 1.0 |
| System | APL (nm2) |
|---|---|
| native | 0.6507 ± 0.0001 |
| nit10–ox40 | 0.7651 ± 0.0001 |
| nit20–ox30 | 0.7448 ± 0.0002 |
| nit25–ox25 | 0.7345 ± 0.0003 |
| nit30–ox20 | 0.7338 ± 0.0001 |
| nit40–ox10 | 0.7216 ± 0.0002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abduvokhidov, D.; Yusupov, M.; Shahzad, A.; Attri, P.; Shiratani, M.; Oliveira, M.C.; Razzokov, J. Unraveling the Transport Properties of RONS across Nitro-Oxidized Membranes. Biomolecules 2023, 13, 1043. https://doi.org/10.3390/biom13071043
Abduvokhidov D, Yusupov M, Shahzad A, Attri P, Shiratani M, Oliveira MC, Razzokov J. Unraveling the Transport Properties of RONS across Nitro-Oxidized Membranes. Biomolecules. 2023; 13(7):1043. https://doi.org/10.3390/biom13071043
Chicago/Turabian StyleAbduvokhidov, Davronjon, Maksudbek Yusupov, Aamir Shahzad, Pankaj Attri, Masaharu Shiratani, Maria C. Oliveira, and Jamoliddin Razzokov. 2023. "Unraveling the Transport Properties of RONS across Nitro-Oxidized Membranes" Biomolecules 13, no. 7: 1043. https://doi.org/10.3390/biom13071043
APA StyleAbduvokhidov, D., Yusupov, M., Shahzad, A., Attri, P., Shiratani, M., Oliveira, M. C., & Razzokov, J. (2023). Unraveling the Transport Properties of RONS across Nitro-Oxidized Membranes. Biomolecules, 13(7), 1043. https://doi.org/10.3390/biom13071043










