Carboxymethylated Rhizoma alismatis Polysaccharides Regulate Calcium Oxalate Crystals Growth and Reduce the Regulated Crystals’ Cytotoxicity
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Apparatus
2.2. Carboxymethylation and Characterization of R. alismatis Polysaccharides
2.2.1. Extraction, Purification and Separation of RAP
2.2.2. Degradation of RAP
2.2.3. Monosaccharide Composition Analysis of RAPD
2.2.4. Carboxymethylation of RAPD
2.2.5. Detection of –COOH Content
2.2.6. FT-IR Characterization of RAPs
2.2.7. 1H NMR and 13C NMR Characterization of RAPs
2.2.8. Zeta Potential Detection of RAPs
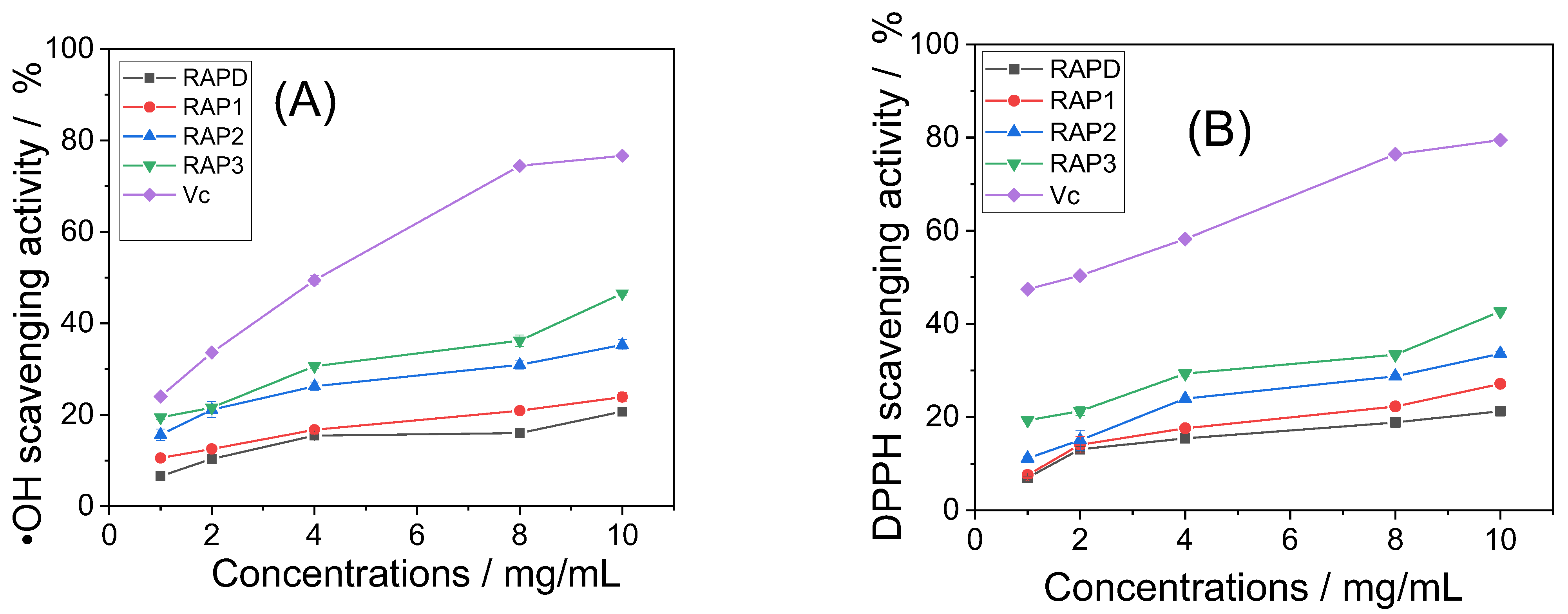
2.3. Antioxidative Activity of RAPs
2.3.1. Ability to Scavenge ·OH Radicals
2.3.2. Ability to Scavenge DPPH Radicals
2.4. RAPs Regulate CaOx Crystal Growth
2.4.1. Crystal Growth and Detection of Soluble Ca2+ Ions in the Supernatant
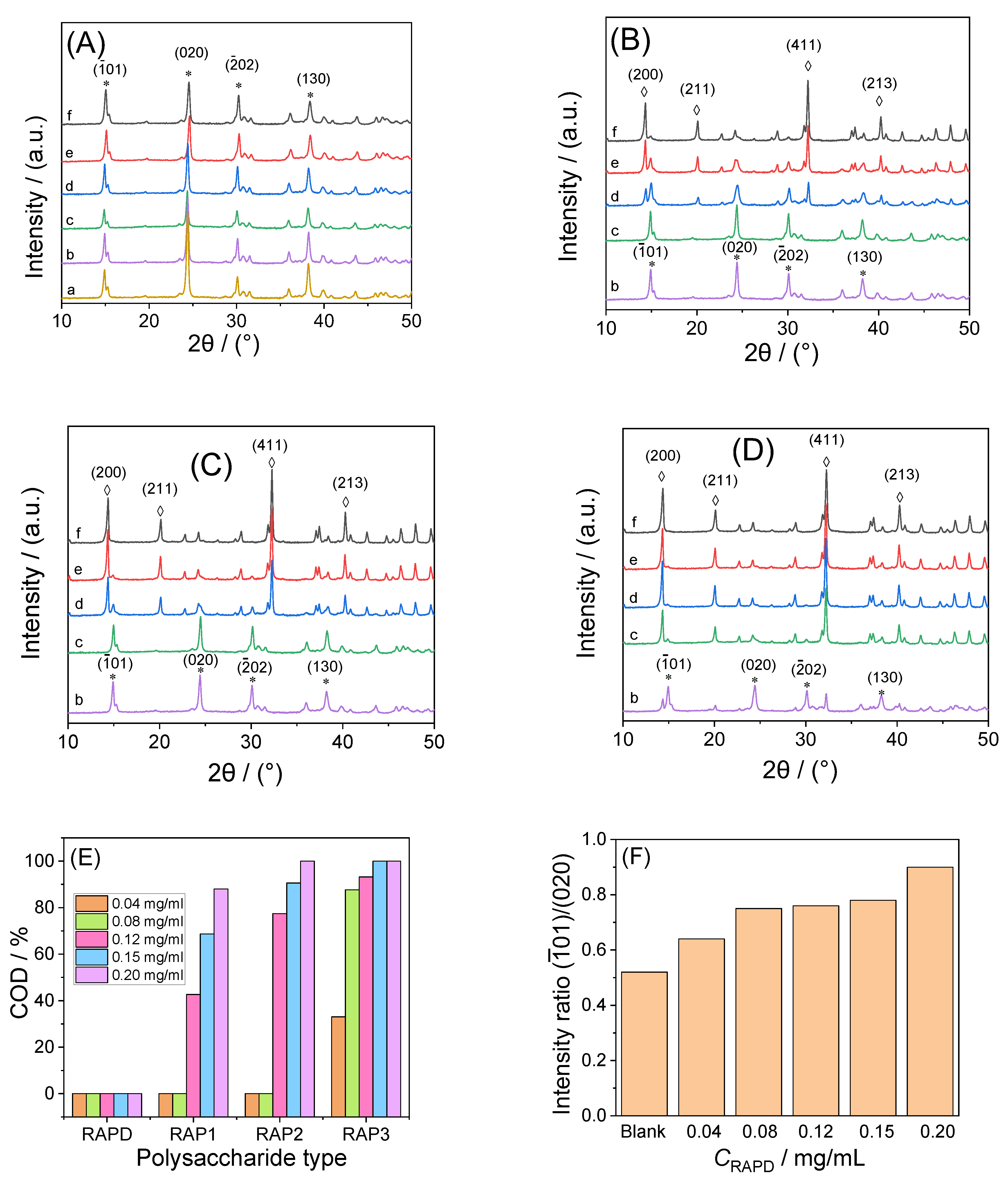
2.4.2. X-ray Diffraction (XRD) Characterization of CaOx Crystals
2.4.3. FT-IR Characterization of CaOx Crystals
2.4.4. Zeta Potential Detection of CaOx Crystals
2.4.5. Thermogravimetric Analysis (TGA) of CaOx Crystals
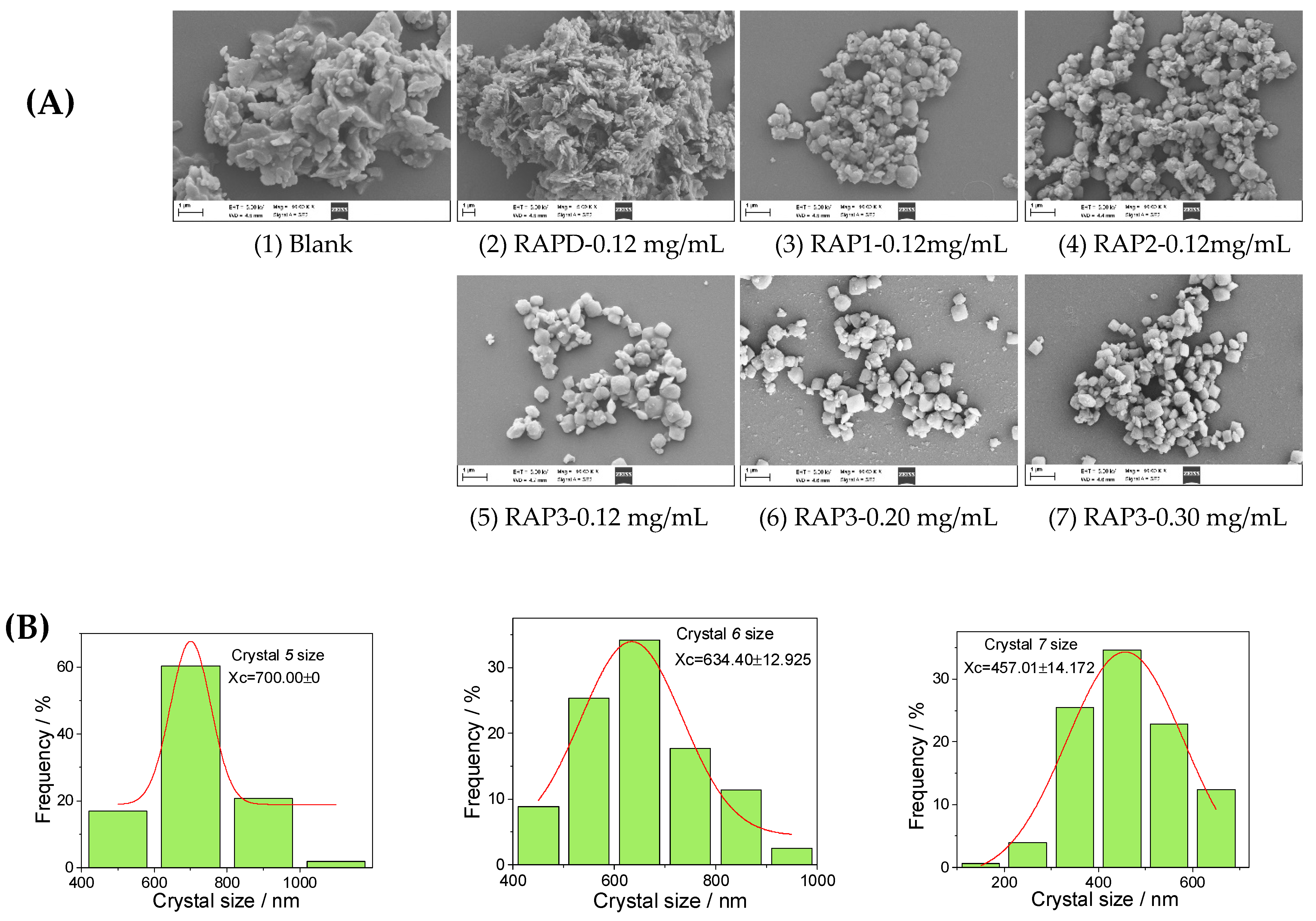
2.4.6. Scanning Electron Microscope (SEM) Observation of CaOx Crystals
2.4.7. Nano Measurer (v1.2.5) Software to Analyze CaOx Crystal Size
2.5. Interaction between FITC-Labeled Polysaccharides and Crystals
2.5.1. Preparation and Characterization of FITC-RAPD
2.5.2. FITC-RAPD Regulates CaOx Crystal Growth and Crystal Characterization
2.6. Cytotoxicity of Crystals Regulated by RAPs
2.6.1. Cell Culture
2.6.2. Cell Viability Test
2.6.3. Lactate Dehydrogenase (LDH) Release Assay
2.6.4. Calcein/Propidium Iodide (PI) Staining Method to Detect Live and Dead Cells
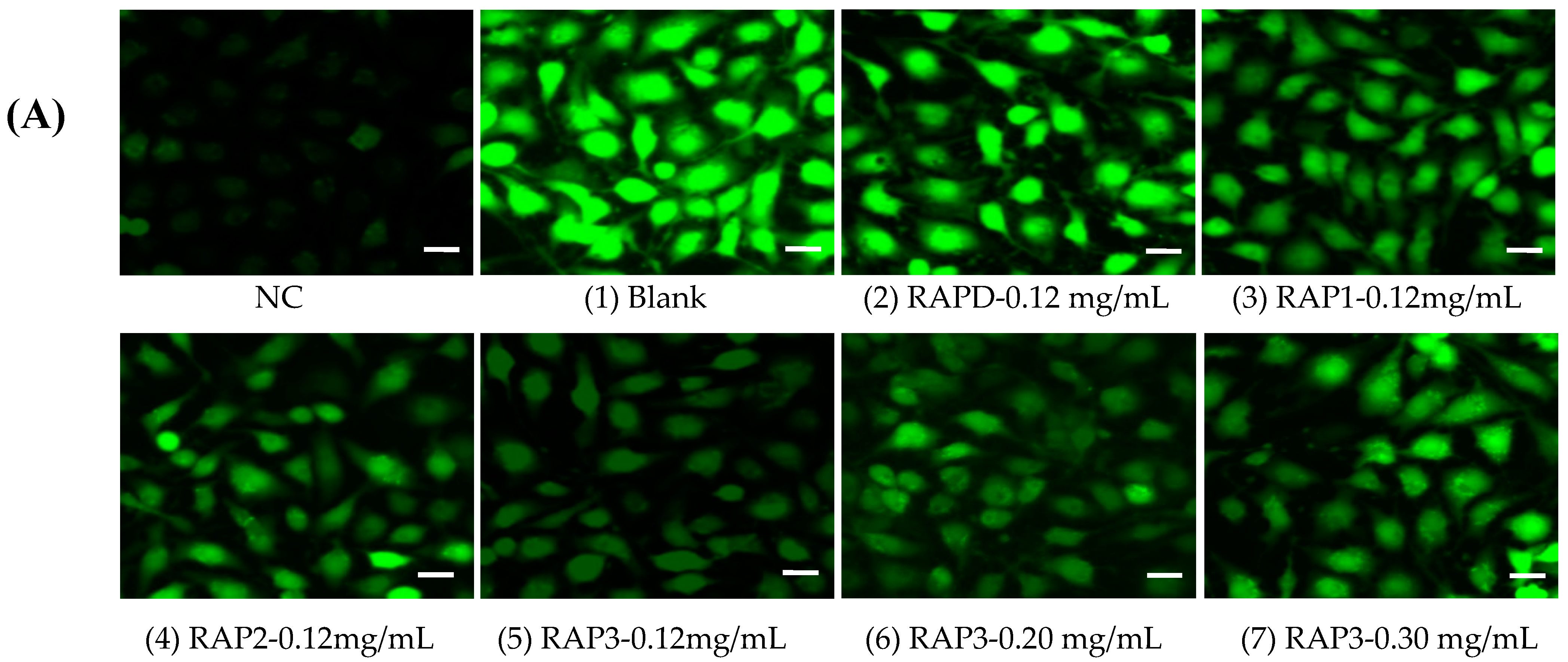
2.6.5. ROS Level Detection
2.7. Statistical Analysis
3. Results
3.1. Carboxymethylation and Characterization of RAPs
3.1.1. Separation, Purification, Degradation, and Monosaccharide Component Analysis of RAPs
3.1.2. Carboxymethylation of RAPD and Detection of –COOH Content
3.1.3. FT-IR Characterization of RAPs
3.1.4. 1H NMR and 13C NMR Characterization of RAPs
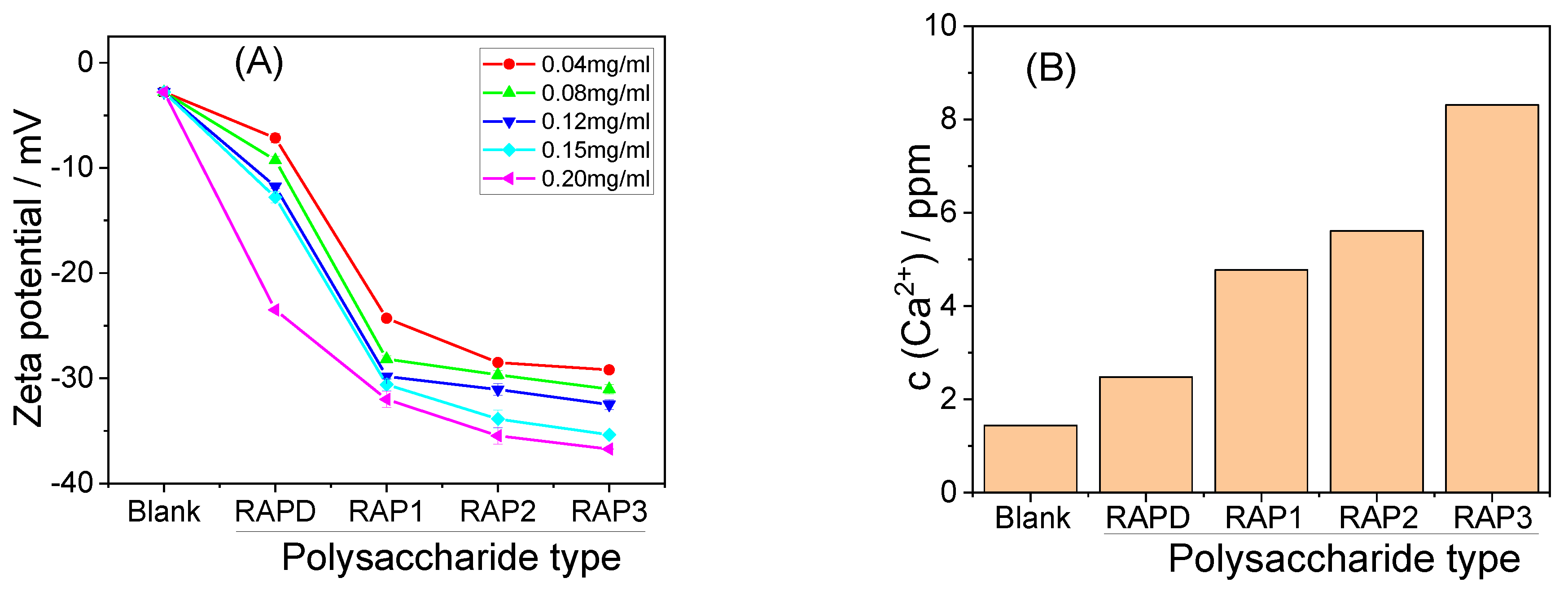
3.1.5. Zeta Potential Detection of RAPs
3.2. Antioxidative Activity of RAPs
3.3. RAPs Regulate CaOx Crystal Growth
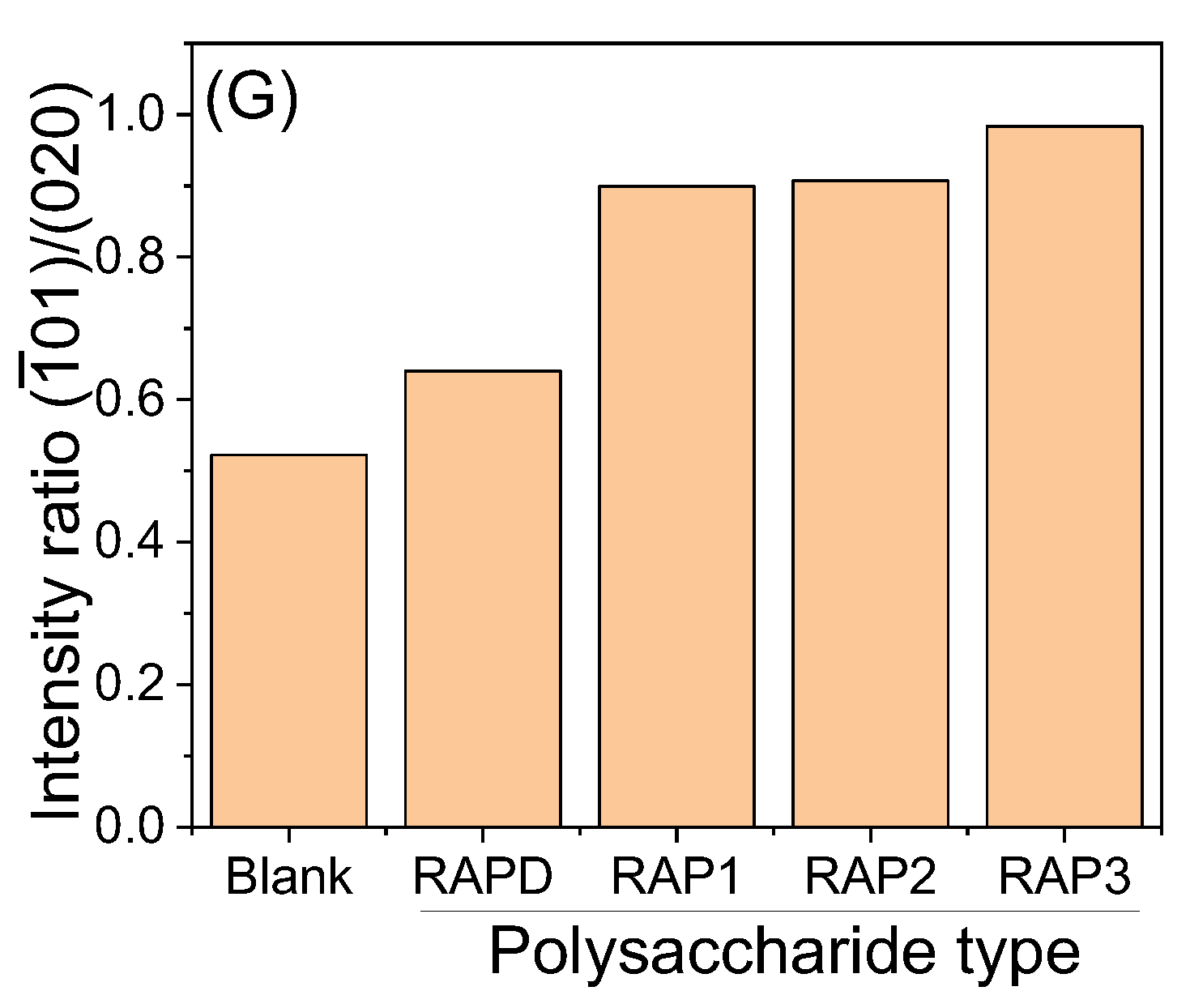
3.3.1. X-ray Diffraction Characterization
3.3.2. SEM Characterization
3.3.3. FT-IR Characterization
3.3.4. Zeta Potential
3.3.5. Soluble Ca2+ Concentration in the Supernatant
3.4. Interaction between RAPs and CaOx Crystals
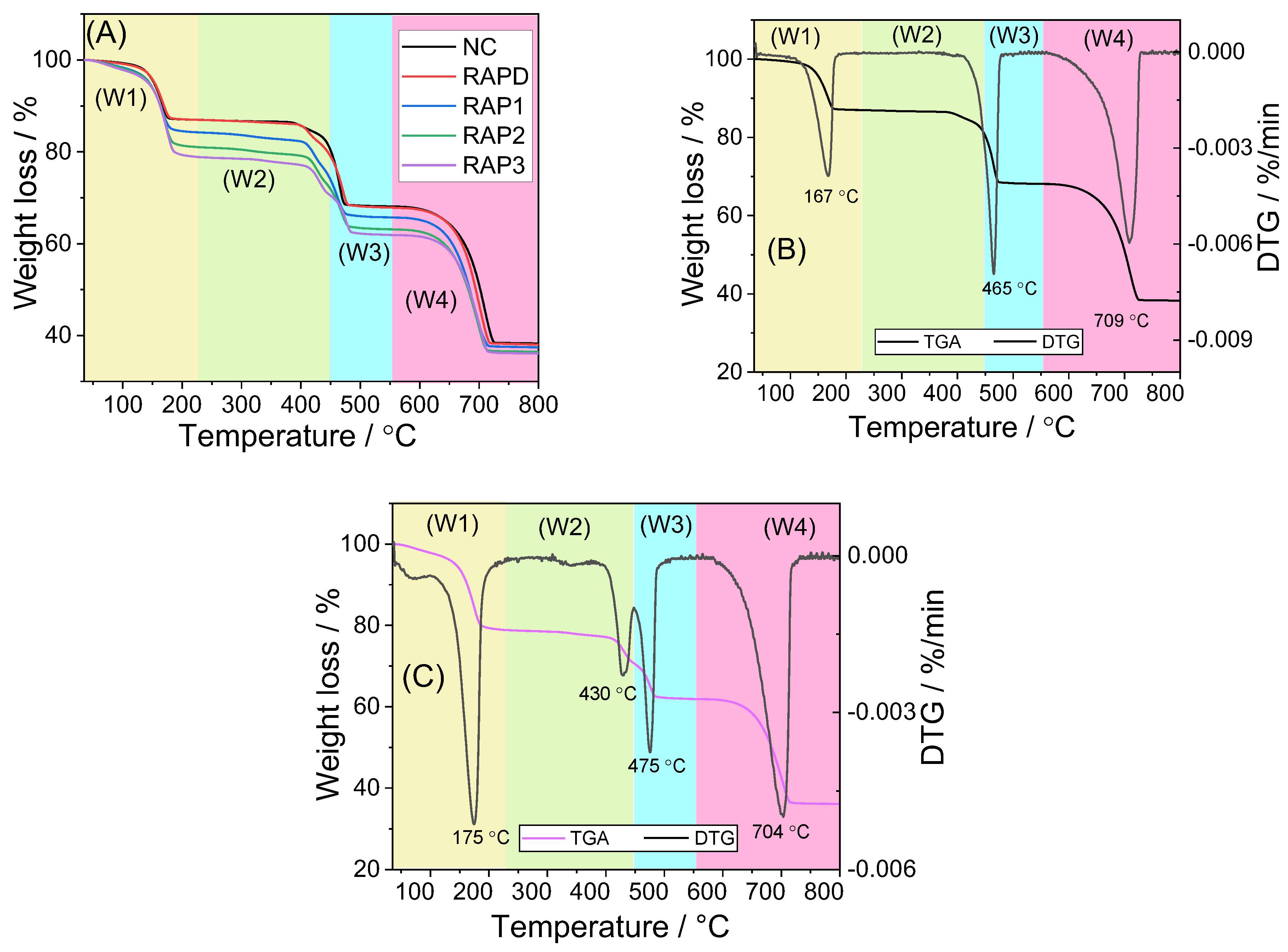
3.4.1. TGA Characterization
- (1)
- CaC2O4.nH2O → CaC2O4 + nH2O (n = 1 or 2)
- (2)
- CaC2O4 → CaCO3 + CO
- (3)
- CaCO3 → CaO + CO2
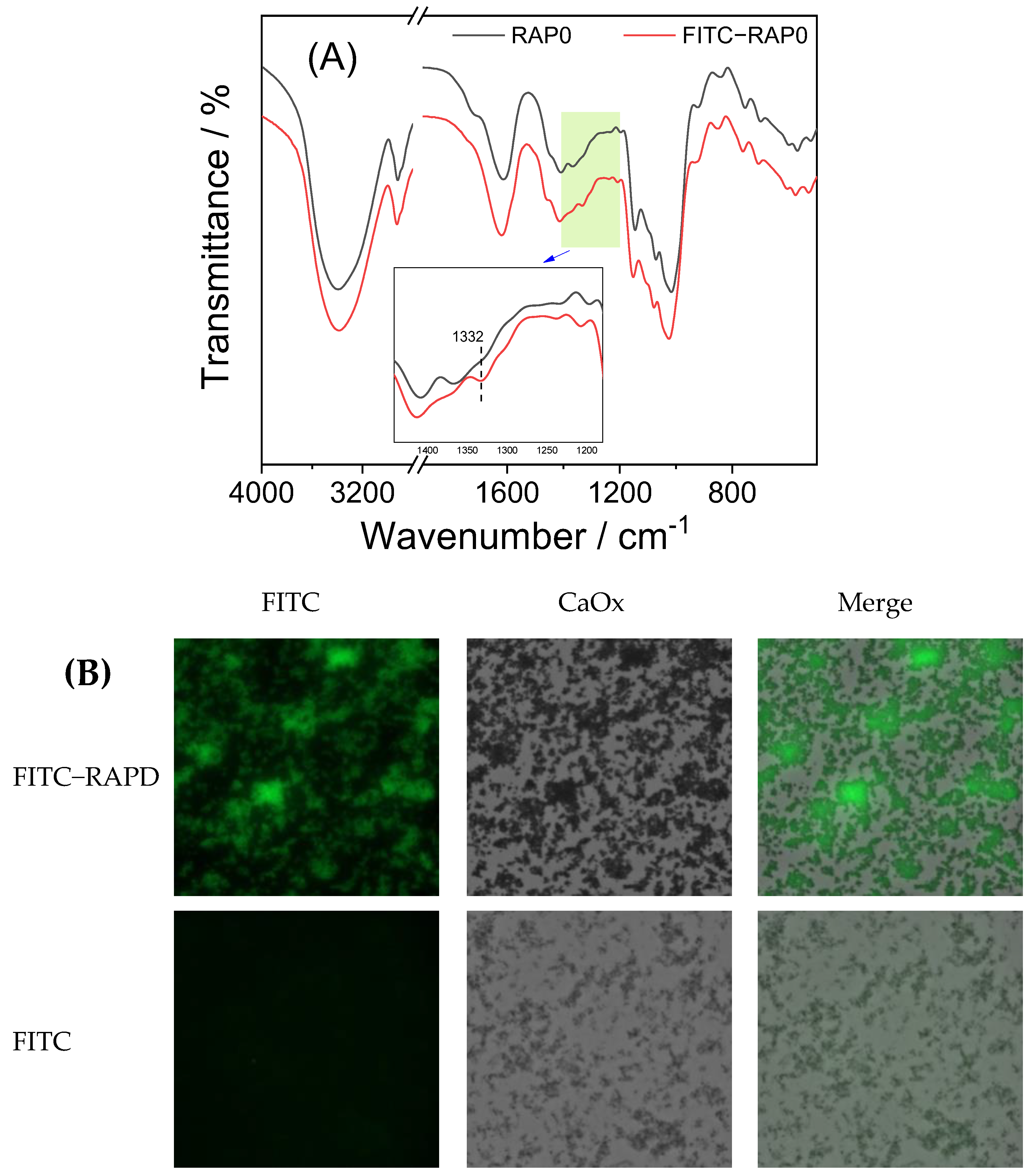
3.4.2. Adsorption of RAPs and Crystals
- (a)
- FT-IR characterization of FITC-RAPD
- (b)
- Fluorescence microscope observation of crystals under FITC-RAPD regulation
3.5. Cytotoxicity of RAP-Regulated Crystals
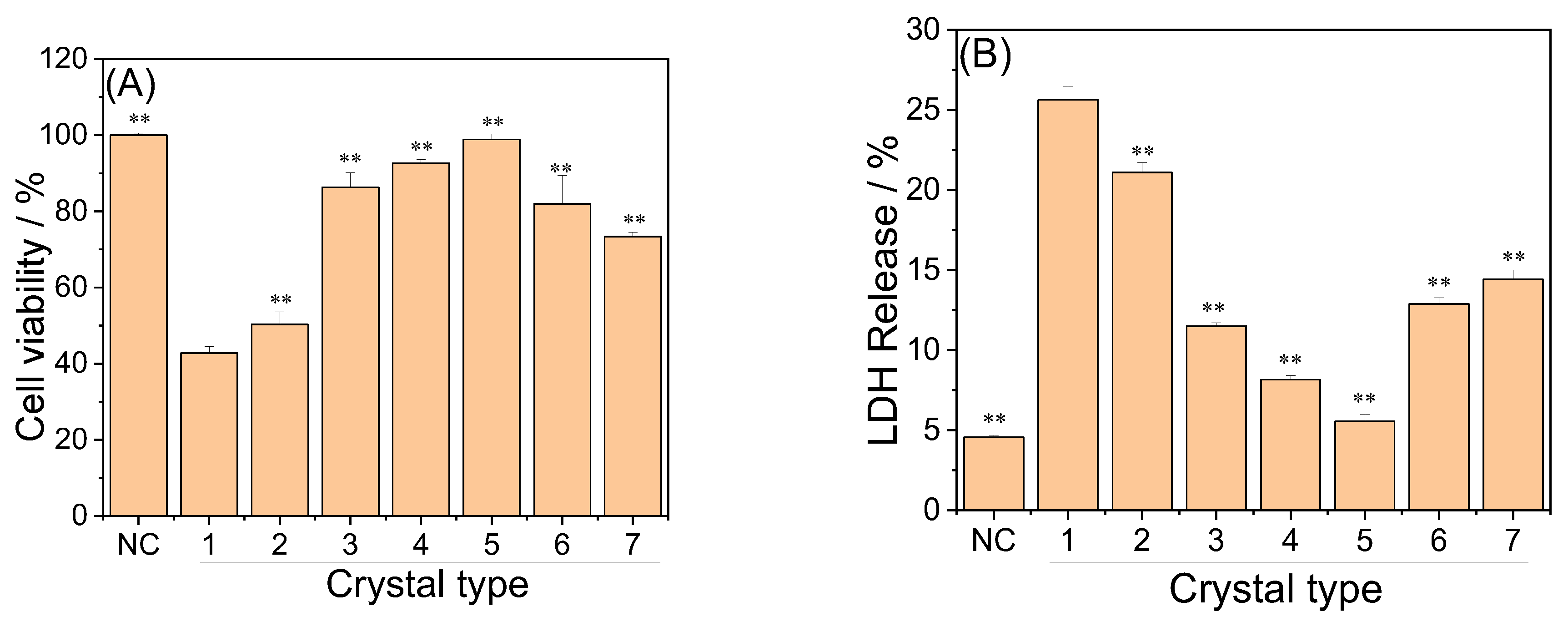
3.5.1. Cell Viability
3.5.2. LDH Release Amount
3.5.3. Calcein/PI Staining to Detect Cell Death
3.5.4. ROS
4. Discussion
4.1. Carboxymethylation and Characterization of RAPs
4.2. Enhanced Antioxidant Capacity of RAPs
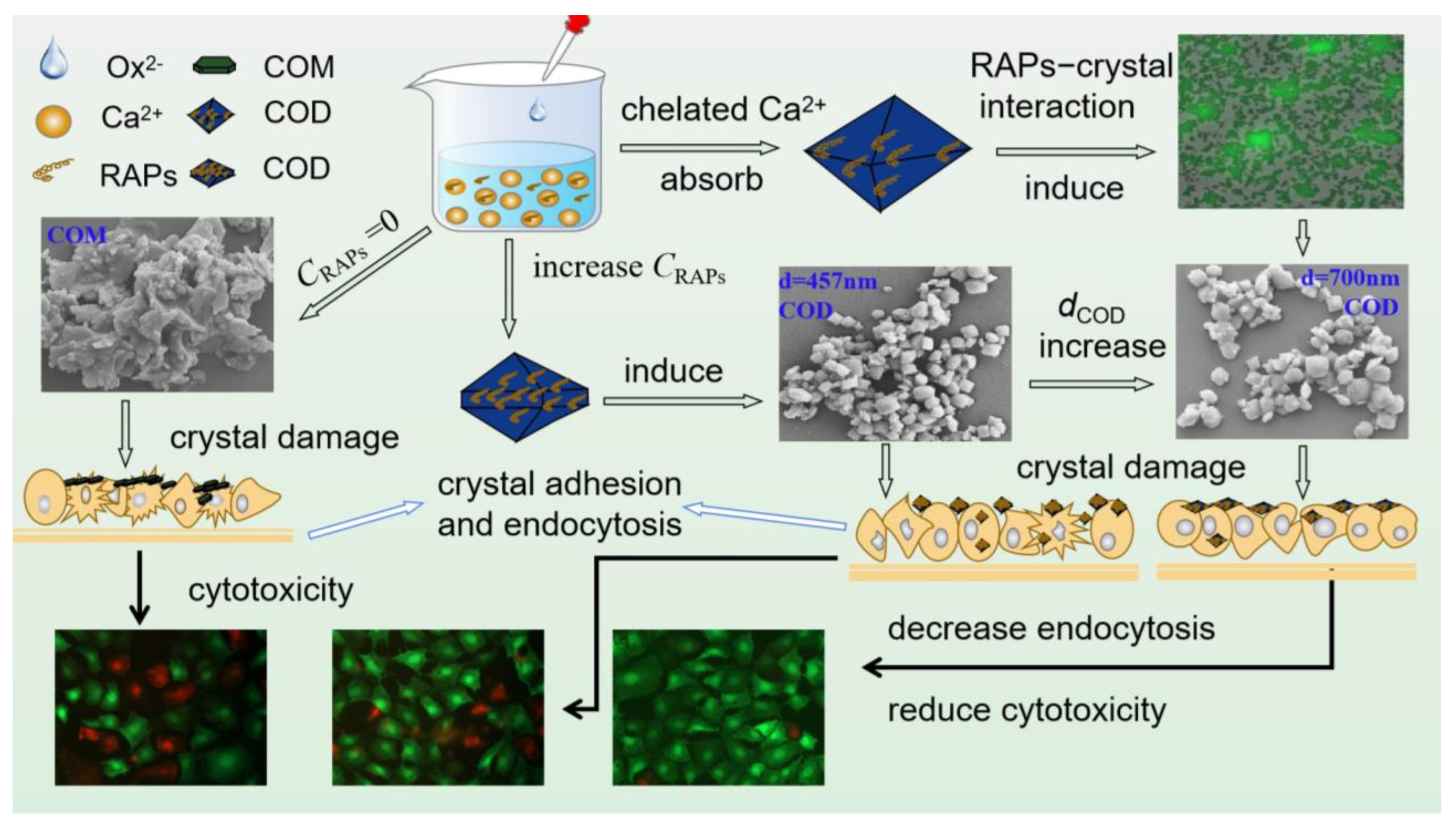
4.3. Ability of RAPs to Regulate the Growth of CaOx Crystals
4.3.1. Induce COD Formation and Inhibit COM Growth
4.3.2. Change the Strength of Different Crystal Planes of COM
4.3.3. Inhibition of Crystal Aggregation
4.4. Reasons for Differences in the Cytotoxicity of CaOx Crystals Regulated by Different RAPs
4.4.1. Reduced Cytotoxicity of Polysaccharide-Regulated Crystals
4.4.2. Differences in Cytotoxicity between COM and COD
4.4.3. Small COD Size Leads to Great Cytotoxicity
4.5. Interaction between RAPs and Crystals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivaguru, M.; Saw, J.J.; Wilson, E.M.; Lieske, J.C.; Krambeck, A.E.; Williams, J.C.; Romero, M.F.; Fouke, K.W.; Curtis, M.W.; Kear-Scott, J.L.; et al. Human kidney stones: A natural record of universal biomineralization. Nat. Rev. Urol. 2021, 18, 404–432. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Pearle, M.S.; Robertson, W.G.; Gambaro, G.; Canales, B.K.; Doizi, S.; Traxer, O.; Tiselius, H.G. Kidney stones. Nat. Rev. Dis. Primers 2016, 2, 16008. [Google Scholar] [CrossRef] [PubMed]
- Shtukenberg, A.G.; Hu, L.; Sahota, A.; Kahr, B.; Ward, M.D. Disrupting crystal growth through molecular recognition: Designer therapies for kidney stone prevention. Acc. Chem. Res. 2022, 55, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Bazin, D.; Frochot, V.; Haymann, J.P.; Letavernier, E.; Daudon, M. Crystal size in µcrystalline pathologies and its clinical implication. Comptes Rendus Chim. 2022, 25, 133–437. [Google Scholar] [CrossRef]
- Thongboonkerd, V. Proteomics of crystal-cell interactions: A model for kidney stone research. Cells 2019, 8, 1076. [Google Scholar] [CrossRef] [PubMed]
- Parvaneh, L.S.; Donadio, D.; Sulpizi, M. Molecular mechanism of crystal growth inhibition at the calcium oxalate/water interfaces. J. Phys. Chem. C 2016, 120, 4410–4417. [Google Scholar] [CrossRef]
- Sheng, X.; Ward, M.D.; Wesson, J.A. Crystal surface adhesion explains the pathological activity of calcium oxalate hydrates in kidney stone formation. J. Am. Soc. Nephrol. 2005, 16, 1904–1908. [Google Scholar] [CrossRef]
- Khan, A.H.; Imran, S.; Talati, J.; Jafri, L. Fourier transform infrared spectroscopy for analysis of kidney stones. Investig. Clin. Urol. 2018, 59, 32–37. [Google Scholar] [CrossRef]
- Ou, Y.; Xue, J.F.; Tan, C.Y.; Gui, B.S.; Sun, X.Y.; Ouyang, J.M. Inhibition of urinary macromolecule heparin on aggregation of nano-COM and nano-COD crystals. Molecules 2015, 20, 1626–1642. [Google Scholar] [CrossRef]
- Huang, F.; Sun, X.Y.; Ouyang, J.M. Preparation and characterization of selenized Astragalus polysaccharide and its inhibitory effect on kidney stones. Mat. Sci. Eng. C 2020, 110, 110732. [Google Scholar] [CrossRef]
- Jung, T.; Kim, W.S.; Choi, C.K. Biomineralization of calcium oxalate for controlling crystal structure and morphology. Mat. Sci. Eng. C 2004, 24, 31–33. [Google Scholar] [CrossRef]
- Liu, J.H.; Zheng, Y.Y.; Ouyang, J.M. Antioxidant Activities and Cytotoxicity of the Regulated Calcium Oxalate Crystals on HK-2 Cells of Polysaccharides from Gracilaria lemaneiformis with Different Molecular Weights. Foods 2023, 12, 1031. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Zhang, H.; Chen, J.Y.; Zeng, G.H.; Ouyang, J.M. Porphyra yezoensis polysaccharide and potassium citrate synergistically inhibit calcium oxalate crystallization induced by renal epithelial cells and cytotoxicity of the formed crystals. Mat. Sci. Eng. C 2021, 119, 111448. [Google Scholar] [CrossRef] [PubMed]
- Semangoen, T.; Sinchaikul, S.; Chen, S.T.; Thongboonkerd, V. Altered proteins in MDCK renal tubular cells in response to calcium oxalate dihydrate crystal adhesion: A proteomics approach. J. Proteome Res. 2008, 7, 2889–2896. [Google Scholar] [CrossRef]
- Vinaiphat, A.; Aluksanasuwan, S.; Manissorn, J.; Sutthimethakorn, S.; Thongboonkerd, V. Response of renal tubular cells to differential types and doses of calcium oxalate crystals: Integrative proteome network analysis and functional investigations. Proteomics 2017, 17, 1700192. [Google Scholar] [CrossRef]
- Aggarwal, K.P.; Narula, S.; Kakkar, M.; Tandon, C. Nephrolithiasis: Molecular mechanism of renal stone formation and the critical role played by modulators. BioMed Res. Int. 2013, 2013, 292953. [Google Scholar] [CrossRef]
- Khan, S.R.; Hackett, R.L. Role of organic matrix in urinary stone formation: An ltrastructural study of crystal matrix interface of calcium oxalate monohydrate stones. J. Urol. 1993, 150, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Doyle, I.R.; Marshall, V.R.; Dawson, C.J.; Ryall, R.L. Calcium oxalate crystal matrix extract: The most potent macromolecular inhibitor of crystal growth and aggregation yet tested in undiluted human urine in vitro. Urol. Res. 1995, 23, 53–62. [Google Scholar] [CrossRef]
- Poon, N.W.; Gohel, M.D.I. Urinary glycosaminoglycans and glycoproteins in a calcium oxalate crystallization system. Carbohyd. Res. 2012, 347, 64–68. [Google Scholar] [CrossRef]
- Duan, C.Y.; Xia, Z.Y.; Zhang, G.N.; Gui, B.S.; Xue, J.F.; Ouyang, J.M. Changes in urinary nanocrystallites in calcium oxalate stone formers before and after potassium citrate intake. Int. J. Nanomed. 2013, 8, 909–918. [Google Scholar]
- Ryall, R.L.; Harnett, R.M.; Marshall, V.R. The effect of urine, pyrophosphate, citrate, magnesium and glycosaminoglycans on the growth and aggregation of calcium oxalate crystals in vitro. Clin. Chim. Acta 1981, 112, 349–356. [Google Scholar] [CrossRef]
- Ahmad, M.M. Recent trends in chemical modification and antioxidant activities of plants-based polysaccharides: A review. Carbohyd. Polym. Technol. Appl. 2021, 2, 100045. [Google Scholar] [CrossRef]
- Feng, Y.; Qiu, Y.; Duan, Y.; He, Y.; Xiang, H.; Sun, W.; Zhang, H.; Ma, H. Characterization, antioxidant, antineoplastic and immune activities of selenium modified Sagittaria sagittifolia L. polysaccharides. Food Res. Int. 2022, 153, 110913. [Google Scholar] [CrossRef]
- Simsek, M.; Asiyanbi-Hammed, T.T.; Rasaq, N.; Hammed, A.M. Progress in Bioactive Polysaccharide-Derivatives: A review. Food Rev. Int. 2023, 39, 1612–1627. [Google Scholar] [CrossRef]
- Chen, T.; Liu, H.; Liu, J.; Li, J.; An, Y.; Zhu, M.; Chen, B.; Liu, F.; Liu, R.; Si, C.; et al. Carboxymethylation of polysaccharide isolated from Alkaline Peroxide Mechanical Pulping (APMP) waste liquor and its bioactivity. Int. J. Biol. Macromol. 2021, 181, 211–220. [Google Scholar] [CrossRef]
- Xie, L.; Shen, M.; Wang, Z.; Xie, J. Structure, function and food applications of carboxymethylated polysaccharides: A comprehensive review. Trends Food Sci. Technol. 2021, 118, 539–557. [Google Scholar] [CrossRef]
- Chen, X.W.; Sun, X.Y.; Tang, G.H.; Ouyang, J.M. Sulfated Undaria pinnatifida polysaccharide inhibits the formation of kidney stones by inhibiting HK-2 cell damage and reducing the adhesion of nano-calcium oxalate crystals. Mat. Sci. Eng. C 2021, 134, 112564. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Huang, W.B.; Sun, X.Y.; Xiong, P.; Ouyang, J.M. Antioxidant activity of sulfated Porphyra yezoensis polysaccharides and their regulating effect on calcium oxalate crystal growth. Mat. Sci. Eng. C 2021, 128, 112338. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Y.; Liu, X.; Han, S.; Fan, S. Triterpenoids from Alisma orientale and their NF-κB inhibitory activity. Planta Med. 2021, 8, e114–e121. [Google Scholar] [CrossRef]
- Liu, S.S.; Guo, J.; Li, Z.A.; Tian, S.S.; Zhu, J.J.; Yan, L.H.; Wang, Z.M.; Gao, L. Advances in studies on chemical compositions of Alismatis Rhizoma and their biological activities. China J. Chin. Mater. Med. 2020, 45, 1578–1595. [Google Scholar]
- Du, J.; Jia, R.; Cao, L.P.; Ding, W.; Xu, P.; Yin, G. Effects of Rhizoma Alismatis extract on biochemical indices and adipose gene expression in oleic acid-induced hepatocyte injury in Jian carp (Cyprinus carpio var. Jian). Fish Physiol. Biochem. 2018, 44, 747–768. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Song, H.H.; Ahn, K.S.; Oh, S.R.; Sadikot, R.T.; Joo, M. Ethanol extract of the tuber of Alisma orientale reduces the pathologic features in a chronic obstructive pulmonary disease mouse model. J. Ethnopharmacol. 2016, 188, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Dou, F.; Miao, H.; Wang, J.W.; Chen, L.; Wang, M.; Chen, H.; Wen, A.D.; Zhao, Y.Y. An integrated lipidomics and phenotype study reveals protective effect and biochemical mechanism of traditionally used Alisma orientale Juzepzuk in chronic kidney disease. Front. Pharmacol. 2018, 9, 53. [Google Scholar] [CrossRef]
- Cao, Z.H.; Su, H.; Zhu, Y.P.; Sun, Y.W.; Dong, X.C.; Wu, B. The auxiliary effect of Chinese medicine Alisma in upper urinary tract calculi. J. Anhui Med. Univ. 2008, 43, 573–575. [Google Scholar]
- Yin, C.P.; Liu, J.H.; Zhang, Y.S. In vitro and animal experiments on prevention of calcium oxalate stone formation by water extract of Alisma orientalis. J. Tongji Med. Univ. 1997, 26, 99–101. [Google Scholar]
- Qian, Z.K.; Cui, F.; Ling, Y.X.; Zhu, W.J.; Li, X.Q.; Mao, Z.; Li, M.T. Alisma orientalis (Sam.) juzep polysaccharide-regulated glucose-lipid metabolism in experimental rats and cell model of diabetes mellitus with regulation of miR-126. Pharmacogn. Mag. 2019, 15, 652. [Google Scholar]
- Zhang, H.; Zou, P.; Zhao, H.; Qiu, J.; Regenstein, J.M.; Yang, X. Isolation, purification, structure and antioxidant activity of polysaccharide from pinecones of Pinus koraiensis. Carbohyd. Polym. 2021, 251, 117078. [Google Scholar] [CrossRef]
- Xia, H.; Yang, C.; Zhou, B.; Tang, H.; Yang, L.; Liao, W.; Sun, G. Pharmacokinetics and Excretion Study of Lycium Barbarum Polysaccharides in Rats by FITC-Fluorescence Labeling. Foods 2021, 10, 2851. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.L.; Melo, K.R.T.; Queiroz, M.F.; Batista, L.A.N.C.; Santos, P.C.; Costa, M.S.S.P.; Almeida-Lima, J.; Camara, R.B.G.; Costa, L.S.; Rocha, H.A.O. In vitro studies reveal antiurolithic effect of antioxidant sulfated polysaccharides from the green seaweed Caulerpa cupressoides var flabellata. Mar. Drugs 2019, 17, 326. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Zhang, Q.; Li, Y.F.; Dong, L.L.; Liu, S.L. Optimization of ultrasound extraction of Alisma orientalis polysaccharides by response surface methodology and their antioxidant activities. Carbohyd. Polym. 2015, 119, 101–109. [Google Scholar] [CrossRef]
- Meena, K.; Muthu, K.; Meenatchi, V.; Rajasekar, M.; Bhagavannarayana, G.; Meenakshisundaram, S.P. Growth, crystalline perfection, spectral, thermal and theoretical studies on imidazolium L-tartrate crystals. Spectrochim. Acta A 2014, 124, 663–669. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and Infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta A 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Shi, X.D.; Li, O.Y.; Yin, J.Y.; Nie, S.P. Structure identification of α-glucans from Dictyophora echinovolvata by methylation and 1D/2D NMR spectroscopy. Food Chem. 2019, 271, 338–344. [Google Scholar] [CrossRef]
- Wang, J.; Nie, S.; Cui, S.W.; Wang, Z.; Phillips, A.O.; Phillips, G.O.; Li, Y.; Xie, M. Structural characterization and immunostimulatory activity of a glucan from natural Cordyceps sinensis. Food Hydrocoll. 2017, 67, 139–147. [Google Scholar] [CrossRef]
- Niu, Y.; Yan, W.; Lv, J.; Yao, W.; Yu, L. Characterization of a novel polysaccharide from tetraploid Gynostemma pentaphyllum makino. J. Agric. Food Chem. 2013, 61, 4882–4889. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.Y.Y.; Wang, J.Q.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. A review of NMR analysis in polysaccharide structure and conformation: Progress, challenge and perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar] [CrossRef]
- Xu, J.; Liu, W.; Yao, W.; Pang, X.; Yin, D.; Gao, X. Carboxymethylation of a polysaccharide extracted from Ganoderma lucidum enhances its antioxidant activities in vitro. Carbohyd. Polym. 2009, 78, 227–234. [Google Scholar] [CrossRef]
- Ren, J.L.; Sun, R.C.; Peng, F. Carboxymethylation of hemicelluloses isolated from sugarcane bagasse. Polym. Degrad. 2008, 93, 786–793. [Google Scholar] [CrossRef]
- Chatterjee, P.; Chakraborty, A.; Mukherjee, A.K. Phase composition and morphological characterization of human kidney stones using IR spectroscopy, scanning electron microscopy and X-ray Rietveld analysis. Spectrochim. Acta A 2018, 200, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Carmona, P.; Bellanato, J.; Escolar, E. Infrared and Raman spectroscopy of urinary calculi: A review. Biospectroscopy 1997, 3, 331–346. [Google Scholar] [CrossRef]
- Shukla, F.; Kikani, T.; Khan, A.; Thakore, S. α-Hydroxy acids modified β-cyclodextrin capped iron nanocatalyst for rapid reduction of nitroaromatics: A sonochemical approach. Int. J. Biol. Macromol. 2022, 209, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Šter, A.; Šafranko, S.; Bilić, K.; Marković, B.; Kralj, D. The effect of hydrodynamic and thermodynamic factors and the addition of citric acid on the precipitation of calcium oxalate dihydrate. Urolithiasis 2018, 46, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Lawson-Wood, K.; Robertson, I. Study of the Decomposition of Calcium Oxalate Monohydrate Using a Hyphenated Thermogravimetric Analyser-FT-IR System (TG-IR); Application Note; PerkinElmer, Inc.: Waltham, MA, USA, 2016. [Google Scholar]
- Li, C.Y.; Liu, L.; Zhao, Y.W.; Chen, J.Y.; Sun, X.Y.; Ouyang, J.M. Inhibition of calcium oxalate formation and antioxidant activity of carboxymethylated Poria cocos polysaccharides. Oxid. Med. Cell. Longev. 2021, 2021, 6653593. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.A.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth. Sci. Rep. 2020, 10, 3011. [Google Scholar] [CrossRef]
- Nawrocka, A.; Szymańska-Chargot, M.; Miś, A.; Wilczewskab, A.Z.; Markiewiczb, K.H. Effect of dietary fibre polysaccharides on structure and thermal properties of gluten proteins-A study on gluten dough with application of FT-Raman spectroscopy, TGA and DSC. Food Hydrocoll. 2017, 69, 410–421. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, M.; Zhao, J.; Zhang, X.; Hu, X.; Goff, H.D.; Guo, Q. Fluorescent labeling affected the structural/conformational properties of arabinoxylans. Carbohyd. Polym. 2021, 265, 118064. [Google Scholar] [CrossRef]
- Laffleur, F.; Psenner, J.; Suchaoin, W. Permeation enhancement via thiolation: In vitro and ex vivo evaluation of hyaluronic acid-cysteine ethyl ester. J. Pharm. Sci. 2015, 104, 2153–2160. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, C.; Du, D.; Li, Y.; Sun, L.; Han, Y.; He, X.; Dai, J.; Shi, L. Metal-organic framework-based hydrogel with structurally dynamic properties as a stimuli-responsive localized drug delivery system for cancer therapy. Acta Biomater. 2022, 145, 43–51. [Google Scholar] [CrossRef]
- An, Y.; Liu, H.T.; Li, X.; Liu, J.; Chen, L.; Jin, X.; Chen, T.; Wang, W.; Liu, Z.; Zhang, M.; et al. Carboxymethylation modification, characterization, antioxidant activity and anti-UVC ability of Sargassum fusiforme polysaccharide. Carbohyd. Res. 2022, 515, 108555. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Wang, X.; Hu, H.; Zhang, Y.; Liu, T.; Zhao, H. Structure characterization and antioxidant activity of carboxymethylated polysaccharide from Pholiota nameko. J. Food Biochem. 2022, 46, e14121. [Google Scholar] [CrossRef]
- Akın, B.; Öner, M.; Bayram, Y.; Demadis, K.D. Effects of carboxylate-modified, “green” inulin biopolymers on the crystal growth of calcium oxalate. Cryst. Growth Des. 2008, 8, 1997–2005. [Google Scholar] [CrossRef]
- Wei, X.X.; Yang, J.; Li, Z.Y.; Su, Y.L.; Wang, D.J. Comparison investigation of the effects of ionic surfactants on the crystallization behavior of calcium oxalate: From cationic to anionic surfactant. Colloids Surf. A 2012, 401, 107–115. [Google Scholar] [CrossRef]
- Viswanathan, P.; Rimer, J.D.; Kolbach, A.M.; Ward, M.D.; Kleinman, J.G.; Wesson, J.A. Calcium oxalate monohydrate aggregation induced by aggregation of desialylated Tamm-Horsfall protein. Urol. Res. 2011, 39, 269–282. [Google Scholar] [CrossRef]
- Xiong, P.; Cheng, X.Y.; Sun, X.Y.; Chen, X.W.; Ouyang, J.M. Interaction between nanometer calcium oxalate and renal epithelial cells repaired with carboxymethylated polysaccharides. Biomater. Adv. 2022, 137, 212854. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.J.; Huang, W.B.; Sun, X.Y.; Tang, G.H.; Ouyang, J.M. Carboxymethylation of corn silk polysaccharide and its inhibition on adhesion of nanocalcium oxalate crystals to damaged renal epithelial cells. ACS Biomater. Sci. Eng. 2021, 7, 3409–3422. [Google Scholar] [CrossRef] [PubMed]
- Elliot, J.S.; Rabinowitz, I.N. Calcium oxalate crystalluria: Crystal size in urine. J. Urol. 1980, 123, 324–327. [Google Scholar] [CrossRef]
- Wesson, J.A.; Worcester, E.M.; Wiessner, J.H.; Mandel, N.S.; Kleinman, J.K. Control of calcium oxalate crystal structure and cell adherence by urinary macromolecules. Kidney Int. 1998, 53, 952–957. [Google Scholar] [CrossRef]
- Sun, X.Y.; Ouyang, J.M.; Li, Y.B.; Wen, X.L. Mechanism of cytotoxicity of micron/nano calcium oxalate monohydrate and dihydrate crystals on renal epithelial cells. RSC Adv. 2015, 5, 45393–45406. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Jin, M.; Du, Z.; Liu, X.; Guo, C.; Li, Y.; Huang, P.; Sun, Z. Size-dependent cytotoxicity of amorphous silica nanoparticles in human hepatoma HepG2 cells. Toxicol. In Vitro 2011, 25, 1343–1352. [Google Scholar] [CrossRef]





| Sample | Reaction Time /h | Chloroacetic Acid /g | Temperature Reflex /°C | –COOH Content /% |
|---|---|---|---|---|
| RAPD | / | / | / | 3.57 |
| RAP1 | 2 | 2.5 | 50 | 7.79 |
| RAP2 | 4 | 3 | 60 | 10.84 |
| RAP3 | 5 | 5 | 60 | 15.33 |
| Sample | –COOH Content/% | Characteristic Absorption Peak/cm−1 | |||||
|---|---|---|---|---|---|---|---|
| O–H | C–H | C=O Asymmetric | C–O | –CH2– from Carboxymethyl | C–O–C | ||
| RAPD | 3.57 | 3403 | 2927 | 1620 | 1415 | / | 1024 |
| RAP1 | 7.79 | 3415 | 2927 | 1604 | 1419 | 1326 | 1026 |
| RAP2 | 10.84 | 3413 | 2925 | 1604 | 1419 | 1326 | 1043 |
| RAP3 | 15.33 | 3421 | 2927 | 1604 | 1421 | 1326 | 1037 |
| Sample | Residue | Chemical Shift (ppm) | |||||
|---|---|---|---|---|---|---|---|
| C1/H1 | C2/H2 | C3/H3 | C4/H4 | C5/H5 | C6/H6 | ||
| RAPD | (1→4)-α-D-Glcp | 99.6/5.32 | 71.5/3.56 | 73.3/3.75 | 76.7/3.57 | 71.5/3.88 | 60.4/3.77, 3.67 |
| α-D-Glcp-(1→) | 98.5/4.89 | 72.9/3.53 | 73.6/3.66 | 71.4/3.34 | 73.4/3.92 | 60.8/3.89 | |
| RAP3 | (1→4)-α-D-Glcp | 96.2/5.70 | 71.0/3.58 | 73.3/3.75 | 76.6/3.58 | 71.0/3.91 | 60.1/3.90, 3.72 |
| α-D-Glcp-(1→) | 92.0/4.91 | 72.5/3.57 | 73.3/3.60 | 71.4/3.38 | 73.4/4.03 | 60.3/4.00 | |
| Polysaccharide Types | Concentration /mg/mL | COD /% | |
|---|---|---|---|
| Blank | / | 0 | 0.52 |
| RAPD | 0.04 | 0 | 0.64 |
| RAPD | 0.08 | 0 | 0.75 |
| RAPD | 0.12 | 0 | 0.76 |
| RAPD | 0.15 | 0 | 0.78 |
| RAPD | 0.20 | 0 | 0.90 |
| Blank | / | 0 | 0.52 |
| RAPD | 0.04 | 0 | 0.64 |
| RAP1 | 0.04 | 0 | 0.90 |
| RAP2 | 0.04 | 0 | 0.91 |
| RAP3 | 0.04 | 33.02 | 0.98 |
| Polysac Charides | COD /% | W1 /% | T1 /°C | W2 * /% | T2 /°C | W3 /% | T3 /°C | W4 /% | T4 /°C | Total Weight Loss / % | Remaining Weight/% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blank | 0 | 12.17 | 193.7 | / | / | 18.12 | 487.9 | 29.69 | 732.8 | 59.99 | 40.01 |
| RAPD | 0 | 12.03 | 196.3 | 2.22 | 431.1 | 15.88 | 495.2 | 29.50 | 730.3 | 59.64 | 40.36 |
| RAP1 | 42.63 | 14.19 | 217.5 | 5.86 | 439.3 | 10.40 | 494.6 | 28.08 | 734.5 | 58.53 | 41.47 |
| RAP2 | 77.46 | 17.16 | 219.5 | 6.06 | 444.6 | 9.91 | 507.9 | 26.46 | 734.5 | 59.58 | 40.42 |
| RAP3 | 93.21 | 18.98 | 224.2 | 6.50 | 449.9 | 8.39 | 514.5 | 25.63 | 745.5 | 59.51 | 40.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.-Y.; Ouyang, J.-M. Carboxymethylated Rhizoma alismatis Polysaccharides Regulate Calcium Oxalate Crystals Growth and Reduce the Regulated Crystals’ Cytotoxicity. Biomolecules 2023, 13, 1044. https://doi.org/10.3390/biom13071044
Cheng X-Y, Ouyang J-M. Carboxymethylated Rhizoma alismatis Polysaccharides Regulate Calcium Oxalate Crystals Growth and Reduce the Regulated Crystals’ Cytotoxicity. Biomolecules. 2023; 13(7):1044. https://doi.org/10.3390/biom13071044
Chicago/Turabian StyleCheng, Xiao-Yan, and Jian-Ming Ouyang. 2023. "Carboxymethylated Rhizoma alismatis Polysaccharides Regulate Calcium Oxalate Crystals Growth and Reduce the Regulated Crystals’ Cytotoxicity" Biomolecules 13, no. 7: 1044. https://doi.org/10.3390/biom13071044
APA StyleCheng, X.-Y., & Ouyang, J.-M. (2023). Carboxymethylated Rhizoma alismatis Polysaccharides Regulate Calcium Oxalate Crystals Growth and Reduce the Regulated Crystals’ Cytotoxicity. Biomolecules, 13(7), 1044. https://doi.org/10.3390/biom13071044





