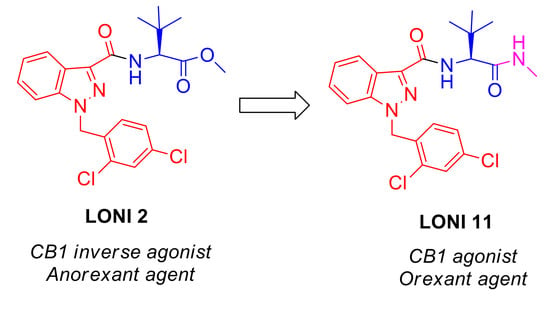
Discovery of Orexant and Anorexant Agents with Indazole Scaffold Endowed with Peripheral Antiedema Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
General Procedure for the N-Methyl Amide Formation
2.2. In Vitro Biological Assays
2.2.1. Preparation of Brain Membrane Homogenates
2.2.2. Radioligand Competition Binding Assay
2.2.3. Ligand Stimulated [35S]GTPγS Binding Assay
2.2.4. Data Analysis
2.3. In Vivo Biological Assays
2.3.1. Animals
2.3.2. Feeding Test
2.3.3. Tail Flick Test
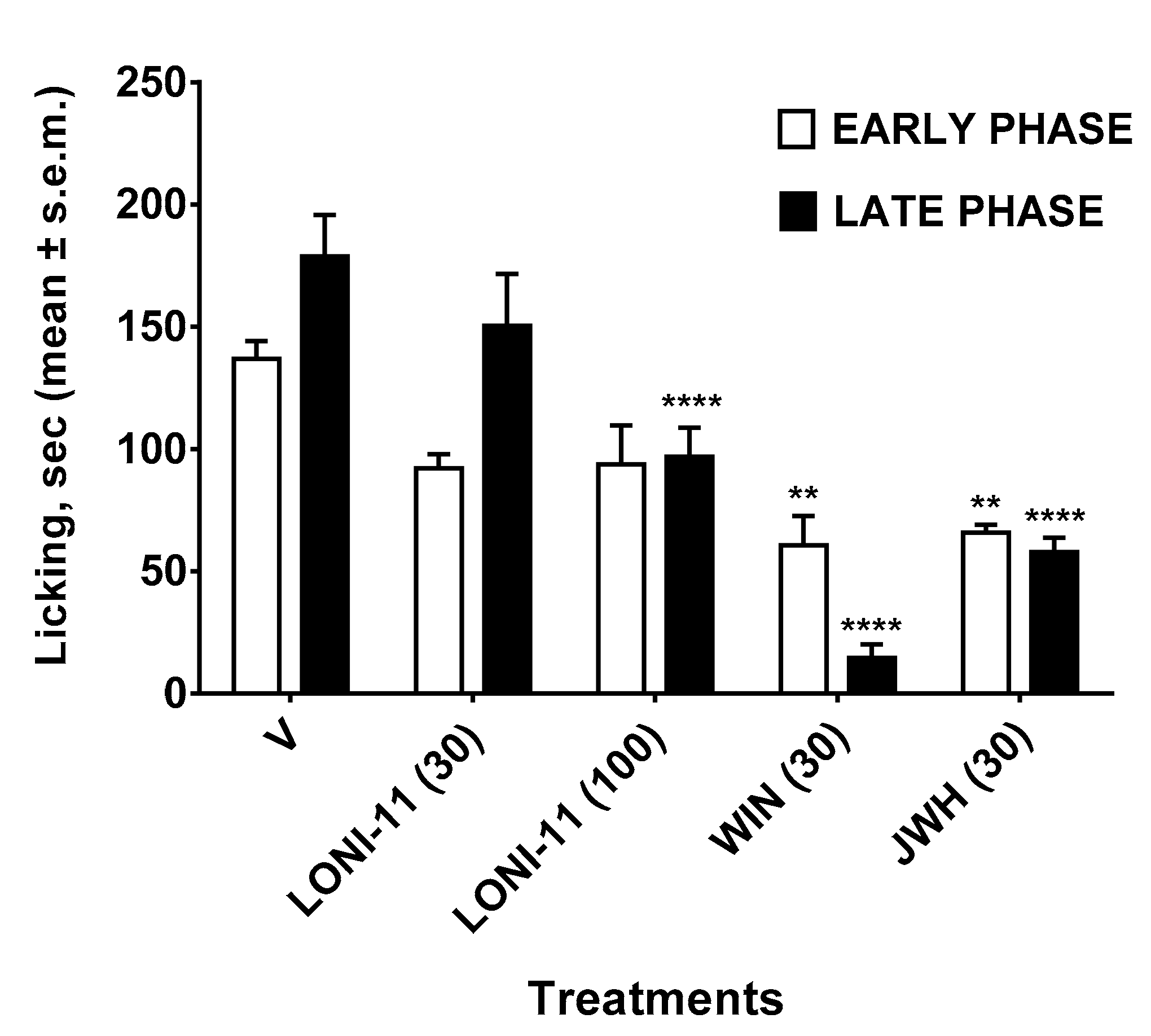
2.3.4. Formalin Test
2.3.5. Edema Induced by Zymosan
2.3.6. Zymosan-Induced Hyperalgesia
2.3.7. Data Analysis and Statistics
2.4. In Silico Experiments
2.4.1. Receptor Preparation
2.4.2. Docking Grid Generation
2.4.3. Self-Docking and Validation Procedure
2.4.4. Ligands Preparation
2.4.5. Ligand Docking Experiments
2.4.6. Molecular Dynamic
3. Results
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Carella, A.M.; Conte, M.; Melfitano, A.; Ponziano, E.; Benvenuto, A. Neuroendocrine Mediators, Food Intake and Obesity: A Narrative Review. Int. J. Cardiol. Lipidol. Res. 2014, 1, 18–32. [Google Scholar] [CrossRef]
- Thomas, B.F. Neuroanatomical basis for the therapeutic applications of cannabinoid receptor 1 antagonists. Drug Dev. Res. 2009, 70, 527–554. [Google Scholar] [CrossRef]
- Foster-Schubert, K.E.; Cummings, D.E. Emerging Therapeutic Strategies for Obesity. Endocr. Rev. 2006, 27, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Soria-Gomez, E.; Matias, I.; Rueda-Orozco, P.E.; Cisneros, M.; Petrosino, S.; Navarro, L.; di Marzo, V.; Prospéro-García, O. Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. Br. J. Pharmacol. 2007, 151, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, T.C.; Williams, C.M.; Fezza, F.; di Marzo, V. Endocannabinoid levels in rat limbic forebrainand hypothalamus in relation to fasting, feeding and satiation: Stimulation of eating by 2-arachidonoyl glycerol. Br. J. Pharmacol. 2002, 136, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Matias, I. Endocannabinoid control of food intake and energy balance. Nat. Neurosci. 2005, 8, 585–589. [Google Scholar] [CrossRef]
- Colombo, G.; Agabio, R.; Diaz, G.; Lobina, C.; Reali, R.; Gessa, G.L. Appetite suppression and weight loss after the cannabinoid antagonist SR141716. Life Sci. 1998, 63, PL113–PL117. [Google Scholar] [CrossRef]
- Lambert, P.; Wilding, J.; Al-Dokhayel, A.; Gilbey, S.; Bloom, S. The effect of central blockade of kappa-opioid receptors on neuropeptide Y-induced feeding in the rat. Brain Res. 1993, 629, 146–148. [Google Scholar] [CrossRef]
- Brunetti, L.; Ferrante, C.; Orlando, G.; Recinella, L.; Leone, S.; Chiavaroli, A.; di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; et al. Orexigenic effects of endomorphin-2 (EM-2) related to decreased CRH gene expression and increased dopamine and norepinephrine activity in the hypothalamus. Peptides 2013, 48, 83–88. [Google Scholar] [CrossRef]
- Crespo, I.; De Heras, R.G.; de Fonseca, F.R.; Navarro, M. Pretreatment with subeffective doses of Rimonabant attenuates orexigenic actions of orexin A-hypocretin 1. Neuropharmacology 2008, 54, 219–225. [Google Scholar] [CrossRef]
- Hilairet, S.; Bouaboula, M.; Carrière, D.; le Fur, G.; Casellas, P. Hypersensitization of the Orexin 1 Receptor by the CB1 Receptor: Evidence For Cross-Talk Blocked by the Specific Cb1 Antagonist, SR141716. J. Biol. Chem. 2003, 278, 23731–23737. [Google Scholar] [CrossRef]
- Scheen, A.J. Sibutramine on Cardiovascular Outcome. Diabetes Care 2001, 34, 114–119. [Google Scholar] [CrossRef]
- Zhang, W.; Roederer, M.W.; Chen, W.-Q.; Fan, L.; Zhou, H.-H. Pharmacogenetics of drugs withdrawn from the market. Pharmacogenomics 2012, 13, 223–231. [Google Scholar] [CrossRef]
- Després, J.-P.; Golay, A.; Sjöstrom, L. Effects of Rimonabant on Metabolic Risk Factors in Overweight Patients with Dyslipidemia. N. Engl. J. Med. 2005, 353, 2121–2134. [Google Scholar] [CrossRef]
- Després, J.P.; Ross, R.; Boka, G.; Alméras, N.; Lemieux, I. Effect of rimonabant on the high-triglyceride/ low-HDL-cholesterol dyslipidemia, intra-abdominal adiposity, and liver fat: The ADAGIO-Lipids trial. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 416–423. [Google Scholar] [CrossRef]
- Sam, A.H.; Salem, V.; Ghatei, M.A. Rimonabant: From RIO to Ban. J. Obes. 2011, 2011, 432607. [Google Scholar] [CrossRef]
- Erkekoglu, P.; Giray, B.; Sahin, G. Toxicological evaluation of Rimonabant, Taranabant, Surinabant and Otenabant in the treatment of obesity: Why the trials on endocannabinoid receptor antagonists and inverse agonists are suspended? FABAD J. Pharm. Sci. 2008, 33, 95–108. [Google Scholar]
- Janero, D.R.; Makriyannis, A. Cannabinoid receptor antagonists: Pharmacological opportunities, clinical experience, and translational prognosis. Expert Opin. Emerg. Drugs 2009, 14, 43–65. [Google Scholar] [CrossRef]
- Ibsen, M.S.; Connor, M.; Glass, M. Cannabinoid CB1 and CB2 Receptor Signaling and Bias. Cannabis Cannabinoid Res. 2017, 2, 48–60. [Google Scholar] [CrossRef]
- Al-Zoubi, R.; Morales, P.; Reggio, P.H. Structural Insights into CB1 Receptor Biased Signaling. Int. J. Mol. Sci. 2019, 20, 1837. [Google Scholar] [CrossRef]
- Al-Zoubi, W.; Salih, A.A.; Duraid, S.A.; Basheer, H.M.; Awad Al-Luhaibi, R.S.; Dib, A.; Young, G.K. Synthesis, characterization, and antioxidant activities ofimine compounds. J. Phys. Org. Chem. 2019, 32, 3916. [Google Scholar]
- Ibsen, D.B.; Laursen, A.S.D.; Lauritzen, L.; Tjønneland, A.; Overvad, K.; Jakobsen, M.U. Substitutions between dairy product subgroups and risk of type 2 diabetes: The Danish Diet, Cancer and Health cohort. Br. J. Nutr. 2017, 118, 989–997. [Google Scholar] [CrossRef]
- Mazier, W.; Saucisse, N.; Gatta-Cherifi, B.; Cota, D. The Endocannabinoid System: Pivotal Orchestrator of Obesity and Metabolic Disease. Trends Endocrinol. Metab. 2015, 26, 524–537. [Google Scholar] [CrossRef]
- Black, A.D.; Car, J.; Pagliari, C.; Anandan, C.; Cresswell, K.; Bokun, T.; McKinstry, B.; Procter, R.; Majeed, A.; Sheikh, A. The Impact of eHealth on the Quality and Safety of Health Care: A Systematic Overview. PLoS Med. 2011, 8, e1000387. [Google Scholar] [CrossRef]
- Rubino, F.; Nathan, D.M.; Eckel, R.H.; Schauer, P.R.; Alberti, K.G.M.; Zimmet, P.Z.; Del Prato, S.; Ji, L.; Sadikot, S.M.; Herman, W.H.; et al. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes Care 2016, 39, 861–877. [Google Scholar] [CrossRef]
- Mallat, S.G.; Abu Samra, S.; Younes, F.; Sawaya, M.-T. Identifying predictors of blood pressure control in the Lebanese population—A national, multicentric survey—I-PREDICT. BMC Public Health 2014, 14, 1142. [Google Scholar] [CrossRef]
- Schindler, K.; Themessl-Huber, M.; Hiesmayr, M.; Kosak, S.; Lainscak, M.; Laviano, A.; Ljungqvist, O.; Mouhieddine, M.; Schneider, S.; de van der Schueren, M.; et al. To eat or not to eat? Indicators for reduced food intake in 91,245 patients hospitalized on nutritionDays 2006–2014 in 56 countries worldwide: A descriptive analysis. Am. J. Clin. Nutr. 2016, 104, 1393–1402. [Google Scholar] [CrossRef]
- Banister, S.D.; Longworth, M.; Kevin, R.; Sachdev, S.; Santiago, M.; Stuart, J.; Mack, J.B.; Glass, M.; McGregor, I.S.; Connor, M.; et al. Pharmacology of Valinate and tert-Leucinate synthetic cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and their analogues. ACS Chem. Neurosci. 2016, 7, 1241–1254. [Google Scholar] [CrossRef]
- Shanks, K.G.; Clark, W.; Behonick, G. Death Associated With the Use of the Synthetic Cannabinoid ADB-FUBINACA. J. Anal. Toxicol. 2016, 40, 236–239. [Google Scholar] [CrossRef]
- Schoeder, C.T.; Hess, C.; Madea, B.; Meiler, J.; Muller, C.E. Pharmacological evaluation of new constituents of “Spice”: Synthetic cannabinoids based on indole, indazole, benzimidazole and carbazole scaffolds. Forensic Toxicol. 2018, 36, 385–403. [Google Scholar] [CrossRef]
- Adamowicz, P.; Meissner, E.; Maślanka, M. Fatal intoxication with new synthetic cannabinoids AMB-FUBINACA and EMB-FUBINACA. Clin. Toxicol. 2019, 26, 1–6. [Google Scholar] [CrossRef]
- Scourfield, A.; Flick, C.; Ross, J.; Wood, D.M.; Thurtle, N.; Stellmach, D.; Dargan, P.I. Synthetic cannabinoid availability on darknet drug markets—Changes during 2016–2017. Toxicol. Commun. 2019, 3, 7–15. [Google Scholar] [CrossRef]
- Stefanucci, A.; Macedonio, G.; Dvorácskó, S.; Tömböly, C.; Mollica, A. Novel Fubinaca/Rimonabant hybrids as endocannabinoid system modulators. Amino Acids 2018, 50, 1595–1605. [Google Scholar] [CrossRef]
- Mollica, A.; Costante, R.; Akdemir, A.; Carradori, S.; Stefanucci, A.; Macedonio, G.; Ceruso, M.; Supuran, C.T. Exploring new Probenecid-based carbonic anhydrase inhibitors: Synthesis, biological evaluation and docking studies. Bioorganic Med. Chem. 2015, 23, 5311–5318. [Google Scholar] [CrossRef]
- Mollica, A.; Pelliccia, S.; Famiglini, V.; Stefanucci, A.; Macedonio, G.; Chiavaroli, A.; Orlando, G.; Brunetti, L.; Ferrante, C.; Pieretti, S.; et al. Exploring the first Rimonabant analog-opioid peptide hybrid compound, as bivalent ligand for CB1 and opioid receptors. J. Enzym. Inhib. Med. Chem. 2017, 32, 444–451. [Google Scholar] [CrossRef]
- Mollica, A.; Pinnen, F.; Stefanucci, A.; Costante, R. The evolution of peptide synthesis: From early days to small molecular machines. Curr. Bioact. Comp. 2013, 9, 184–202. [Google Scholar] [CrossRef]
- Dvorácskó, S.; Keresztes, A.; Mollica, A.; Stefanucci, A.; Macedonio, G.; Pieretti, S.; Zádor, F.; Walter, F.R.; Deli, M.A.; Kékesi, G.; et al. Preparation of bivalent agonists for targeting the mu opioid and cannabinoid receptors. Eur. J. Med. Chem. 2019, 178, 571–588. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef]
- Wiley, J.L.; Burston, J.J.; Leggett, D.C.; Alekseeva, O.O.; Razdan, R.K.; Mahadevan, A.; Martin, B.R. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br. J. Pharmacol. 2005, 145, 293–300. [Google Scholar] [CrossRef]
- Pieretti, S.; di Giannuario, A.; de Felice, M.; Perretti, M.; Cirino, G. Stimulus-dependent specificity for annexin 1 inhibition of the inflammatory nociceptive response: The involvement of the receptor for formylated peptides. Pain 2004, 109, 52–63. [Google Scholar] [CrossRef]
- Stefanucci, A.; Lei, W.; Pieretti, S.; Novellino, E.; Dimmito, M.P.; Marzoli, F.; Streicher, J.M.; Mollica, A. On resin click-chemistry-mediated synthesis of novel enkephalin analogues with potent anti-nociceptive activity. Sci. Rep. 2019, 9, 5771. [Google Scholar] [CrossRef]
- Maione, F.; Minosi, P.; di Giannuario, A.; Raucci, F.; Chini, M.G.; de Vita, S.; Bifulco, G.; Mascolo, N.; Pieretti, S. Long-Lasting Anti-Inflammatory and Antinociceptive Effects of Acute Ammonium Glycyrrhizinate Administration: Pharmacological, Biochemical, and Docking Studies. Molecules 2019, 24, 2453. [Google Scholar] [CrossRef]
- Pieretti, S.; Dominici, L.; di Giannuario, A.; Cesari, N.; Piaz, V.D. Local anti-inflammatory effect and behavioral studies on new PDE4 inhibitors. Life Sci. 2006, 79, 791–800. [Google Scholar] [CrossRef]
- Niederberger, E.; Schmidtko, A.; Gao, W.; Kühlein, H.; Ehnert, C.; Geisslinger, G. Impaired acute and inflammatory nociception in mice lacking the p50 subunit of NF-kappaB. Eur. J. Pharmacol. 2007, 559, 55–60. [Google Scholar] [CrossRef]
- Curtis, M.J.; Bond, R.A.; Spina, D.; Ahluwalia, A.; Alexander, S.P.A.; Giembycz, M.A.; Gilchrist, A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; et al. Experimental design and analysis and their reporting: New guidance for publication in BJP. Br. J. Pharmacol. 2015, 172, 3461–3471. [Google Scholar] [CrossRef]
- Kumar, K.K.; Shalev-Benami, M.; Robertson, M.J.; Hu, H.; Banister, S.D.; Hollingsworth, S.A.; Latorraca, N.R.; Kato, H.E.; Hilger, D.; Maeda, S.; et al. Structure of a Signaling Cannabinoid Receptor 1-G Protein Complex. Cell 2019, 176, 448–458.e12. [Google Scholar] [CrossRef]
- Schrödinger Release 2019-3: Schrödinger Suite 2019-2 Protein Preparation Wizard; Epik, Schrödinger, LLC: New York, NY, USA; Impact, Schrödinger, LLC: New York, NY, USA; Prime, Schrödinger, LLC: New York, NY, USA, 2019.
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.; Honig, B.; Shaw, D.E.; Friesner, R.A. A hierarchical approach to all-atom protein loop prediction. Proteins Struct. Funct. Bioinform. 2004, 55, 351–367. [Google Scholar] [CrossRef]
- Stefanucci, A.; Novellino, E.; Mirzaie, S.; Macedonio, G.; Pieretti, S.; Minosi, P.; Szűcs, E.; Erdei, A.I.; Zádor, F.; Benyhe, S.; et al. Opioid Receptor Activity and Analgesic Potency of DPDPE Peptide Analogues Containing a Xylene Bridge. ACS Med. Chem. Lett. 2017, 8, 449–454. [Google Scholar] [CrossRef]
- Schrödinger Release 2019-3: Glide; Schrödinger, LLC: New York, NY, USA, 2019.
- Schrödinger Release 2019-3: LigPrep; Schrödinger, LLC: New York, NY, USA, 2019.
- Schrödinger Release 2019-3: Desmond Molecular Dynamics System; D. E. Shaw Research: New York, NY, USA; Maestro-Desmond Interoperability Tools, Schrödinger: New York, NY, USA, 2019.
- Chandrasekhar, J.; Impey, R.W.; Jorgensen, W.L.; Madura, J.D.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926. [Google Scholar]
- Martyna, G.J.; Tobias, D.J.; Klein, M.L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 1994, 101, 4177–4189. [Google Scholar] [CrossRef]
- Hünenberger, P.H. Thermostat Algorithms for Molecular Dynamics Simulations. Adv. Polym. Sci. 2005, 173, 105–149. [Google Scholar]
- Matsubara, K.; Kagawa, M.; Fukui, Y. In vivo and in vitro studies on cocaine metabolism: Ecgonine methyl ester as a major metabolite of cocaine. Forensic Sci. Int. 1984, 26, 169–180. [Google Scholar] [CrossRef]
- Slomski, A. THC for Chronic Pain. JAMA 2018, 320, 1631. [Google Scholar] [CrossRef]
- Kaneko, S. Motor vehicle collisions caused by the ‘super-strength’ synthetic cannabinoids, MAM-2201, 5F-PB-22, 5F-AB-PINACA, 5F-AMB and 5F-ADB in Japan experienced from 2012 to 2014. Forensic Toxicol. 2017, 35, 244–251. [Google Scholar] [CrossRef]
- Castaneto, M.S.; Gorelick, D.A.; Desrosiers, N.A.; Hartman, R.L.; Pirard, S.; Huestis, M.A. Synthetic cannabinoids: Epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014, 144, 12–41. [Google Scholar] [CrossRef]
- Deng, H.; Verrico, C.D.; Kosten, T.R.; Nielsen, D.A. Psychosis and synthetic cannabinoids. Psychiatry Res. 2018, 268, 400–412. [Google Scholar] [CrossRef]
- Abouchedid, R.; Ho, J.H.; Hudson, S.; Dines, A.; Archer, J.R.H.; Wood, D.M.; Dargan, P.I. Acute Toxicity Associated with Use of 5F-Derivations of Synthetic Cannabinoid Receptor Agonists with Analytical Confirmation. J. Med. Toxicol. 2016, 12, 396–401. [Google Scholar] [CrossRef]
- Langford, A.M.; Bolton, J.R. Synthetic cannabinoids: Variety is definitely not the spice of life. J. Forensic Leg. Med. 2018, 59, 36–38. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. EMCDDA–Europol Joint Report on a New Psychoactive Substance: 1-(4-Cyanobutyl)-N-(2-Phenylpropan-2-yl)Indazole-3-Carboxamide (CUMYL-4CN-BINACA); Joint Reports; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar]
- Van Hout, M.C.; Benschop, A.; Bujalski, M.; Dabrowska, K.; Demetrovics, Z.; Felvinczi, K.; Herne, E.; Henriques, S.; Kaló, Z.; Kamphausen, G.; et al. Health and social problems associated with recent novel psychoactive substance (NPS) use amongst marginalised, nightlife and online users in six European countries. Int. J. Ment. Health Addict. 2018, 16, 480–495. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. Report on the Risk Assessment of 1-(4-Cyanobutyl)-N-(2-Phenylpropan-2-yl)-1H-Indazole-3-Carboxamide (CUMYL-4CN-BINACA) in the Framework of the Council Decision on New Psychoactive Substances; Risk Assessments; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: Temporary placement of six synthetic cannabinoids (5F-ADB, 5F-AMB, 5F-APINACA, ADB-FUBINACA, MDMB-CHMICA and MDMB-FUBINACA) into Schedule I. Fed. Regist. 2017, 82, 17119–17124. [Google Scholar]
- Michel, J.; Tirado-Rives, J.; Jorgensen, W.L. Prediction of the Water Content in Protein Binding Sites. J. Phys. Chem. B 2009, 113, 13337–13346. [Google Scholar] [CrossRef]
- Huffman, J.W.; Dai, D.; Martin, B.R.; Compton, D.R. Design, Synthesis and Pharmacology of Cannabimimetic Indoles. Bioorganic Med. Chem. Lett. 1994, 4, 563–566. [Google Scholar] [CrossRef]
- Huffman, J.W.; Zengin, G.; Wu, M.J.; Lu, J.; Hynd, G.; Bushell, K.; Thompson, A.L.; Bushell, S.; Tartal, C.; Hurst, D.P.; et al. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: Steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorg. Med. Chem. 2005, 13, 89–112. [Google Scholar] [CrossRef]


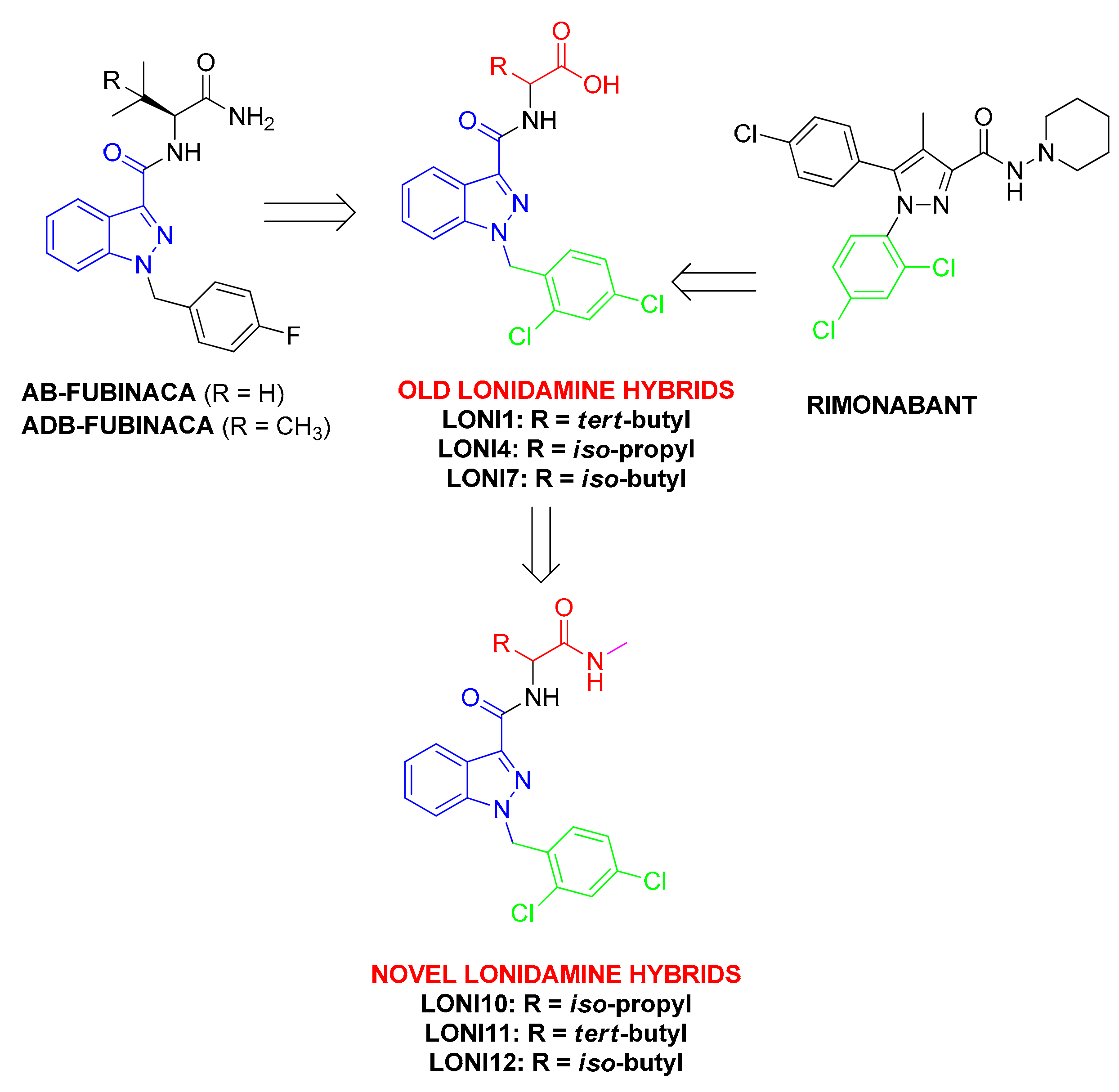








| Compounds | Sequence | Ki (nM) | [35S]GTPγS Binding | |
|---|---|---|---|---|
| Emax (%) | EC50 (nM) | |||
| WIN55,212-2 | 10 ± 1 | 173 ± 11 | 56 ± 3.8 | |
| JWH-018 [33] | 3.5 ± 1 | 163 ± 5.2 | 16 ± 3 | |
| LONI1 [33] | Lonidamine-tert-Leu-OH | 0.08 * | 84 ± 6.6 | >1 μM |
| LONI2 [33] | Lonidamine-tert-Leu-OCH3 | 3.1 * | 143 ± 5.7 | 8.4 |
| LONI3 [33] | Lonidamine-tert-Leu-NH2 | 17 * | 139 ± 4.5 | 126 |
| LONI4 [33] | Lonidamine-Val-OH | 2.6 * | 82 ± 10.6 | >1 μM |
| LONI10 | Lonidamine-Val-NHMe | 84 ± 3.4 | 100 ± 1.7 | n.r. |
| LONI11 | Lonidamine-tert-Leu-NHMe | 11 ± 1.2 | 151 ± 3 | 200 ± 13 |
| LONI12 | Lonidamine-Leu-NHMe | 320 ± 16 | 101 ± 1 | n.r. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimmito, M.P.; Stefanucci, A.; Pieretti, S.; Minosi, P.; Dvorácskó, S.; Tömböly, C.; Zengin, G.; Mollica, A. Discovery of Orexant and Anorexant Agents with Indazole Scaffold Endowed with Peripheral Antiedema Activity. Biomolecules 2019, 9, 492. https://doi.org/10.3390/biom9090492
Dimmito MP, Stefanucci A, Pieretti S, Minosi P, Dvorácskó S, Tömböly C, Zengin G, Mollica A. Discovery of Orexant and Anorexant Agents with Indazole Scaffold Endowed with Peripheral Antiedema Activity. Biomolecules. 2019; 9(9):492. https://doi.org/10.3390/biom9090492
Chicago/Turabian StyleDimmito, Marilisa P., Azzurra Stefanucci, Stefano Pieretti, Paola Minosi, Szabolcs Dvorácskó, Csaba Tömböly, Gokhan Zengin, and Adriano Mollica. 2019. "Discovery of Orexant and Anorexant Agents with Indazole Scaffold Endowed with Peripheral Antiedema Activity" Biomolecules 9, no. 9: 492. https://doi.org/10.3390/biom9090492
APA StyleDimmito, M. P., Stefanucci, A., Pieretti, S., Minosi, P., Dvorácskó, S., Tömböly, C., Zengin, G., & Mollica, A. (2019). Discovery of Orexant and Anorexant Agents with Indazole Scaffold Endowed with Peripheral Antiedema Activity. Biomolecules, 9(9), 492. https://doi.org/10.3390/biom9090492