Quercetin Interrupts the Positive Feedback Loop Between STAT3 and IL-6, Promotes Autophagy, and Reduces ROS, Preventing EBV-Driven B Cell Immortalization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Virus Preparation
2.3. Cell Viability
2.4. Antibodies
2.5. Western Blot Analysis
2.6. Endogenous Reactive Oxygen Species Detection
2.7. ELISA Assay
2.8. Densitometric Analysis
2.9. Statistical Analysis
3. Results
3.1. Quercetin Counteracts B Cell Immortalization into LCLs Driven by EBV
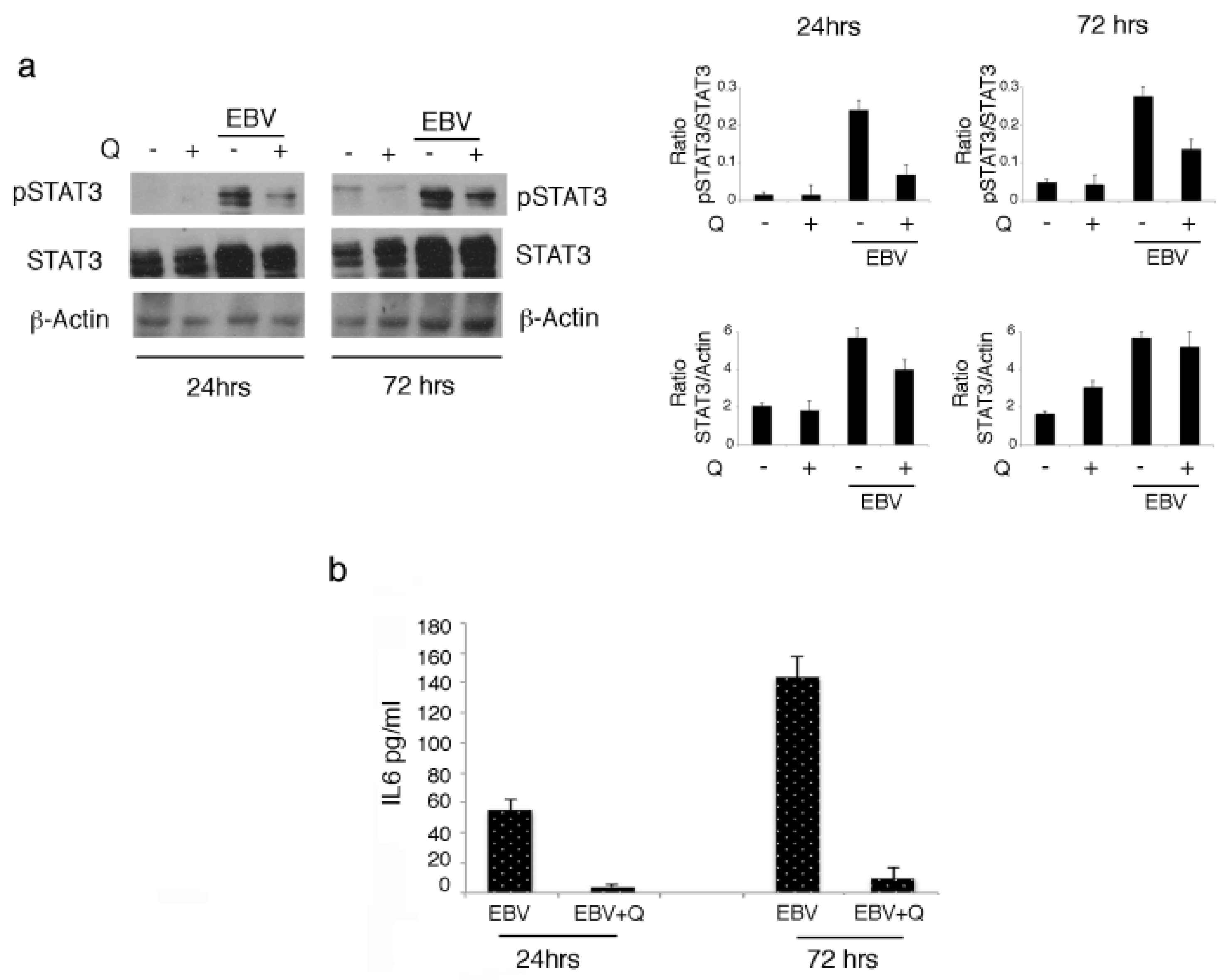
3.2. Quercetin Interrupts the Positive Feedback Loop Between STAT3 and IL-6 in EBV-Infected B Lymphocytes
3.3. Quercetin Restores the Autophagic Flux Blocked by EBV Infection
3.4. Quercetin Counteracts ROS Increase But Does Not Affect EBV’s BRLF1 and EBNA1 Expression in EBV-Infected B Cells
4. Discussion
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Kamranvar, S.A.; Masucci, M.G. Oxidative Stress Enables Epstein-Barr Virus-Induced B-Cell Transformation by Posttranscriptional Regulation of Viral and Cellular Growth-Promoting Factors. Oncogene 2016, 35, 3807–3816. [Google Scholar] [CrossRef] [PubMed]
- Guasparri, I.; Bubman, D.; Cesarman, E. EBV LMP2a Affects LMP1-Mediated NF-κB Signaling and Survival of Lymphoma Cells by Regulating Traf2 Expression. Blood 2008, 111, 3813–3820. [Google Scholar] [CrossRef] [PubMed]
- Krams, S.M.; Martinez, O.M. Epstein-Barr Virus, Rapamycin, and Host Immune Responses. Curr. Opin. Organ. Transpl. 2008, 13, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bhaduri-Mcintosh, S. A Central Role for STAT3 in Gammaherpesvirus-Life Cycle and -Diseases. Front. Microbiol. 2016, 7, 1052. [Google Scholar] [CrossRef] [PubMed]
- Koganti, S.; Hui-Yuen, J.; Mcallister, S.; Gardner, B.; Grasser, F.; Palendira, U.; Tangye, S.G.; Freeman, A.F.; Bhaduri-Mcintosh, S. STAT3 Interrupts ATR-Chk1 Signaling to Allow Oncovirus-Mediated Cell Proliferation. Proc. Natl. Acad. Sci. USA 2014, 111, 4946–4951. [Google Scholar] [CrossRef]
- Koganti, S.; De La Paz, A.; Freeman, A.F.; Bhaduri-Mcintosh, S. B Lymphocytes from Patients with a Hypomorphic Mutation in STAT3 Resist Epstein-Barr Virus-Driven Cell Proliferation. J. Virol. 2014, 88, 516–524. [Google Scholar] [CrossRef]
- Yokoi, T.; Miyawaki, T.; Yachie, A.; Kato, K.; Kasahara, Y.; Taniguchi, N. Epstein-Barr Virus-Immortalized B Cells Produce IL-6 as an Autocrine Growth Factor. Immunology 1990, 70, 100–105. [Google Scholar]
- Hirano, T.; Ishihara, K.; Hibi, M. Roles of STAT3 In Mediating the Cell Growth, Differentiation and Survival Signals Relayed Through The IL-6 Family of Cytokine Receptors. Oncogene 2000, 19, 2548–2556. [Google Scholar] [CrossRef]
- Granato, M.; Rizzello, C.; Gilardini Montani, M.S.; Cuomo, L.; Vitillo, M.; Santarelli, R.; Gonnella, R.; D’orazi, G.; Faggioni, A.; Cirone, M. Quercetin Induces Apoptosis and Autophagy in Primary Effusion Lymphoma Cells By Inhibiting PI3K/Akt/mTOR And STAT3 Signaling Pathways. J. Nutr. Biochem. 2017, 41, 124–136. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Karin, M. Dangerous Liaisons: STAT3 and NF-κB Collaboration and Crosstalk In Cancer. Cytokine Growth Factor Rev. 2010, 21, 11–19. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Zitvogel, L. STAT3 Inhibition for Cancer Therapy: Cell-Autonomous Effects Only? Oncoimmunology 2016, 5, E1126063. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, R.; Gonnella, R.; Di Giovenale, G.; Cuomo, L.; Capobianchi, A.; Granato, M.; Gentile, G.; Faggioni, A.; Cirone, M. Stat3 Activation By Kshv Correlates With IL-10, IL-6 And IL-23 Release and an Autophagic Block in Dendritic Cells. Sci. Rep. 2014, 4, 4241. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Zhou, Z.; He, J.; Yan, C.; Ding, S. IL-6 Inhibits Starvation-Induced Autophagy Via the STAT3/Bcl-2 Signaling Pathway. Sci. Rep. 2015, 5, 15701. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for The Use and Interpretation of Assays for Monitoring Autophagy (3rd Edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [PubMed]
- Cirone, M.; Gilardini Montani, M.S.; Granato, M.; Garufi, A.; Faggioni, A.; D’orazi, G. Autophagy Manipulation as a Strategy for Efficient Anticancer Therapies: Possible Consequences. J. Exp. Clin. Cancer Res. 2019, 38, 262. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Sugden, B. The Latent Membrane Protein 1 Oncogene Modifies B-Cell Physiology by Regulating Autophagy. Oncogene 2008, 27, 2833–2842. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Bose, P.; Patel, K.; Roy, S.G.; Gain, C.; Gowda, H.; Robertson, E.S.; Saha, A. Transcriptional and Epigenetic Modulation of Autophagy Promotes Ebv Oncoprotein EBNA 3c Induced B-Cell Survival. Cell Death Dis. 2018, 9, 605. [Google Scholar] [CrossRef]
- Granato, M.; Santarelli, R.; Farina, A.; Gonnella, R.; Lotti, L.V.; Faggioni, A.; Cirone, M. Epstein-Barr Virus Blocks the Autophagic Flux And Appropriates the Autophagic Machinery to Enhance Viral Replication. J. Virol. 2014, 88, 12715–12726. [Google Scholar] [CrossRef]
- Cirone, M. EBV and Kshv Infection Dysregulates Autophagy to Optimize Viral Replication, Prevent Immune Recognition And Promote Tumorigenesis. Viruses 2018, 10, 599. [Google Scholar] [CrossRef]
- Kumari, S.; Badana, A.K.; Gavara, M.M.; Gugalavath, S.; Malla, R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.; Rizzello, C.; Romeo, M.A.; Yadav, S.; Santarelli, R.; D’orazi, G.; Faggioni, A.; Cirone, M. Concomitant Reduction of C-Myc Expression and PI3K/Akt/mTOR Signaling by Quercetin Induces A Strong Cytotoxic Effect Against Burkitt’s Lymphoma. Int. J. Biochem. Cell Biol. 2016, 79, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Borboni, P.; Di Cola, G.; Sesti, G.; Marini, M.A.; Del Porto, P.; Gilardini Montani, M.S.; Lauro, R.; De Pirro, R. Beta-Endorphin Receptors on Cultured and Freshly Isolated Lymphocytes from Normal Subjects. Biochem. Biophys. Res. Commun. 1989, 163, 642–648. [Google Scholar] [CrossRef]
- Miller, G.; Robinson, J.; Heston, L.; Lipman, M. Differences Between Laboratory Strains of Epstein-Barr Virus Based on Immortalization, Abortive Infection and Interference. IARC Sci. Publ. 1975, 11 Pt 1, 395–408. [Google Scholar] [CrossRef]
- Granato, M.; Feederle, R.; Farina, A.; Gonnella, R.; Santarelli, R.; Hub, B.; Faggioni, A.; Delecluse, H.J. Deletion of Epstein-Barr Virus BFLF2 Leads to Impaired Viral DNA Packaging and Primary Egress as Well as to the Production of Defective Viral Particles. J. Virol. 2008, 82, 4042–4051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granato, M.; Gilardini Montani, M.S.; Santarelli, R.; D’orazi, G.; Faggioni, A.; Cirone, M. Apigenin, By Activating p53 And Inhibiting STAT3, Modulates the Balance Between Pro-Apoptotic and Pro-Survival Pathways to Induce Pel Cell Death. J. Exp. Clin. Cancer Res. 2017, 36, 167. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Gao, Y.; Wang, W.; Cheng, Y.; Lu, P.; Ma, C.; Zhang, Y. Optimization of The Extraction of Polysaccharides from Tobacco Waste and Their Biological Activities. Int. J. Biol. Macromol. 2016, 91, 188–197. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, E47. [Google Scholar] [CrossRef]
- Garufi, A.; Pistritto, G.; Cirone, M.; D’orazi, G. Reactivation of Mutant p53 By Capsaicin, The Major Constituent of Peppers. J. Exp. Clin. Cancer Res. 2016, 35, 136. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer Potential of Quercetin: A Comprehensive Review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Son, M.; Ryu, E.; Shin, Y.S.; Kim, J.G.; Kang, B.W.; Cho, H.; Kang, H. Quercetin-Induced Apoptosis Prevents EBV Infection. Oncotarget 2015, 6, 12603–12624. [Google Scholar] [CrossRef]
- Kim, H.J.; Ko, Y.H.; Kim, J.E.; Lee, S.S.; Lee, H.; Park, G.; Paik, J.H.; Cha, H.J.; Choi, Y.D.; Han, J.H.; et al. Epstein-Barr Virus-Associated Lymphoproliferative Disorders: Review and Update On 2016 Who Classification. J. Pathol. Transl. Med. 2017, 51, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Mcfadden, K.; Hafez, A.Y.; Kishton, R.; Messinger, J.E.; Nikitin, P.A.; Rathmell, J.C.; Luftig, M.A. Metabolic Stress Is A Barrier to Epstein-Barr Virus-Mediated B-Cell Immortalization. Proc. Natl. Acad. Sci. USA 2016, 113, E782–E790. [Google Scholar] [CrossRef] [PubMed]
- Moscat, J.; Diaz-Meco, M.T.; Wooten, M.W. Signal Integration and Diversification Through the p62 Scaffold Protein. Trends Biochem. Sci. 2007, 32, 95–100. [Google Scholar] [CrossRef]
- Mathew, R.; Karp, C.M.; Beaudoin, B.; Vuong, N.; Chen, G.; Chen, H.Y.; Bray, K.; Reddy, A.; Bhanot, G.; Gelinas, C.; et al. Autophagy Suppresses Tumorigenesis Through Elimination of p62. Cell 2009, 137, 1062–1075. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, D.; Dallaglio, K.; Torquato, P.; Piroddi, M.; Galli, F. NRF2-p62 Autophagy Pathway and Its Response to Oxidative Stress in Hepatocellular Carcinoma. Transl. Res. J. Lab. Clin. Med. 2018, 193, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Howell, M.E.A.; Sparks-Wallace, A.; Hawkins, C.; Nicksic, C.A.; Kohne, C.; Hall, K.H.; Moorman, J.P.; Yao, Z.Q.; Ning, S. p62-Mediated Selective Autophagy Endows Virus-Transformed Cells with Insusceptibility to DNA Damage Under Oxidative Stress. PLoS Pathog. 2019, 15, e1007541. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granato, M.; Gilardini Montani, M.S.; Zompetta, C.; Santarelli, R.; Gonnella, R.; Romeo, M.A.; D’Orazi, G.; Faggioni, A.; Cirone, M. Quercetin Interrupts the Positive Feedback Loop Between STAT3 and IL-6, Promotes Autophagy, and Reduces ROS, Preventing EBV-Driven B Cell Immortalization. Biomolecules 2019, 9, 482. https://doi.org/10.3390/biom9090482
Granato M, Gilardini Montani MS, Zompetta C, Santarelli R, Gonnella R, Romeo MA, D’Orazi G, Faggioni A, Cirone M. Quercetin Interrupts the Positive Feedback Loop Between STAT3 and IL-6, Promotes Autophagy, and Reduces ROS, Preventing EBV-Driven B Cell Immortalization. Biomolecules. 2019; 9(9):482. https://doi.org/10.3390/biom9090482
Chicago/Turabian StyleGranato, Marisa, Maria Saveria Gilardini Montani, Claudia Zompetta, Roberta Santarelli, Roberta Gonnella, Maria Anele Romeo, Gabriella D’Orazi, Alberto Faggioni, and Mara Cirone. 2019. "Quercetin Interrupts the Positive Feedback Loop Between STAT3 and IL-6, Promotes Autophagy, and Reduces ROS, Preventing EBV-Driven B Cell Immortalization" Biomolecules 9, no. 9: 482. https://doi.org/10.3390/biom9090482
APA StyleGranato, M., Gilardini Montani, M. S., Zompetta, C., Santarelli, R., Gonnella, R., Romeo, M. A., D’Orazi, G., Faggioni, A., & Cirone, M. (2019). Quercetin Interrupts the Positive Feedback Loop Between STAT3 and IL-6, Promotes Autophagy, and Reduces ROS, Preventing EBV-Driven B Cell Immortalization. Biomolecules, 9(9), 482. https://doi.org/10.3390/biom9090482





