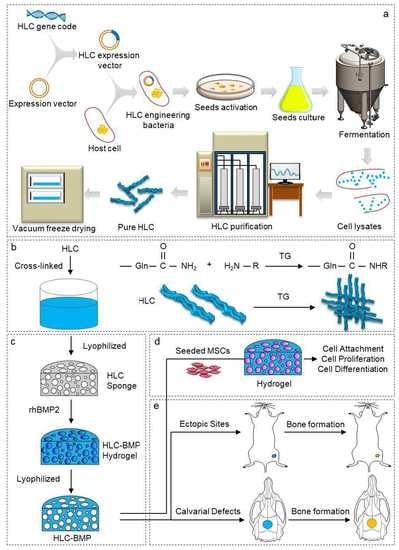
Newly Designed Human-Like Collagen to Maximize Sensitive Release of BMP-2 for Remarkable Repairing of Bone Defects
Abstract
1. Introduction
2. Materials and Methods
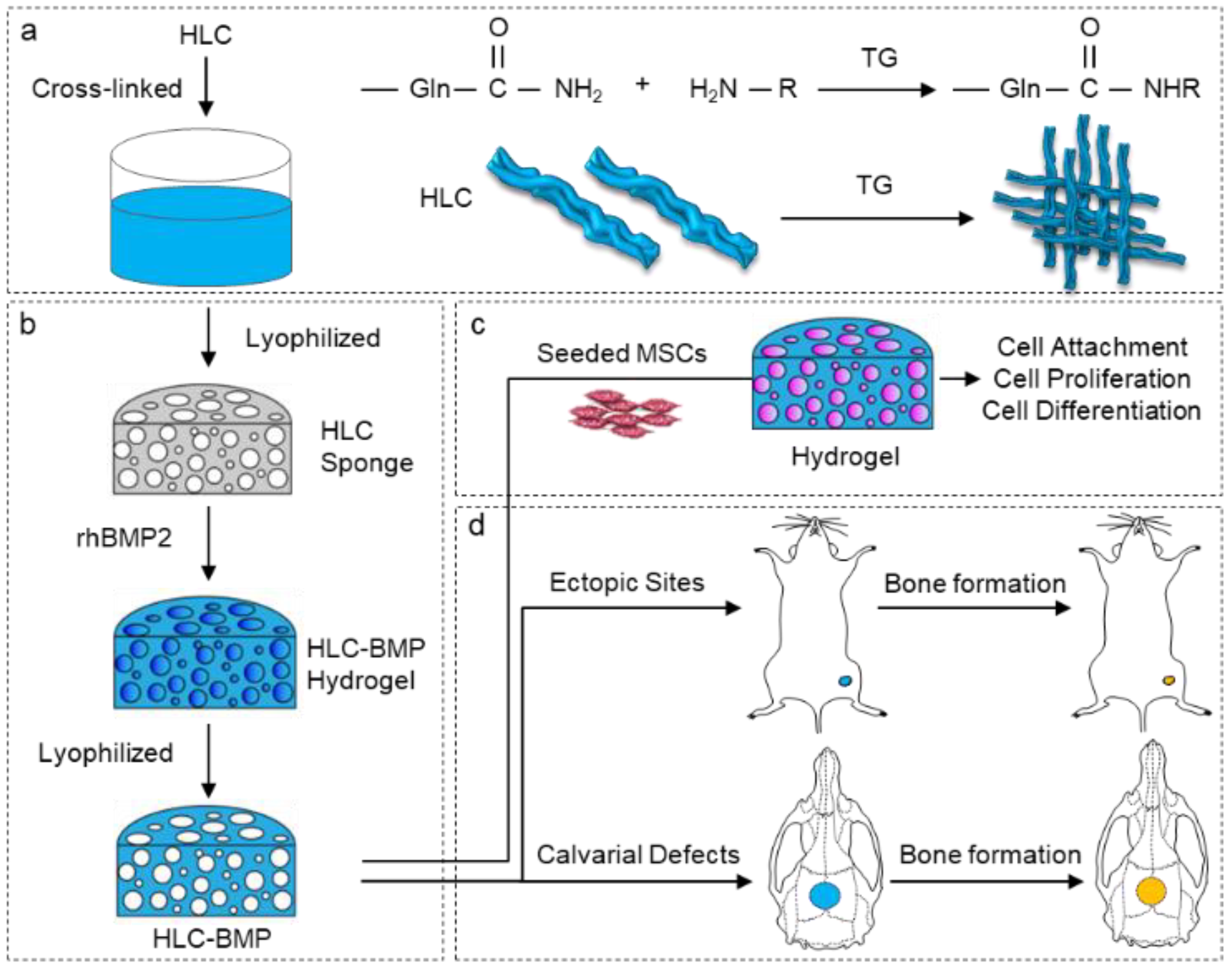
2.1. Fabrication of HLC-BMP2 Sponge
2.2. Morphology Characterization of Cross-Linked HLC Sponge
2.3. Biomechanical Evaluation of HLC/HLC-BMP Sponges
2.4. Kinetics of Releasing rhBMP-2 from the HLC-BMP Hydrogel
2.5. In Vitro Studies
2.5.1. Isolation and Culture of Rat MSCs
2.5.2. MSCs Attachment and Proliferation in the HLC/HLC-BMP Sponges
2.5.3. Osteoblast Differentiation Gene Levels in the MSCs Cultured in the HLC/HLC-BMP Sponges
2.5.4. Osteo-Related Protein Expression in the HLC/HLC-BMP Sponges Cultured with MSCs
2.6. Ectopic Bone Regeneration of HLC/HLC-BMP Sponges
2.6.1. Animals and Surgery
2.6.2. Histology of HLC/HLC-BMP Implant
2.7. Rat Cranial Defect Repair Model
2.7.1. Animals and Surgery
2.7.2. Micro-CT Evaluation of Cranial Defect Repairing Using HLC/HLC-BMP Sponges
2.7.3. Histological and Immune-Histological Analysis of Cranial Defect Repairing Using HLC/HLC-BMP Sponges
2.8. Statistical Analysis
3. Results
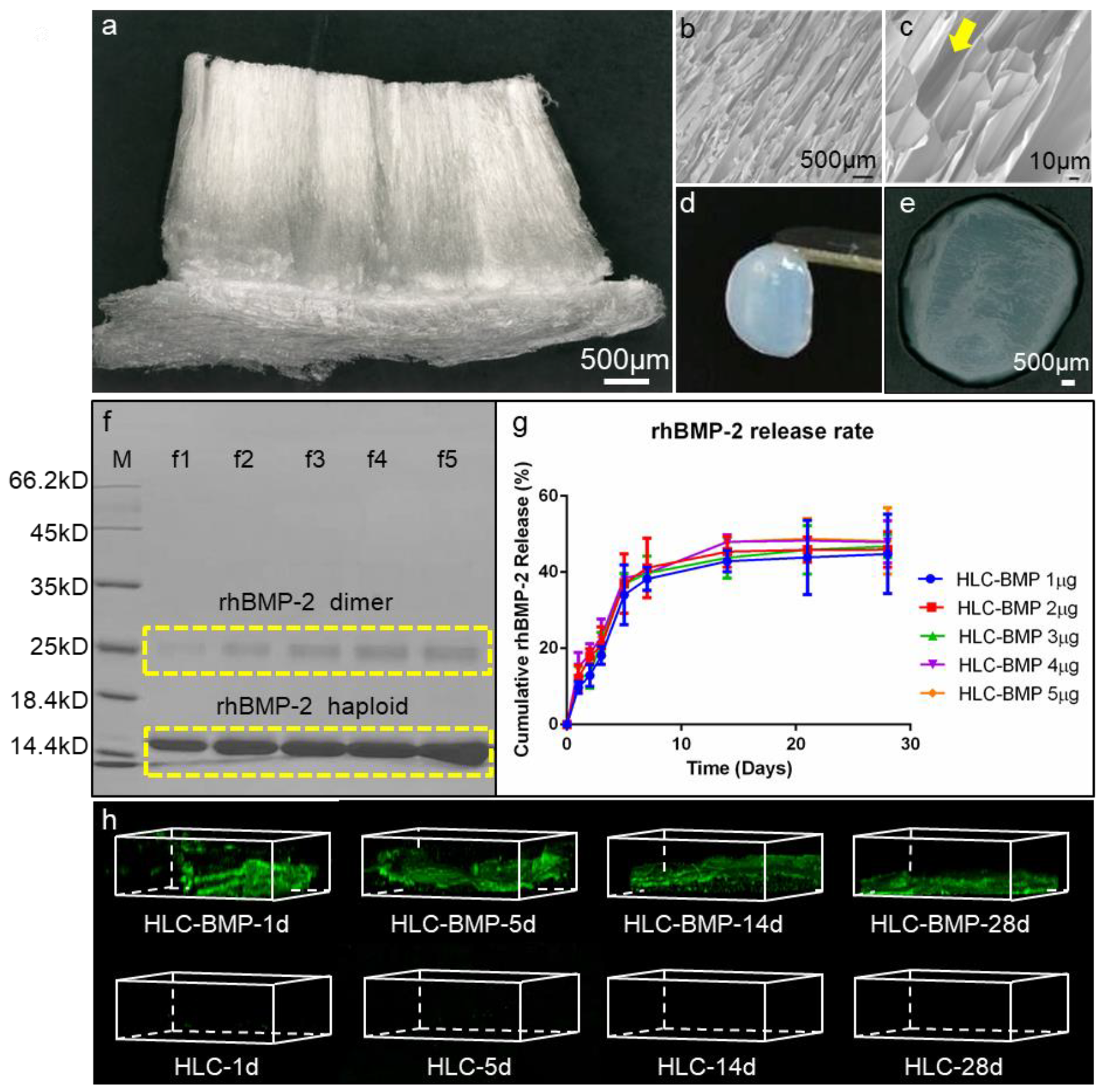
3.1. Morphology and Mechanical Properties of the HLC/HLC-BMP, and rhBMP-2 Release Kinetics
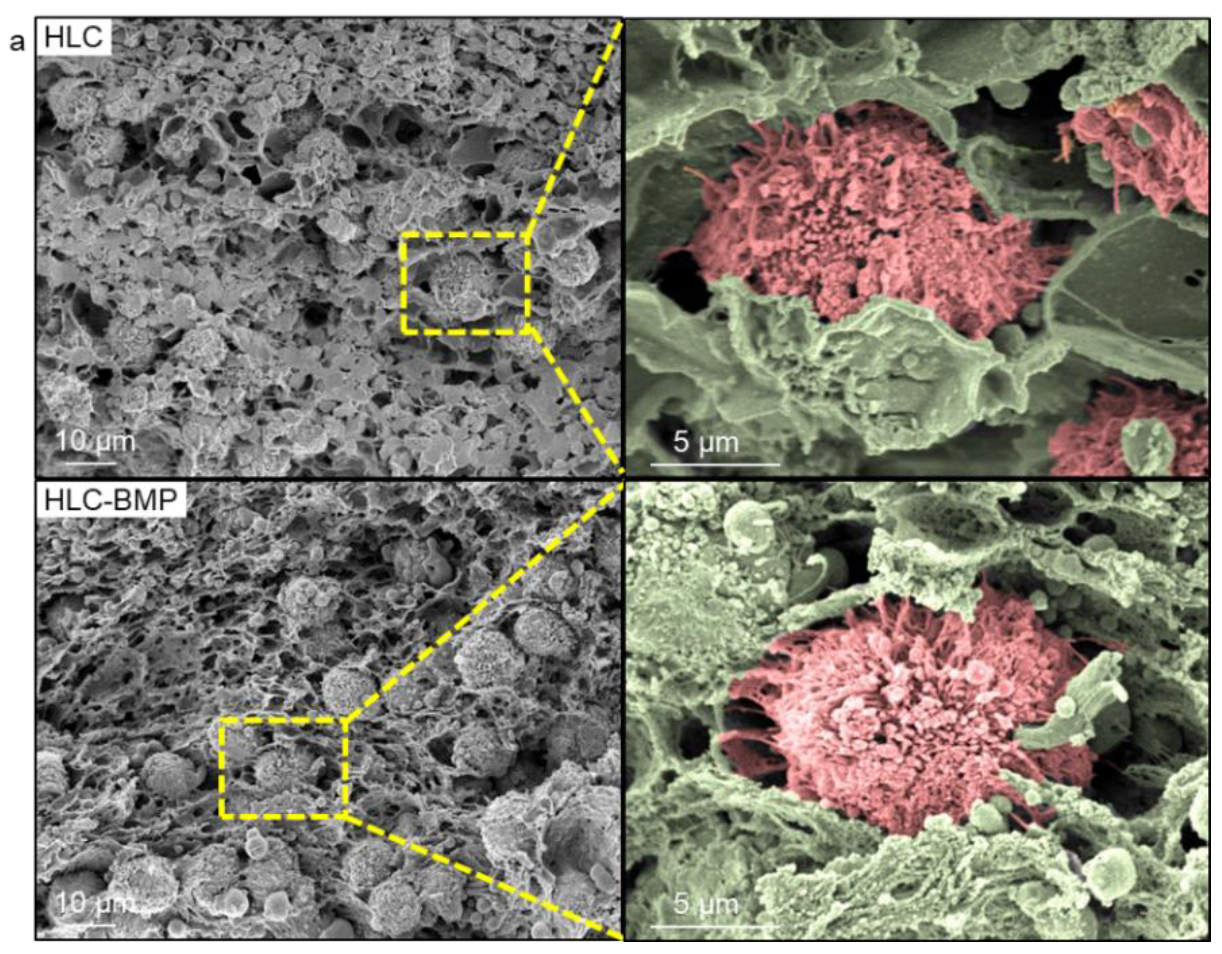
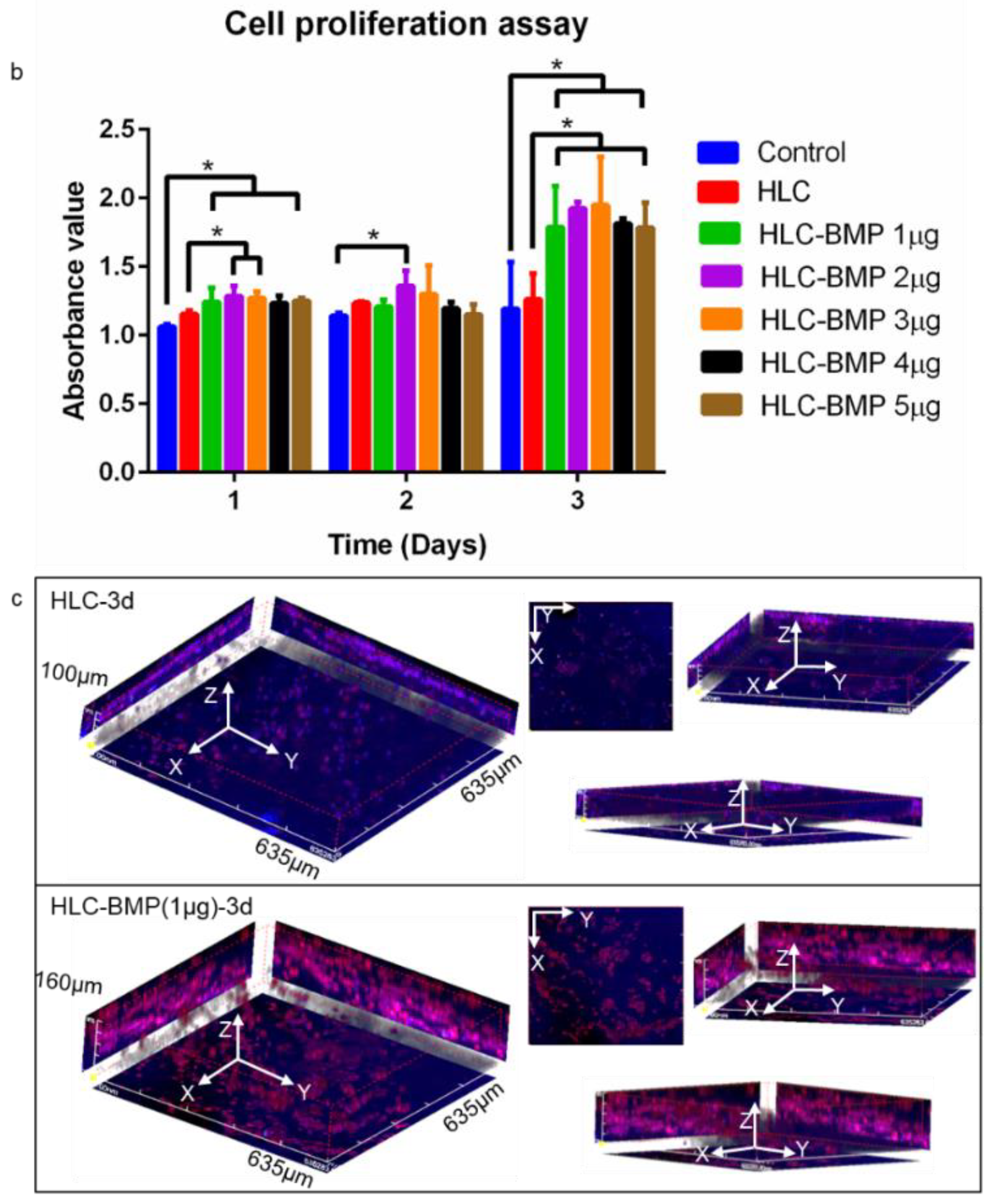
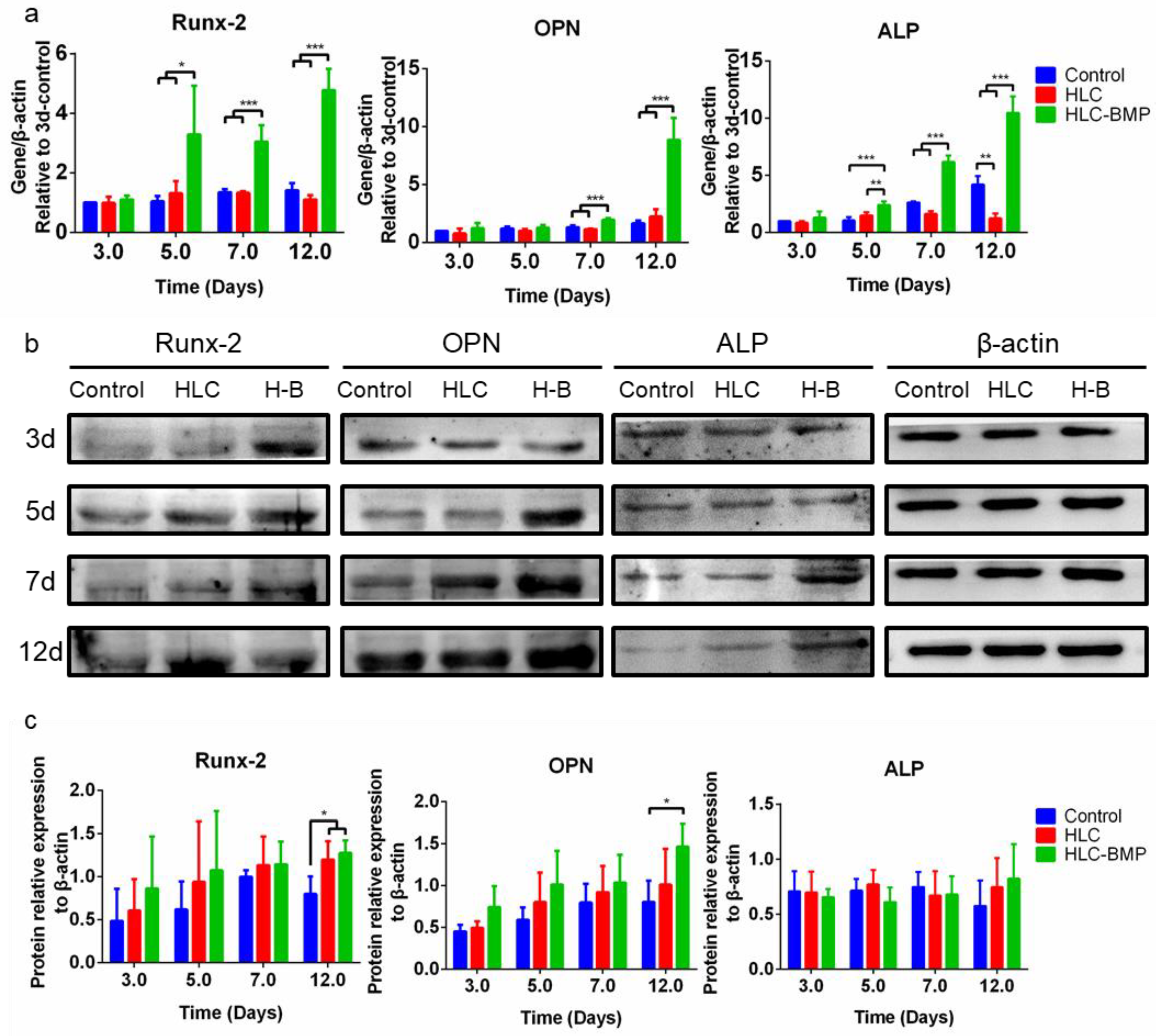
3.2. MSCs Attachment, Proliferation and Differentiation in the HLC/HLC-BMP Sponges
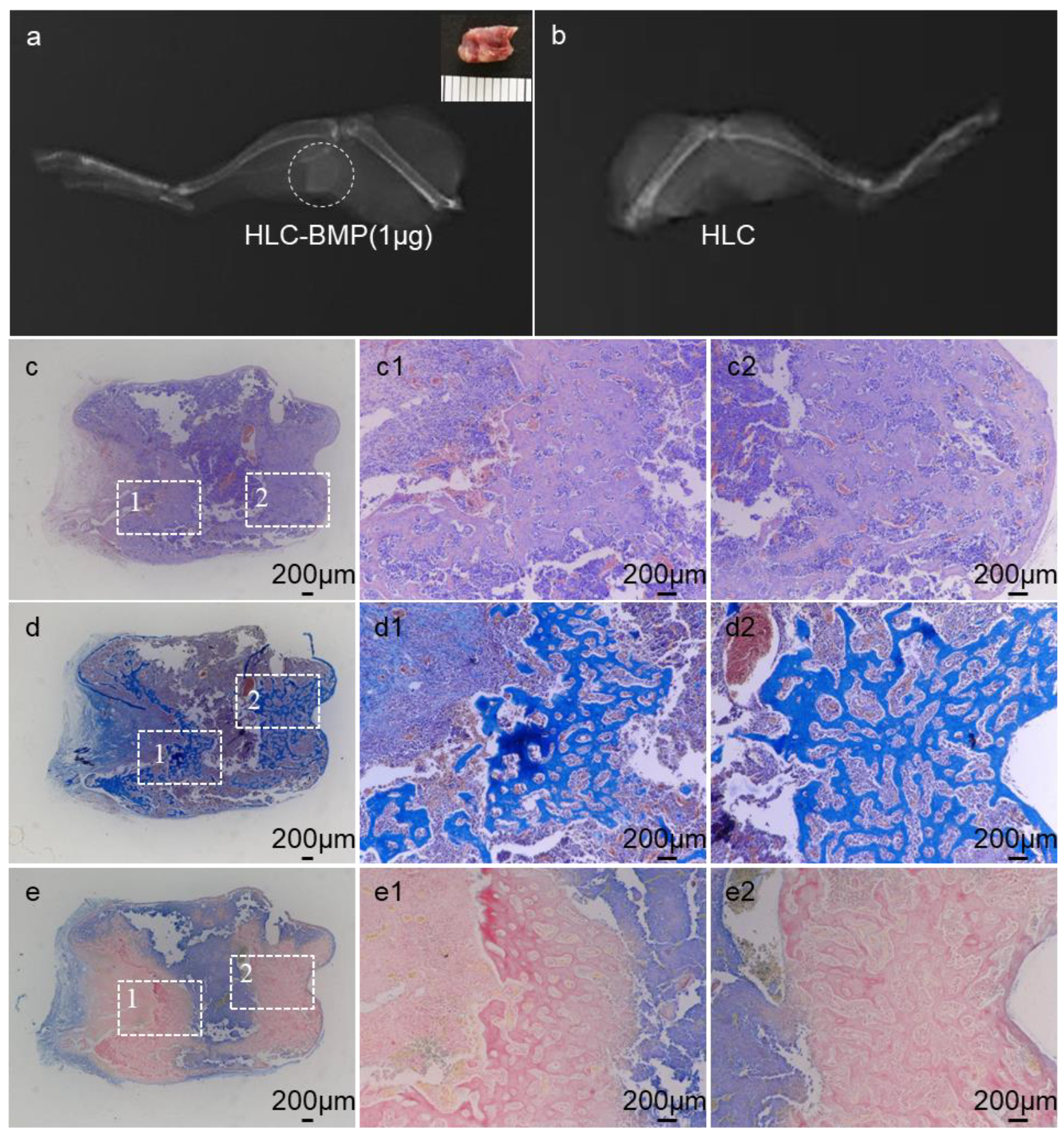
3.3. Ectopic Bone Formation by HLC/HLC-BMP Sponges
3.4. Osteogenesis In Situ of HLC-BMP Sponge in Rat Cranial Defect Repair Model
3.5. Distinguish the Effects of the Different Dose of BMP-2 Release from the HLC-BMP Sponge in Rat Cranial Defect Repair Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Poon, B.; Kha, T.; Tran, S.; Dass, C.R. Bone Morphogenetic Protein-2 and Bone Therapy: Successes and Pitfalls. J. Pharm. Pharmacol. 2016, 68, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Bougioukli, S.; Jain, A.; Sugiyama, O.; Tinsley, B.A.; Tang, A.H.; Tan, M.H.; Adams, D.J.; Kostenuik, P.J.; Lieberman, J.R. Combination Therapy with BMP-2 and a Systemic RANKL Inhibitor Enhances Bone Healing in a Mouse Critical-Sized Femoral Defect. Bone 2016, 84, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP Signalling in Skeletal Development, Disease and Repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.K.; Jung, U.W.; Thoma, D.S.; Hämmerle, C.; Jung, R.E. Osteogenic Efficacy of BMP-2 Mixed with Hydrogel and Bone Substitute in Peri-Implant Dehiscence Defects in Dogs: 16 Weeks of Healing. Clin. Oral Implant. Res. 2018, 29, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.; Sass, F.A.; Duda, G.N.; Schmidt-Bleek, K. A Review of Biomaterials in Bone Defect Healing, Remaining Shortcomings and Future Opportunities for Bone Tissue Engineering: The Unsolved Challenge. Bone Jt. Res. 2018, 7, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, G.S.; Roffi, A.; Reale, D.; Kon, E.; Filardo, G. Clinical Application of Bone Morphogenetic Proteins for Bone Healing: A Systematic Review. Int. Orthop. 2017, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Begam, H.; Nandi, S.K.; Kundu, B.; Chanda, A. Strategies for Delivering Bone Morphogenetic Protein for Bone Healing. Mater. Sci. Eng. C 2017, 70, 856–869. [Google Scholar] [CrossRef]
- Brigaud, I.; Agniel, R.; Leroy-Dudal, J.; Kellouche, S.; Ponche, A.; Bouceba, T.; Mihailescu, N.; Sopronyi, M.; Viguier, E.; Ristoscu, C.; et al. Synergistic Effects of BMP-2, BMP-6 Or BMP-7 with Human Plasma Fibronectin Onto Hydroxyapatite Coatings: A Comparative Study. Acta Biomater. 2017, 55, 481–492. [Google Scholar] [CrossRef]
- Lee, G.H.; Makkar, P.; Paul, K.; Lee, B. Development of BMP-2 Immobilized Polydopamine Mediated Multichannelled Biphasic Calcium Phosphate Granules for Improved Bone Regeneration. Mater. Lett. 2017, 208, 122–125. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Zhang, Z.; Feng, J.T.; Guo, Y.Y.; Yu, Y.; Cui, J.H.; Li, H.M.; Shang, L.J. The Influence of Mussel-Derived Bioactive BMP-2 Decorated PLA on MSCs Behavior in Vitro and Verification with Osteogenicity at Ectopic Sites in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 11961–11971. [Google Scholar] [CrossRef]
- Kirby, G.T.S.; White, L.J.; Rahman, C.V.; Cox, H.C.; Qutachi, O.; Rose, F.R.A.J.; Hutmacher, D.W.; Shakesheff, K.M.; Woodruff, M.A. PLGA-Based Microparticles for the Sustained Release of BMP-2. Polymers 2011, 3, 571–586. [Google Scholar] [CrossRef]
- Lee, S.S.; Huang, B.J.; Kaltz, S.R.; Sur, S.; Newcomb, C.J.; Stock, S.R.; Shah, R.N.; Stupp, S.I. Bone Regeneration with Low Dose BMP-2 Amplified by Biomimetic Supramolecular Nanofibers within Collagen Scaffolds. Biomaterials 2013, 34, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Kirker-Head, C.; Karageorgiou, V.; Hofmann, S.; Fajardo, R.; Betz, O.; Merkle, H.P.; Hilbe, M.; Rechenberg, B.V.; Mccool, J.; Abrahamsen, L. BMP-silk Composite Matrices Heal Critically Sized Femoral Defects. Bone 2007, 41, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Priddy, L.B.; Chaudhuri, O.; Stevens, H.Y.; Krishnan, L.; Uhrig, B.A.; Willett, N.J.; Guldberg, R.E. Oxidized Alginate Hydrogels for BMP-2 Delivery in Long Bone Defects. Acta Biomater. 2014, 10, 4390–4399. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.; Yu, X.; Liu, J.; Wu, J.; Wan, Y. Chitosan-Based Hydrogels Embedded with Hyaluronic Acid Complex Nanoparticles for Controlled Delivery of Bone Morphogenetic Protein-2. Pharmaceutics 2019, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, L.; Fu, C.; Yang, F.; Jiao, Z.; Shi, X.; Ito, Y.; Wang, Z.; Liu, Q.; Zhang, P. Micro-Porous Polyetheretherketone Implants Decorated with BMP-2 Via Phosphorylated Gelatin Coating for Enhancing Cell Adhesion and Osteogenic Differentiation. Colloids Surf. B Biointerfaces 2018, 169, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Hokugo, A.; Takahashi, Y.; Nakano, T.; Hiraoka, M.; Tabata, Y. Combination of BMP-2-releasing gelatin/β-TCP Sponges with Autologous Bone Marrow for Bone Regeneration of X-ray-irradiated Rabbit Ulnar Defects. Biomaterials 2015, 56, 18–25. [Google Scholar] [CrossRef]
- Jo, J.Y.; Jeong, S.I.; Shin, Y.M.; Kang, S.S.; Kim, S.E.; Jeong, C.M.; Huh, J.B. Sequential Delivery of BMP-2 and BMP-7 for Bone Regeneration Using a Heparinized Collagen Membrane. Int. J. Oral Maxillofac. Surg. 2015, 44, 921–928. [Google Scholar] [CrossRef]
- Zétola, A.; Ferreira, F.M.; Larson, R.; Shibli, J.A. Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) in the Treatment of Mandibular Sequelae after Tumor Resection. Oral Maxillofac. Surg. 2011, 15, 169–174. [Google Scholar] [CrossRef]
- Rasi Ghaemi, S.; Delalat, B.; Cetó, X.; Harding, F.J.; Tuke, J.; Voelcker, N.H. Synergistic Influence of Collagen I and BMP 2 Drives Osteogenic Differentiation of Mesenchymal Stem Cells: A Cell Microarray Analysis. Acta Biomater. 2016, 34, 41–52. [Google Scholar] [CrossRef]
- Bhakta, G.; Lim, Z.X.H.; Rai, B.; Lin, T.; Hui, J.H.; Prestwich, G.D.; Wijnen, A.J.V.; Nurcombe, V.; Cool, S.M. The Influence of Collagen and Hyaluronan Matrices on the Delivery and Bioactivity of Bone Morphogenetic Protein-2 and Ectopic Bone Formation. Acta Biomater. 2013, 9, 9098–9106. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Cai, X.; Huang, J.; Xu, F.; Liu, X.; Wang, Q. Recombinant Human BMP-2 for the Treatment of Open Tibial Fractures. Orthopedics 2012, 35, e847–e854. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, J.D.; Salehi, K.; Kanim, L.; Sheyn, D.; Napier, Z.; Behrens, P.; Garcia, L.; Cuéllar, J.M.; Bae, H.W. Anti-Inflammatory Peptide Attenuates Edema and Promotes BMP-2-induced Bone Formation in Spine Fusion. Tissue Eng. Part A 2018, 24, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Lin, Y.S.; Brodsky, B. Collagen Interactions: Drug Design and Delivery. Adv. Drug Deliv. Rev. 2016, 97, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shinkai, M.; Takezawa, T.; Ohba, S.; Chung, U.; Nagamune, T. Bone Regeneration Using Collagen Type I Vitrigel with Bone Morphogenetic Protein-2. J. Biosci. Bioeng. 2009, 107, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Bulleid, N.J.; John, D.C.; Kadler, K.E. Recombinant Expression Systems for the Production of Collagen. Biochem. Soc. Trans. 2000, 28, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Myllyharju, J.; Nokelainen, M.; Vuorela, A.; Kivirikko, K.I. Expression of Recombinant Human Type I-III Collagens in the Yeast Pichia Pastoris. Biochem. Soc. Trans. 2000, 28, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Báez, J.; Olsen, D.; Polarek, J.W. Recombinant Microbial Systems for the Production of Human Collagen and Gelatin. Appl. Microbiol. Biotechnol. 2005, 69, 245–252. [Google Scholar] [CrossRef]
- Que, R.A.; Arulmoli, J.; Silva, N.A.D.; Flanagan, L.A.; Wang, S.W. Recombinant Collagen Scaffolds as Substrates for Human Neural Stem/Progenitor Cells. J. Biomed. Mater. Res. Part A 2018, 106, 1363–1372. [Google Scholar] [CrossRef]
- Woodley, D.T.; Wang, X.; Amir, M.; Hwang, B.; Remington, J.; Hou, Y.; Uitto, J.; Keene, D.; Chen, M. Intravenously Injected Recombinant Human Type VII Collagen Homes to Skin Wounds and Restores Skin Integrity of Dystrophic Epidermolysis Bullosa. J. Investig. Dermatol. 2013, 133, 1910–1913. [Google Scholar] [CrossRef]
- Podrebarac, J. Development of Recombinant Human Collagen Type I and Type III Injectable Hydrogels for Cardiac Therapy. Ph.D. Thesis, University of Ottawa, Ottawa, ON, Canada, 2017. [Google Scholar]
- Fan, D.D.; Luo, Y.; Mi, Y.; Ma, X.X.; Shang, L. Characteristics of Fed-Batch Cultures of Recombinant Escherichia Coli Containing Human-Like Collagen cDNA at Different Specific Growth Rates. Biotechnol. Lett. 2005, 27, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhu, C.; Fan, D.; Mi, Y.; Li, X.; Fu, R.; Duan, Z.; Wang, Y.; Feng, R. A Novel Human-Like Collagen Hydrogel Scaffold with Porous Structure and Sponge-Like Properties. Polymers 2017, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, Y.; Fan, D.; Zhu, C.; Liu, L.; Duan, Z. A Novel Human-Like Collagen Hemostatic Sponge with Uniform Morphology, Good Biodegradability and Biocompatibility. J. Biomater. Appl. 2017, 31, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, X.; Hang, B.; Li, J.; Huang, L.; Huang, F.; Xu, Z. Efficient Production of Hydroxylated Human-Like Collagen Via the Co-Expression of Three Key Genes in Escherichia coli Origami (DE3). Appl. Biochem. Biotechnol. 2016, 178, 1458–1470. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, J.Y.; Wang, M.; Wu, S.M.; Ma, Y.; Wang, J.F. Expression, Purification, and Functional Characterization of Recombinant Human-Like Type I Collagen Peptide in E. coli. Mod. Food Sci. Technol. 2016, 32, 60–65. [Google Scholar]
- Zhao, L.; Xian, L.; Zhao, J.; Ma, S.; Ma, X.; Fan, D.; Zhu, C.; Liu, Y. A Novel Smart Injectable Hydrogel Prepared by Microbial Transglutaminase and Human-Like Collagen: Its Characterization and Biocompatibility. Mater. Sci. Eng. C 2016, 68, 317–326. [Google Scholar] [CrossRef]
- Zhu, C.; Fan, D.; Wang, Y. Human-Like Collagen/Hyaluronic Acid 3D Scaffolds for Vascular Tissue Engineering. Mater. Sci. Eng. C 2014, 34, 393–401. [Google Scholar] [CrossRef]
- Zhu, C.; Fan, D.; Duan, Z.; Xue, W.; Shang, L.; Chen, F.; Luo, Y. Initial Investigation of Novel Human-Like Collagen/Chitosan Scaffold for Vascular Tissue Engineering. J. Biomed. Mater. Res. Part A 2010, 89A, 829–840. [Google Scholar] [CrossRef]
- Huang, G.P.; Shanmugasundaram, S.; Masih, P.; Pandya, D.; Amara, S.; Collins, G.; Arinzeh, T.L. An Investigation of Common Crosslinking Agents on the Stability of Electrospun Collagen Scaffolds. J. Biomed. Mater. Res. Part A 2015, 103, 762–771. [Google Scholar] [CrossRef]
- Delgado, L.; Fuller, K.; Zeugolis, D.I. Collagen Cross-Linking—Biophysical, Biochemical and Biological Response Analysis. Tissue Eng. Part A 2017, 23, 1064–1077. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Husmann, A.; Best, S.M.; Cameron, R.E. A Design Protocol for Tailoring Ice-Templated Scaffold Structure. J. R. Soc. Interface 2014, 11, 20130958. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wei, J.; Zhu, J.; Liu, W.; Cui, J.; Li, H.; Chen, F. Chm-1 Gene-Modified Bone Marrow Mesenchymal Stem Cells Maintain the Chondrogenic Phenotype of Tissue-Engineered Cartilage. Stem Cell Res. Ther. 2016, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yue, S.; Jing, Z.; Wei, L.; Cui, J.; Li, H.; Chen, F. Laminated Electrospun nHA/PHB-composite Scaffolds Mimicking Bone Extracellular Matrix for Bone Tissue Engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Y.; Wu, Y.; Zhao, Y.; Chen, H.; Yuan, Y.; Xu, K.; Zhang, H.; Lu, Y.; Wang, J. Heparin-Poloxamer Thermosensitive Hydrogel Loaded with bFGF and NGF Enhances Peripheral Nerve Regeneration in Diabetic Rats. Biomaterials 2018, 168, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Knopf, F.; Hammond, C.; Chekuru, A.; Kurth, T.; Hans, S.; Weber, C.W.; Mahatma, G.; Fisher, S.; Brand, M.; Schulte-Merker, S.; et al. Bone Regenerates Via Dedifferentiation of Osteoblasts in the Zebrafish Fin. Dev. Cell 2011, 20, 713–724. [Google Scholar] [CrossRef]
- Ben-David, D.; Srouji, S.; Shapira-Schweitzer, K.; Kossover, O.; Ivanir, E.; Kuhn, G.; Müller, R.; Seliktar, D.; Livne, E. Low Dose BMP-2 Treatment for Bone Repair Using a PEGylated Fibrinogen Hydrogel Matrix. Biomaterials 2013, 34, 2902–2910. [Google Scholar] [CrossRef]
- Bao, M.; Xie, J.; Piruska, A.; Huck, W.T.S. 3D Microniches Reveal the Importance of Cell Size and Shape. Nat. Commun. 2017, 8, 1962. [Google Scholar] [CrossRef]
- Pobloth, A.M.; Duda, G.N.; Giesecke, M.T.; Dienelt, A.; Schwabe, P. High-Dose Recombinant Human Bone Morphogenetic Protein-2 Impacts Histological and Biomechanical Properties of a Cervical Spine Fusion Segment: Results from a Sheep Model. J. Tissue Eng. Regen. Med. 2017, 11, 1514–1523. [Google Scholar] [CrossRef]
- Krishnan, L.; Priddy, L.B.; Esancy, C.; Klosterhoff, B.S.; Stevens, H.Y.; Tran, L.; Guldberg, R.E. Delivery Vehicle Effects On Bone Regeneration and Heterotopic Ossification Induced by High Dose BMP-2. Acta Biomater. 2016, 49, 101–112. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, X.; Zheng, Q.; Wu, Y.; Cui, F.; Wu, B. Improving Osteogenesis of Three-Dimensional Porous Scaffold Based on Mineralized Recombinant Human-Like Collagen Via Mussel-Inspired Polydopamine and Effective Immobilization of BMP-2-derived Peptide. Colloids Surf. B Biointerfaces 2017, 152, 124–132. [Google Scholar] [CrossRef]
- Pan, H.; Zheng, Q.; Guo, X.; Wu, Y.; Wu, B. Polydopamine-Assisted BMP-2-derived Peptides Immobilization on Biomimetic Copolymer Scaffold for Enhanced Bone Induction in Vitro and in Vivo. Colloids Surf. B Biointerfaces 2016, 142, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, D.; Yoon, T.R.; Kim, H.K.; Jo, H.H.; Park, J.S.; Lee, J.H.; Kim, W.D.; Kwon, I.K.; Park, S.A. Surface Modification of 3D-printed Porous Scaffolds Via Mussel-Inspired Polydopamine and Effective Immobilization of rhBMP-2 to Promote Osteogenic Differentiation for Bone Tissue Engineering. Acta Biomater. 2016, 40, 182–191. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primers | Product Size (bp) |
|---|---|---|
| Runx-2 | 5′-GCCACTTACCACAGAGCTATTA-3′(F) | 106 |
| 5′-GGCGGTCAGAGAACAAACTA-3′(R) | ||
| OPN | 5′-AGGAGTTTCCCTGTTTCTGATG-3′(F) | 110 |
| 5′-GCAACTGGGATGACCTTGATA-3′(R) | ||
| ALP | 5′-ACAAGTGTGGCAGTGGTATT-3′(F) | 104 |
| 5′-CTGCTTGAGGTTGAGGTTACA-3′(R) | ||
| β-actin | 5′-CTGTGCTATGTTGCCCTAGAC-3′(F) | 115 |
| 5′-GCTCATTGCCGATAGTGATGA-3′(R) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Zhang, Z.; Ma, X.; Duan, Z.; Hui, J.; Zhu, C.; Zhang, D.; Fan, D.; Shang, L.; Chen, F. Newly Designed Human-Like Collagen to Maximize Sensitive Release of BMP-2 for Remarkable Repairing of Bone Defects. Biomolecules 2019, 9, 450. https://doi.org/10.3390/biom9090450
Chen Z, Zhang Z, Ma X, Duan Z, Hui J, Zhu C, Zhang D, Fan D, Shang L, Chen F. Newly Designed Human-Like Collagen to Maximize Sensitive Release of BMP-2 for Remarkable Repairing of Bone Defects. Biomolecules. 2019; 9(9):450. https://doi.org/10.3390/biom9090450
Chicago/Turabian StyleChen, Zhuoyue, Zhen Zhang, Xiaoxuan Ma, Zhiguang Duan, Junfeng Hui, Chenhui Zhu, Donggang Zhang, Daidi Fan, Lijun Shang, and Fulin Chen. 2019. "Newly Designed Human-Like Collagen to Maximize Sensitive Release of BMP-2 for Remarkable Repairing of Bone Defects" Biomolecules 9, no. 9: 450. https://doi.org/10.3390/biom9090450
APA StyleChen, Z., Zhang, Z., Ma, X., Duan, Z., Hui, J., Zhu, C., Zhang, D., Fan, D., Shang, L., & Chen, F. (2019). Newly Designed Human-Like Collagen to Maximize Sensitive Release of BMP-2 for Remarkable Repairing of Bone Defects. Biomolecules, 9(9), 450. https://doi.org/10.3390/biom9090450