Orientin Induces G0/G1 Cell Cycle Arrest and Mitochondria Mediated Intrinsic Apoptosis in Human Colorectal Carcinoma HT29 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cell Culture Maintenance and Treatment
2.3. Tetrazolium Based Cell Viability Assay
2.4. Morphological Observation and Cell-Cycle Analysis
2.5. Annexin V-FITC/PI Apoptotic Assay
2.6. Measurement of Intracellular ROS
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
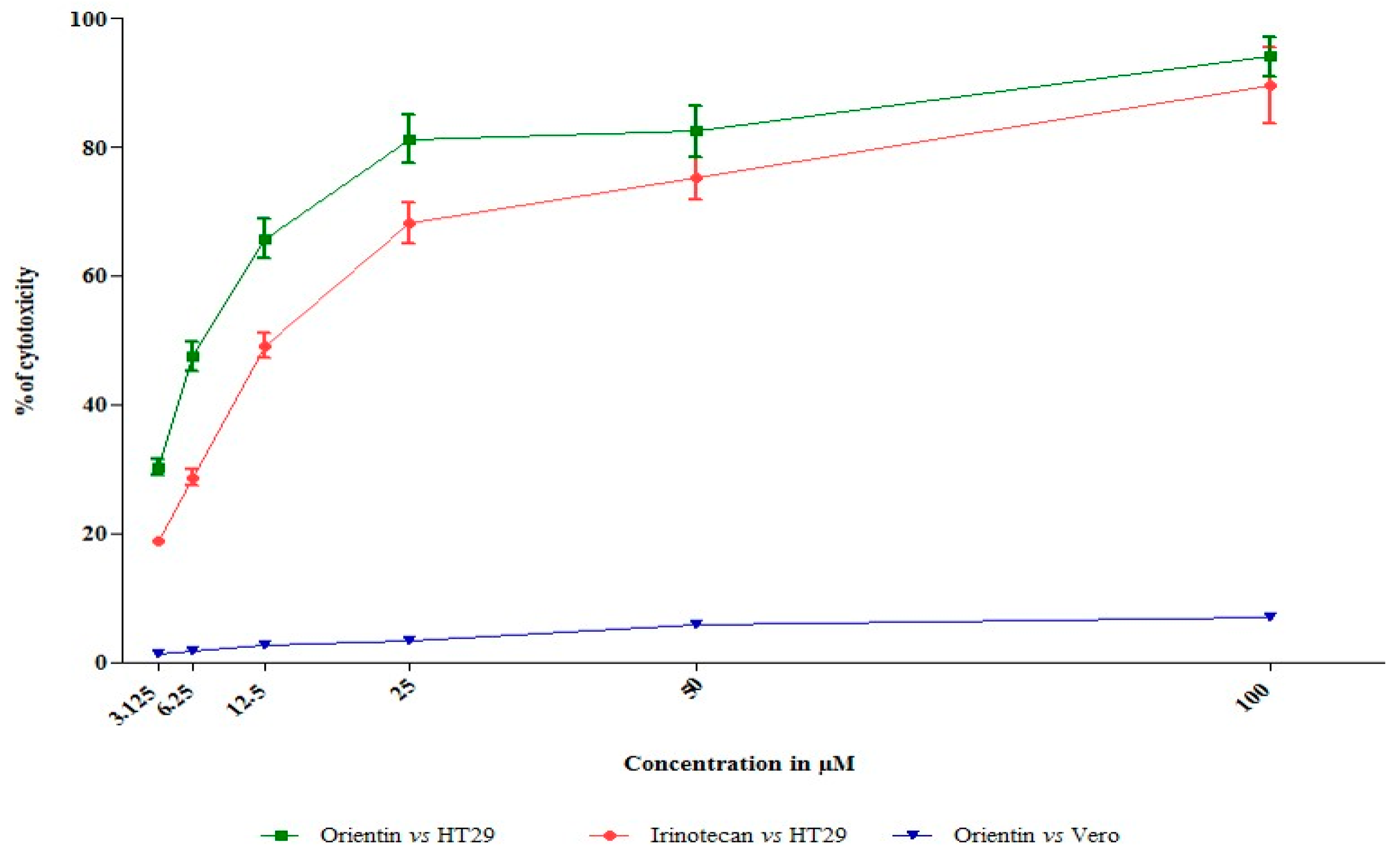
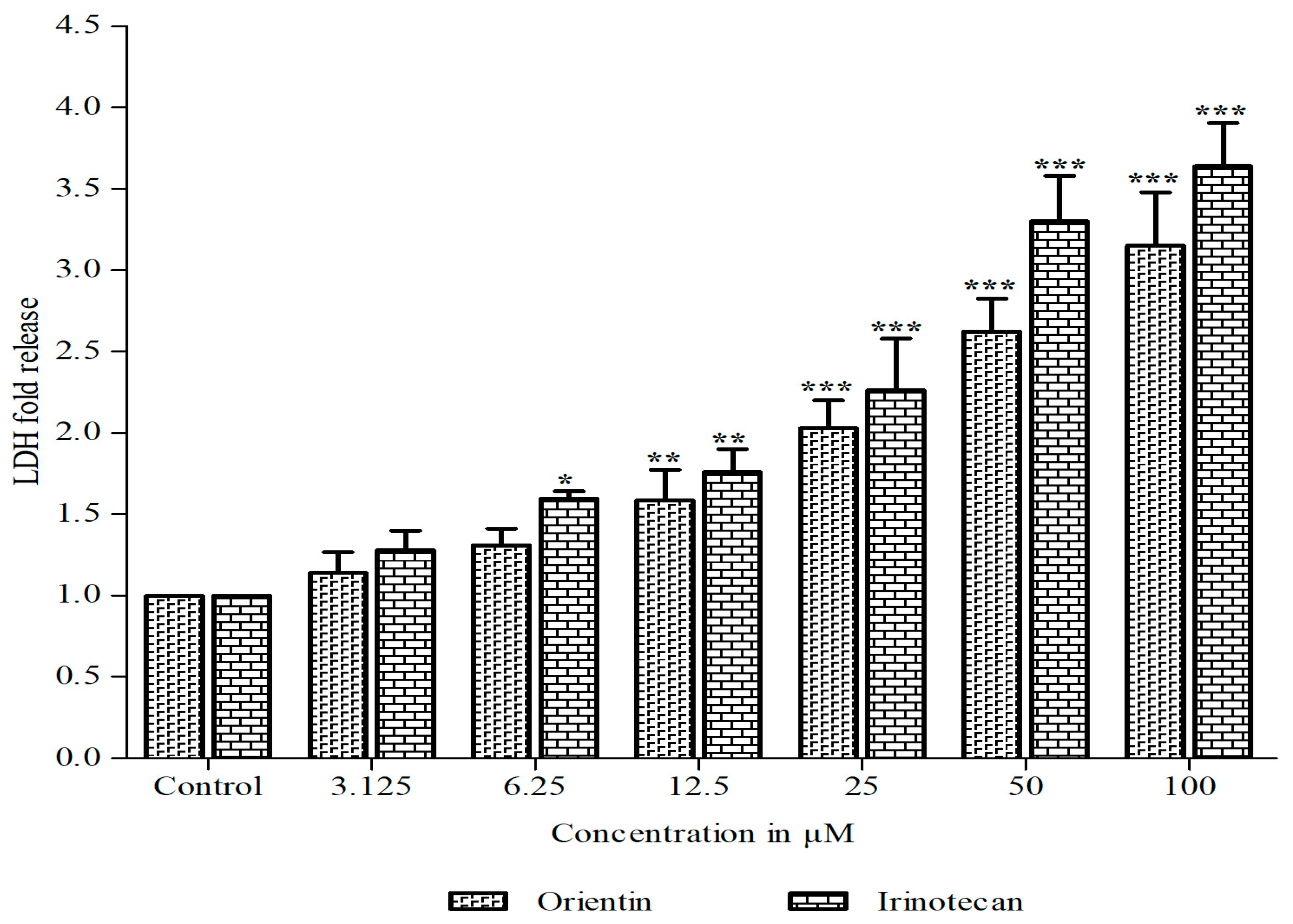
3.1. Orientin Exhibits Cytotoxicity in HT29 Cells
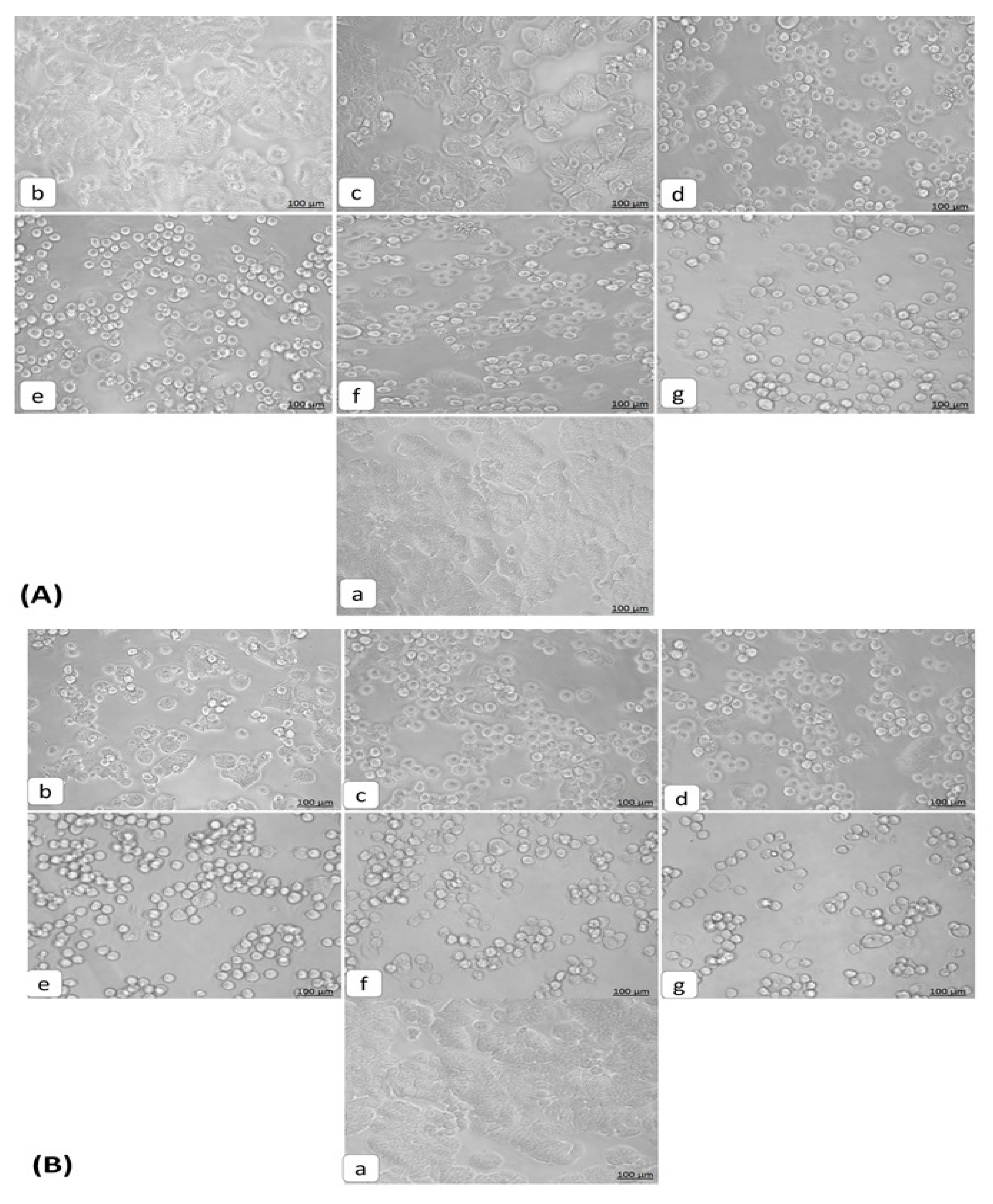
3.2. Morphological Changes Induced in HT29 Cells by Orientin
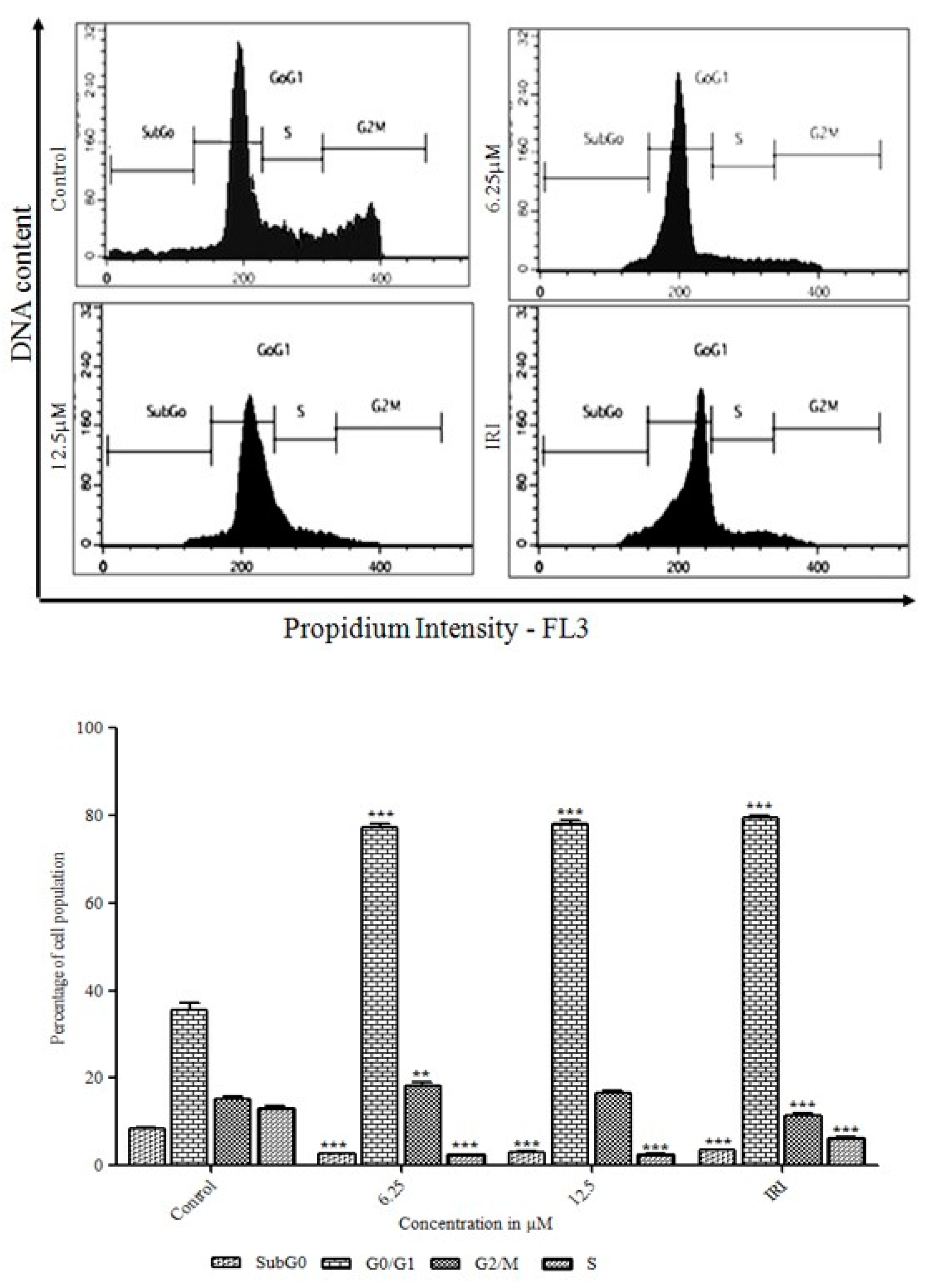
3.3. Initiation of the G0/G1 Phase Cell Cycle Arrest by Orientin
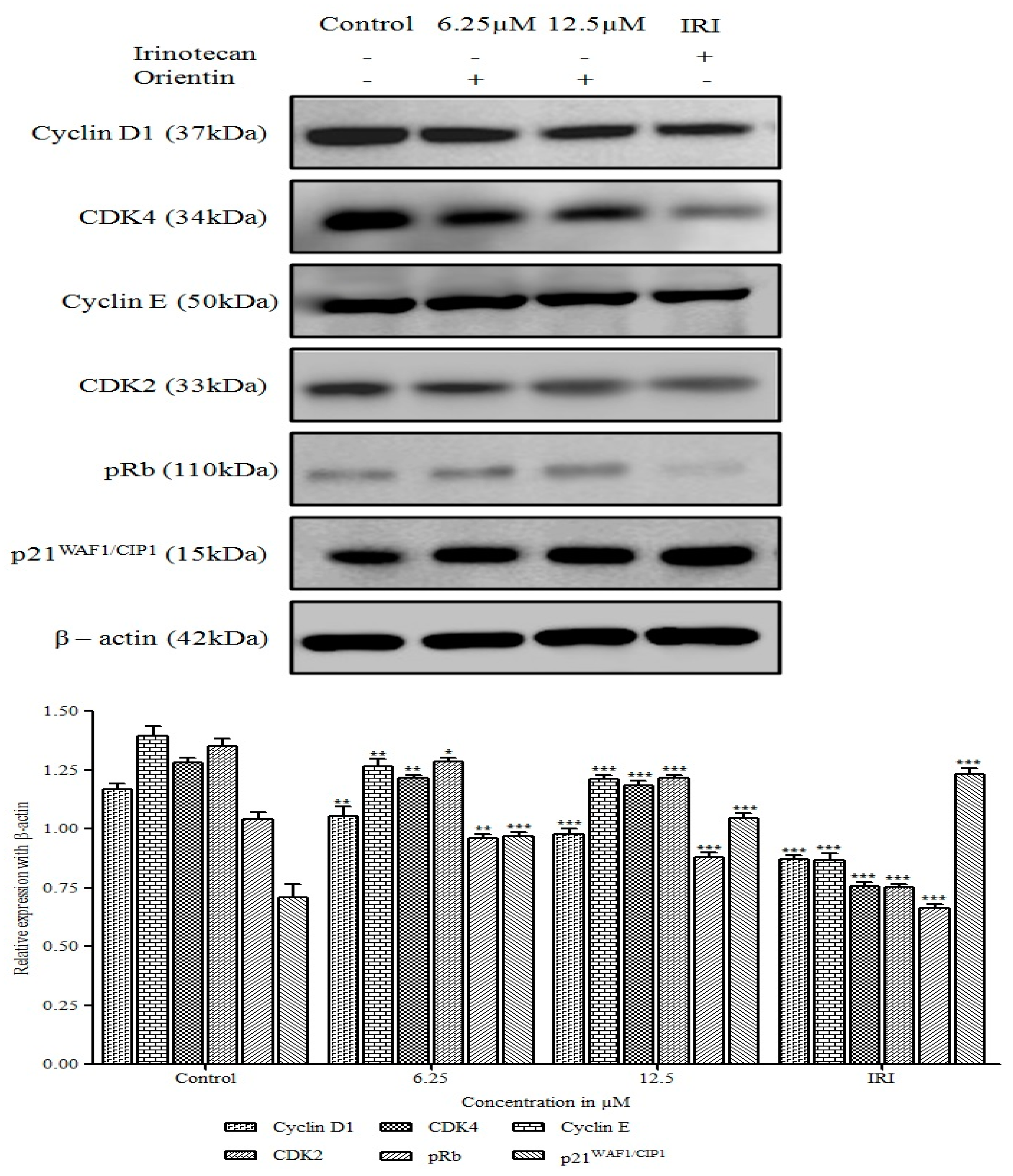
3.4. Orientin Induced p21WAF1/CIP1 Mediated G0/G1 Arrest in HT29 Cells
3.5. Orientin Induces Apoptosis in HT29 Cells
3.6. Intracellular Accumulation of ROS by Orientin in HT29 Cells
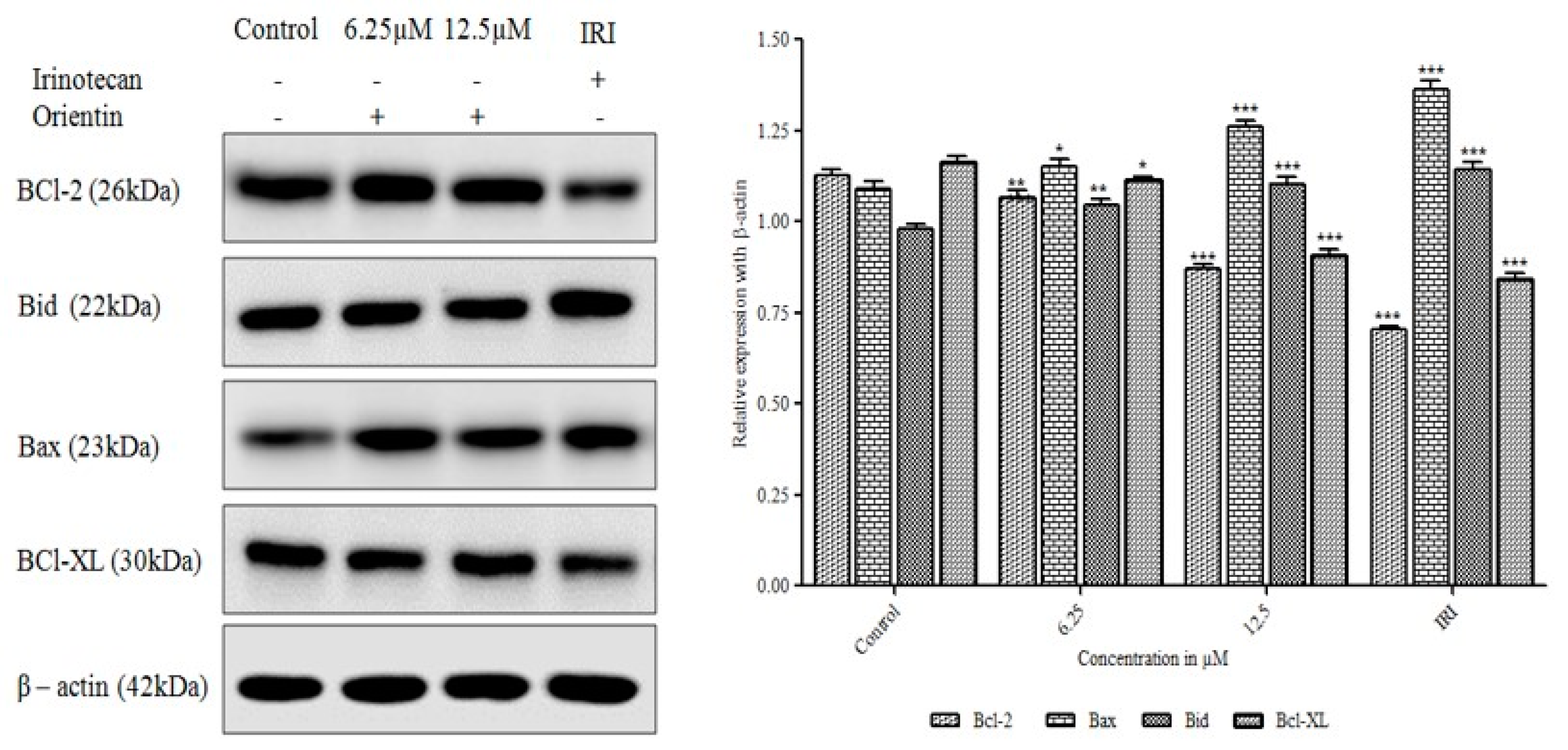
3.7. Orientin Modulates Bcl-2 Family Proteins
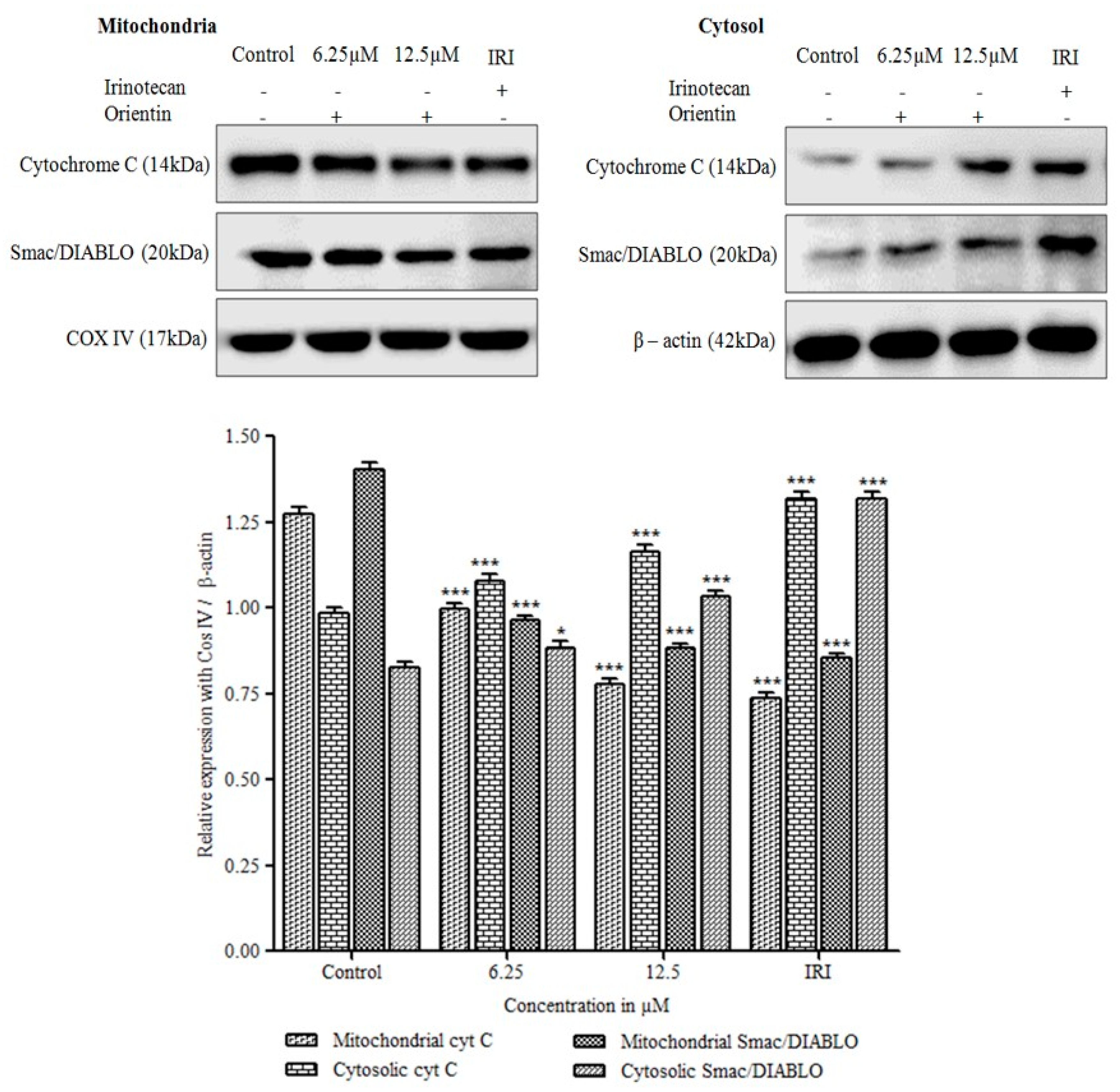
3.8. Cytochrome C Release and Translocation of Smac/DIABLO by Orientin
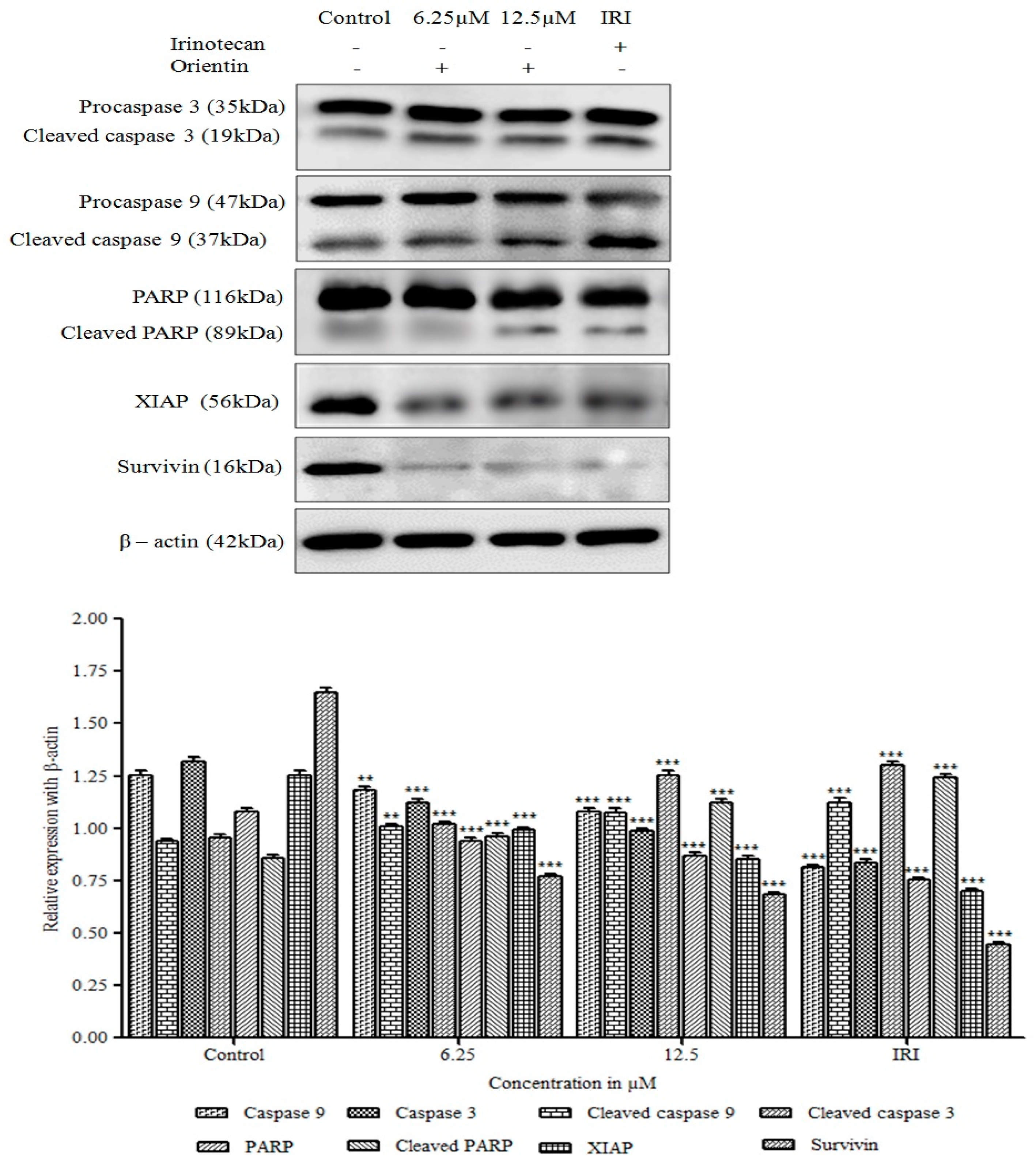
3.9. Orientin Activates Caspase Cascade and Induces PARP Cleavage
3.10. Orientin Blocks the Inhibitor of Apoptotic Proteins (IAP)
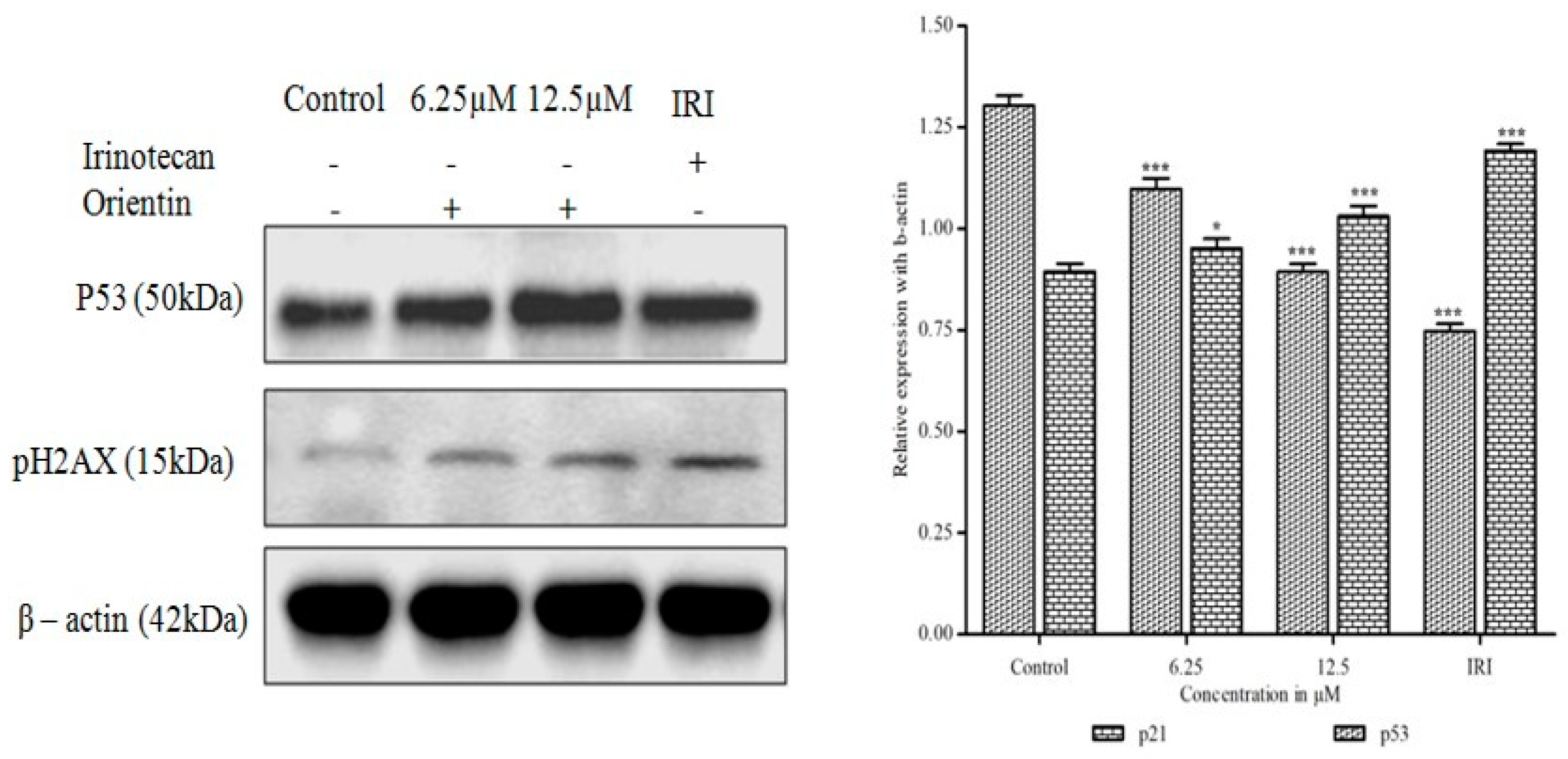
3.11. Orientin Induces p53 Expression and DNA Damage
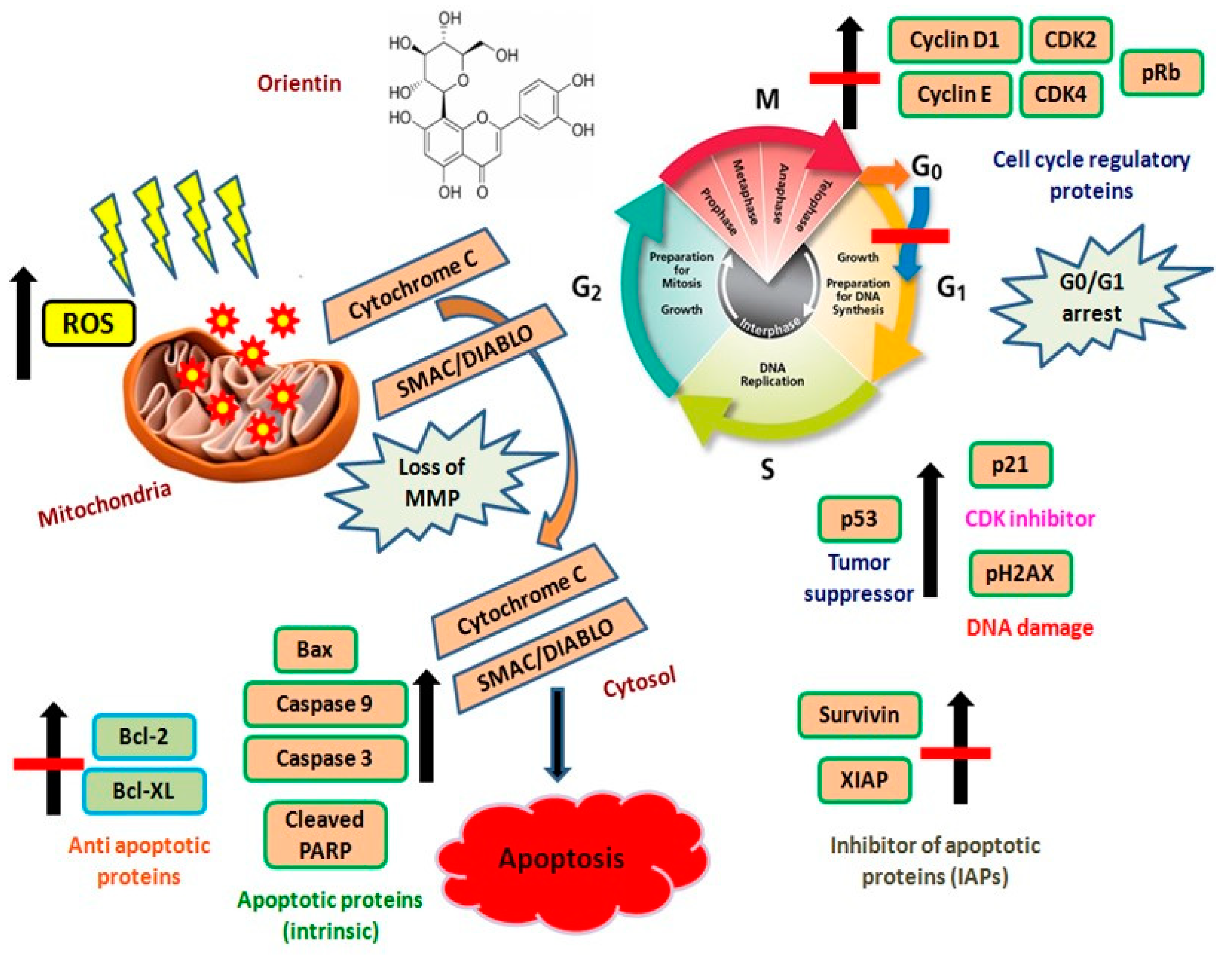
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Okada, K.; Hakata, S.; Terashima, J.; Gamou, T.; Habano, W.; Ozawa, S. Combination of the histone deacetylase inhibitor depsipeptide and 5-fluorouracil upregulates major histocompatibility complex class II and p21 genes and activates caspase-3/7 in human colon cancer HCT-116 cells. Oncol. Rep. 2016, 36, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Thelin, C.; Sikka, S. Epidemiology of colorectal cancer—Incidence, lifetime risk factors statistics and temporal trends. Lung 2015, 1, 13–20. [Google Scholar]
- Soo, H.C.; Chung, F.F.; Lim, K.H.; Yap, V.A.; Bradshaw, T.D.; Hii, L.W.; Tan, S.H.; See, S.J.; Tan, Y.F.; Leong, C.O.; et al. Cudraflavone C induces tumor-specific apoptosis in colorectal cancer cells through inhibition of the phosphoinositide 3-kinase (PI3K)-AKT pathway. PLoS ONE 2017, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Lei, L.; Zhou, Y.; Ye, F.; Zhao, G. Dietary Flavonoids and the Risk of Colorectal Cancer: An Updated Meta-Analysis of Epidemiological Studies. Nutrients 2018, 10, 950. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T.; Chen, G.Y. Flavonoids and Colorectal Cancer Prevention. Antioxidants 2018, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Khan, A.Q.; Qamar, W.; Lateef, A.; Tahir, M.; Rehman, M.U.; Ali, F.; Sultana, S. Chrysin protects against cisplatin-induced colon toxicity via amelioration of oxidative stress and apoptosis: Probable role of p38MAPK and p53. Toxicol. Appl. Pharmacol. 2012, 258, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Clawson, G.A. Histone deacetylase inhibitors as cancer therapeutics. Ann. Transl. Med. 2016, 4, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Yi, J.; Wang, Z.; Bai, H.; Li, L.; Zhao, H.; Cheng, C.; Zhang, H.; Li, J. Polyphenols from pinecones of Pinus koraiensis induce apoptosis in colon cancer cells through the activation of caspase in vitro. RSC Adv. 2016, 6, 5278–5287. [Google Scholar] [CrossRef]
- Sergeeva, T.F.; Shirmanova, M.V.; Zlobovskaya, O.A.; Gavrina, A.I.; Dudenkova, V.V.; Lukina, M.M.; Lukyanov, K.A.; Zagaynova, E.V. Relationship between intracellular pH, metabolic co-factors and caspase-3 activation in cancer cells during apoptosis. BBA-Mol. Cell. Res. 2017, 864, 604–611. [Google Scholar] [CrossRef]
- Baig, S.; Seevasant, I.; Mohamad, J.; Mukheem, A.; Huri, H.Z.; Kamarul, T. Potential of apoptotic pathway-targeted cancer therapeutic research: Where do we stand? Cell Death Dis. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jo, A.; Baek, S.; Lim, D.; Park, S.Y.; Cho, S.K.; Chung, J.W.; Yoon, J. Synergistically enhanced selective intracellular uptake of anticancer drug carrier comprising folic acid-conjugated hydrogels containing magnetite nanoparticles. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ye, T.; Liu, Z.; Fang, A.; Luo, Y.; Wei, W.; Li, Y.; Li, Y.; Zeng, A.; Deng, Y.; et al. Niclosamide induces colorectal cancer apoptosis, impairs metastasis and reduces immunosuppressive cells in vivo. RSC Adv. 2016, 6, 106019–106030. [Google Scholar] [CrossRef]
- Hata, A.N.; Engelman, J.A.; Faber, A.C. The BCL2 family: Key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov. 2015, 5, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Besbes, S.; Mirshahi, M.; Pocard, M.; Billard, C. New dimension in therapeutic targeting of BCL-2 family proteins. Oncotarget 2015, 6, 12862–12871. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.Y.; Ling, A.P.; Koh, R.Y.; Wong, Y.P.; Say, Y.H. A review on medicinal properties of orientin. Adv. Pharmacol. Sci. 2016, 4104595, 1–9. [Google Scholar]
- Ku, S.K.; Kwak, S.; Bae, J.S. Orientin inhibits high glucose-induced vascular inflammation in vitro and in vivo. Inflammation 2014, 37, 2164–21673. [Google Scholar] [CrossRef] [PubMed]
- Law, B.N.; Ling, A.P.; Koh, R.Y.; Chye, S.M.; Wong, Y.P. Neuroprotective effects of orientin on hydrogen peroxide-induced apoptosis in SH-SY5Y cells. Mol. Med. Rep. 2014, 9, 947–954. [Google Scholar] [CrossRef]
- An, F.; Yang, G.; Tian, J.; Wang, S. Antioxidant effects of the orientin and vitexin in Trollius chinensis Bunge in D-galactose-aged mice. Neural Regen. Res. 2012, 7, 2565–2575. [Google Scholar]
- Czemplik, M.; Mierziak, J.; Szopa, J.; Kulma, A. Flavonoid C-glucosides derived from flax straw extracts reduce human breast cancer cell growth in vitro and induce apoptosis. Front. Pharmacol. 2016, 7, 282. [Google Scholar] [CrossRef]
- Karthi, N.; Kalaiyarasu, T.; Kandakumar, S.; Mariyappan, P.; Manju, V. Pelargonidin induces apoptosis and cell cycle arrest via a mitochondria mediated intrinsic apoptotic pathway in HT29 cells. RSC Adv. 2016, 6, 45064–45076. [Google Scholar] [CrossRef]
- Phang, C.W.; Karsani, S.A.; Sethi, G.; Malek, S.N. Flavokawain C inhibits cell cycle and promotes apoptosis, associated with endoplasmic reticulum stress and regulation of MAPKs and Akt signaling pathways in HCT 116 human colon carcinoma cells. PLoS ONE 2016, 11, e0148775. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Park, C.; Jin, C.Y.; Kim, G.Y.; Chang, Y.C.; Moon, S.K.; Kim, W.J.; Choi, Y.H. Apoptosis induction of human bladder cancer cells by sanguinarine through reactive oxygen species-mediated up-regulation of early growth response gene-1. PLoS ONE 2013, 8, e63425. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, K.; Vaiyapuri, M. Orientin, a C-glycosyl dietary flavone, suppresses colonic cell proliferation and mitigates NF-κB mediated inflammatory response in 1, 2-dimethylhydrazine induced colorectal carcinogenesis. Biomed. Pharmacother. 2017, 96, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Song, M.; Qiu, P.; Li, F.; Wang, M.; Zheng, J.; Wang, Q.; Xu, F.; Xiao, H. A metabolite of nobiletin, 4′-demethylnobiletin and atorvastatin synergistically inhibits human colon cancer cell growth by inducing G0/G1 cell cycle arrest and apoptosis. Food Funct. 2018, 9, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Song, X.L.; Zhang, Y.J.; Wang, X.F.; Zhang, W.J.; Wang, Z.; Zhang, F.; Zhang, Y.J.; Lu, J.H.; Mei, J.W.; Hu, Y.P.; et al. Casticin induces apoptosis and G0/G1 cell cycle arrest in gallbladder cancer cells. Cancer Cell Int. 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Peyressatre, M.; Prevel, C.; Pellerano, M.; Morris, M.C. Targeting cyclin-dependent kinases in human cancers: From small molecules to peptide inhibitors. Cancers 2015, 7, 179–237. [Google Scholar] [CrossRef]
- Kan, W.L.; Yin, C.; Xu, H.X.; Xu, G.; To, K.K.; Cho, C.H.; Rudd, J.A.; Lin, G. Antitumor effects of novel compound, guttiferone K., on colon cancer by p21Waf1/Cip1-mediated G0/G1 cell cycle arrest and apoptosis. Int. J. Cancer 2013, 132, 707–716. [Google Scholar] [CrossRef]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef]
- Hajiaghaalipour, F.; Faraj, F.L.; Bagheri, E.; Ali, H.M.; Abdulla, M.A.; Majid, N.A. Synthesis and Characterization of a New Benzoindole Derivative with Apoptotic Activity Against Colon Cancer Cells. Curr. Pharma Des. 2017, 23, 6358–6365. [Google Scholar] [CrossRef]
- Khodapasand, E.; Jafarzadeh, N.; Farrokhi, F.; Kamalidehghan, B.; Houshmand, M. Is Bax/Bcl-2 ratio considered as a prognostic marker with age and tumor location in colorectal cancer? Iran. Biomed. J. 2015, 19, 69–75. [Google Scholar] [PubMed]
- Bleicken, S.; Zeth, K. Conformational changes and protein stability of the pro-apoptotic protein Bax. J. Bioenerg. Biomembr. 2009, 41, 29–40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dewson, G.K.; Kluck, R.M. Bcl-2 family-regulated apoptosis in health and disease. Cell Health Cytoskel. 2010, 2, 9–22. [Google Scholar]
- Zhang, M.; Zheng, J.; Nussinov, R.; Ma, B. Release of cytochrome C from Bax pores at the mitochondrial membrane. Sci. Rep. 2017, 7, 2635. [Google Scholar] [CrossRef] [PubMed]
- Srinivasula, S.M.; Datta, P.; Fan, X.J.; Fernandes-Alnemri, T.; Huang, Z.; Alnemri, E.S. Molecular determinants of the caspase-promoting activity of Smac/DIABLO and its role in the death receptor pathway. J. Biol. Chem. 2000, 275, 36152–36157. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Kohnoe, S.; Watanabe, A.; Tashiro, H.; Sakata, H.; Morita, M.; Kakeji, Y.; Maehara, Y. Clinical significance of Smac/DIABLO expression in colorectal cancer. Oncol. Rep. 2009, 21, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Magid, A.F. Modulation of the Inhibitors of Apoptosis Proteins (IAPs) Activities for Cancer Treatment. ACS Med. Chem. Lett. 2017, 8, 471–473. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Omer, F.A.A.; Hashim, N.B.M.; Ibrahim, M.Y.; Dehghan, F.; Yahayu, M.; Karimian, H.; Salim, L.Z.A.; Mohan, S. Beta-mangostin from Cratoxylum arborescens activates the intrinsic apoptosis pathway through reactive oxygen species with downregulation of the HSP70 gene in the HL60 cells associated with a G0/G1 cell-cycle arrest. Tumor Biol. 2017, 39, 1–12. [Google Scholar] [CrossRef]
- Li, M.; Song, L.H.; Yue, G.G.; Lee, J.K.; Zhao, L.M.; Li, L.; Zhou, X.; Tsui, S.K.; Ng, S.S.; Fung, K.P.; et al. Bigelovin triggered apoptosis in colorectal cancer in vitro and in vivo via upregulating death receptor 5 and reactive oxidative species. Sci. Rep. 2017, 7, 42176. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, X.; Hu, D. Furanodienone induces G0/G1 arrest and causes apoptosis via the ROS/MAPKs-mediated caspase-dependent pathway in human colorectal cancer cells: A study in vitro and in vivo. Cell Death Dis. 2017, 8, 2815. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangaraj, K.; Balasubramanian, B.; Park, S.; Natesan, K.; Liu, W.; Manju, V. Orientin Induces G0/G1 Cell Cycle Arrest and Mitochondria Mediated Intrinsic Apoptosis in Human Colorectal Carcinoma HT29 Cells. Biomolecules 2019, 9, 418. https://doi.org/10.3390/biom9090418
Thangaraj K, Balasubramanian B, Park S, Natesan K, Liu W, Manju V. Orientin Induces G0/G1 Cell Cycle Arrest and Mitochondria Mediated Intrinsic Apoptosis in Human Colorectal Carcinoma HT29 Cells. Biomolecules. 2019; 9(9):418. https://doi.org/10.3390/biom9090418
Chicago/Turabian StyleThangaraj, Kalaiyarasu, Balamuralikrishnan Balasubramanian, Sungkwon Park, Karthi Natesan, Wenchao Liu, and Vaiyapuri Manju. 2019. "Orientin Induces G0/G1 Cell Cycle Arrest and Mitochondria Mediated Intrinsic Apoptosis in Human Colorectal Carcinoma HT29 Cells" Biomolecules 9, no. 9: 418. https://doi.org/10.3390/biom9090418
APA StyleThangaraj, K., Balasubramanian, B., Park, S., Natesan, K., Liu, W., & Manju, V. (2019). Orientin Induces G0/G1 Cell Cycle Arrest and Mitochondria Mediated Intrinsic Apoptosis in Human Colorectal Carcinoma HT29 Cells. Biomolecules, 9(9), 418. https://doi.org/10.3390/biom9090418





