The Inhibitory Effect of Cordycepin on the Proliferation of MCF-7 Breast Cancer Cells, and Its Mechanism: An Investigation Using Network Pharmacology-Based Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cordyceps Militaris Concentrate
2.2. Cell Culture
2.3. Determination of Cell Viability
2.4. Cell Staining with Hoechst 33342
2.5. Image-Based Cytometric Assay
2.6. Western Blot Analysis
2.7. Network Pharmacological Analysis
2.8. Statistical Analysis
3. Results
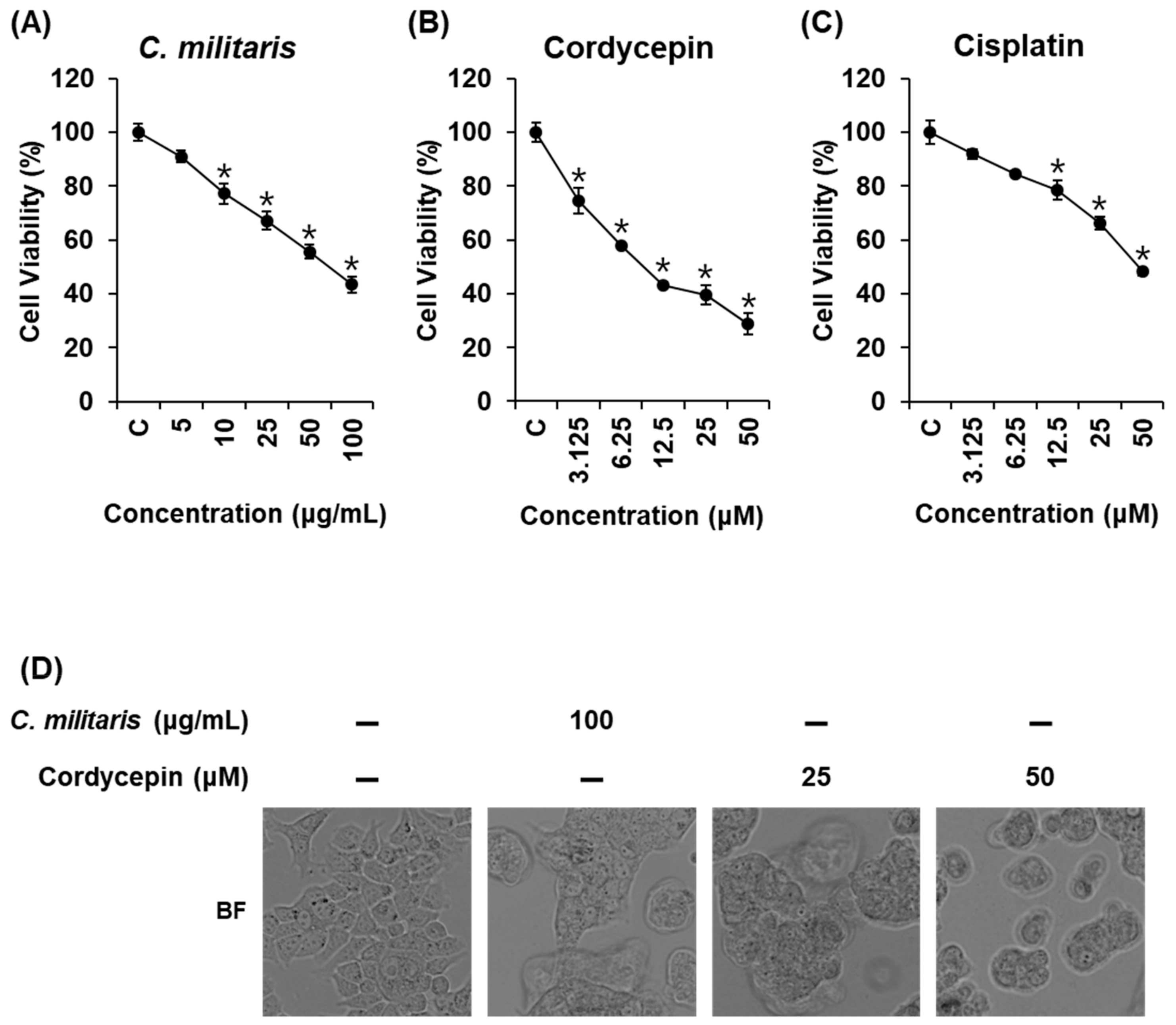
3.1. Effects of Cordyceps Militaris Concentrate and Cordycepin on MCF-7 Breast Cancer Cell Viability
3.2. Network Pharmacological Analysis of Cordycepin
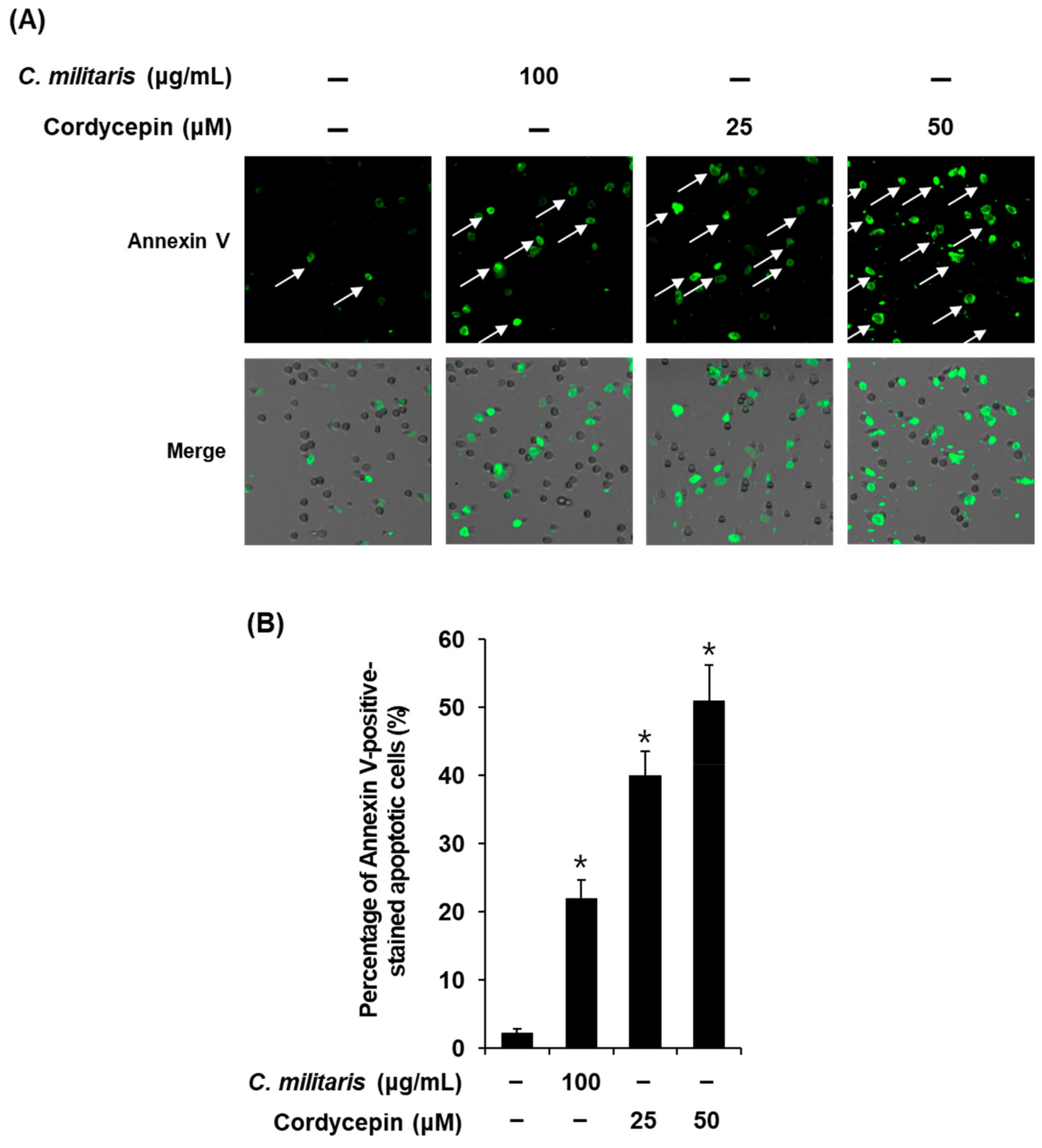
3.3. Effects of the Cordyceps Militaris Concentrate and Cordycepin on Apoptosis
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. Jama 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Meng, X.; Gan, R.Y.; Zhang, J.J.; Li, H.B. Dietary Natural Products for Prevention and Treatment of Breast Cancer. Nutrients 2017, 9, 728. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jing, Z.; Li, Y.; Mao, W. Berberine in combination with cisplatin suppresses breast cancer cell growth through induction of DNA breaks and caspase-3-dependent apoptosis. Oncol. Rep. 2016, 36, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Luo, Q.; Zhang, Y.; Jia, F.; Zhao, Y.; Wang, F. Advances in Toxicological Research of the Anticancer Drug Cisplatin. Chem. Res. Toxicol. 2019, 32, 469–1486. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.L.; Nasrollahi, S.; Shah, K.N.; Soltisz, A.; Paruchuri, S.; Yun, Y.H.; Luker, G.D.; Bishayee, A.; Tavana, H. Phytochemicals potently inhibit migration of metastatic breast cancer cells. Integr. Biol. (Camb) 2015, 7, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.J.; Das Gupta, S.; Wahler, J.; Suh, N. Role of dietary bioactive natural products in estrogen receptor-positive breast cancer. Semin. Cancer Biol. 2016, 40–41, 170–191. [Google Scholar] [CrossRef]
- Goodarzi, S.; Tabatabaei, M.J.; Mohammad Jafari, R.; Shemirani, F.; Tavakoli, S.; Mofasseri, M.; Tofighi, Z. Cuminum cyminum fruits as source of luteolin-7-O-glucoside, potent cytotoxic flavonoid against breast cancer cell lines. Nat. Prod. Res. 2018, 22, 1–5. [Google Scholar] [CrossRef]
- Le, T.V.T.; Nguyen, P.H.; Choi, H.S.; Yang, J.L.; Kang, K.W.; Ahn, S.G.; Oh, W.K. Diarylbutane-type Lignans from Myristica fragrans (Nutmeg) show the Cytotoxicity against Breast Cancer Cells through Activation of AMP-activated Protein Kinase. Nat. Prod. Sci. 2017, 23, 21–28. [Google Scholar] [CrossRef]
- Yang, E.J.; An, J.H.; Son, Y.K.; Yeo, J.H.; Song, K.S. The Cytotoxic Constituents of Betula platyphylla and their Effects on Human Lung A549 Cancer Cells. Nat. Prod. Sci. 2018, 24, 219–224. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, R.; Tian, S.; Wang, Y.; Lou, S.; Zhao, H. Jatamanvaltrate P induces cell cycle arrest, apoptosis and autophagy in human breast cancer cells in vitro and in vivo. Biomed. Pharmacother. 2017, 89, 1027–1036. [Google Scholar] [CrossRef]
- Li, J.Y.; Zou, L.; Chen, W.; Zhu, B.B.; Shen, N.; Ke, J.T.; Lou, J.; Song, R.R.; Zhong, R.; Miao, X.P. Dietary Mushroom Intake May Reduce the Risk of Breast Cancer: Evidence from a Meta-Analysis of Observational Studies. PLoS ONE 2014, 9, e93437. [Google Scholar] [CrossRef]
- Tuli, H.S.; Sandhu, S.S.; Sharma, A.K. Pharmacological and therapeutic potential of Cordyceps with special reference to Cordycepin. 3 Biotech 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Ouyang, Z.; Su, Z.; Wang, D.; Yu, X. Neuroprotective effects of adenosine isolated from Cordyceps cicadae against oxidative and ER stress damages induced by glutamate in PC12 cells. Environ. Toxicol. Pharmacol. 2016, 44, 53–61. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Wei, Y.; Ouyang, Z.; Su, Z. Polysaccharides purified from Cordyceps cicadae protects PC12 cells against glutamate-induced oxidative damage. Carbohydr. Polym. 2016, 153, 187–195. [Google Scholar] [CrossRef]
- Joshi, M.; Sagar, A.; Kanwar, S.S.; Singh, S. Anticancer, antibacterial and antioxidant activities of Cordyceps militaris. Indian J. Exp. Biol. 2019, 57, 15–20. [Google Scholar]
- Mehrotra, S.; Kirar, V.; Vats, P.; Nandi, S.P.; Negi, P.; Misra, K. Phytochemical and antimicrobial activities of Himalayan Cordyceps sinensis (Berk.) Sacc. Indian J. Exp. Biol. 2015, 53, 36–43. [Google Scholar]
- Chiu, C.P.; Liu, S.C.; Tang, C.H.; Chan, Y.; El-Shazly, M.; Lee, C.L.; Du, Y.C.; Wu, T.Y.; Chang, F.R.; Wu, Y.C. Anti-inflammatory cerebrosides from cultivated Cordyceps militaris. J. Agric. Food Chem. 2016, 64, 1540–1548. [Google Scholar] [CrossRef]
- Kim, Y.S.; Shin, W.B.; Dong, X.; Kim, E.K.; Nawarathna, W.P.A.S.; Kim, H.; Park, P.J. Anti-inflammatory effect of the extract from fermented Asterina pectinifera with Cordyceps militaris mycelia in LPS-induced RAW264. 7 macrophages. Food Sci. Biotechnol. 2017, 26, 1633–1640. [Google Scholar] [CrossRef]
- Jin, Y.; Meng, X.; Qiu, Z.; Su, Y.; Yu, P.; Qu, P. Anti-tumor and anti-metastatic roles of cordycepin, one bioactive compound of Cordyceps militaris. Saudi J. Biol. Sci. 2018, 25, 991–995. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, E.K.; Tang, Y.; Hwang, J.W.; Natarajan, S.B.; Kim, W.S.; Moon, S.H.; Jeon, B.T.; Park, P.J. Antioxidant and anticancer effects of extracts from fermented Haliotis discus hannai with Cordyceps militaris mycelia. Food Sci. Biotechnol. 2016, 25, 1775–1782. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Teng, M.; Zhang, S.; Yin, M.; Lu, J.; Liu, Y.; Lee, R.J.; Wang, D.; Teng, L. Cordyceps militaris induces tumor cell death via the caspase-dependent mitochondrial pathway in HepG2 and MCF-7 cells. Mol. Med. Rep. 2016, 13, 5132–5140. [Google Scholar] [CrossRef]
- Chen, C.; Wang, M.L.; Jin, C.; Chen, H.J.; Li, S.H.; Li, S.Y.; Dou, X.F.; Jia, J.Q.; Gui, Z.Z. Cordyceps militaris polysaccharide triggers apoptosis and G0/G1 cell arrest in cancer cells. J. Asia Pac. Entomol. 2015, 18, 433–438. [Google Scholar] [CrossRef]
- Uhrinová, A.; Poľančíková, N. Antioxidant Activity of the Fungus Cordyceps sinensis Grown on Two Different Media. Folia Vet. 2018, 62, 68–73. [Google Scholar]
- Wu, H.C.; Chen, S.T.; Chang, J.C.; Hsu, T.Y.; Cheng, C.C.; Chang, H.S.; Liu, C.S.; Wu, Y.C.; Liang, Z.C. Radical Scavenging and Antiproliferative Effects of Cordycepin-Rich Ethanol Extract from Brown Rice− Cultivated Cordyceps militaris (Ascomycetes) Mycelium on Breast Cancer Cell Lines. Int. J. Med. Mushrooms 2019, 21, 657–669. [Google Scholar] [CrossRef]
- Hsiao-Ping, K.; Lin, Y.S.; Huang, S.T.; An-Chi, W.; Lai, J.T. Method for Cultivating Cordyceps Militaris Fruiting Body. USA Patent 20190053438A1, 21 February 2019. [Google Scholar]
- Liu, S.C.; Chiu, C.P.; Tsai, C.H.; Hung, C.Y.; Li, T.M.; Wu, Y.C.; Tang, C.H. Soya-cerebroside, an extract of Cordyceps militaris, suppresses monocyte migration and prevents cartilage degradation in inflammatory animal models. Sci. Rep. 2017, 7, 43205. [Google Scholar] [CrossRef]
- Chiang, S.S.; Liang, Z.C.; Wang, Y.C.; Liang, C.H. Effect of light-emitting diodes on the production of cordycepin, mannitol and adenosine in solid-state fermented rice by Cordyceps militaris. J. Food Compos. Anal. 2017, 60, 51–56. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Li, F.; Cai, H.; Sun, W.; Chen, X.; Gao, H.; Shen, W. Determination of cordycepin content of Cordyceps militaris recombinant rice by high performance liquid chromatography. Trop. J. Pharm. Res. 2016, 15, 2235–2239. [Google Scholar] [CrossRef][Green Version]
- Eguchi, F.; Kalaw, S.P.; Dulay, R.M.R.; Miyasawa, N.; Yoshimoto, H.; Seyama, T.; Reyes, R.G. Nutrient composition and functional activity of different stages in the fruiting body development of Philippine paddy straw mushroom, Volvariella volvacea (Bull.: Fr.) Sing. Adv. Environ. Biol. 2015, 9, 54–66. [Google Scholar]
- Olatunji, O.J.; Tang, J.; Tola, A.; Auberon, F.; Oluwaniyi, O.; Ouyang, Z. The genus Cordyceps: An extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2018, 129, 293–316. [Google Scholar] [CrossRef]
- Amirullah, N.A.; Abidin, N.Z.; Abdullah, N. The potential applications of mushrooms against some facets of atherosclerosis: A review. Food Res. Int. 2018, 105, 517–536. [Google Scholar] [CrossRef]
- Khan, M.; Tania, M. Cordycepin in Anticancer Research: Molecular Mechanism of Therapeutic Effects. Curr. Med. Chem. 2019. [Google Scholar] [CrossRef]
- Tuli, H.S.; Kashyap, D.; Sharma, A.K. Cordycepin: A cordyceps metabolite with promising therapeutic potential. In Fungal Metabolites; Springer: Cham, Switzerland, 2017; pp. 761–782. [Google Scholar]
- Kang, N.; Lee, H.H.; Park, I.; Seo, Y.S. Development of High Cordycepin-Producing Cordyceps militaris Strains. Mycobiology 2017, 45, 31–38. [Google Scholar] [CrossRef]
- Tian, X.; Li, Y.; Shen, Y.; Li, Q.; Wang, Q.; Feng, L. Apoptosis and inhibition of proliferation of cancer cells induced by cordycepin. Oncol. Lett. 2015, 10, 595–599. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, M.; Wang, H.; Li, S.; Wang, X.; Li, Y.; Wang, D.; Li, S. Network Pharmacology to Unveil the Biological Basis of Health-Strengthening Herbal Medicine in Cancer Treatment. Cancers 2018, 10, 461. [Google Scholar] [CrossRef]
- Poornima, P.; Kumar, J.D.; Zhao, Q.; Blunder, M.; Efferth, T. Network pharmacology of cancer: From understanding of complex interactomes to the design of multi-target specific therapeutics from nature. Pharmacol. Res. 2016, 111, 290–302. [Google Scholar] [CrossRef]
- Fang, J.; Cai, C.; Wang, Q.; Lin, P.; Zhao, Z.; Cheng, F. Systems Pharmacology-Based Discovery of Natural Products for Precision Oncology Through Targeting Cancer Mutated Genes. CPT Pharm. Syst. Pharmacol. 2017, 6, 177–187. [Google Scholar] [CrossRef]
- Hu, J.X.; Thomas, C.E.; Brunak, S. Network biology concepts in complex disease comorbidities. Nat. Rev. Genet. 2016, 17, 615. [Google Scholar] [CrossRef]
- Camacho, D.M.; Collins, K.M.; Powers, R.K.; Costello, J.C.; Collins, J.J. Next-generation machine learning for biological networks. Cell 2018, 173, 1581–1592. [Google Scholar] [CrossRef]
- Park, M.; Park, S.Y.; Lee, H.J.; Kim, C.E. A Systems-Level Analysis of Mechanisms of Platycodon grandiflorum Based on A Network Pharmacological Approach. Molecules 2018, 23, 2841. [Google Scholar] [CrossRef]
- Lee, W.Y.; Lee, C.Y.; Kim, Y.S.; Kim, C.E. The Methodological Trends of Traditional Herbal Medicine Employing Network Pharmacology. Biomolecules 2019, 9, 362. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Yu, S.J.; Bai, H.; Ning, K. TCM-Mesh: The database and analytical system for network pharmacology analysis for TCM preparations. Sci. Rep. 2017, 7, 2821. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Santos, A.; von Mering, C.; Jensen, L.J.; Bork, P.; Kuhn, M. STITCH 5: Augmenting protein–chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2015, 44, D380–D384. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Ziegler, U.; Groscurth, P. Morphological features of cell death. Physiology 2004, 19, 124–128. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Rese. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Lipponen, P.; Aaltomaa, S.; Kosma, V.M.; Syrjänen, K. Apoptosis in breast cancer as related to histopathological characteristics and prognosis. Eur. J. Cancer 1994, 30, 2068–2073. [Google Scholar] [CrossRef]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Crown, J.P. The platinum agents: A role in breast cancer treatment? Semin. Oncol. 2001, 28 (Suppl. 3), 28–37. [Google Scholar] [CrossRef]
- Cai, H.; Li, J.; Gu, B.; Xiao, Y.; Chen, R.; Liu, X.; Xie, X.; Cao, L. Extracts of Cordyceps sinensis inhibit breast cancer cell metastasis via down-regulation of metastasis-related cytokines expression. J. Ethnopharmacol. 2018, 214, 106–112. [Google Scholar] [CrossRef]
- Lee, H.J.; Burger, P.; Vogel, M.; Friese, K.; Brüning, A. The nucleoside antagonist cordycepin causes DNA double strand breaks in breast cancer cells. Investig. New Drugs 2012, 30, 1917–1925. [Google Scholar] [CrossRef]
- Staring, K.E. Comparison of Wild and Cultivated Extracts of Cordyceps Sinensis Apoptotic Potential. 2013. Available online: https://pdfs.semanticscholar.org/b35d/82573910f61b555d0d502be0dc741602f4a8.pdf (accessed on 15 August 2019).
- Cohen, G.; Sun, X.; Snowden, R.; Dinsdale, D.; Skilleter, D. Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem. J. 1992, 286, 331–334. [Google Scholar] [CrossRef]
- Koc, Y.; Urbano, A.; Sweeney, E.; McCaffrey, R. Induction of apoptosis by cordycepin in ADA-inhibited TdT-positive leukemia cells. Leukemia 1996, 10, 1019–1024. [Google Scholar]
- Chaicharoenaudomrung, N.; Jaroonwitchawan, T.; Noisa, P. Cordycepin induces apoptotic cell death of human brain cancer through the modulation of autophagy. Toxicol. Vitr. 2018, 46, 113–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.X.; Yuan, R.Y.; Ren, T.; Shao, Z.Y.; Wang, H.F.; Cai, W.L.; Chen, L.T.; Wang, X.A.; Wang, P. cordycepin induces apoptosis in human pancreatic cancer cells via the mitochondrial-mediated intrinsic pathway and suppresses tumor growth in vivo. OncoTargets Ther. 2018, 11, 4479. [Google Scholar] [CrossRef]
- Zhang, C.; Zhong, Q.; Zhang, X.; Hu, D.; He, X.; Li, Q.; Feng, T. Effects of Cordycepin on Proliferation, Apoptosis and NF-κB Signaling Pathway in A549 Cells. J. Chin. Med. Mater. 2015, 38, 786–789. [Google Scholar]
- Chen, Y.H.; Wang, J.Y.; Pan, B.S.; Mu, Y.F.; Lai, M.S.; So, E.C.; Wong, T.S.; Huang, B.M. Cordycepin enhances cisplatin apoptotic effect through caspase/MAPK pathways in human head and neck tumor cells. OncoTargets Ther. 2013, 6, 983. [Google Scholar]
- Garber, K. New apoptosis drugs face critical test. Nat. Biotechnol. 2005, 23, 409–411. [Google Scholar] [CrossRef]
- Jänicke, R.U. MCF-7 breast carcinoma cells do not express caspase-3. Breast Cancer Res. Treat. 2009, 117, 219–221. [Google Scholar] [CrossRef]
- Wang, S.; He, M.; Li, L.; Liang, Z.; Zou, Z.; Tao, A. Cell-in-cell death is not restricted by caspase-3 deficiency in MCF-7 cells. J. Breast Cancer 2016, 19, 231–241. [Google Scholar] [CrossRef]



| Term | Overlap | Adjusted p-Value | Z-Score | Combined Score | Genes |
|---|---|---|---|---|---|
| Hedgehog signaling pathway | 2/47 | 0.002 | −55.763 | 410.509 | SHH, BCL2 |
| Apoptosis | 5/143 | >0.001 | −4.979 | 81.932 | CASP8, BCL2, BAX, XIAP, TNF |
| p53 signaling pathway | 3/72 | >0.001 | −6.464 | 68.700 | CASP8, BCL2, BAX |
| Estrogen signaling pathway | 2/137 | 0.011 | −0.946 | 4.964 | BCL2, MMP9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Lee, W.-Y.; Jung, K.; Kwon, Y.S.; Kim, D.; Hwang, G.S.; Kim, C.-E.; Lee, S.; Kang, K.S. The Inhibitory Effect of Cordycepin on the Proliferation of MCF-7 Breast Cancer Cells, and Its Mechanism: An Investigation Using Network Pharmacology-Based Analysis. Biomolecules 2019, 9, 414. https://doi.org/10.3390/biom9090414
Lee D, Lee W-Y, Jung K, Kwon YS, Kim D, Hwang GS, Kim C-E, Lee S, Kang KS. The Inhibitory Effect of Cordycepin on the Proliferation of MCF-7 Breast Cancer Cells, and Its Mechanism: An Investigation Using Network Pharmacology-Based Analysis. Biomolecules. 2019; 9(9):414. https://doi.org/10.3390/biom9090414
Chicago/Turabian StyleLee, Dahae, Won-Yung Lee, Kiwon Jung, Yong Sam Kwon, Daeyoung Kim, Gwi Seo Hwang, Chang-Eop Kim, Sullim Lee, and Ki Sung Kang. 2019. "The Inhibitory Effect of Cordycepin on the Proliferation of MCF-7 Breast Cancer Cells, and Its Mechanism: An Investigation Using Network Pharmacology-Based Analysis" Biomolecules 9, no. 9: 414. https://doi.org/10.3390/biom9090414
APA StyleLee, D., Lee, W.-Y., Jung, K., Kwon, Y. S., Kim, D., Hwang, G. S., Kim, C.-E., Lee, S., & Kang, K. S. (2019). The Inhibitory Effect of Cordycepin on the Proliferation of MCF-7 Breast Cancer Cells, and Its Mechanism: An Investigation Using Network Pharmacology-Based Analysis. Biomolecules, 9(9), 414. https://doi.org/10.3390/biom9090414








