Study of the Combination of Self-Activating Photodynamic Therapy and Chemotherapy for Cancer Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Coelenterazine-Based Photosensitizers
2.2. Photophysical Properties and Detection of Singlet Oxygen
2.3. Cell Lines and Reagents
2.4. Cell Treatment
2.5. Cell Viability Assay
2.6. Statistical Analyzes
3. Results and Discussion
3.1. Photophysical and Photochemical Characterization of the Photosensitizers
3.2. In Vitro Cytotoxicity of the Photosensitizers
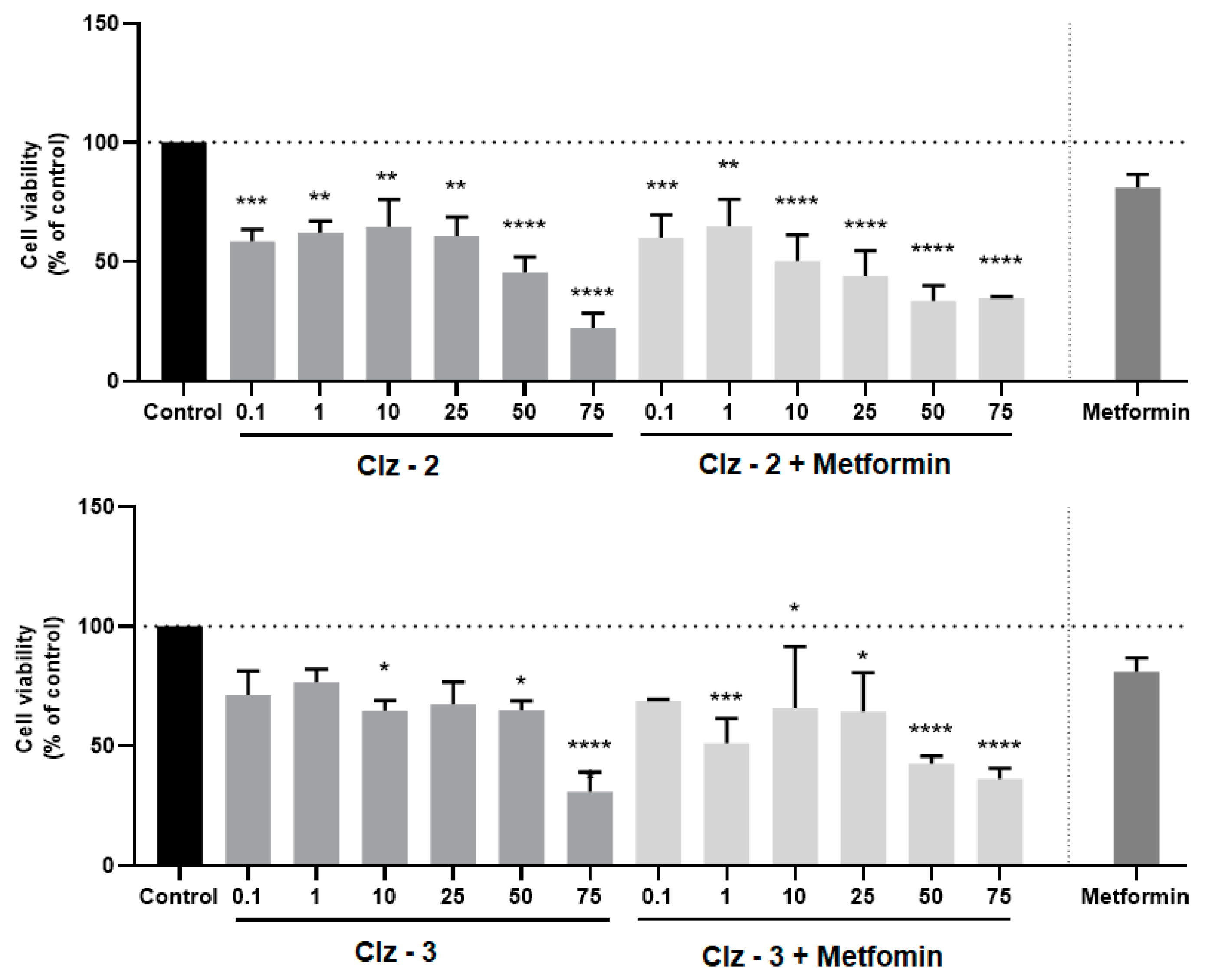
3.3. Co-Treatment Studies
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cancer Research UK. Worldwide Cancer Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer#heading-One (accessed on 8 July 2019).
- Malani, P.N. Harrison’s Principles of Internal Medicine. JAMA 2012, 308, 1813–1814. [Google Scholar] [CrossRef]
- Dawood, S.; Austin, L.; Cristofanilli, M. Cancer stem cells: Implications for cancer therapy. Oncology (Williston Park) 2014, 28, 1101–1107. [Google Scholar]
- Kreso, A.; Dick, J.E. Evolution of the cancer stem cell model. Cell Stem Cell 2014, 14, 275–291. [Google Scholar] [CrossRef]
- Foucquier, J.; Guedj, M. Analysis of drug combinations: Current methodological landscape. Pharmacol. Res. Perspect. 2015, 3, e00149. [Google Scholar] [CrossRef]
- Silva, S.; Almeida, A.J.; Vale, N. Combination of Cell-Penetrating Peptides with Nanoparticles for Therapeutic Application: A Review. Biomolecules 2019, 9, 22. [Google Scholar] [CrossRef]
- Correia, A.; Silva, D.; Correira, A.; Vilanova, M.; Gartner, F.; Vale, N. Study of New Therapeutic Strategies to Combat Breast Cancer Using Drug Combinations. Biomolecules 2018, 8, 175. [Google Scholar] [CrossRef]
- Lee, M.J.; Ye, A.S.; Gardino, A.K.; Heijink, A.M.; Sorger, P.K.; MacBeath, G.; Yaffe, M.B. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell 2012, 149, 780–794. [Google Scholar] [CrossRef]
- Cao, P.; Bae, Y. Polymer nanoparticulate drug delivery and combination cancer therapy. Future Oncol. 2012, 8, 1471–1480. [Google Scholar] [CrossRef]
- Han, K.; Jeng, E.E.; Hess, G.T.; Morgens, D.W.; Li, A.; Bassik, M.C. Synergistic drug combinations for cancer identified in a CRISPR screen for pairwise genetic interactions. Nat. Biotechnol. 2017, 35, 463–474. [Google Scholar] [CrossRef]
- National Cancer Institute. Combining Therapies to Improve Outcomes. Available online: https://www.cancer.gov/about-nci/budget/plan/treating/combining-therapies (accessed on 4 August 2018).
- Lu, D.-Y. Drug combinations. In Personalized Cancer Chemotherapy; Woodhead Publishing: Oxford, UK, 2015; pp. 37–41. [Google Scholar]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.-I.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef]
- Zang, Q.; Yu, J.; Yu, W.; Qian, J.; Hu, R.; Tang, B.Z. Red-emissive azabenzanthrone derivatives for photodynamic therapy irradiated with ultralow light power density and two-photon imaging. Chem. Sci. 2018, 9, 5165–5171. [Google Scholar] [CrossRef]
- Li, B.; Lin, L.; Lin, H.; Wilson, B.C. Photosensitized singlet oxygen generation and detection: Recent advances and future perspectives in cancer photodynamic therapy. J. Biophotonics 2016, 9, 1314–1325. [Google Scholar] [CrossRef]
- Magalhães, C.M.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Chemiluminescence and Bioluminescence as an Excitation Source in the Photodynamic Therapy of Cancer: A Critical Review. ChemPhysChem 2016, 4, 2286–2294. [Google Scholar] [CrossRef]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef]
- Pinto da Silva, L.; Esteves da Silva, J.C.G. Firefly chemiluminescence and bioluminescence: Efficient generation of excited states. ChemPhysChem 2012, 13, 2257–2262. [Google Scholar] [CrossRef]
- Vacher, M.; Fdez Galván, I.; Ding, B.W.; Schramm, S.; Berraud-Pache, R.; Naumov, P.; Ferré, N.; Liu, Y.J.; Navizet, I.; Roca-Sanjuán, D.; et al. Chemi-and Bioluminescence of Cyclic Peroxides. Chem. Rev. 2018, 118, 6927–6974. [Google Scholar] [CrossRef]
- Vieira, J.; Pinto da Silva, L.; Esteves da Silva, J.C.G. Advances in the knowledge of light emission by firefly luciferin and oxyluciferin. J. Photochem. Photobiol. B 2012, 117, 33–39. [Google Scholar] [CrossRef]
- Kaskova, Z.M.; Tsarkova, A.S.; Yampolsky, I.V. 1001 lights: Luciferins, luciferases, their mechanisms of action and applications in chemical analysis, biology and medicine. Chem. Soc. Rev. 2016, 45, 6048–6077. [Google Scholar] [CrossRef]
- Pinto da Silva, L.; Simkovitch, R.; Huppert, D.; Esteves da Silva, J.C.G. Oxyluciferin photoacidity: The missing element for solving the keto-enol mystery? ChemPhysChem 2013, 14, 3441–3446. [Google Scholar] [CrossRef]
- Mao, D.; Wu, W.; Ji, S.; Chen, C.; Hy, F.; Kong, D.; Ding, D.; Liu, B. Chemiluminescence-Guided Cancer Therapy Using a Chemiexcited Photosensitizer. Chem 2017, 3, 991–1007. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, P.; Pan, W.; Li, N.; Tang, B. A biomimetic nanoreator for synergistic chemiexcited photodynamic therapy and starvation therapy against tumor metastasis. Nat. Commun. 2018, 9, 5044. [Google Scholar] [CrossRef]
- Yuan, H.; Chong, H.; Wang, B.; Zhu, C.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Chemical Molecule-Induced Light-Activated System for Anticancer and Antifungal Activities. J. Am. Chem. Soc. 2012, 134, 13184–13187. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chen, C.W.; Yu, H.P.; Ling, Y.F.; Lai, P.S. Bioluminescence resonance energy transfer using luciferase-immobilized quantum dots for self-illuminated photodynamic therapy. Biomaterials 2013, 34, 1204–1212. [Google Scholar] [CrossRef]
- Zhang, Y.; Pang, L.; Ma, C.; Tu, Q.; Zhang, R.; Saeed, E.; Mahmoud, A.E.; Wang, J. Small Molecule-Initiated Light-Activated Semiconducting Polymer Dots: An Integrated Nanoplatform for Targeted Photodynamic Therapy and Imaging of Cancer Cells. Anal. Chem. 2014, 86, 3092–3099. [Google Scholar] [CrossRef]
- Kervinen, M.; Patsi, J.; Finel, M.; Hassinen, I.E. Lucigenin and coelenterazine as superoxide probes in mitochondrial and bacterial membranes. Anal. Biochem. 2004, 324, 45–51. [Google Scholar] [CrossRef]
- Teranishi, K. Luminescence of Imidazo [1, 2-a] pyrazin-3(7H)-one compounds. Bioorg. Chem. 2007, 35, 82–111. [Google Scholar] [CrossRef]
- De Wael, F.; Jeanjot, P.; Moens, C.; Verbeuren, T.; Cordi, A.; Bouskela, E.; Rees, J.F.; Marchand-Brynaert, J. In vitro and in vivo studies of 6, 8-(diaryl) Imidazo [1, 2-a] pyrazin-3(7H)-ones as new antioxidants. Bioorg. Med. Chem. 2009, 17, 4336–4344. [Google Scholar] [CrossRef]
- Figueira, T.R.; Barros, M.H.; Camargo, A.A.; Castilho, R.F.; Ferreira, J.C.; Kowaltowski, A.J.; Sluse, F.E.; Souza-Pinto, N.C.; Vercesi, A.E. Mitochondria as a source of reactive oxygen and nitrogen species: From molecular mechanisms to human health. Antioxid. Redox Signal. 2013, 18, 2029–2074. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Chandel, N.S. Mitochondria reactive oxygen species and cancer. Cancer Metab. 2014, 2, 17. [Google Scholar] [CrossRef]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updates 2004, 7, 97–110. [Google Scholar] [CrossRef]
- Ahmad, I. Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives. Eur. J. Med. Chem. 2018, 143, 515–531. [Google Scholar]
- Whitburn, J.; Edwards, C.M.; Sooriakumaran, P. Metformin and prostate cancer: A new role for an old drug. Curr. Urol. Rep. 2017, 18, 46. [Google Scholar] [CrossRef]
- Adamczyk, M.; Akireddy, S.R.; Johnson, D.; Mattingly, P.G.; Pan, Y.; Reddy, R.E. Synthesis of 3, 7-dihydroimidazo [1, 2a] pyrazine-3-ones and their chemiluminescent properties. Tetrahedron 2003, 59, 8129–8142. [Google Scholar] [CrossRef]
- Dong, C.; Liu, Z.; Wang, S.; Zheng, B.; Guo, W.; Yang, W.; Gong, X.; Wu, X.; Wang, H.; Chang, J.A. Protein-Polymer Bioconjugate-Coated Upconversion Nanosystem for Simultaneous Tumor Cell Imaging, Photodynamic Therapy, and Chemotherapy. ACS Appl. Mater. Interfaces 2016, 8, 32688–32698. [Google Scholar] [CrossRef]
- Magalhães, C.M.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Study of Coelenterazine luminescence: Electrostatic interactions as the controlling factor for efficient chemiexcitation. J. Lumin. 2018, 199, 339–347. [Google Scholar] [CrossRef]
- Magalhães, C.M.; Esteves da Silva, J.C.G.; Pinto da Silva, L. Comparative study of the chemiluminescence of Coelenterazine, Coelenterazine-e and Cypridina luciferin. J. Photochem. Photobiol. B 2019, 190, 21–31. [Google Scholar] [CrossRef]
- Pinto da Silva, L.; Pereira, R.F.J.; Magalhães, C.M.; Esteves da Silva, J.C.G. Mechanistic Insight into Cypridina Bioluminescence with a Combined Experimental and Theoretical Chemiluminescent Approach. J. Phys. Chem. B 2017, 121, 7862–7871. [Google Scholar] [CrossRef]
- Pinto da Silva, L.; Magalhães, C.M.; Esteves da Silva, J.C.G. Interstate Crossing-Induced Chemiexcitation Mechanism as the Basis for Imidazopyrazinone Bioluminescence. ChemistrySelect 2016, 1, 3343–3356. [Google Scholar] [CrossRef]
- Pinto da Silva, L.; Magalhães, C.M.; Crista, D.M.A.; Esteves da Silva, J.C.G. Theoretical modulation of singlet/triplet chemiexcitation of chemiluminescent imidazopyrazinone dioxetanone via C8 substitution. Photochem. Photobiol. Sci. 2017, 16, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Pinto da Silva, L.; Magalhães, C.M. Mechanistic insights into the efficient intramolecular chemiexcitation of dioxetanones from TD-DFT and multireference calculations. Int. J. Quantum Chem. 2019, 119, e25881. [Google Scholar] [CrossRef]
- Zou, J.; Yin, Z.; Ding, K.; Tang, K.; Li, J.; Si, W.; Shao, J.; Zhang, Q.; Huang, W.; Dong, K. BODIPY Derivatives for Photodynamic Therapy: Influence of Configuration versus Heavy Atom Effect. ACS Appl. Mater. Interfaces 2017, 9, 32475–32481. [Google Scholar] [CrossRef] [PubMed]








| MCF-7 IC50 a | PC-3 IC50 a | |||
|---|---|---|---|---|
| 24 h | 72 h | 24 h | 72 h | |
| Metformin | N.D. | N.D. | 1.270 ± 0.416 | 0.813 ± 0.261 |
| Tamoxifen | 2.219 ± 0.194 | 11.07 ± 0.02 | N.D. | N.D. |
| Clz–1 | >100 | 12.18 ± 0.06 | 0.048 ± 0.426 | 12.11 ± 0.15 |
| Clz–2 | 47.31 b | 3.00 ± 0.08 | 0.388 ± 0.459 | 1.647 ± 0.366 |
| Clz–3 | >100 | 49.59 b | 0.530 ± 0.525 | 3.949 ± 0.362 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto da Silva, L.; Magalhães, C.M.; Núñez-Montenegro, A.; Ferreira, P.J.O.; Duarte, D.; Rodríguez-Borges, J.E.; Vale, N.; Esteves da Silva, J.C.G. Study of the Combination of Self-Activating Photodynamic Therapy and Chemotherapy for Cancer Treatment. Biomolecules 2019, 9, 384. https://doi.org/10.3390/biom9080384
Pinto da Silva L, Magalhães CM, Núñez-Montenegro A, Ferreira PJO, Duarte D, Rodríguez-Borges JE, Vale N, Esteves da Silva JCG. Study of the Combination of Self-Activating Photodynamic Therapy and Chemotherapy for Cancer Treatment. Biomolecules. 2019; 9(8):384. https://doi.org/10.3390/biom9080384
Chicago/Turabian StylePinto da Silva, Luís, Carla M. Magalhães, Ara Núñez-Montenegro, Paulo J.O. Ferreira, Diana Duarte, José E. Rodríguez-Borges, Nuno Vale, and Joaquim C.G. Esteves da Silva. 2019. "Study of the Combination of Self-Activating Photodynamic Therapy and Chemotherapy for Cancer Treatment" Biomolecules 9, no. 8: 384. https://doi.org/10.3390/biom9080384
APA StylePinto da Silva, L., Magalhães, C. M., Núñez-Montenegro, A., Ferreira, P. J. O., Duarte, D., Rodríguez-Borges, J. E., Vale, N., & Esteves da Silva, J. C. G. (2019). Study of the Combination of Self-Activating Photodynamic Therapy and Chemotherapy for Cancer Treatment. Biomolecules, 9(8), 384. https://doi.org/10.3390/biom9080384









