Bioactivities and Medicinal Value of Solanesol and Its Accumulation, Extraction Technology, and Determination Methods
Abstract
1. Introduction
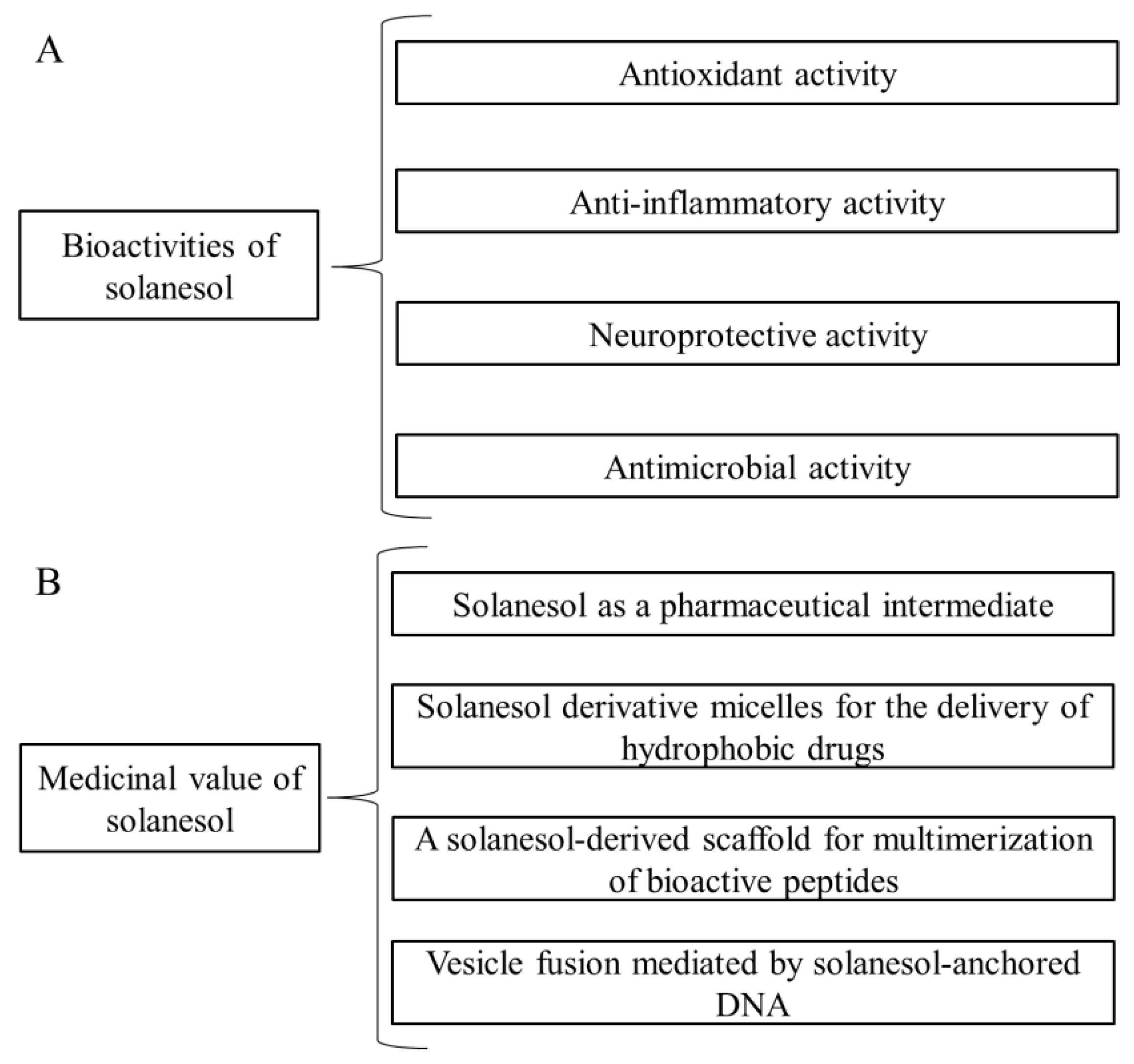
2. Bioactivities of Solanesol
2.1. Antioxidant Activity
2.2. Anti-Inflammatory Activity
2.3. Neuroprotective Activity
2.4. Antimicrobial Activity
3. Medicinal Value of Solanesol
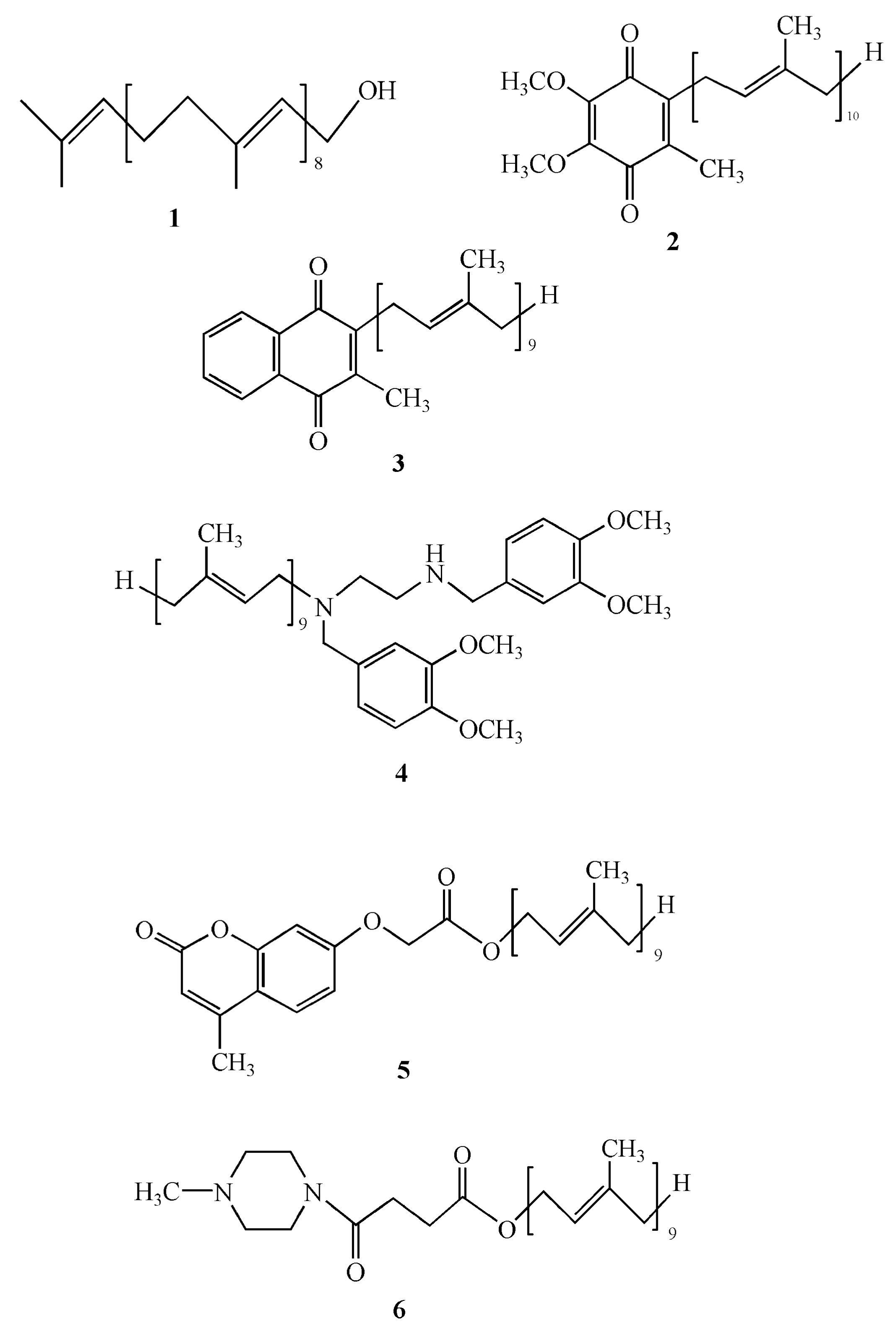
3.1. Solanesol as a Pharmaceutical Intermediate
3.2. Solanesol Derivative Micelles for the Delivery of Hydrophobic Drugs
3.3. Other Values of Solanesol
3.3.1. A Solanesol-Derived Scaffold for Multimerization of Bioactive Peptides
3.3.2. Vesicle Fusion Mediated by Solanesol-Anchored DNA
4. Factors Associated with Solanesol Accumulation in Plants
4.1. Genetic Factors
4.2. Environmental Factors
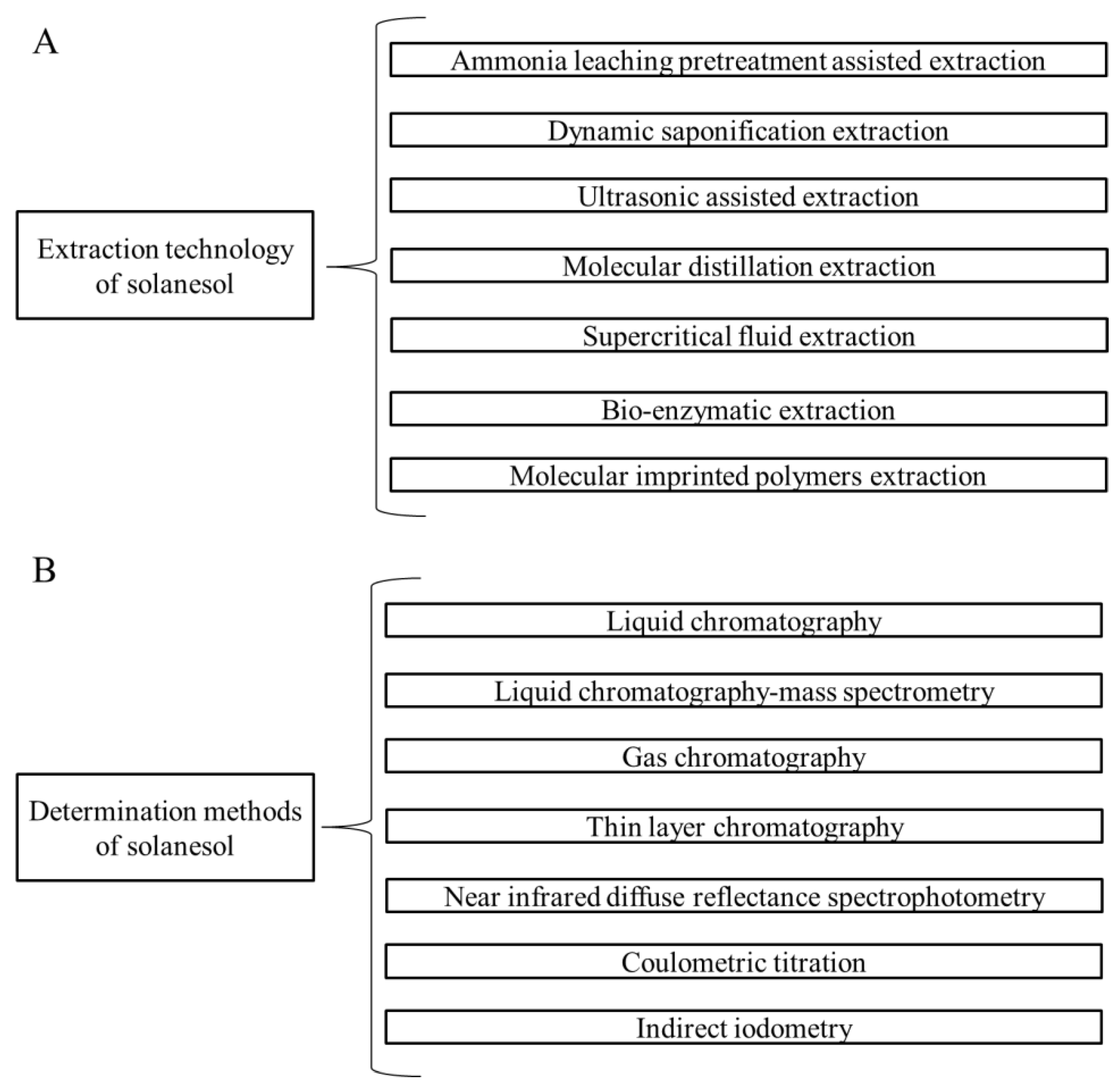
5. Extraction Technology of Solanesol
5.1. Ammonia Leaching Pretreatment Assisted Extraction
5.2. Dynamic Saponification Extraction
5.3. Ultrasonic Assisted Extraction
5.4. Molecular Distillation Extraction
5.5. Supercritical Fluid Extraction
5.6. Bio-Enzymatic Extraction
5.7. Molecular Imprinted Polymers Extraction
6. Determination Methods of Solanesol
6.1. Liquid Chromatography
6.1.1. Ultraviolet Detector
6.1.2. Refractive Index Detector
6.1.3. Evaporative Light Scattering Detector
6.2. Liquid Chromatography-Mass Spectrometry
6.3. Gas Chromatography
6.4. Thin Layer Chromatography
6.5. Near Infrared Diffuse Reflectance Spectrophotometry
6.6. Coulometric Titration
6.7. Indirect Iodometry
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Yan, N.; Liu, Y.; Gong, D.; Du, Y.; Zhang, H.; Zhang, Z. Solanesol: A review of its resources, derivatives, bioactivities, medicinal applications, and biosynthesis. Phytochem. Rev. 2015, 14, 403–417. [Google Scholar] [CrossRef]
- Yan, N.; Zhang, H.; Zhang, Z.; Shi, J.; Timko, M.P.; Du, Y.; Liu, X.; Liu, Y. Organ-and growing stage-specific expression of solanesol biosynthesis genes in Nicotiana tabacum reveals their association with solanesol content. Molecules 2016, 21, 1536. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Liu, Y.; Zhang, H.; Du, Y.; Liu, X.; Zhang, Z. Solanesol biosynthesis in plants. Molecules 2017, 22, 510. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Du, Y.; Zhang, H.; Zhang, Z.; Liu, X.; Shi, J.; Liu, Y. RNA sequencing provides insights into the regulation of solanesol biosynthesis in Nicotiana tabacum induced by moderately high temperature. Biomolecules 2018, 8, 165. [Google Scholar] [CrossRef] [PubMed]
- Arab, M.; Bahramian, B.; Schindeler, A.; Fathi, A.; Valtchev, P.; McConchie, R.; Dehghani, F. A benign process for the recovery of solanesol from tomato leaf waste. Heliyon 2019, 5, e01523. [Google Scholar] [CrossRef]
- Roe, S.J.; Oldfield, M.F.; Geach, N.; Baxter, A. A convergent stereocontrolled synthesis of [3-14C] solanesol. J. Label. Compound. Radiopharm. 2013, 56, 485–491. [Google Scholar] [CrossRef]
- Yan, N.; Zhao, T.; Xiang, D.; Gong, D.; Zhang, H.; Du, Y.; Liu, X.; Zhang, Z.; Liu, Y. Cloning and expression analysis of solanesyl diphosphate synthase (NtSPS) genes in Nicotiana tabacum. Chin. Tob. Sci. 2016, 37, 45–51. [Google Scholar]
- Taylor, M.A.; Fraser, P.D. Solanesol: Added value from Solanaceous waste. Phytochemistry 2011, 72, 1323–1327. [Google Scholar] [CrossRef]
- Campbell, R.; Freitag, S.; Bryan, G.J.; Stewart, D.; Taylor, M.A. Environmental and genetic factors associated with solanesol accumulation in potato leaves. Front. Plant Sci. 2016, 7, 1263. [Google Scholar] [CrossRef]
- Xiang, D.; Yao, Z.; Liu, Y.; Gai, X.; Du, Y.; Zhang, Z.; Yan, N.; Wang, A.; Fu, Q. Analysis on solanesol content and genetic diversity of Chinese flue-cured tobacco (Nicotiana tabacum L.). Crop Sci. 2017, 57, 847–855. [Google Scholar] [CrossRef]
- Rowland, R.L.; Latimer, P.H.; Giles, J.A. Flue-cured tobacco. I. Isolation of solanesol, an unsaturated alcohol. J. Am. Chem. Soc. 1956, 78, 4680–4683. [Google Scholar] [CrossRef]
- Huang, W.; Li, Z.; Niu, H.; Wang, J.; Qin, Y. Bioactivity of solanesol extracted from tobacco leaves with carbon dioxide–ethanol fluids. Biochem. Eng. J. 2008, 42, 92–96. [Google Scholar] [CrossRef]
- Yao, X.; Bai, Q.; Yan, D.; Li, G.; Lü, C.; Xu, H. Solanesol protects human hepatic L02 cells from ethanol-induced oxidative injury via upregulation of HO-1 and Hsp70. Toxicol. In Vitro 2015, 29, 600–608. [Google Scholar] [CrossRef]
- Yao, X.; Lu, B.; Lü, C.; Bai, Q.; Yan, D.; Wu, Y.; Hong, Z.; Xu, H. Solanesol induces the expression of heme oxygenase-1 via p38 and Akt and suppresses the production of proinflammatory cytokines in RAW264.7 cells. Food Funct. 2017, 8, 132–141. [Google Scholar] [CrossRef]
- Mehan, S.; Rajput, M.; Dudi, R.; Ghimire, K. Neuroprotective strategies of solanesol in mitochondrial impairment in experimentally induced Huntington disease. J. Pharm. Toxicol. 2018, 1, 3–7. [Google Scholar]
- Li, J.; Chase, H.A. Development of adsorptive (non-ionic) macroporous resins and their uses in the purification of pharmacologically-active natural products from plant sources. Nat. Prod. Rep. 2010, 27, 1493–1510. [Google Scholar] [CrossRef]
- Qin, B.; Liu, L.; Wu, X.; Liang, F.; Hou, T.; Pan, Y.; Song, S. mPEGylated solanesol micelles as redox-responsive nanocarriers with synergistic anticancer effect. Acta Biomater. 2017, 64, 211–222. [Google Scholar] [CrossRef]
- Qin, B.; Liu, L.; Pan, Y.; Zhu, Y.; Wu, X.; Song, S.; Han, G. PEGylated solanesol for oral delivery of coenzyme Q10. J. Agric. Food Chem. 2017, 65, 3360–3367. [Google Scholar] [CrossRef]
- Ma, Y.; Hou, T.; Zhang, J.; Zhou, Y.; Xu, R.; Liu, Y.; Wang, Y. Solanesol antioxidation. Food Res. Dev. 2011, 32, 8–12. [Google Scholar]
- Bai, Q.; Yu, J.; Su, M.; Bai, R.; Katumata, G.; Katumata, M.; Chen, X. Antioxidant function of solanesol and its inhibitory effect on tyrosinase. J. Biomed. Eng. 2014, 31, 833–836, 841. [Google Scholar]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef]
- Mayer, M.P. Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem. Sci. 2013, 38, 507–514. [Google Scholar] [CrossRef]
- Banožić, M.; Banjari, I.; Jakovljević, M.; Šubarić, D.; Tomas, S.; Babić, J.; Jokić, S. Optimization of ultrasound-assisted extraction of some bioactive compounds from tobacco waste. Molecules 2019, 24, 1611. [Google Scholar] [CrossRef]
- Sridevi, P.; Vijayanand, P.; Raju, M.B. Formulation and evaluation of anti-inflammatory herbal gel containing isolated solanesol. Ann. Phytomed. 2017, 6, 127–131. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Ren, X.; Li, Q.; Tang, J.; Tang, L.; Hua, Y.; Liu, J.; Wang, H. The effect of solanesol on experimental models of periodontitis in rats. Chin. J. Ethnomed. Ethnopharm. 2018, 27, 31–40. [Google Scholar]
- Gao, Y.; Zhou, X.; Wang, F.; Zhang, H. Interaction between solanesol and bovine serum albumin. Chem. Ind. For. Prod. 2011, 31, 60–64. [Google Scholar]
- Zheng, Z.; Diamond, M.I. Huntington disease and the huntingtin protein. Prog. Mol. Biol. Transl. Sci. 2012, 107, 189–214. [Google Scholar]
- Mehan, S.; Monga, V.; Rani, M.; Dudi, R.; Ghimire, K. Neuroprotective effect of solanesol against 3-nitropropionic acid-induced Huntington’s disease-like behavioral, biochemical, and cellular alterations: Restoration of coenzyme-Q10-mediated mitochondrial dysfunction. Indian J. Pharmacol. 2018, 50, 309–319. [Google Scholar] [CrossRef]
- Chen, H.B.; Zhang, J.; Yu, H.X.; Hu, Q.L. In vitro study on the antibacterial of a medicinal intermediate, solanesol. Qilu Pharm. Aff. 2007, 26, 558–559. [Google Scholar]
- Duan, S.; Du, Y.; Hou, X.; Li, D.; Ren, X.; Dong, W.; Zhao, W.; Zhang, Z. Inhibitory effects of tobacco extracts on eleven phytopathogenic fungi. Nat. Prod. Res. Dev. 2015, 27, 470–474. [Google Scholar]
- Bajda, A.; Konopka-Postupolska, D.; Krzymowska, M.; Hennig, J.; Skorupinska-Tudek, K.; Surmacz, L.; Wojcik, J.; Matysiak, Z.; Chojnacki, T.; Skorzynska-Polit, E.; et al. Role of polyisoprenoids in tobacco resistance against biotic stresses. Physiol. Plant. 2009, 135, 351–364. [Google Scholar] [CrossRef]
- Bentinger, M.; Tekle, M.; Dallner, G. Coenzyme Q-Biosynthesis and functions. Biochem. Biophys. Res. Commun. 2010, 396, 74–79. [Google Scholar] [CrossRef]
- Sarmiento, A.; Diaz-Castro, J.; Pulido-Moran, M.; Kajarabille, N.; Guisado, R.; Ochoa, J.J. Coenzyme Q10 supplementation and exercise in healthy humans: A systematic review. Curr. Drug Metab. 2016, 17, 345–358. [Google Scholar] [CrossRef]
- Mezawa, M.; Takemoto, M.; Onishi, S.; Ishibashi, R.; Ishikawa, T.; Yamaga, M.; Fujimoto, M.; Okabe, E.; He, P.; Kobayashi, K.; et al. The reduced form of coenzyme Q10 improves glycemic control in patients with type 2 diabetes: An open label pilot study. BioFactors 2012, 38, 416–421. [Google Scholar] [CrossRef]
- Hamidi, M.S.; Gajic-Veljanoski, O.; Cheung, A.M. Vitamin K and bone health. J. Clin. Densitom. 2013, 16, 409–413. [Google Scholar] [CrossRef]
- Villa, J.K.D.; Diaz, M.A.N.; Pizziolo, V.R.; Martino, H.S.D. Effect of vitamin K in bone metabolism and vascular calcification: A review of mechanisms of action and evidences. Crit. Rev. Food Sci. 2017, 57, 3959–3970. [Google Scholar] [CrossRef]
- Enokida, H.; Gotanda, T.; Oku, S.; Imazono, Y.; Kubo, H.; Hanada, T.; Suzuki, S.; Inomata, K.; Kishiye, T.; Tahara, Y.; et al. Reversal of P-glycoprotein-mediated paclitaxel resistance by new synthetic isoprenoids in human bladder cancer cell line. Jpn. J. Cancer Res. 2002, 93, 1037–1046. [Google Scholar] [CrossRef]
- Sidorova, T.A.; Nigmatov, A.G.; Kakpakova, E.S.; Stavrovskaya, A.A.; Gerassimova, G.K.; Shtil, A.A.; Serebryakov, E.P. Effects of isoprenoid analogues of SDB-ethylenediamine on multidrug resistant tumour cells alone and in combination with chemotherapeutic drugs. J. Med. Chem. 2002, 45, 5330–5339. [Google Scholar] [CrossRef]
- Xiao, K.; Dai, Y.; Shi, W.; Li, J.; Li, Y.; Yin, S. Synthesis and antitumor activities of the diacid solanesyl 5-fluorouracil esters derivatives. Chin. J. Org. Chem. 2012, 32, 169–173. [Google Scholar] [CrossRef]
- Daneial, B.; Joseph, J.P.V.; Ramakrishna, G. Molecular dynamics simulation analysis of Focal Adhesive Kinase (FAK) docked with solanesol as an anti-cancer agent. Bioinformation 2017, 13, 274–283. [Google Scholar] [CrossRef]
- Srivastava, S.; Raj, K.; Khare, P.; Bhaduri, A.P.; Chander, R.; Raghubir, R.; Mahendra, K.; Rao, C.V.N.; Prabhu, S.R. Novel hybrid natural products derived from solanesol as wound healing agents. Indian J. Chem. 2009, 48, 237–247. [Google Scholar]
- Alleti, R.; Rao, V.; Xu, L.; Gillies, R.J.; Mash, E.A. A solanesol-derived scaffold for multimerization of bioactive peptides. J. Org. Chem. 2010, 75, 5895–5903. [Google Scholar] [CrossRef]
- Flavier, K.M.; Boxer, S.G. Vesicle fusion mediated by solanesol-anchored DNA. Biophys. J. 2017, 113, 1260–1268. [Google Scholar] [CrossRef]
- Gai, X.; Liu, Y.; Yao, Z.; Du, Y.; Yan, N.; Zhang, H.; Dai, P. Study on the correlation between solanesol accumulation and expression of gene encoding terpenoid synthetic enzymes in tobacco. Chin. Tob. Sci. 2017, 38, 8–14. [Google Scholar]
- Xiang, D.; Zhao, T.; Du, Y.; Zhang, Z.; Yan, N.; Huang, W.; Wang, A.; Fu, Q.; Gong, Y.; Liu, Y. Genetic analysis on solanesol content of tobacco. Chin. Tob. Sci. 2015, 36, 1–7. [Google Scholar]
- Qiao, R.; Xia, L.; Liu, L.; Yue, C. Effect of illumination intensity on solanesol content in tobacco. Guizhou Agric. Sci. 2011, 39, 49–50, 53. [Google Scholar]
- Schepartz, A.I.; Ellington, J.J.; Burk, L.G. Catalase activity and lipid contents of leaves from normal and sterile-flowered tobacco plants. Phytochemistry 1978, 17, 1787–1788. [Google Scholar] [CrossRef]
- Burton, H.R.; Leggett, E.; Philips, R.E. Factors influencing the concentration of solanesol in burley tobacco. Beit. Tabakforsch. Int. 1989, 14, 313–320. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, R.; Xing, X.; Qi, W.; Su, R.; He, Z. Enhanced extraction of solanesol from tobacco leaves by a new ammonia leaching pretreatment method. Fine Chem. 2013, 30, 32–35, 50. [Google Scholar]
- Zhao, C.; Yang, L.; Zu, Y.; Xia, L.; Xiao, C. Extraction of solanesol by dynamic saponification method. Chem. Eng. 2007, 35, 9–12. [Google Scholar]
- Zhang, Z.; Feng, X. Study on ultrasonic assisted extraction of solanesol from tobacco leaves. J. Food Sci. Biotechnol. 2007, 26, 51–53. [Google Scholar]
- Qian, C.; Zhang, J. The study of refining solanesol by molecular distillation. J. Chem. Eng. Chin. Univ. 2009, 23, 275–278. [Google Scholar]
- Wei, H.; Mi, H.; Liu, Z. Extraction and separation of solanesol from Nicotiana tobacum by supercritical fluid extraction and silica gel column chromatography. Chin. Tradit. Herb. Drugs 2005, 36, 690–692. [Google Scholar]
- Wang, Y.; Gu, W. Study on supercritical fluid extraction of solanesol from industrial tobacco waste. J. Supercrit. Fluids 2018, 138, 228–237. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhang, G.; Yin, Z. Improved extraction of solanesol from tobacco waste by enzymatic cell wall breaking. Chin. J. Biotechnol. 2013, 29, 1706–1710. [Google Scholar]
- Long, J.P.; Chen, Z.B.; Liu, X.J.; Du, X.Y. Preparation and adsorption property of solanesol molecular imprinted polymers. Des. Monomers Polym. 2015, 18, 641–649. [Google Scholar] [CrossRef]
- Ma, X.; Meng, Z.; Qiu, L.; Chen, J.; Guo, Y.; Yi, D.; Ji, T.; Jia, H.; Xue, M. Solanesol extraction from tobacco leaves by Flash chromatography based on molecularly imprinted polymers. J. Chromatogr. B 2016, 1020, 1–5. [Google Scholar] [CrossRef]
- Duan, C.; Chen, Z.; Liu, X.; Li, K.; Wang, X.; Jia, W.; Tang, Z.; Ruso, J.M.; Liu, Z. Noble surface molecularly imprinted polymer modified titanium dioxide toward solanesol adsorption selectivity study. J. Mater. Res. 2019. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.; Du, Y.; Hou, X.; Li, D.; Yan, N. The simultaneous extraction and saponification of tobacco solanesol using ultra performance liquid chromatography. Chin. Tob. Sci. 2015, 36, 79–84. [Google Scholar]
- Zhao, Y.; Du, Q. Separation of solanesol in tobacco leaves extract by slow rotary counter-current chromatography using a novel non-aqueous two-phase solvent system. J. Chromatogr. A 2007, 1151, 193–196. [Google Scholar] [CrossRef]
- Rao, R.N.; Talluri, M.K.; Krishna, T.M.; Ravindranath, K. Continuous counter current extraction, isolation and determination of solanesol in Nicotiana tobacum L. by non-aqueous reversed phase high performance liquid chromatography. J. Pharmaceut. Biomed. 2008, 46, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Chen, F.; Zhou, Y.; Chen, Z.; Li, X. Research progress of determination methods of solanesol in tobacco. Chin. Agric. Sci. Bull. 2015, 31, 279–284. [Google Scholar]
- Zhao, J.; Wang, C.; Sun, X. Determination of solanesol in the extracts of tobacco leaves by high performance liquid chromatography (HPLC). Chin. J. Chromatogr. 1997, 15, 544–545. [Google Scholar]
- Zhang, H.; Wang, H.; Chen, S. Extraction and determination of the solanesol in discarded tobacco leaf. J. Henan Univ. Technol. 2005, 26, 44–47. [Google Scholar]
- Li, L.; Qian, Q. Determination of solanesol in Nicotiana tobacum L. by HPLC-ELSD. Chin. J. Pharm. Anal. 2002, 22, 103–105. [Google Scholar]
- Zhou, H.; Liu, C. Rapid determination of solanesol in tobacco by high-performance liquid chromatography with evaporative light scattering detection following microwave-assisted extraction. J. Chromatogr. B 2006, 835, 119–122. [Google Scholar] [CrossRef]
- Zhao, C.; Li, C.; Zu, Y. Rapid and quantitative determination of solanesol in Nicotiana tabacum by liquid chromatography–tandem mass spectrometry. J. Pharmaceut. Biomed. 2007, 44, 35–40. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, R. Determination of solanesol in the extracts of tobacco leaves by electrospray ionization mass spectrometry. J. Jiangsu Polytech. Univ. 2008, 20, 56–58. [Google Scholar]
- Chen, J.; Liu, J.; Lee, F.S.C.; Wang, X. Optimization of HPLC-APCI-MS conditions for the qualitative and quantitative determination of total solanesol in tobacco leaves. J. Sep. Sci. 2008, 31, 137–142. [Google Scholar] [CrossRef]
- Severson, R.F.; Ellington, J.J.; Schlotzhauer, P.F.; Arrendale, R.F.; Schepartz, A.I. Gas chromatographic method for the determination of free and total solanesol in tobacco. J. Chromatogr. A 1977, 139, 269–282. [Google Scholar] [CrossRef]
- Sheen, S.J.; Davis, D.L.; DeJong, D.W.; Chaplin, J.F. Gas-liquid chromatographic quantification of solanesol in chlorophyll mutants of tobacco. J. Agric. Food Chem. 1978, 26, 259–262. [Google Scholar] [CrossRef]
- Chaberlain, W.J.; Severson, R.F.; Chortyk, O.T.; Sisson, V.E. Determination of solanesol in tobacco by capillary gas chromatography. J. Chromatogr. A 1990, 513, 55–60. [Google Scholar] [CrossRef]
- Liu, Y.; Yong, G.; Xu, Y.; Zhu, D.; Tong, H.; Liu, S. Simultaneous determination of free and esterified fatty alcohols, phytosterols and solanesol in tobacco leaves by GC. Chromatographia 2010, 71, 727–732. [Google Scholar] [CrossRef]
- Woollen, B.H.; Jones, D.H. Analytical methods for tobacco lipids: 1. A rapid method for the estimation of solanesol by thin-layer densitometry. J. Chromatogr. A 1971, 61, 180–182. [Google Scholar] [CrossRef]
- Li, L.; Ma, Z.; Qian, Q.; Cong, X. Determination of solanesol in tobacco leaves by TLC scanning. Chin. Tradit. Herb. Drugs 2002, 33, 420–421. [Google Scholar]
- Yu, H.; You, W.; Rao, L.; Cao, Y.; Li, G.; Zhang, A. Detecting content of solanesol in tobacco leaves by TLC. Sichuan Food Ferment. 2006, 42, 58–60. [Google Scholar]
- Fu, Q.; Du, Y.; Zhang, H.; Hou, X.; Li, Y.; Li, D. Determination of solanesol content of flue-cured tobacco leaves by near-infrared diffuse reflectance technology. Nat. Prod. Res. Dev. 2015, 27, 84–88. [Google Scholar]
- Liu, K.; Liu, M.; Li, D. Determination of solanesol by coulometric titration. Anal. Lett. 1998, 31, 1947–1954. [Google Scholar]
- Zhao, G.; Qu, J.; Liu, M.; Liu, K.; Du, Z. Application of chemical modified electrode in coulometric titration for determination of solanesol. Anal. Lett. 2002, 35, 785–795. [Google Scholar] [CrossRef]
- Zheng, K. One kind of method separating purification and measuring solanesol content from matter distilled of tobacco leaf. J. Guizhou Norm. Univ. 2003, 21, 7–9. [Google Scholar]
- Wei, Y.; Liu, J. Test solanesol with indirect iodimetry. Appl. Chem. Ind. 2009, 38, 1688–1691. [Google Scholar]
- Duan, S.; Du, Y.; Hou, X.; Yan, N.; Dong, W.; Mao, X.; Zhang, Z. Chemical basis of the fungicidal activity of tobacco extracts against Valsa mali. Molecules 2016, 21, 1743. [Google Scholar] [CrossRef]
- Yan, N.; Du, Y.; Liu, X.; Zhang, H.; Liu, Y.; Zhang, P.; Gong, D.; Zhang, Z. Chemical structures, biosynthesis, bioactivities, biocatalysis and semisynthesis of tobacco cembranoids: An overview. Ind. Crops Prod. 2016, 83, 66–80. [Google Scholar] [CrossRef]
- Yan, N.; Du, Y.; Liu, X.; Zhang, H.; Liu, Y.; Shi, J.; Xue, S.J.; Zhang, Z. Analyses of effects of α-cembratrien-diol on cell morphology and transcriptome of Valsa mali var. mali. Food Chem. 2017, 214, 110–118. [Google Scholar] [CrossRef]
- Yan, N.; Du, Y.; Liu, X.; Zhang, H.; Liu, Y.; Zhang, Z. A review on bioactivities of tobacco cembranoid diterpenes. Biomolecules 2019, 9, 30. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, N.; Liu, Y.; Liu, L.; Du, Y.; Liu, X.; Zhang, H.; Zhang, Z. Bioactivities and Medicinal Value of Solanesol and Its Accumulation, Extraction Technology, and Determination Methods. Biomolecules 2019, 9, 334. https://doi.org/10.3390/biom9080334
Yan N, Liu Y, Liu L, Du Y, Liu X, Zhang H, Zhang Z. Bioactivities and Medicinal Value of Solanesol and Its Accumulation, Extraction Technology, and Determination Methods. Biomolecules. 2019; 9(8):334. https://doi.org/10.3390/biom9080334
Chicago/Turabian StyleYan, Ning, Yanhua Liu, Linqing Liu, Yongmei Du, Xinmin Liu, Hongbo Zhang, and Zhongfeng Zhang. 2019. "Bioactivities and Medicinal Value of Solanesol and Its Accumulation, Extraction Technology, and Determination Methods" Biomolecules 9, no. 8: 334. https://doi.org/10.3390/biom9080334
APA StyleYan, N., Liu, Y., Liu, L., Du, Y., Liu, X., Zhang, H., & Zhang, Z. (2019). Bioactivities and Medicinal Value of Solanesol and Its Accumulation, Extraction Technology, and Determination Methods. Biomolecules, 9(8), 334. https://doi.org/10.3390/biom9080334




