Hypoxylonol F Isolated from Annulohypoxylon annulatum Improves Insulin Secretion by Regulating Pancreatic β-cell Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Fungal Material
2.3. Extraction and Isolation
2.4. Cell Culture
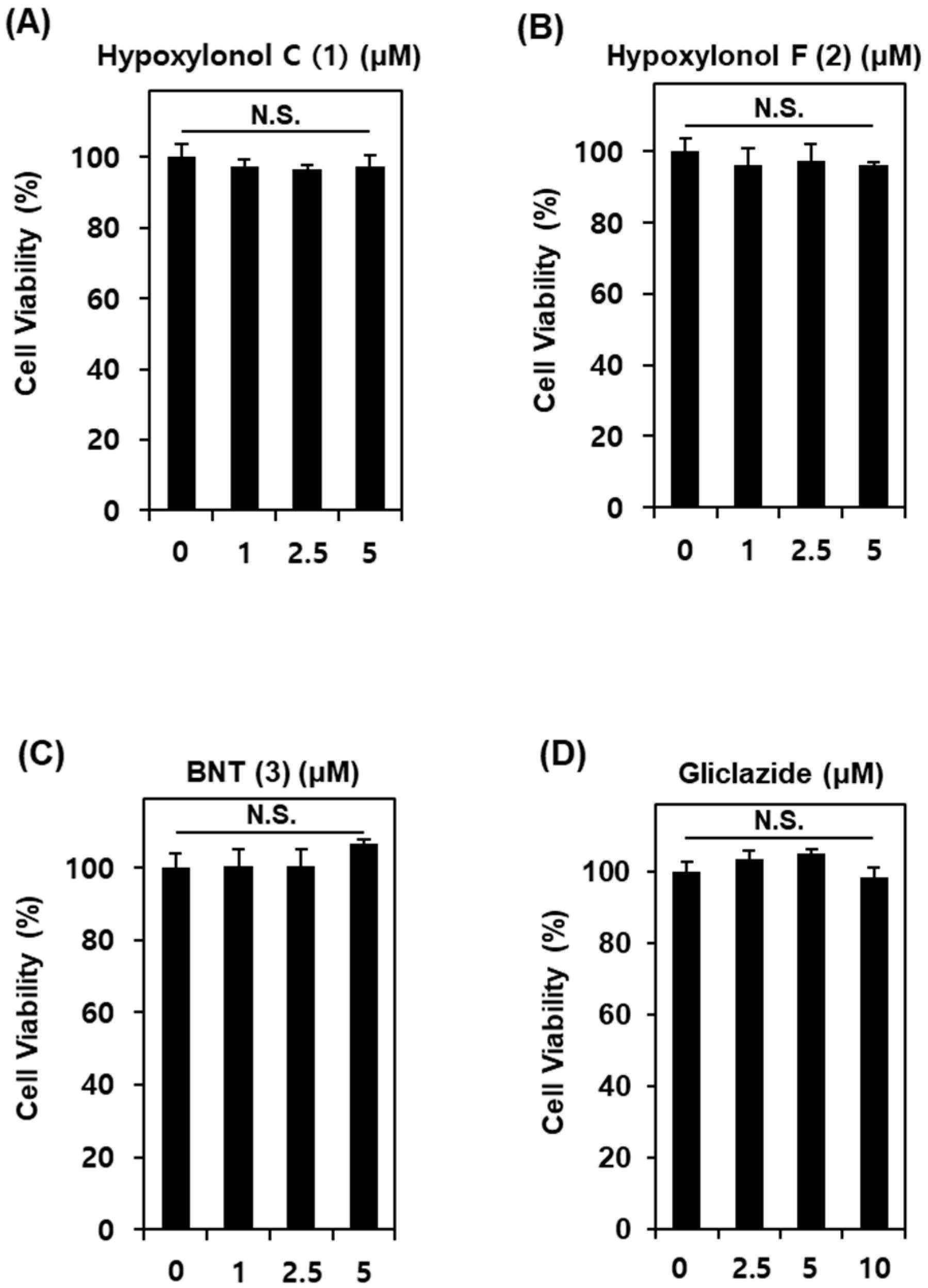
2.5. Cell Viability Assay
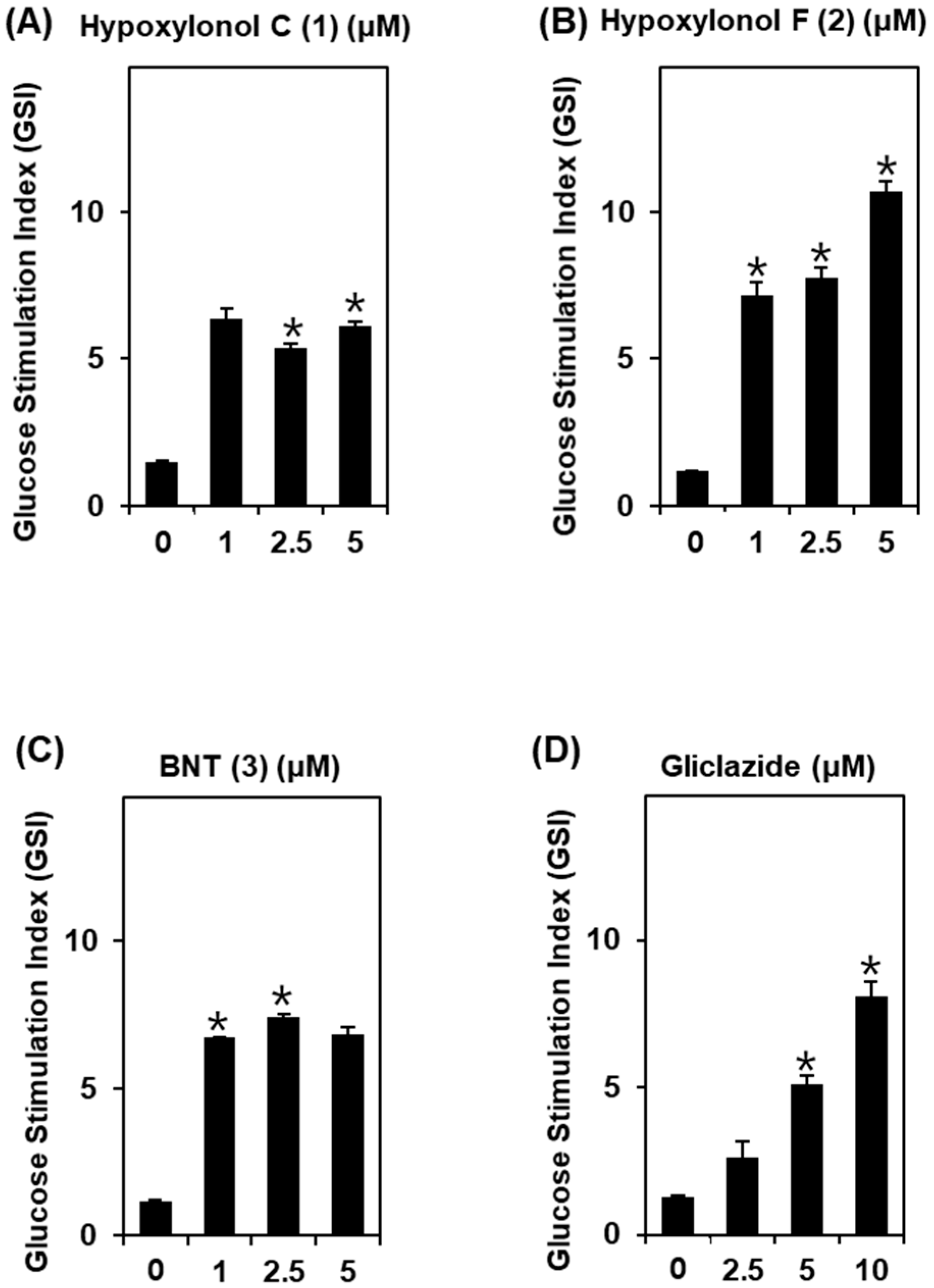
2.6. Insulin Secretion Assay
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
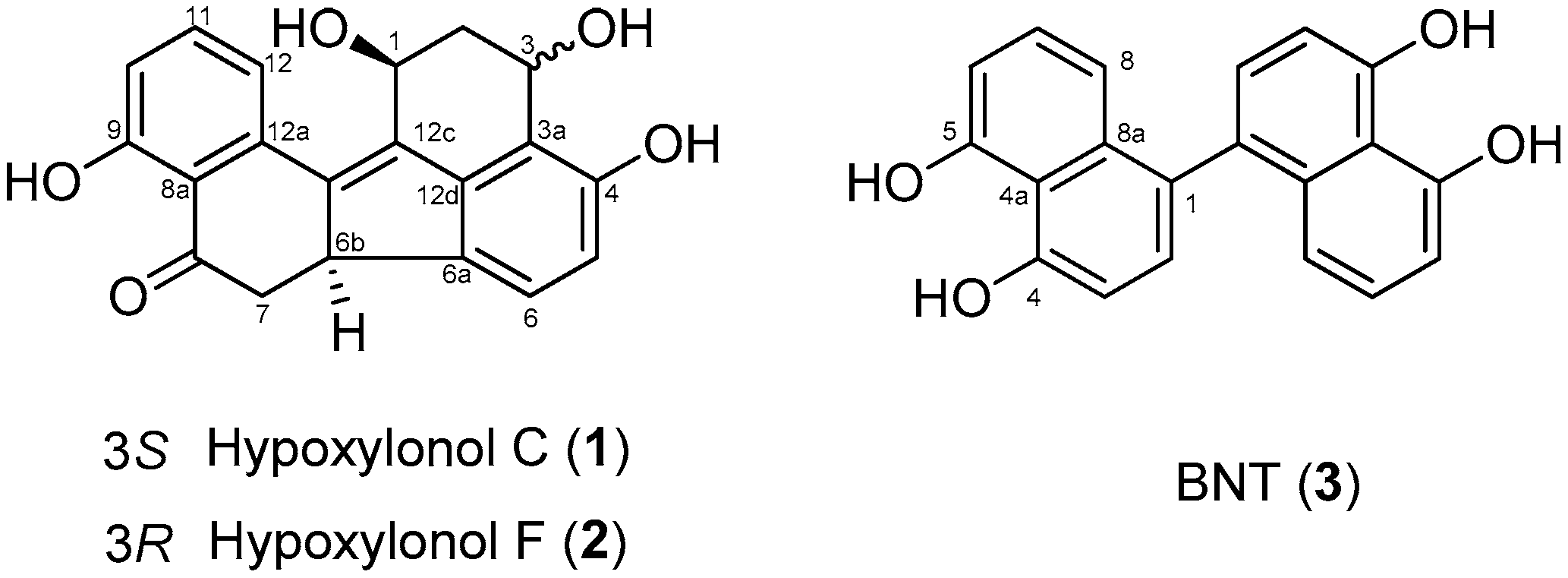
3.1. Effect of Compounds 1–3 Isolated from A. annulatum on Glucose-Stimulated Insulin Secretion
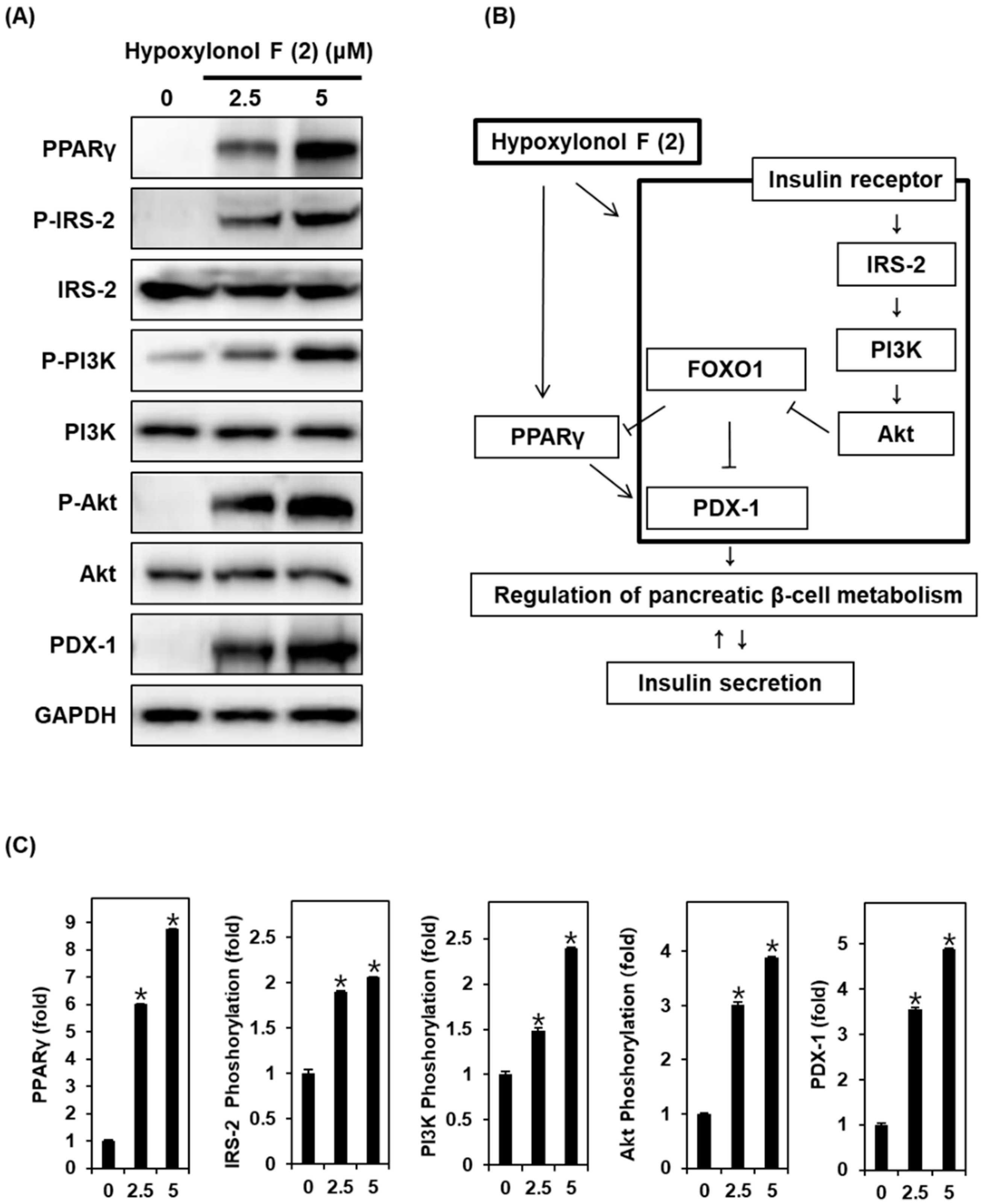
3.2. Effect of Hypoxylonol F (2) on the Protein Expression of PPARγ, P-IRS-2, IRS-2, P-PI3K, PI3K, P-Akt (Ser473), Akt, and PDX-1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shi, B.-Y. The importance and strategy of diabetes prevention. Chronic. Dis. Transl. Med. 2016, 2, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L. Investigation of pancreatic β-cell insulin receptor regulation of β-cell growth, function, and survival via a temporal conditional knockout. Electron. Thesis Diss. Repos. 2015, 2989. [Google Scholar]
- Lamontagne, J.; Al-Mass, A.; Nolan, C.J.; Corkey, B.E.; Madiraju, S.M.; Joly, E.; Prentki, M. Identification of the signals for glucose-induced insulin secretion in INS1 (832/13) β-cells using metformin-induced metabolic deceleration as a model. J. Biol. Chem. 2017, 292, 19458–19468. [Google Scholar] [CrossRef] [PubMed]
- Valeron, P.F.; de Pablos-Velasco, P.L. Limitations of insulin-dependent drugs in the treatment of type 2 diabetes mellitus. Med. Clin. 2013, 141 (Suppl. 2), 20–25. [Google Scholar] [CrossRef]
- Medina, M.C.; Souza, L.C.; Caperuto, L.C.; Anhê, G.F.; Amanso, A.M.; Teixeira, V.P.; Bordin, S.; Carpinelli, Â.R.; Britto, L.R.; Barbieri, R.L.; et al. Dehydroepiandrosterone increases β-cell mass and improves the glucose-induced insulin secretion by pancreatic islets from aged rats. FEBS Lett. 2006, 580, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.G.; Chen, F.; Li, P.; Quan, L.; Chen, J.; Yu, L.; Ding, H.; Li, C.; Chen, L.; Gao, Z.; et al. Natural product vindoline stimulates insulin secretion and efficiently ameliorates glucose homeostasis in diabetic murine models. J. Ethnopharmacol. 2013, 150, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Torii, S.; Kubota, C.; Saito, N.; Kawano, A.; Hou, N.; Kobayashi, M.; Torii, R.; Hosaka, M.; Kitamura, T.; Takeuchi, T.; et al. The pseudophosphatase phogrin enables glucose-stimulated insulin signaling in pancreatic β cells. J. Biol. Chem. 2018, 293, 5920–5933. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef]
- Kim, H.-S.; Hwang, Y.-C.; Koo, S.-H.; Park, K.S.; Lee, M.-S.; Kim, K.-W.; Lee, M.-K. PPAR-γ activation increases insulin secretion through the up-regulation of the free fatty acid receptor GPR40 in pancreatic β-cells. PLoS ONE 2013, 8, e50128. [Google Scholar] [CrossRef]
- Kitamura, T.; Nakae, J.; Kitamura, Y.; Kido, Y.; Biggs, W.H., 3rd; Wright, C.V.; White, M.F.; Arden, K.C.; Accili, D. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J. Clin. Invest. 2002, 110, 1839–1847. [Google Scholar] [CrossRef]
- Zhang, T.; Kim, D.H.; Xiao, X.; Lee, S.; Gong, Z.; Muzumdar, R.; Calabuig-Navarro, V.; Yamauchi, J.; Harashima, H.; Wang, R.; et al. FoxO1 plays an important role in regulating β-cell compensation for insulin resistance in male mice. Endocrinology 2016, 157, 1055–1070. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Herrera, I.; Martin, M.A.; Bravo, L.; Goya, L.; Ramos, S. Cocoa flavonoids improve insulin signalling and modulate glucose production via AKT and AMPK in HepG2 cells. Mol. Nutr. Food Res. 2013, 57, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.A.; Lamos, E.M.; Davis, S.N. A review of the efficacy and safety of oral antidiabetic drugs. Expert Opin. Drug Saf. 2013, 12, 153–175. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Tang, Y.H.; Zhao, G.; Yang, X.; Wang, D.; Zhang, Y. Pioglitazone prescription increases risk of bladder cancer in patients with type 2 diabetes: An updated meta-analysis. Tumour Biol. 2014, 35, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Wolski, K.; Nicholls, S.J.; Nissen, S.E. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. JAMA. 2007, 298, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Mizushige, K.; Tsuji, T.; Noma, T. Pioglitazone: Cardiovascular effects in prediabetic patients. Cardiovasc. Drug Rev. 2002, 20, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Bone, H.G.; Lindsay, R.; McClung, M.R.; Perez, A.T.; Raanan, M.G.; Spanheimer, R.G. Effects of pioglitazone on bone in postmenopausal women with impaired fasting glucose or impaired glucose tolerance: A randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 2013, 98, 4691–4701. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Derosa, G.; Tinelli, C.; Maffioli, P. Effects of pioglitazone and rosiglitazone combined with metformin on body weight in people with diabetes. Diabetes Obes. Metab. 2009, 11, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Ruano, G.; Bernene, J.; Windemuth, A.; Bower, B.; Wencker, D.; Seip, R.L.; Kocherla, M.; Holford, T.R.; Petit, W.A.; Hanks, S. Physiogenomic comparison of edema and BMI in patients receiving rosiglitazone or pioglitazone. Clin. Chim. Acta. 2009, 400, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Mudaliar, S. Pioglitazone: Side effect and safety profile. Expert Opin. Drug Saf. 2010, 9, 347–354. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S.; et al. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J. Tradit. Complement. Med. 2018, 8, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Fukai, M.; Tsukada, M.; Miki, K.; Suzuki, T.; Sugita, T.; Kinoshita, K.; Takahashi, K.; Shiro, M.; Koyama, K. Hypoxylonols C–F, Benzo [j] fluoranthenes from Hypoxylon truncatum. J. Nat. Prod. 2012, 75, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.S.; Lee, D.; Choi, P.; Kim, K.S.; Choi, S.-J.; Song, B.G.; Kim, T.; Song, J.H.; Kang, K.S.; Ham, J. Renoprotective effects of hypoxylonol C and F isolated from hypoxylon truncatum against cisplatin-induced cytotoxicity in LLC-PK1 cells. Int. J. Mol. Sci. 2018, 19, 948. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Wollweber, H.; Muhlbauer, A.; Henkel, T.; Asakawa, Y.; Hashimoto, T.; Ju, Y.-M.; Rogers, J.D.; Wetzstein, H.-G.; Thchy, H.-V. Secondary metabolite profiles, genetic fingerprints and taxonomy of Daldinia and allies. Mycotaxon 2001, 77, 379–429. [Google Scholar]
- Lee, H.; Kim, J.; Park, J.Y.; Kang, K.S.; Park, J.H.; Hwang, G.S. Processed Panax ginseng, sun ginseng, inhibits the differentiation and proliferation of 3T3-L1 preadipocytes and fat accumulation in Caenorhabditis elegans. J. Ginseng Res. 2017, 41, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Guon, T.; Chung, H.S. Induction of apoptosis with Moringa oleifera fruits in HCT116 human colon cancer cells via intrinsic pathway. Nat. Prod. Sci. 2017, 23, 227–234. [Google Scholar] [CrossRef]
- Shim, S.; Lee, S.; Kim, M.; Lee, J.W.; Hwang, B.Y.; Lee, M. Falcarindiol from Angelica koreana down-regulated IL-8 and up-regulated IL-10 in colon epithelial cells. Nat. Prod. Sci. 2017, 23, 103–107. [Google Scholar] [CrossRef]
- Park, R.; Lee, K.I.; Kim, H.; Jang, M.; Ha, T.K.Q.; Oh, W.K.; Park, J. Reserpine treatment activates AMP activated protein kinase (AMPK). Nat. Prod. Sci. 2017, 23, 157–161. [Google Scholar] [CrossRef]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar]
- Rhodes, C.J. Type 2 diabetes-a matter of beta-cell life and death? Science 2005, 307, 380–384. [Google Scholar] [CrossRef]
- Fujimoto, K.; Polonsky, K.S. Pdx1 and other factors that regulate pancreatic β-cell survival. Diabetes Obes. Metab. 2009, 11, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Otani, K.; Kulkarni, R.N.; Baldwin, A.C.; Krutzfeldt, J.; Ueki, K.; Stoffel, M.; Kahn, C.R.; Polonsky, K.S. Reduced β-cell mass and altered glucose sensing impair insulin-secretory function in βIRKO mice. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E41–E49. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Huang, Z.; Guan, H.; Liu, J.; Yao, B.; Xiao, H.; Li, Y. The Akt/FoxO1/p27 pathway mediates the proliferative action of liraglutide in β cells. Mol. Med. Rep. 2012, 5, 233–238. [Google Scholar] [PubMed]
- Kittl, M.; Beyreis, M.; Tumurkhuu, M.; Furst, J.; Helm, K.; Pitschmann, A.; Gaisberger, M.; Glasl, S.; Ritter, M.; Jakab, M. Quercetin stimulates insulin secretion and reduces the viability of rat INS-1 beta-cells. Cell. Physiol. Biochem. 2016, 39, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Y.; Zheng, D.; Ju, S.; Shen, Y.; Guo, L. Orexin a affects INS-1 rat insulinoma cell proliferation via orexin receptor 1 and the AKT signaling pathway. Int. J. Endocrinol. 2013, 854623. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Guo, L.X.; Yin, F.; Zhang, Y.L.; Liu, Z.X.; Wang, Y.W. Geniposide regulates glucose-stimulated insulin secretion possibly through controlling glucose metabolism in INS-1 cells. PLoS ONE 2013, 8, e78315. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, X.; Wu, Y.; Duan, R.; Zhang, J.; Du, F.; Zhang, Q.; Li, Y.; Li, N. Effects of vaspin on pancreatic β cell secretion via PI3K/Akt and NF-κB signaling pathways. PLoS ONE 2017, 12, e0189722. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Leahy, A.A.; Monga, N.; Peshavaria, M.; Jetton, T.L.; Leahy, J.L. Peroxisome proliferator-activated receptor γ (PPARγ) and its target genes are downstream effectors of FoxO1 protein in islet β-cells: Mechanism of β-cell compensation and failure. J. Biol. Chem. 2013, 288, 25440–25449. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Hwang, B.S.; Choi, P.; Kim, T.; Kim, Y.; Song, B.G.; Yamabe, N.; Hwang, G.S.; Kang, K.S.; Ham, J. Hypoxylonol F Isolated from Annulohypoxylon annulatum Improves Insulin Secretion by Regulating Pancreatic β-cell Metabolism. Biomolecules 2019, 9, 335. https://doi.org/10.3390/biom9080335
Lee D, Hwang BS, Choi P, Kim T, Kim Y, Song BG, Yamabe N, Hwang GS, Kang KS, Ham J. Hypoxylonol F Isolated from Annulohypoxylon annulatum Improves Insulin Secretion by Regulating Pancreatic β-cell Metabolism. Biomolecules. 2019; 9(8):335. https://doi.org/10.3390/biom9080335
Chicago/Turabian StyleLee, Dahae, Buyng Su Hwang, Pilju Choi, Taejung Kim, Youngseok Kim, Bong Geun Song, Noriko Yamabe, Gwi Seo Hwang, Ki Sung Kang, and Jungyeob Ham. 2019. "Hypoxylonol F Isolated from Annulohypoxylon annulatum Improves Insulin Secretion by Regulating Pancreatic β-cell Metabolism" Biomolecules 9, no. 8: 335. https://doi.org/10.3390/biom9080335
APA StyleLee, D., Hwang, B. S., Choi, P., Kim, T., Kim, Y., Song, B. G., Yamabe, N., Hwang, G. S., Kang, K. S., & Ham, J. (2019). Hypoxylonol F Isolated from Annulohypoxylon annulatum Improves Insulin Secretion by Regulating Pancreatic β-cell Metabolism. Biomolecules, 9(8), 335. https://doi.org/10.3390/biom9080335






