Metabolic Innovations Underpinning the Origin and Diversification of the Diatom Chloroplast
Abstract
1. Diatoms: Powerhouses and Bellweathers in the Contemporary Ocean
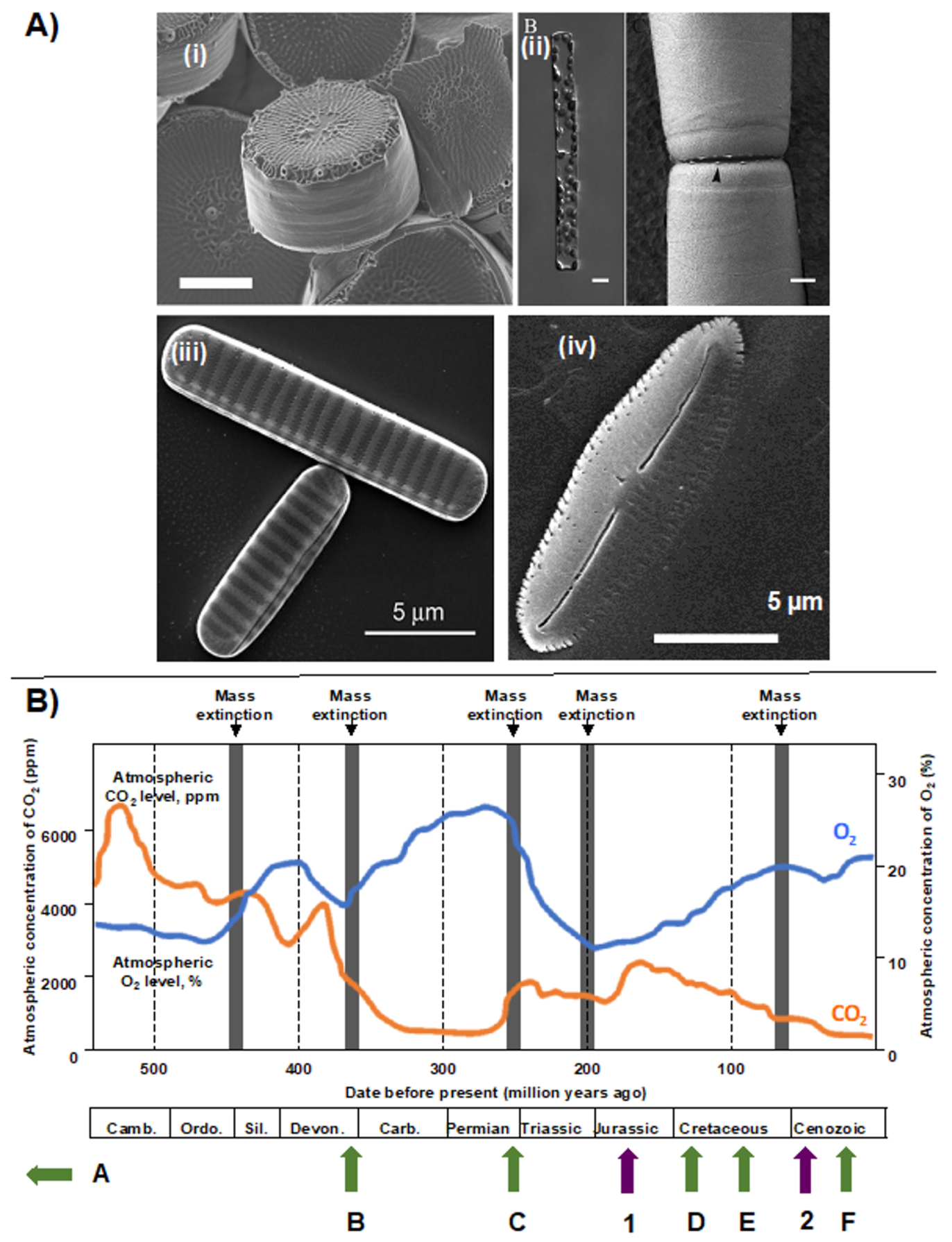
2. Taxonomic and Ecological Diversity of Diatoms
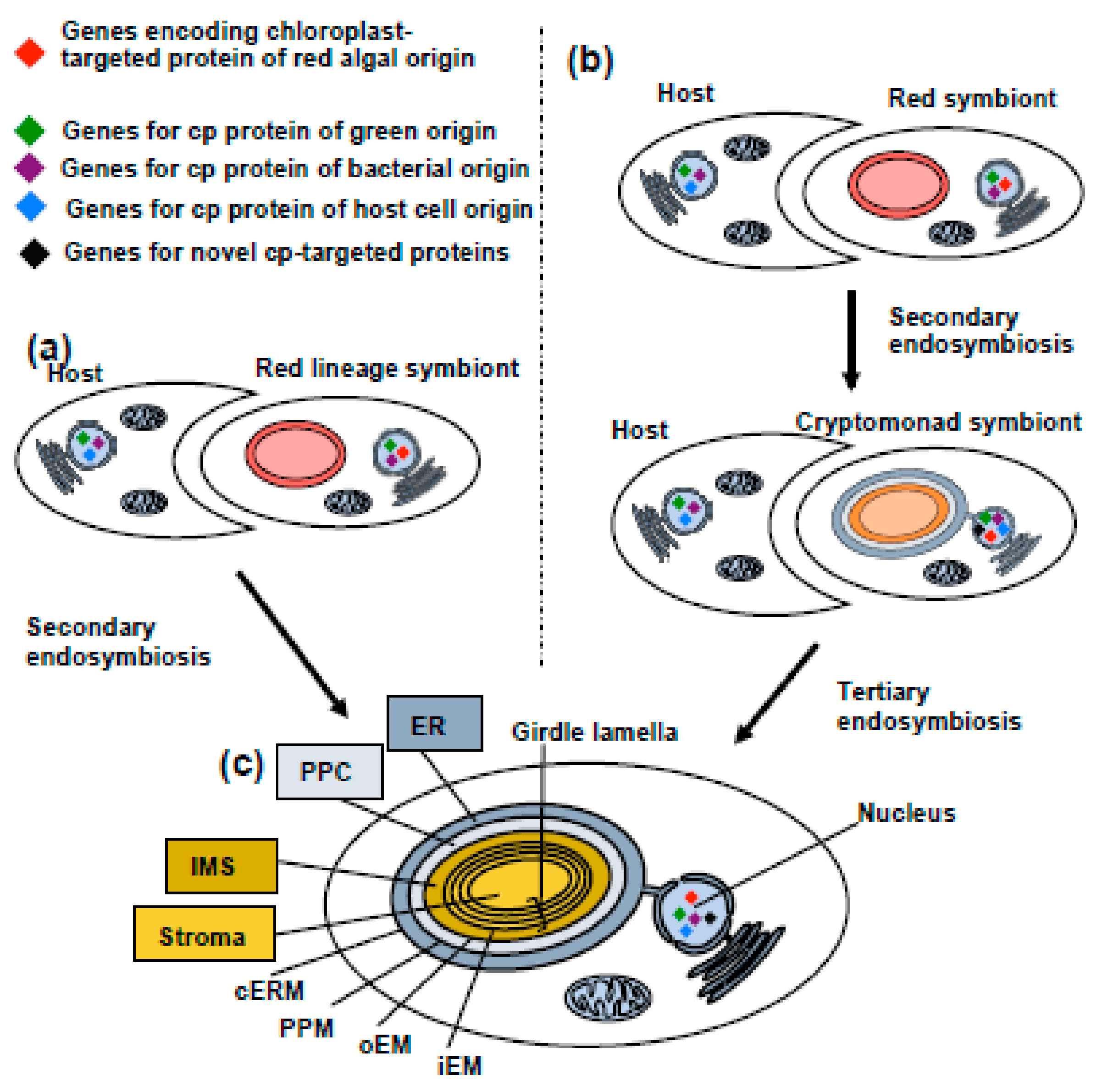
3. Diatom Chloroplast Structure and Genomes
4. Import and Mosaic Origin of Diatom Nucleus-Encoded Chloroplast Proteins
5. Metabolic and Evolutionary Complexity of the Diatom Chloroplast
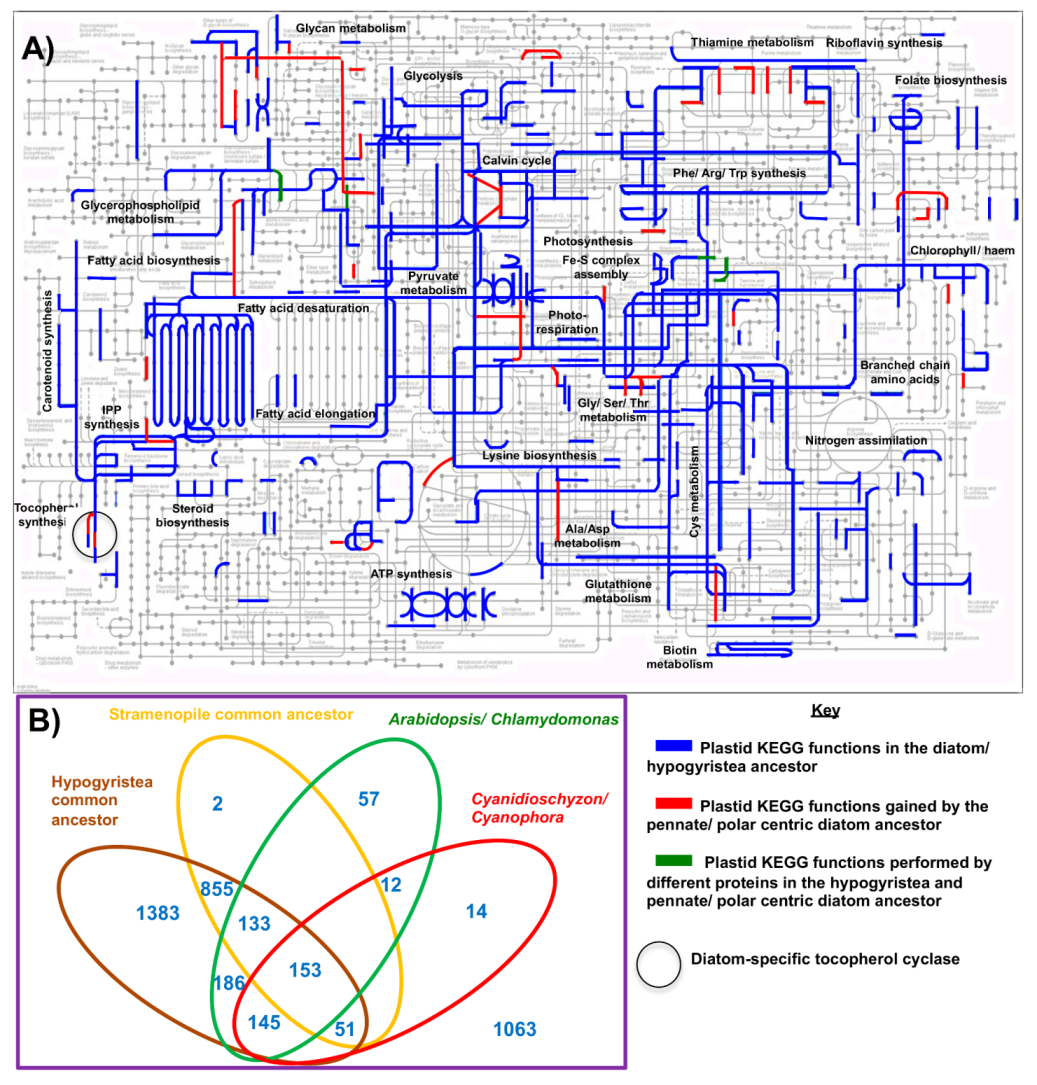
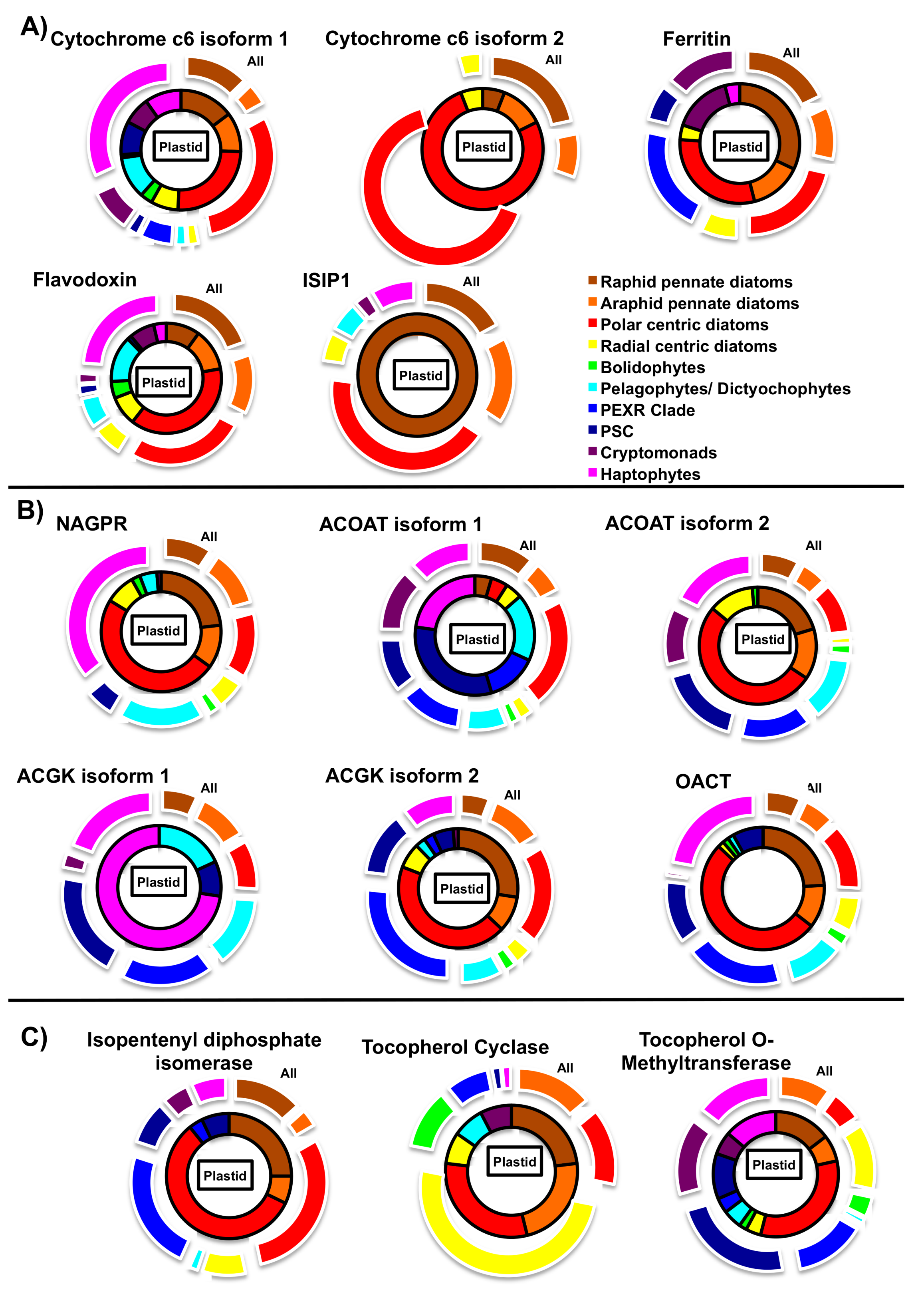
5.1. The Chloroplast Proteome of the Diatom Common Ancestor
5.2. Iron Metabolism
5.3. Nitrogen Metabolism
5.4. Photoprotection
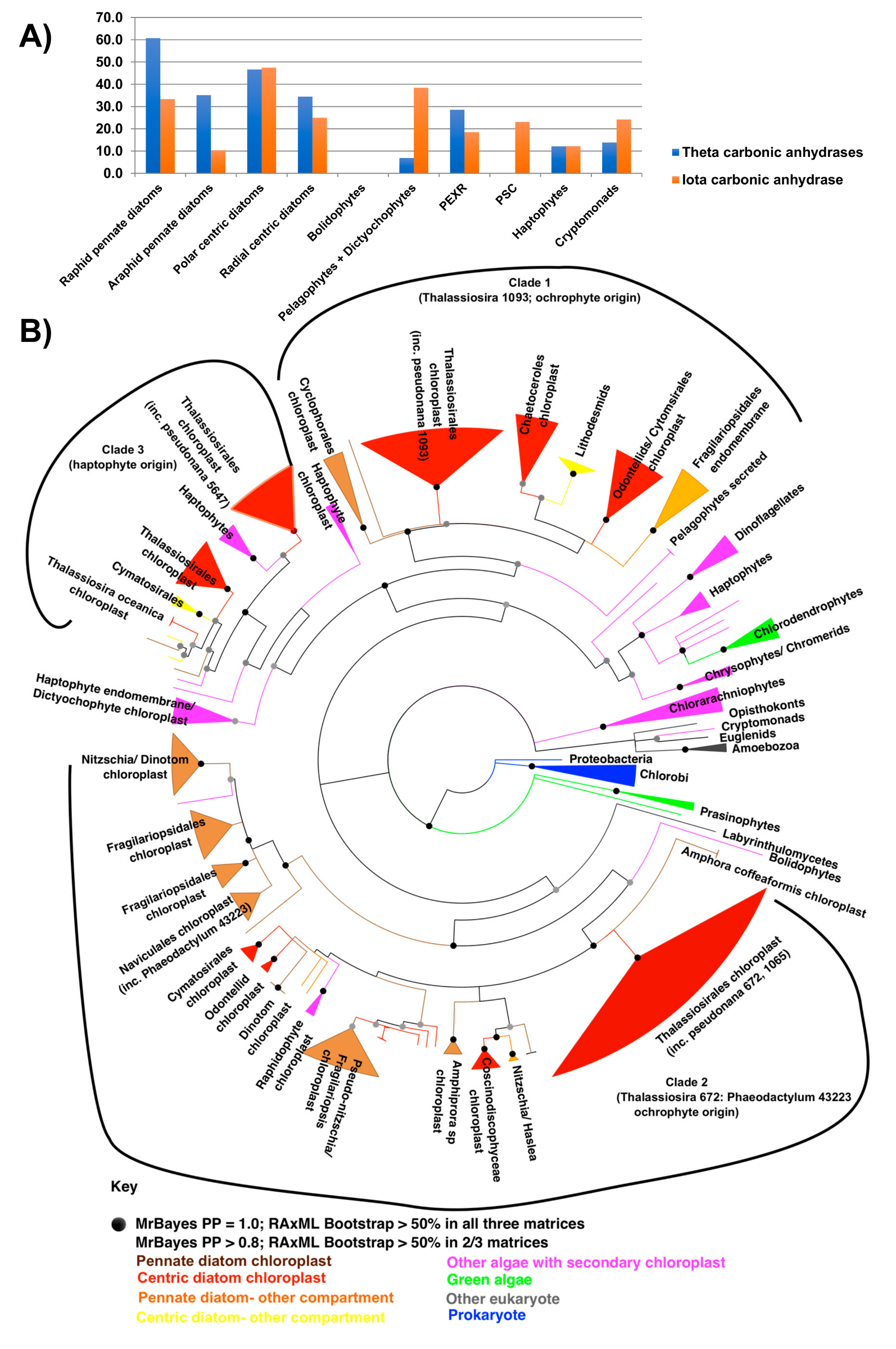
5.5. CO2 Concentrating Mechanisms (CCM)
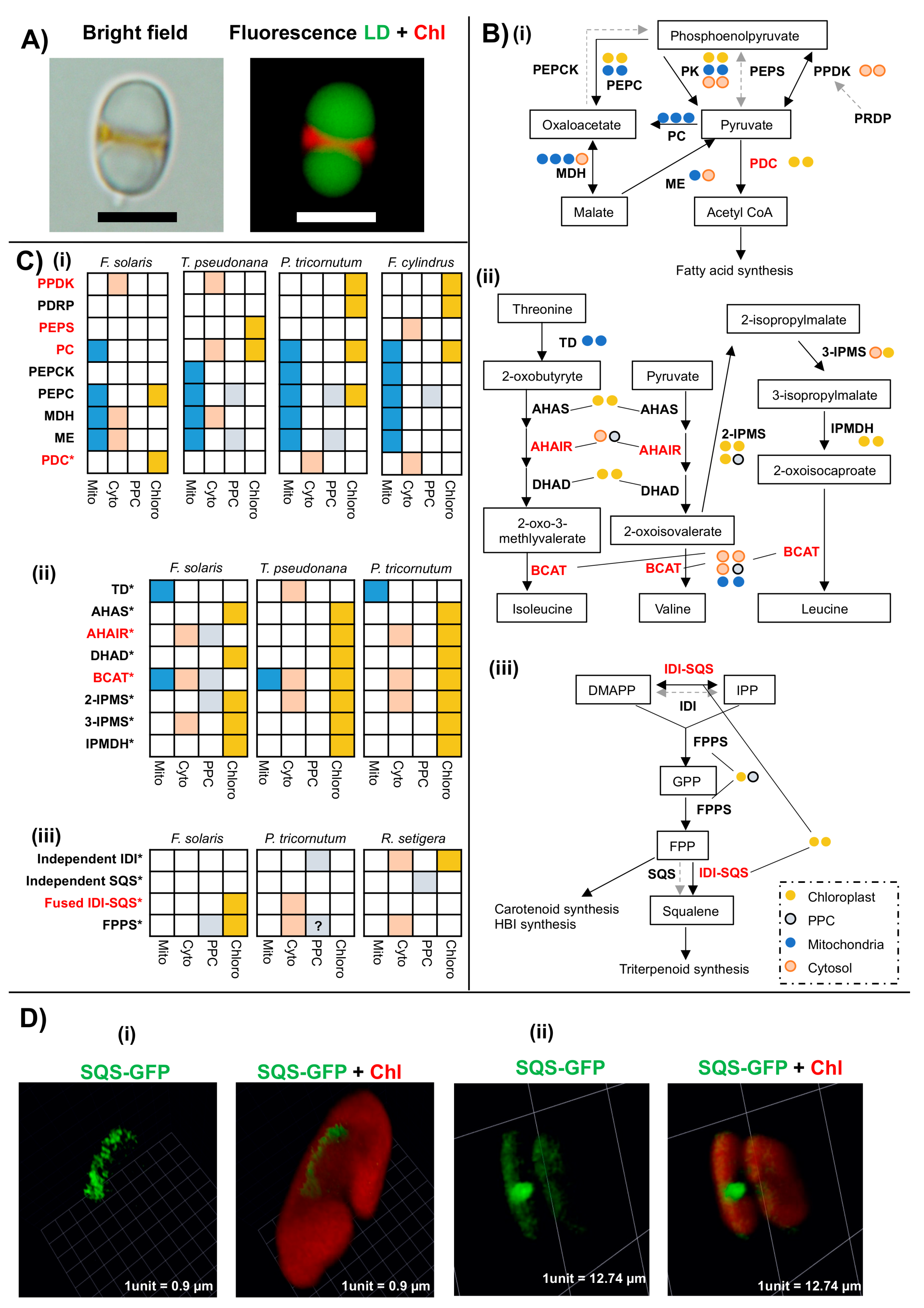
6. Industrial Futures: Remodelling Chloroplast Metabolism in the Oil-Producing Diatom Fistulifera solaris
6.1. Remodelling of Lipid and Amino Acid Metabolism in the F. solaris Chloroplast
6.2. Modified Chloroplast Isoprenoid Synthesis in Specific Diatoms
7. Concluding Remarks
Supplementary Materials
Funding
Conflicts of Interest
References
- Hinder, S.L.; Hays, G.C.; Edwards, M.; Roberts, E.C.; Waine, A.W.; Gravenor, M.B. Changes in marine dinoflagellate and diatom abundance under climate change. Nat. Clim. Chang. 2012, 2, 271–275. [Google Scholar] [CrossRef]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P.A. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 4. [Google Scholar] [CrossRef] [PubMed]
- Malviya, S.; Scalco, E.; Audic, S.; Vincent, F.; Veluchamy, A.; Poulain, J.; Wincker, P.; Iudicone, D.; de Vargas, C.; Bittner, L.; et al. Insights into global diatom distribution and diversity in the world’s ocean. Proc. Natl. Acad. Sci. USA 2016, 113, E1516–E1525. [Google Scholar] [CrossRef] [PubMed]
- Dorrell, R.G.; Bowler, C. Secondary Plastids of Stramenopiles. In Adv Bot Res: Secondary Endosymbiosis; Hirakawa, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 84, pp. 59–104. [Google Scholar]
- Ichinomiya, M.; Dos Santos, A.L.; Gourvil, P.; Yoshikawa, S.; Kamiya, M.; Ohki, K.; Audic, S.; de Vargas, C.; Noël, M.H.; Vaulot, D.; et al. Diversity and oceanic distribution of the Parmales (Bolidophyceae), a picoplanktonic group closely related to diatoms. ISME J. 2016, 10, 2419–2434. [Google Scholar] [CrossRef] [PubMed]
- Lepetit, B.; Sturm, S.; Rogato, A.; Gruber, A.; Sachse, M.; Falciatore, A.; Kroth, P.G.; Lavaud, J. High light acclimation in the secondary plastids containing diatom Phaeodactylum tricornutum is triggered by the redox state of the plastoquinone pool. Plant Physiol. 2013, 161, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Bailleul, B.; Berne, N.; Murik, O.; Petroutsos, D.; Prihoda, J.; Tanaka, A.; Villanova, V.; Bligny, R.; Flori, S.; Falconet, D.; et al. Energetic coupling between plastids and mitochondria drives CO2 assimilation in diatoms. Nature 2015, 524, 366. [Google Scholar] [CrossRef] [PubMed]
- Levering, J.; Broddrick, J.; Dupont, C.L.; Peers, G.; Beeri, K.; Mayers, J.; Gallina, A.A.; Allen, A.E.; Palsson, B.O.; Zengler, K. Genome-scale model reveals metabolic basis of biomass partitioning in a model diatom. PLoS ONE 2016, 11, e0155038. [Google Scholar] [CrossRef]
- McQuaid, J.B.; Kustka, A.B.; Oborník, M.; Horák, A.; McCrow, J.P.; Karas, B.J.; Zheng, H.; Kindeberg, T.; Andersson, A.J.; Barbeau, K.A.; et al. Carbonate-sensitive phytotransferrin controls high-affinity iron uptake in diatoms. Nature 2018, 555, 534–537. [Google Scholar] [CrossRef]
- Kazamia, E.; Sutak, R.; Paz-Yepes, J.; Dorrell, R.G.; Vieiria, F.R.J.; Mach, J.; Morrissey, J.; Leon, S.; Lam, F.; Pelletier, E.; et al. Endocytosis-mediated siderophore uptake as a strategy for Fe acquisition in diatoms. Sci. Adv. 2018, 4, 4536. [Google Scholar]
- Li, W.K.; McLaughlin, F.A.; Lovejoy, C.; Carmack, E.C. Smallest algae thrive as the Arctic Ocean freshens. Science 2009, 326, 539. [Google Scholar] [CrossRef]
- Yarnold, J.; Karan, H.; Oey, M.; Hankamer, B. Microalgal aquafeeds as part of a circular bioeconomy. Trends Plant Sci. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Sprague, M.; Betancor, M.B.; Tocher, D.R. Microbial and genetically engineered oils as replacements for fish oil in aquaculture feeds. Biotechnol. Lett. 2017, 39, 1599–1609. [Google Scholar] [CrossRef]
- Sørensen, M.; Gong, Y.; Bjarnason, F.; Vasanth, G.K.; Dahle, D.; Huntley, M.; Kiron, V. Nannochloropsis oceania-derived defatted meal as an alternative to fishmeal in Atlantic salmon feeds. PLoS ONE 2017, 12, e0179907. [Google Scholar] [CrossRef] [PubMed]
- Abida, H.; Ruchaud, S.; Rios, L.; Humeau, A.; Probert, I.; De Vargas, C.; Bach, S.; Bowler, C. Bioprospecting marine plankton. Mar. Drugs 2013, 11, 4594–4611. [Google Scholar] [CrossRef]
- Hamilton, M.L.; Warwick, J.; Terry, A.; Allen, M.J.; Napier, J.A.; Sayanova, O. Towards the industrial production of omega-3 long chain polyunsaturated fatty acids from a genetically modified diatom Phaeodactylum tricornutum. PLoS ONE 2015, 10, e0144054. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, Y.J.; Kwon, O.N.; Cha, K.H.; Um, B.H.; Chung, D.; Pan, C.H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef]
- Kuczynska, P.; Jemiola-Rzeminska, M.; Strzalka, K. Photosynthetic pigments in diatoms. Mar. Drugs 2015, 13, 5847–5881. [Google Scholar] [CrossRef] [PubMed]
- Allert, S.; Wulff, A. Frustules Extracted from Benthic Pennate Diatoms Harvested from an Industrial Biofilm Process. U.S. Patent US20190106672A1, 18 April 2019. [Google Scholar]
- Kang, J.S.; Raymond, J.A. Reduction of freeze-thaw-induced hemolysis of red blood cells by an algal ice-binding protein. Cryoletters 2004, 25, 307–310. [Google Scholar]
- Tanaka, T.; Maeda, Y.; Veluchamy, A.; Tanaka, M.; Abida, H.; Marechal, E.; Bowler, C.; Muto, M.; Sunaga, Y.; Yoshino, T.; et al. Oil accumulation by the oleaginous diatom Fistulifera solaris as revealed by the genome and transcriptome. Plant Cell 2015, 27, 162–176. [Google Scholar] [CrossRef]
- Tanaka, T.; Yabuuchi, T.; Maeda, Y.; Nojima, D.; Matsumoto, M.; Yoshino, T. Production of eicosapentaenoic acid by high cell density cultivation of the marine oleaginous diatom Fistulifera solaris. Bioresour. Technol. 2017, 245 (Pt A), 567–572. [Google Scholar] [CrossRef]
- Matsumoto, M.; Nojima, D.; Nonoyama, T.; Ikeda, K.; Maeda, Y.; Yoshino, T.; Tanaka, T. Outdoor cultivation of marine diatoms for year-round production of biofuels. Mar. Drugs 2017, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Maheswari, U.; Dorrell, R.G.; Vieira, F.R.J.; Maumus, F.; Kustka, A.; McCarthy, J.; Allen, A.E.; Kersey, P.; Bowler, C.; et al. Integrative analysis of large scale transcriptome data draws a comprehensive landscape of Phaeodactylum tricornutum genome and evolutionary origin of diatoms. Sci. Rep. 2018, 8, 4834. [Google Scholar] [CrossRef]
- Dorrell, R.G.; Gile, G.; McCallum, G.; Méheust, R.; Bapteste, E.P.; Klinger, C.M.; Brillet-Guéguen, L.; Freeman, K.D.; Richter, D.J.; Bowler, C. Chimeric origins of ochrophytes and haptophytes revealed through an ancient plastid proteome. eLife 2017, 6, 23717. [Google Scholar] [CrossRef] [PubMed]
- McNair, H.M.; Brzezinski, M.A.; Krause, J.W. Diatom populations in an upwelling environment decrease silica content to avoid growth limitation. Environ. Microbiol. 2018, 20, 4184–4193. [Google Scholar] [CrossRef] [PubMed]
- De Tommasi, E.; Gielis, J.; Rogato, A. Diatom Frustule Morphogenesis and Function: A Multidisciplinary Survey. Mar. Genom. 2017, 35, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Armbrust, E.V.; Chisholm, S.W.; Olson, R.J. Role of light and the cell cycle on the induction of spermatogenesis in a centric diatom. J. Phycol. 1990, 26, 470–478. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Katz, M.E.; Wright, J.D.; Schofield, O.M.; Falkowski, P.G. Climatically driven macroevolutionary patterns in the size of marine diatoms over the Cenozoic. Proc. Natl. Acad. Sci. USA 2005, 102, 8927–8932. [Google Scholar] [CrossRef]
- Brunner, E.; Richthammer, P.; Ehrlich, H.; Paasch, S.; Simon, P.; Ueberlein, S.; van Pée, K.H. Chitin-based organic networks: An integral part of cell wall biosilica in the diatom Thalassiosira pseudonana. Angew. Chem. Int. Ed. Engl. 2009, 48, 9724–9727. [Google Scholar] [CrossRef]
- Mock, T.; Otillar, R.P.; Strauss, J.; McMullan, M.; Paajanen, P.; Schmutz, J.; Salamov, A.; Sanges, R.; Toseland, A.; Ward, B.J.; et al. Evolutionary genomics of the cold-adapted diatom Fragilariopsis cylindrus. Nature 2017, 541, 536–540. [Google Scholar] [CrossRef]
- Dorrell, R.G.; Smith, A.G. Do red and green make brown?: Perspectives on plastid acquisitions within chromalveolates. Eukaryot. Cell 2011, 10, 856–868. [Google Scholar] [CrossRef]
- Lewitus, E.; Bittner, L.; Malviya, S.; Bowler, C.; Morlon, H. Clade-specific diversification dynamics of marine diatoms since the Jurassic. Nat. Ecol. Evol. 2018, 2, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.W.; Sorhannus, U. A molecular genetic timescale for the diversification of autotrophic stramenopiles (Ochrophyta): Substantive underestimation of putative fossil ages. PLoS ONE 2010, 5, 12759. [Google Scholar] [CrossRef] [PubMed]
- Kooistra, W.; Forlani, G.; De Stefano, M. Adaptations of araphid pennate diatoms to a planktonic existence. Mar. Ecol. 2009, 30, 1–15. [Google Scholar] [CrossRef]
- Parks, M.B.; Wickett, N.J.; Alverson, A.J. Signal, uncertainty, and conflict in phylogenomic data for a diverse lineage of microbial eukaryotes (Diatoms, Bacillariophyta). Mol. Biol. Evol. 2018, 18, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Kooistra, W.; Medlin, L.K. Evolution of the diatoms (bacillariophyta): IV. Reconstruction of their age from small subunit rRNA coding regions and the fossil record. Mol. Phylogenet. Evol. 1996, 6, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.; Chaffron, S.; Bittner, L.; Eveillard, D.; Larhlimi, A.; Roux, S.; Darzi, Y.; Audic, S.; Berline, L.; Brum, J.R.; et al. Plankton networks driving carbon export in the oligotrophic ocean. Nature 2016, 532, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Lommer, M.; Specht, M.; Roy, A.S.; Kraemer, L.; Andreson, R.; Gutowska, M.A.; Wolf, J.; Bergner, S.V.; Schilhabel, M.B.; Klostermeier, U.C.; et al. Genome and low-iron response of an oceanic diatom adapted to chronic iron limitation. Genom. Biol. 2012, 13, R66. [Google Scholar] [CrossRef] [PubMed]
- Armbrust, E.V.; Berges, J.A.; Bowler, C.; Green, B.R.; Martinez, D.; Putnam, N.H.; Zhou, S.G.; Allen, A.E.; Apt, K.E.; Bechner, M.; et al. The genome of the diatom Thalassiosira pseudonana: Ecology, evolution, and metabolism. Science 2004, 306, 79–86. [Google Scholar] [CrossRef]
- Andersen, R.A. Biology and systematics of heterokont and haptophyte algae. Am. J. Bot. 2004, 91, 1508–1522. [Google Scholar] [CrossRef]
- Stiller, J.W.; Schreiber, J.; Yue, J.; Guo, H.; Ding, Q.; Huang, J. The evolution of photosynthesis in chromist algae through serial endosymbioses. Nat. Commun. 2014, 5, 5764. [Google Scholar] [CrossRef]
- Moustafa, A.; Beszteri, B.; Maier, U.G.; Bowler, C.; Valentin, K.; Bhattacharya, D. Genomic footprints of a cryptic plastid endosymbiosis in diatoms. Science 2009, 324, 1724–1726. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, P.; Moreira, D. Re-evaluating the green contribution to diatom genomes. Genome Biol. Evol. 2012, 4, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Burki, F.; Flegontov, P.; Oborník, M.; Cihlár, J.; Pain, A.; Lukes, J.; Keeling, P.J. Re-evaluating the green versus red signal in eukaryotes with secondary plastid of red algal origin. Genome Biol. Evol. 2012, 4, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Ashworth, M.; Hajrah, N.; Khiyami, M.; Sabir, M.; Alhebshi, A.; Al-Malki, A.; Sabir, J.; Theriot, E.C.; Jansen, R. Evolution of the Plastid Genomes in Diatoms. In Plastid Genome Evolution; Chaw, S., Jansen, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 85, pp. 129–155. [Google Scholar]
- Sabir, J.S.M.; Yu, M.J.; Ashworth, M.P.; Baeshen, N.A.; Baeshen, M.N.; Bahieldin, A.; Theriot, E.C.; Jansen, R.K. Conserved gene order and expanded inverted repeats characterize plastid genomes of Thalassiosirales. PLoS ONE 2014, 9, 107854. [Google Scholar] [CrossRef] [PubMed]
- Ruck, E.C.; Linard, S.R.; Nakov, T.; Theriot, E.C.; Alverson, A.J. Hoarding and horizontal transfer led to an expanded gene and intron repertoire in the plastid genome of the diatom, Toxarium undulatum (Bacillariophyta). Curr. Genet. 2017, 63, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Kamikawa, R.; Yubuki, N.; Yoshida, M.; Taira, M.; Nakamura, N.; Ishida, K.; Leander, B.S.; Miyashita, H.; Hashimoto, T.; Mayama, S.; et al. Multiple losses of photosynthesis in Nitzschia (Bacillariophyceae). Phycol. Res. 2015, 63, 19–28. [Google Scholar] [CrossRef]
- Kamikawa, R.; Tanifuji, G.; Ishikawa, S.A.; Ishii, K.I.; Matsuno, Y.; Onodera, N.T.; Ishida, K.I.; Hashimoto, T.; Miyashita, H.; Mayama, S.; et al. Proposal of a twin arginine translocator system-mediated constraint against loss of ATP synthase genes from nonphotosynthetic plastid genomes. Mol. Biol. Evol. 2015, 32, 2598–2604. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.; Rocap, G.; Kroth, P.G.; Armbrust, E.V.; Mock, T. Plastid proteome prediction for diatoms and other algae with secondary plastids of the red lineage. Plant J. 2015, 81, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Felsner, G.; Sommer, M.S.; Gruenheit, N.; Hempel, F.; Moog, D.; Zauner, S.; Martin, W.; Maier, U.G. ERAD components in organisms with complex red plastids suggest recruitment of a preexisting protein transport pathway for the periplastid membrane. Genom. Biol. Evol. 2011, 3, 140–150. [Google Scholar] [CrossRef]
- Gould, S.B.; Sommer, M.S.; Kroth, P.G.; Gile, G.H.; Keeling, P.J.; Maier, U.G. Nucleus-to-nucleus gene transfer and protein retargeting into a remnant cytoplasm of cryptophytes and diatoms. Mol. Biol. Evol. 2006, 23, 2413–2422. [Google Scholar] [CrossRef]
- Kikutani, S.; Nakajima, K.; Nagasato, C.; Tsuji, Y.; Miyatake, A.; Matsuda, Y. Thylakoid luminal theta-carbonic anhydrase critical for growth and photosynthesis in the marine diatom Phaeodactylum tricornutum. Proc. Natl. Acad. Sci. USA 2016, 113, 9828–9833. [Google Scholar] [CrossRef] [PubMed]
- Peschke, M.; Moog, D.; Klingl, A.; Maier, U.G.; Hempel, F. Evidence for glycoprotein transport into complex plastids. Proc. Natl. Acad. Sci. USA 2013, 110, 10860–10865. [Google Scholar] [CrossRef] [PubMed]
- Gile, G.H.; Moog, D.; Slamovits, C.H.; Maier, U.G.; Archibald, J.M. Dual organellar targeting of aminoacyl-tRNA synthetases in diatoms and cryptophytes. Genom. Biol. Evol. 2015, 7, 1728–1742. [Google Scholar] [CrossRef] [PubMed]
- Dautermann, O.; Lohr, M. A functional zeaxanthin epoxidase from red algae shedding light on the evolution of light-harvesting carotenoids and the xanthophyll cycle in photosynthetic eukaryotes. Plant. J. 2017, 92, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Dorrell, R.G.; Azuma, T.; Nomura, M.; Audren de Kerdrel, G.; Paoli, L.; Yang, S.; Bowler, C.; Ishii, K.I.; Miyashita, H.; Gile, G.H.; et al. Principles of plastid reductive evolution illuminated by nonphotosynthetic chrysophytes. Proc. Natl. Acad. Sci. USA 2019, 116, 6914–6923. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Price, D.C.; Weber, A.P.; Facchinelli, F.; Yoon, H.S.; Bhattacharya, D. Assessing the bacterial contribution to the plastid proteome. Trends Plant Sci. 2013, 18, 680–687. [Google Scholar] [CrossRef]
- Kleffmann, T.; Russenberger, D.; von Zychlinski, A.; Christopher, W.; Sjolander, K.; Gruissem, W.; Baginsky, S. The Arabidopsis thaliana chloroplast proteome reveals pathway abundance and novel protein functions. Curr. Biol. 2004, 14, 354–362. [Google Scholar] [CrossRef]
- Terashima, M.; Specht, M.; Hippler, M. The chloroplast proteome: A survey from the Chlamydomonas reinhardtii perspective with a focus on distinctive features. Curr. Genet. 2011, 57, 151–168. [Google Scholar] [CrossRef]
- Suzuki, K.; Miyagishima, S. Eukaryotic and eubacterial contributions to the establishment of plastid proteome estimated by large-scale phylogenetic analyses. Mol. Biol. Evol. 2010, 27, 581–590. [Google Scholar] [CrossRef]
- Karlsen, E.; Schulz, C.; Almaas, E. Automated generation of genome-scale metabolic draft reconstructions based on KEGG. BMC Bioinformat. 2018, 19, 467. [Google Scholar] [CrossRef]
- Marchetti, A.; Schruth, D.M.; Durkin, C.A.; Parker, M.S.; Kodner, R.B.; Berthiaume, C.T.; Morales, R.; Allen, A.E.; Armbrust, E.V. Comparative metatranscriptomics identifies molecular bases for the physiological responses of phytoplankton to varying iron availability. Proc. Natl. Acad. Sci. USA 2012, 109, E317–E325. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.; Bowler, C. Iron utilization in marine cyanobacteria and eukaryotic algae. Front. Microbiol. 2012, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Peers, G.; Price, N.M. Copper-containing plastocyanin used for electron transport by an oceanic diatom. Nature 2006, 441, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Groussman, R.D.; Parker, M.S.; Armbrust, E.V. Diversity and Evolutionary History of Iron Metabolism Genes in Diatoms. PLoS ONE 2015, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Lommer, M.; Roy, A.S.; Schilhabel, M.; Schreiber, S.; Rosenstiel, P.; LaRoche, J. Recent transfer of an iron-regulated gene from the plastid to the nuclear genome in an oceanic diatom adapted to chronic iron limitation. BMC Genom. 2010, 11, 718. [Google Scholar] [CrossRef] [PubMed]
- Burki, F.; Kaplan, M.; Tikhonenkov, D.V.; Zlatogursky, V.; Minh, B.Q.; Radaykina, L.V.; Smirnov, A.; Mylnikov, A.P.; Keeling, P.J. Untangling the early diversification of eukaryotes: A phylogenomic study of the evolutionary origins of Centrohelida, Haptophyta and Cryptista. Proc. Biol. Sci. 2016, 283, 20152802. [Google Scholar] [CrossRef] [PubMed]
- Boyd, P.W.; Jickells, T.; Law, C.S.; Blain, S.; Boyle, E.A.; Buesseler, K.O.; Coale, K.H.; Cullen, J.J.; de Baar, H.J.; Follows, M.; et al. Mesoscale iron enrichment experiments 1993–2005: Synthesis and future directions. Science 2007, 315, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Gledhill, M.; Buck, K.N. The organic complexation of iron in the marine environment: A review. Front. Microbiol. 2012, 3, 69. [Google Scholar] [CrossRef]
- Allen, A.E.; Laroche, J.; Maheswari, U.; Lommer, M.; Schauer, N.; Lopez, P.J.; Finazzi, G.; Fernie, A.R.; Bowler, C. Whole-cell response of the pennate diatom Phaeodactylum tricornutum to iron starvation. Proc. Natl. Acad. Sci. USA 2008, 105, 10438–10443. [Google Scholar] [CrossRef]
- Lampe, R.H.; Mann, E.L.; Cohen, N.R.; Till, C.P.; Thamatrakoln, K.; Brzezinski, M.A.; Bruland, K.W.; Twining, B.S.; Marchetti, A. Different iron storage strategies among bloom-forming diatoms. Proc. Natl. Acad. Sci. USA 2018, 115, E12275–E12284. [Google Scholar] [CrossRef]
- Allen, A.E.; Dupont, C.L.; Obornik, M.; Horak, A.; Nunes-Nesi, A.; McCrow, J.P.; Zheng, H.; Johnson, D.A.; Hu, H.; Fernie, A.R.; et al. Evolution and metabolic significance of the urea cycle in photosynthetic diatoms. Nature 2011, 473, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Broddrick, J.T.; Du, N.; Smith, S.R.; Tsuji, Y.; Jallet, D.; Ware, M.A.; Peers, G.; Matsuda, Y.; Dupont, C.L.; Mitchell, B.G.; et al. Cross-compartment metabolic coupling enables flexible photoprotective mechanisms in the diatom Phaeodactylum tricornutum. New Phytol. 2019, 222, 1364–1379. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.K.; Smith, S.R.; McCrow, J.P.; Tan, M.; Zheng, H.; Beeri, K.; Roth, R.; Lichtle, C.; Goodenough, U.; Bowler, C.P.; et al. Nitrate reductase knockout uncouples nitrate transport from nitrate assimilation and drives repartitioning of carbon flux in a model pennate diatom. Plant Cell 2017, 29, 2047–2070. [Google Scholar] [CrossRef] [PubMed]
- Coesel, S.; Obornik, M.; Varela, J.; Falciatore, A.; Bowler, C. Evolutionary origins and functions of the carotenoid biosynthetic pathway in marine diatoms. PLoS ONE 2008, 3, e2896. [Google Scholar] [CrossRef] [PubMed]
- Lepetit, B.; Gelin, G.; Lepetit, M.; Sturm, S.; Vugrinec, S.; Rogato, A.; Kroth, P.G.; Falciatore, A.; Lavaud, J. The diatom Phaeodactylum tricornutum adjusts nonphotochemical fluorescence quenching capacity in response to dynamic light via fine-tuned Lhcx and xanthophyll cycle pigment synthesis. New Phytol. 2017, 214, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Büchel, C. Evolution and function of light harvesting proteins. J. Plant Physiol. 2015, 172, 62–75. [Google Scholar] [CrossRef]
- Murik, O.; Tirichine, L.; Prihoda, J.; Thomas, Y.; Araújo, W.L.; Allen, A.E.; Fernie, A.R.; Bowler, C. Downregulation of mitochondrial alternative oxidase affects chloroplast function, redox status and stress response in a marine diatom. New Phytol. 2019, 221, 1303–1316. [Google Scholar] [CrossRef]
- Dang, K.V.; Plet, J.; Tolleter, D.; Jokel, M.; Cuiné, S.; Carrier, P.; Auroy, P.; Richaud, P.; Johnson, X.; Alric, J.; et al. Combined increases in mitochondrial cooperation and oxygen photoreduction compensate for deficiency in cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell 2014, 26, 3036–3050. [Google Scholar] [CrossRef]
- Dłużewska, J.; Szymańska, R.; Gabruk, M.; Kós, P.; Nowicka, B.; Kruk, J. Tocopherol Cyclases—substrate specificity and phylogenetic Relations. PLoS ONE 2016, 11, 0159629. [Google Scholar] [CrossRef]
- Roberts, K.; Granum, E.; Leegood, R.C.; Raven, J.A. Carbon acquisition by diatoms. Photosynth. Res. 2007, 93, 79–88. [Google Scholar] [CrossRef]
- Holdsworth, E.S.; Colbeck, J. The pattern of carbon fixation in the marine unicellular alga Phaeodactylum tricornutum. Mar. Biol. 1976, 3, 11. [Google Scholar] [CrossRef]
- Reinfelder, J.R.; Kraepiel, A.M.; Morel, F.M. Unicellular C4 photosynthesis in a marine diatom. Nature 2000, 407, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nakatsuma, D.; Harada, H.; Ishida, M.; Matsuda, Y. Localization of soluble beta-carbonic anhydrase in the marine diatom Phaeodactylum tricornutum. Sorting to the chloroplast and cluster formation on the girdle lamellae. Plant Physiol. 2005, 138, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Samukawa, M.; Shen, C.; Hopkinson, B.M.; Matsuda, Y. Localization of putative carbonic anhydrases in the marine diatom, Thalassiosira pseudonana. Photosynth Res. 2014, 121, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Allen, A.E.; Kikutani, S.; Endo, Y.; Bowler, C.; Matsuda, Y. Localization of putative carbonic anhydrases in two marine diatoms, Phaeodactylum tricornutum and Thalassiosira pseudonana. Photosynth Res. 2011, 109, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.L.; Clement, R.; Kosta, A.; Maberly, S.C.; Gontero, B.A. A new widespread subclass of carbonic anhydrase in marine phytoplankton. ISME J. 2019, 13, 2094–2106. [Google Scholar] [CrossRef]
- Shtaida, N.; Khozin-Goldberg, I.; Boussiba, S. The role of pyruvate hub enzymes in supplying carbon precursors for fatty acid synthesis in photosynthetic microalgae. Photosynth. Res. 2015, 125, 407–422. [Google Scholar] [CrossRef]
- Bromke, M.A. Amino Acid biosynthesis pathways in diatoms. Metabolites 2013, 3, 294–311. [Google Scholar] [CrossRef]
- Osada, K.; Maeda, Y.; Yoshino, T.; Nojima, D.; Bowler, C.; Tanaka, T. Enhanced NADPH production in the pentose phosphate pathway accelerates lipid accumulation in the oleaginous diatom Fistulifera solaris. Algal Res. 2017, 23, 126–134. [Google Scholar] [CrossRef]
- Ge, F.; Huang, W.; Chen, Z.; Zhang, C.; Xiong, Q.; Bowler, C.; Yang, J.; Xu, J.; Hu, H. Methylcrotonyl-coA carboxylase regulates triacylglycerol accumulation in the model diatom Phaeodactylum tricornutum. Plant Cell 2014, 26, 1681–1697. [Google Scholar] [CrossRef]
- Smith, S.R.; Abbriano, R.M.; Hildebrand, M. Comparative analysis of diatom genomes reveals substantial differences in the organization of carbon partitioning pathways. Algal Res. 2012, 1, 2–16. [Google Scholar] [CrossRef]
- Ferriols, V.M.E.N.; Yaginuma-Suzuki, R.; Fukunaga, K.; Kadono, T.; Adachi, M.; Matsunaga, S.; Okada, S. An exception among diatoms: Unique organization of genes involved in isoprenoid biosynthesis in Rhizosolenia setigera CCMP 1694. Plant J. 2017, 92, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Fukuda, Y.; Nemoto, M.; Yoshino, T.; Matsunaga, T.; Tanaka, T. Establishment of a genetic transformation system for the marine pennate diatom Fistulifera sp. strain JPCC DA0580--a high triglyceride producer. Mar. Biotechnol. 2013, 15, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Hempel, F.; Stork, S.; Bolte, K.; Moog, D.; Heimerl, T.; Maier, U.G.; Zauner, S. Addressing various compartments of the diatom model organism Phaeodactylum tricornutum via sub-cellular marker proteins. Algal Res. 2016, 20, 249–257. [Google Scholar] [CrossRef]
- Szkopiñska, A.; Plochocka, D. Farnesyl diphosphate synthase; regulation of product specificity. Acta Biochim. Pol. 2005, 52, 45–55. [Google Scholar] [PubMed]
- Busquets, A.; Keim, V.; Closa, M.; Del Arco, A.; Boronat, A.; Arró, M.; Ferrer, A. Arabidopsis thaliana contains a single gene encoding squalene synthase. Plant Mol. Biol. 2008, 67, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Metzger, P.; Largeau, C. Botryococcus braunii: A rich source for hydrocarbons and related ether lipids. Appl Microbiol. Biotechnol. 2005, 66, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.A.; Belt, S.T.; Tatarek, A.; Mundy, C.J. Source identification of the Arctic sea ice proxy IP25. Nat. Commun. 2014, 5, 4197. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nonoyama, T.; Kazamia, E.; Nawaly, H.; Gao, X.; Tsuji, Y.; Matsuda, Y.; Bowler, C.; Tanaka, T.; G. Dorrell, R. Metabolic Innovations Underpinning the Origin and Diversification of the Diatom Chloroplast. Biomolecules 2019, 9, 322. https://doi.org/10.3390/biom9080322
Nonoyama T, Kazamia E, Nawaly H, Gao X, Tsuji Y, Matsuda Y, Bowler C, Tanaka T, G. Dorrell R. Metabolic Innovations Underpinning the Origin and Diversification of the Diatom Chloroplast. Biomolecules. 2019; 9(8):322. https://doi.org/10.3390/biom9080322
Chicago/Turabian StyleNonoyama, Tomomi, Elena Kazamia, Hermanus Nawaly, Xia Gao, Yoshinori Tsuji, Yusuke Matsuda, Chris Bowler, Tsuyoshi Tanaka, and Richard G. Dorrell. 2019. "Metabolic Innovations Underpinning the Origin and Diversification of the Diatom Chloroplast" Biomolecules 9, no. 8: 322. https://doi.org/10.3390/biom9080322
APA StyleNonoyama, T., Kazamia, E., Nawaly, H., Gao, X., Tsuji, Y., Matsuda, Y., Bowler, C., Tanaka, T., & G. Dorrell, R. (2019). Metabolic Innovations Underpinning the Origin and Diversification of the Diatom Chloroplast. Biomolecules, 9(8), 322. https://doi.org/10.3390/biom9080322




