Discovery and Rational Design of a Novel Bowman-Birk Related Protease Inhibitor
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Biodata and Secretion Harvesting
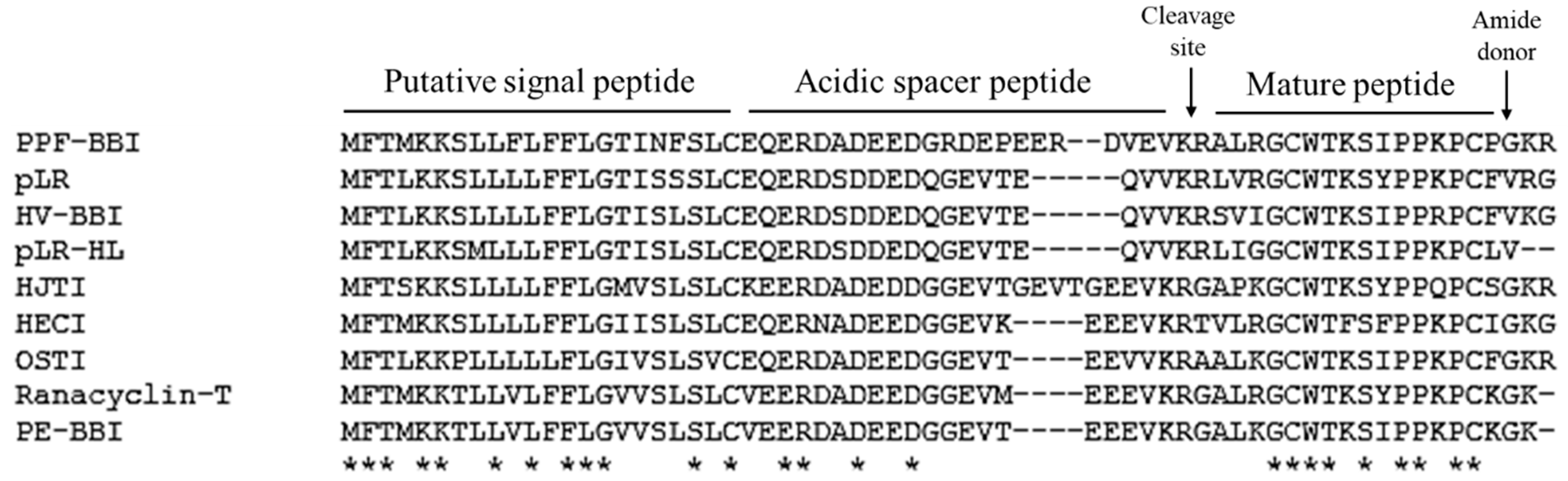
2.2. Identification of Precursor-Encoding cDNA from the Skin Secretion
2.3. Isolation and Identification of PPF-BBI from Skin Secretion
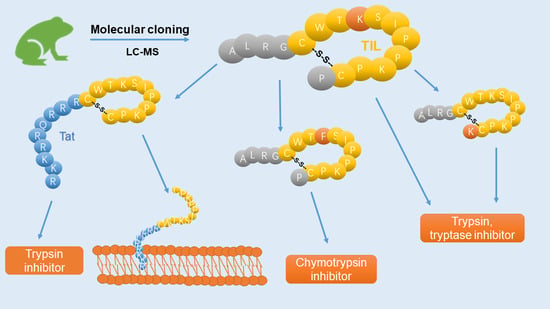
2.4. Peptide Design and Solid Phase Peptide Synthesis of PPF-BBI, F8-PPF-BBI, K16-PPF-BBI, Tat-loop, Tat and Trypsin Inhibitory Loop
2.5. Trypsin, Chymotrypsin and Tryptase Inhibition Assay
2.6. Minimal Inhibitory Concentration (MIC) Assay and Minimal Batericidal Concentration (MBC) Assay
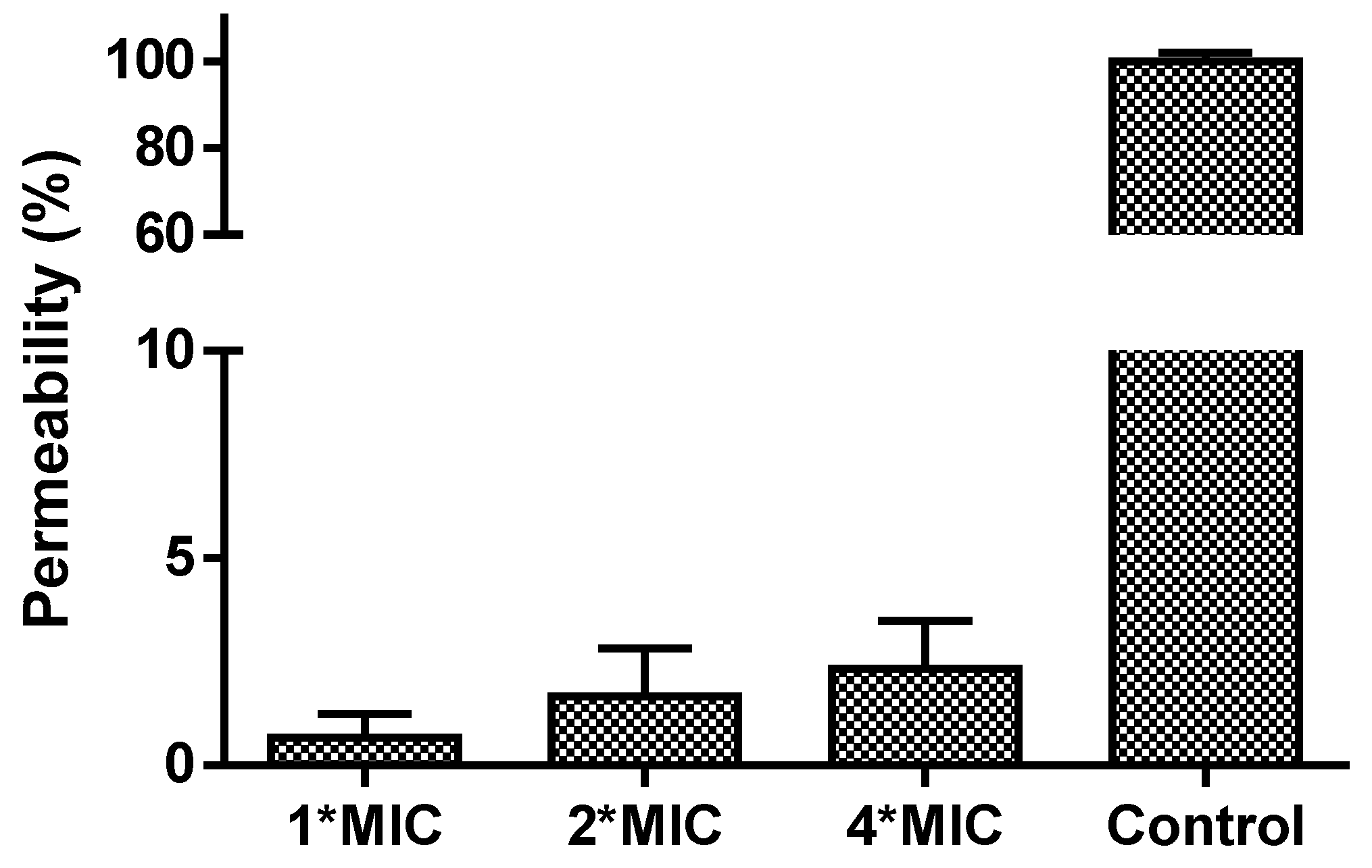
2.7. Membrane Permeability Assay
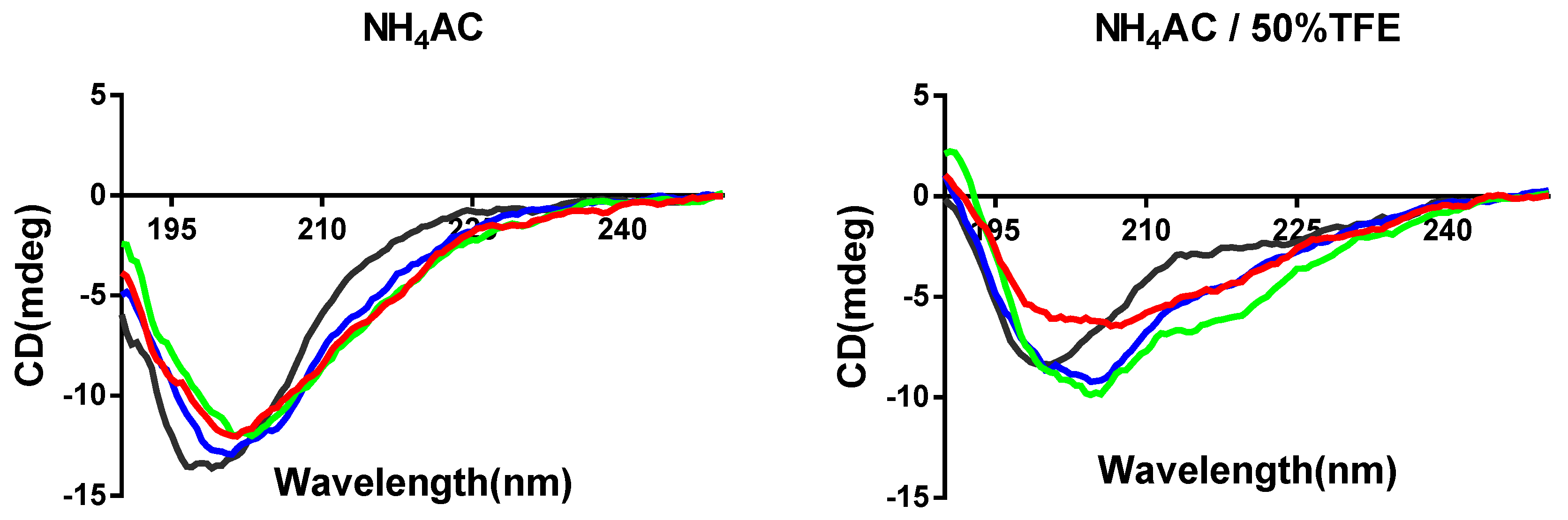
2.8. Secondary Structure Analysis through Circular Dichroism (CD)
2.9. MTT Assay
2.10. Haemolysis Test
2.11. Statitical Analysis
3. Results
3.1. Identification and Structural Determination of PPF-BBI
3.2. Peptide Design
3.3. Synthesis and Secondary Structure Analysis of PPF-BBI and its Analogues
3.4. Trypsin, Chymotrypsin and Tryptase Inhibitory Activity of PPF-BBI and its Analogues
3.5. Antimicrobial Activity
3.6. Membrane Permeability
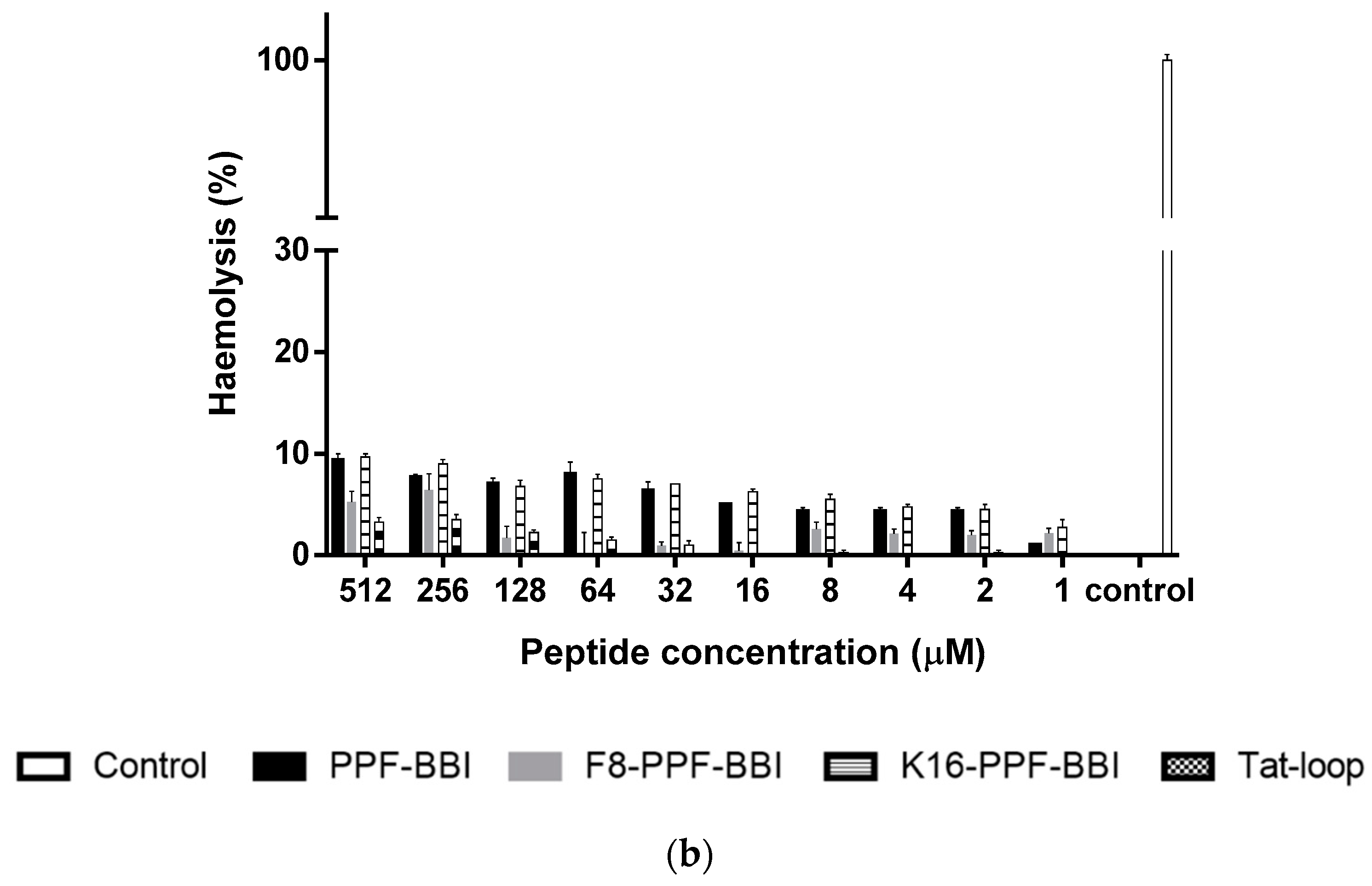
3.7. Anti-Cancer and Haemolytic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stein, P.E.; Carrell, R.W. What do dysfunctional serpins tell us about molecular mobility and disease? Nat. Struct. Mol. Boil. 1995, 2, 96–113. [Google Scholar] [CrossRef]
- Soualmia, F.; El Amri, C. Serine protease inhibitors to treat inflammation: A patent review (2011–2016). Expert Opin. Ther. Patents 2017, 28, 93–110. [Google Scholar] [CrossRef]
- Norioka, S.; Ikenaka, T. Amino Acid Sequences of Trypsin-Chymotrypsin Inhibitors (A-I, A-II, B-I, and B-II) from Peanut (Arachis hypogaea)1: A Discussion on the Molecular Evolution of Legume Bowman-Birk Type Inhibitors. J. Biochem. 1983, 94, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Chen, Z. Plant Protease Inhibitors in Therapeutics-Focus on Cancer Therapy. Front. Pharmacol. 2016, 7, 470. [Google Scholar] [CrossRef]
- Dantzger, M.; Vasconcelos, I.M.; Scorsato, V.; Aparicio, R.; Marangoni, S.; Macedo, M.L.R. Bowman–Birk proteinase inhibitor from Clitoria fairchildiana seeds: Isolation, biochemical properties and insecticidal potential. Phytochem. 2015, 118, 224–235. [Google Scholar] [CrossRef]
- Ma, J.; Luo, Y.; Ge, L.; Wang, L.; Zhou, M.; Zhang, Y.; Duan, J.; Chen, T.; Shaw, C. Ranakinestatin-PPF from the skin secretion of the Fukien gold-striped pond frog, Pelophylax plancyi fukienensis: A prototype of a novel class of bradykinin B2 receptor antagonist peptide from ranid frogs. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Salmon, A.L.; Cross, L.J.; Irvine, A.E.; Lappin, T.R.; Dathe, M.; Krause, G.; Canning, P.; Thim, L.; Beyermann, M.; Rothemund, S.; et al. Peptide leucine arginine, a potent immunomodulatory peptide isolated and structurally characterized from the skin of the Northern Leopard frog, Rana pipiens. J. Biol. Chem. 2001, 276, 10145–10152. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.; Irvine, A.E.; McClean, S.; Richter, S.C.; Flatt, P.R.; Shaw, C. Peptide Tyrosine Arginine, a potent immunomodulatory peptide isolated and structurally characterized from the skin secretions of the dusky gopher frog, Rana sevosa. Peptide 2005, 26, 737–743. [Google Scholar] [CrossRef]
- Song, G.; Zhou, M.; Chen, W.; Chen, T.; Walker, B.; Shaw, C. HV-BBI—A novel amphibian skin Bowman–Birk-like trypsin inhibitor. Biochem. Biophys. Res. Commun. 2008, 372, 191–196. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Chen, T.; Walker, B.; Zhou, M.; Sui, D.; Conlon, J.M.; Shaw, C. Identification and molecular cloning of a novel amphibian Bowman Birk-type trypsin inhibitor from the skin of the Hejiang Odorous Frog; Odorrana hejiangensis. Peptide 2012, 33, 245–250. [Google Scholar] [CrossRef]
- Wu, Y.; Long, Q.; Xu, Y.; Guo, S.; Chen, T.; Wang, L.; Zhou, M.; Zhang, Y.; Shaw, C.; Walker, B. A structural and functional analogue of a Bowman–Birk-type protease inhibitor from Odorrana schmackeri. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.; Wu, Y.; Zhou, M.; Ma, C.; Xi, X.; Chen, T.; Walker, B.; Shaw, C.; Wang, L. A Bowman-Birk type chymotrypsin inhibitor peptide from the amphibian, Hylarana erythraea. Sci. Rep. 2018, 8, 5851. [Google Scholar] [CrossRef] [PubMed]
- Lyu, P.; Ge, L.; Ma, R.; Wei, R.; McCrudden, C.M.; Chen, T.; Shaw, C.; Kwok, H.F. Identification and pharmaceutical evaluation of novel frog skin-derived serine proteinase inhibitor peptide-PE-BBI (Pelophylax esculentus Bowman-Birk inhibitor) for the potential treatment of cancer. Sci. Rep. 2018, 8, 14502. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, C.; Xu, X.; Wang, J.; Yu, H.; Lai, R.; Gong, W. Trypsin inhibitory loop is an excellent lead structure to design serine protease inhibitors and antimicrobial peptides. FASEB J. 2007, 21, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, H.; Yang, X.; Che, Q.; Liu, R.; Yang, H.; Liu, X.; You, D.; Wang, A.; Li, J.; et al. Bi-functional peptides with both trypsin-inhibitory and antimicrobial activities are frequent defensive molecules in Ranidae amphibian skins. Amino Acids 2012, 43, 309–316. [Google Scholar] [CrossRef]
- Tyler, M.J.; Stone, D.J.; Bowie, J.H. A novel method for the release and collection of dermal, glandular secretions from the skin of frogs. J. Pharmacol. Toxicol. Methods 1992, 28, 199–200. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, D.; Wang, L.; Lin, C.; Ma, C.; Xi, X.; Zhou, M.; Duan, J.; Bininda-Emonds, O.R.P.; Chen, T.; et al. Targeted Modification of a Novel Amphibian Antimicrobial Peptide from Phyllomedusa tarsius to Enhance Its Activity against MRSA and Microbial Biofilm. Front. Microbiol. 2017, 8, 951. [Google Scholar] [CrossRef]
- Wu, D.; Gao, Y.; Wang, L.; Xi, X.; Wu, Y.; Zhou, M.; Zhang, Y.; Ma, C.; Chen, T.; Shaw, C. A Combined Molecular Cloning and Mass Spectrometric Method to Identify, Characterize, and Design Frenatin Peptides from the Skin Secretion of Litoria infrafrenata. Mol. 2016, 21, 1429. [Google Scholar] [CrossRef]
- Brooks, H.; LeBleu, B.; Vivès, E. Tat peptide-mediated cellular delivery: Back to basics. Adv. Drug Deliv. Rev. 2005, 57, 559–577. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Wu, Y.; Wang, L.; Ma, C.; Xi, X.; Bininda-Emonds, O.R.; Shaw, C.; Chen, T.; Zhou, M. Evaluation of the bioactivity of a mastoparan peptide from wasp venom and of its analogues designed through targeted engineering. Int. J. Boil. Sci. 2018, 14, 599–607. [Google Scholar] [CrossRef]
- Zhu, H.; Ding, X.; Li, W.; Lu, T.; Ma, C.; Xi, X.; Wang, L.; Zhou, M.; Burden, R.; Chen, T.; et al. Discovery of two skin-derived dermaseptins and design of a TAT-fusion analogue with broad-spectrum antimicrobial activity and low cytotoxicity on healthy cells. PeerJ 2018, 6, e5635. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hang, H.; Chen, T.; Zhou, M.; Wang, L.; Shaw, C. pLR-HL: A Novel Amphibian Bowman-Birk-type Trypsin Inhibitor from the Skin Secretion of the Broad-folded Frog, Hylarana latouchii. Chem. Biol. Drug Des. 2016, 87, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.-H.; Goto, Y.; Réfrégiers, M.; Kardos, J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095–E3103. [Google Scholar] [CrossRef] [PubMed]
- Anandalakshmi, V.; Murugan, E.; Leng, E.G.T.; Ting, L.W.; Chaurasia, S.S.; Yamazaki, T.; Nagashima, T.; George, B.L.; Peh, G.S.L.; Pervushin, K.; et al. Effect of position-specific single-point mutations and biophysical characterization of amyloidogenic peptide fragments identified from lattice corneal dystrophy patients. Biochem. J. 2017, 474, 1705–1725. [Google Scholar] [CrossRef] [PubMed]
- Mulvenna, J.P.; Foley, F.M.; Craik, D.J. Discovery, Structural Determination, and Putative Processing of the Precursor Protein That Produces the Cyclic Trypsin Inhibitor Sunflower Trypsin Inhibitor 1. J. Boil. Chem. 2005, 280, 32245–32253. [Google Scholar] [CrossRef]
- Eiríksdóttir, E.; Konate, K.; Langel, Ü.; Divita, G.; Deshayes, S. Secondary structure of cell-penetrating peptides controls membrane interaction and insertion. Biochim. Biophys. Acta (BBA) Biomembr. 2010, 1798, 1119–1128. [Google Scholar]
- McBride, J.D.; Leatherbarrow, R.J. Synthetic peptide mimics of the Bowman-Birk inhibitor protein. Curr. Med. Chem. 2001, 8, 909–917. [Google Scholar] [CrossRef]
- Scarpi, D.; McBride, J.D.; Leatherbarrow, R.J. Inhibition of human beta-tryptase by Bowman-Birk inhibitor derived peptides: Creation of a new tri-functional inhibitor. Bioorg. Med. Chem. 2004, 12, 6045–6052. [Google Scholar] [CrossRef]
- Pereira, P.J.; Bergner, A.; Macedo-Ribeiro, S.; Huber, R.; Matschiner, G.; Fritz, H.; Sommerhoff, C.P.; Bode, W. Human beta-tryptase is a ring-like tetramer with active sites facing a central pore. Nature 1998, 392, 306–311. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Papo, N.; Mignogna, G.; Andreu, D.; Shai, Y.; Barra, D.; Simmaco, M. Ranacyclins, a New Family of Short Cyclic Antimicrobial Peptides: Biological Function, Mode of Action, and Parameters Involved in Target Specificity. Biochemistry 2003, 42, 14023–14035. [Google Scholar] [CrossRef]
- Yu, H.; Wang, C.; Feng, L.; Cai, S.; Liu, X.; Qiao, X.; Shi, N.; Wang, H.; Wang, Y. Cathelicidin-trypsin inhibitor loop conjugate represents a promising antibiotic candidate with protease stability. Sci. Rep. 2017, 7, 2600. [Google Scholar] [CrossRef] [PubMed]
- Chilosi, G.; Caruso, C.; Caporale, C.; Leonardi, L.; Bertini, L.; Buzi, A.; Nobile, M.; Buonocore, V.; Magro, P. Antifungal Activity of a Bowman-Birk-type Trypsin Inhibitor from Wheat Kernel. J. Phytopathol. 2000, 148, 477–481. [Google Scholar] [CrossRef]
- Roccatano, D.; Colombo, G.; Fioroni, M.; Mark, A.E. Mechanism by which 2,2,2-trifluoroethanol/water mixtures stabilize secondary-structure formation in peptides: A molecular dynamics study. Proc. Natl. Acad. Sci. USA 2002, 99, 12179–12184. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Uemura, S.; Mihara, H. Fabrication of Nanofibers with Uniform Morphology by Self-Assembly of Designed Peptides. Chem. A Eur. J. 2004, 10, 2789–2794. [Google Scholar] [CrossRef] [PubMed]

| Peptide Name | Sequence | Positive Charge |
|---|---|---|
| PPF-BBI | ALRGCWTKSIPPKPCP-amide | +4 |
| F8-PPF-BBI | ALRGCWTKSIPPKPCP-amide | +3 |
| K16-PPF-BBI | ALRGCWTKSIPPKPCK-amide | +5 |
| Tat-loop | RKKRRQRRRCWTKSIPPKPC | +10 |
| Peptide | Name | Ki (µM) of Trypsin | Ki (µM) of Tryptase | Ki (µM) of Chymotrypsin |
|---|---|---|---|---|
| ALRGCWTKSIPPKPCP-amide | PPF-BBI | 0.17 | 30.73 | N.I.* |
| ALRGCWTKSIPPKPCP-amide | F8-PPF-BBI | N.I.* | N.I.* | 0.85 |
| ALRGCWTKSIPPKPCK-amide | K16-PPF-BBI | 0.112 | 9.67 | N.I.* |
| RKKRRQRRRCWTKSIPPKPC | Tat-loop | 0.607 | N.I.* | N.I.* |
| CWTKSIPPKPC | TIL | 0.741 | N.I.* | N.I.* |
| Microorganisms | MIC/MBC (µM) | |||||
|---|---|---|---|---|---|---|
| PPF-BBI | K16-PPF-BBI | F8-PPF-BBI | Tat | Tat-loop | TIL | |
| S. aureus | 128/128 | 64/64 | >512 | 512/512 | 128/128 | >512 |
| E. coli | 128/128 | 128/128 | >512 | 256/256 | 128/128 | >512 |
| C. albicans | 512/512 | 128/128 | >512 | >512 | 4/8 | >512 |
| MRSA | >512 | 512/512 | >512 | >512 | 256/512 | >512 |
| P. aeruginosa | >512 | 512/512 | >512 | >512 | 256/256 | >512 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, Y.; Chen, G.; Xi, X.; Ma, C.; Wang, L.; Burrows, J.F.; Duan, J.; Zhou, M.; Chen, T. Discovery and Rational Design of a Novel Bowman-Birk Related Protease Inhibitor. Biomolecules 2019, 9, 280. https://doi.org/10.3390/biom9070280
Miao Y, Chen G, Xi X, Ma C, Wang L, Burrows JF, Duan J, Zhou M, Chen T. Discovery and Rational Design of a Novel Bowman-Birk Related Protease Inhibitor. Biomolecules. 2019; 9(7):280. https://doi.org/10.3390/biom9070280
Chicago/Turabian StyleMiao, Yuxi, Guanzhu Chen, Xinping Xi, Chengbang Ma, Lei Wang, James F. Burrows, Jinao Duan, Mei Zhou, and Tianbao Chen. 2019. "Discovery and Rational Design of a Novel Bowman-Birk Related Protease Inhibitor" Biomolecules 9, no. 7: 280. https://doi.org/10.3390/biom9070280
APA StyleMiao, Y., Chen, G., Xi, X., Ma, C., Wang, L., Burrows, J. F., Duan, J., Zhou, M., & Chen, T. (2019). Discovery and Rational Design of a Novel Bowman-Birk Related Protease Inhibitor. Biomolecules, 9(7), 280. https://doi.org/10.3390/biom9070280